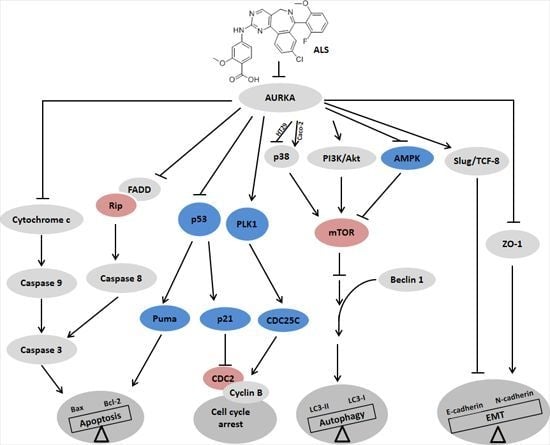
Alisertib Induces Cell Cycle Arrest, Apoptosis, Autophagy and Suppresses EMT in HT29 and Caco-2 Cells
Abstract
:1. Introduction
2. Results
2.1. Alisertib (ALS) Inhibits the Proliferation of HT29 and Caco-2 Cells
2.2. Overview of Proteomic Response to ALS Treatment in HT29 and Caco-2 Cells
| ID | Molecules in Network | Score | Focus Molecules | Top Diseases and Functions |
|---|---|---|---|---|
| 1 | 60S ribosomal ubunit, DDX1, DDX5, DDX17, DDX21, DDX3X, DHX9, EIF3E, HDGF, HNRNPU, ILF2, ILF3, KPNA6, LYAR, MTDH, NEDD8, RAD23B, RBM39, Rnr, RPL3, RPL5, RPL8, RPL9, RPL10, RPL14, RPL18, RPL24, RPL28, RPL30, RPL27A, RPLP0, RPS2, RPS15A, Vegf, XRCC5 | 52 | 32 | Protein synthesis, infectious disease, gene expression |
| 2 | AHSA1, ALYREF, CAND1, ERK1/2, FUBP1, GIGYF2, HNRNPK, HNRNPL, LARS, LOC102724594/U2AF1, MARS, NOLC1, NOP56, NOP58, OLA1, PABPC1, PCBP1, PCBP2, PDE6H, Pki, PTBP1, PUF60, QARS, RALY, RNA polymerase I, SF3B1, SF3B2, Srp30, SRSF1, SRSF2, SRSF3, Tap, TOP1, U2af, U2AF2 | 44 | 29 | RNA post-transcriptional modification, protein synthesis, cancer |
| 3 | CALU, CLIC4, creatine kinase, CUTA, EFTUD2, FUS, Histone H1, HNRNPC, HNRNPD, HNRNPF, HNRNPH1, HNRNPM, HNRNPUL1, Importin α, Importin β, IPO5, IPO7, P38 MAPK, PQBP1, PRMT1, PRMT5, PRPF19, PTMA, PYCR1, RAN, RANBP1, SF3B4, snRNP, SNRPD3, SYNC, TNPO1, Transportin, TRAP/Media, UBA2, WDR1 | 40 | 27 | RNA post-transcriptional modification, amino acid metabolism, post-translational modification |
| 4 | AGR2, Akt, ANXA4, Calcineurin A, CALR, Collagen α1, Collagen type VI, COP I, COPA, COPB1, COPG1, Cytoplasmic Dynein, DSTN, DYNC1LI2, FKBP4, Mre11, PA2G4, PDIA3, PDIA4, PDIA6, peptidylprolyl isomerase, peroxidase (miscellaneous), PPIA, PPIB, PPT1, PRDX1, PRDX2, PRDX4, PRDX5, PRDX6, RRBP1, SAE1, TMED9, TXNDC5, ZYX | 38 | 26 | Cancer, endocrine system disorders, organismal injury and abnormalities |
| 5 | ACTN1, ACTN4, ANP32B, ASPH, ATP synthase, C1QBP, CAMK2D, CaMKII, COX5A, CSRP1, CTNNA1, Cytochrome bc1, cytochrome C, ERP44, Filamin, ITPR, KLK3, LONP1, MAP4, Mitochondrial complex 1, MRPL12, MT-CO1, MT-CO2, MT-CYB, NDUFA5, Pde, PDS5A, PDXDC1, PHB, PHB2, Pka, PPP2R1A, PRDX3, PYGB, STMN1 | 38 | 26 | Drug metabolism, lipid metabolism, small molecule biochemistry |
| 6 | 19S proteasome, 20s proteasome, ADRM1, BUB3, COTL1, CPNE1, DAD1, FLII, Immunoproteasome Pa28/20s, MHC CLASS I (family), NFkB (complex), OTUB1, Proteasome PA700/20s, PSMA, PSMA4, PSMA7, PSMB5, PSMC2, PSMD, PSMD2, PSMD6, PSMD8, PSMD14, PSME1, PSME2, RNF13, S100, S100A2, S100P, UBA1, UBE2, UBE2L3, UBE2N, USMG5, USP14 | 35 | 25 | Cellular movement, protein degradation, protein synthesis |
| 7 | adenosine-tetraphosphatase, α tubulin, ATP5H, ATP5J, ATP5J2, β Tubulin, BZW1, BZW2, CCT4, CCT5, CCT8, CCT6A, DCTN1, Dynein, Ephb, ERP29, F0 ATP synthase, FAM162A, H2AFV, KDELR, LGALS3BP, MAPK1, OGFR, PGAM1, SAR1A, Sec23, TBCA, TSPO, TUBA4A, TUBB6, tubulin (family), VBP1, Vdac, VDAC1, VDAC2 | 35 | 25 | Cellular assembly and organization, cell-to-cell signaling and interaction, reproductive system development and function |
| 8 | CD9, chymotrypsin, CTSH, EIF1, EIF3, EIF3A, EIF3B, EIF3F, EIF3G, EIF3J, EIF3L, EIF4B, Eif4g, Erm, EZR, G6PD, GLRX3, GPIIB-IIIA, IPO9, Lamin b, LBR, Lh, PEBP1, Pkc(s), Pkg, PLCB3, PLIN3, PSIP1, RAB1A, RBM3, Rho gdi, SERPINB1, SLC12A2, TLN1, VAT1 | 35 | 25 | Gene expression, protein synthesis, cellular growth and proliferation |
| 9 | ABCB8, Aconitase, Adaptor protein 1, Aldose Reductase, Angiotensin II receptor type 1, AP1B1, Arf, ARF1, ARF4, ARF5, ARL1, COPB2, CRIP2, cytochrome-c oxidase, DAB2IP, DHCR24, Gef, glutathione peroxidase, glutathione transferase, GST, GSTK1, GSTO1, GSTP1, Jnk, MGST1, PARK7, RAB1B, RPN2, SFXN1, SLC25A3, SLC25A11, SOD2, SRP54, SSR3, USO1 | 33 | 24 | Drug metabolism, protein synthesis, DNA replication, recombination, and repair |
| 10 | ABCC1, AK2, ALDH2, ANXA3, ATPase, atypical protein kinase C, CAPG, Caveolin, CLTA, DBNL, DNPEP, Dynamin, ETFA, ETFB, GANAB, IL-2R, Integrin α 3 β 1, MTCH2, NSF, NUDC, OSBP, PAK2, PCMT1, PHGDH, PI3K (complex), Ptk, Raf, RAP1B, SEPT11, SHMT2, SLC3A2, TPP2, trypsin, VAPA, Vla-4 | 33 | 24 | Developmental disorder, hereditary disorder, metabolic disease |
| 11 | ALDOA, AP2B1, C11orf54, CAD, CBX3, CYP, DHCR7, DPP3, Focal adhesion kinase, GGCT, Histone h4, HMGCS1, IDI1, Ldh (complex), LDHA, LDHB, MAT2A, NPC2, NQO1, PI3K (family), POR, Rar, RBBP4, RBP2, RPN1, Rxr, SLC16A1, Sod, Sos, STAT5a/b, TGM2, thymidine kinase, TPP1, TXNRD1, UGDH | 33 | 24 | DNA replication, recombination, and repair, energy production, nucleic acid metabolism |
| 12 | ACAA1, APEX1, BTF3, BTF3L4, Cbp/p300, CPSF6, Cyclin A, Cyclin D, Cyclin E, E2f, EDF1, EEF2, EPCAM, FEN1, Hat, HAT1, Holo RNA polymerase II, HSD17B4, Ku, Mcm, MCM3, MCM7, MTHFD1, NASP, NONO, PCNA, POLβ-POLepsilon-POLγ-XRCC1-LIGI-PARP1-PCNA-FEN1, POLR2H, PRKDC, RAD50, Ras, Rb, SSRP1, TIP60, XRCC6 | 29 | 22 | DNA replication, recombination, and repair, cellular response to therapeutics, cell morphology |
| 13 | ANXA5, BCR (complex), CAPZA2, CAPZB, caspase, CDC37, DENR, EIF4G1, FAS, FLNA, FLNB, Hsp27, HSPB1, IFIH1, JINK1/2, Lamin, LMNB1, MAP2K1/2, MCTS1, Mlc, MTORC2, MTPN, Pak, PARP, PARP1, PDCD6, PLEC, PP2A, PYGL, RHOA, Rsk, RTN3, RTN4, Sapk, SRP72 | 29 | 22 | Cellular compromise, cell morphology, cellular movement |
| 14 | ACTG1, ATP5C1, Calmodulin, Caspase 3/7, CD3, CD44, CSTF2, DDX19A, ECH1, EEF1D, Eif2, HNRNPR, HSPA5, Ifn γ, LMNA, MYH9, PGK1, PPM1G, Proinsulin, RAB10, RAB8A, Ribosomal 40s subunit, RPS7, RPS24, RPS3A, RPSA, Secretase γ, SRPRB, TCF, TCR, Tgf β, TSTA3, TUBB, tubulin (complex), tyrosine kinase | 29 | 22 | Hematological disease, immunological disease, inflammatory disease |
| 15 | 26s Proteasome, AMPK, CD59, CS, CTBP1, CTBP2, DDB1, GFPT1, HDL, hemoglobin, HISTONE, Hsp70, Hsp90, HUWE1, IDH3A, IL1, LMAN1, MIF, NADPH oxidase, Nos, NSUN2, PRKAA, Pro-inflammatory Cytokine, PTGES3, Ras homolog, SDHA, SGTA, SSBP1, succinate dehydrogenase, SURF4, TMED2, TRAP1, Ubiquitin, UBXN1, UCHL3 | 25 | 20 | Cellular assembly and organization, cellular function and maintenance, cellular development |
| 16 | ABCE1, ACLY, Ap1, ATP1A1, Calcineurin protein(s), CK1, Ck2, Gsk3, HMBS, HMGA1, HMGB1, HMGB2, HMGB3, HNRNPAB, MEF2, Mek, MVP, NAP1L1, NFAT (complex), Nfat (family), NMDA Receptor, NUDT5, p70 S6k, PDAP1, Pdgf (complex), phosphatase, PICALM, PP1 protein complex group, PP1-C, PPP1R7, Ppp2c, STARD10, TECR, WARS, XPO1 | 24 | 19 | DNA replication, recombination, and repair, gene expression, nucleic acid metabolism |
| 17 | ACTB, Actin, ACTR2, aldo, α actin, α Actinin, α catenin, API5, Arp2/3, ARPC2, ARPC3, Cadherin, CAP1, CLIC1, CNN2, Cofilin, DIAPH1, DPYSL2, ERK, F Actin, FCGR1A/2A/3A, G-Actin, GOT1, LASP1, LTA4H, MYH14, Profilin, Rock, Talin, TPM3, TPM4, Tropomyosin, Troponin t, TWF1, VASP | 22 | 18 | Cellular assembly and organization, cellular function and maintenance, cell morphology |
| 18 | Adaptor protein 2, AHR, AKR1A1, APP, ATIC, BIN3, BOLA2/BOLA2B, C11orf54, C14orf93, Clathrin, CORO1C, CPN2, CUL3, DDX55, DNAAF2, ERH, EWSR1, FAM98B, GCSH, HEATR5A, MYC, NLE1, NUDT21, OARD1, OLFML2A, PAICS, RAB7A, SLC25A1, SRSF10, TKT, TMEM183A, TPGS2, UBC, UBL4A, UGT1A9 (includes others) | 22 | 19 | Cell cycle, hepatic system development and function, cell morphology |
| 19 | ACY1, APMAP, APRT, BBS7, BCKDK, C21orf33/LOC102724023, CARHSP1, CLN5, CMPK1, CNN3, CNPY2, CRYZ, CS, EFHD2, FAM98B, GLS, IARS2, KIF20A, MARCH8, MDH1, MTAP, MYLIP, NME3, NME4, NME7, PADI2, PGM3, RAB6B, RABGAP1, RASA4, REXO4, SCT, SLC25A22, TTC1, UBC | 20 | 20 | Nucleic acid metabolism, small molecule biochemistry, cell-to-cell signaling and interaction |
| 20 | ACSL3, ADCY, ADRB, Alp, ANP32E, ANXA2, Creb, CTNNB1, DPY30, estrogen receptor, G protein, G protein α, G protein β γ, GTPase, Hdac, Histone h3, HSPA9, Insulin, MATR3, Mmp, NOMO1 (includes others), p85 (pik3r), Pdgfr, PLC, PMM2, RCC2, RNA polymerase II, SFPQ, Shc, SRC (family), STIP1, SUMO2, TOP2A, WDR12, WDR36 | 18 | 16 | Cell cycle, hair and skin development and function, cancer |
| 21 | APPBP2, ARIH2, ARL4D, CD70, CDV3, COQ6, EDNRA, ELF4, EML4, GALE, GMDS, H32, HKDC1, ISOC2, KIF6, LSM8, MAGEA11, NME4, NME1-NME2, NUP210L, OARD1, PCSK5, PODXL, RBM47, RRS1, SCFD1, SELL, SYT11, TBL3, TCIRG1, TNKS, TRMT1, UBC, YBX2, ZCCHC12 | 15 | 15 | Cell-to-cell signaling and interaction, hematological system development and function, immune cell trafficking |
| 22 | AAMP, ANXA1, calpain, Casein, Collagen type I, Collagen type III, Collagen type IV, Collagen(s), COMT, Cpla2, DDAH1, Fc γ receptor, Fibrin, Fibrinogen, GADD45, Growth hormone, Integrin, Laminin, LAMP1, LDL, Mac1, Mapk, MCFD2, NAMPT, PDGF BB, PLCE1, Pld, Rap1, SEC13, SERPINH1, SLIRP, SYK, thyroid hormone receptor, TSH, VAV | 12 | 12 | Lipid metabolism, molecular transport, small molecule biochemistry |
| 23 | AChR, ALDOC, FABP5, Fcer1, Gm-csf, GOT, HINT1, HLA-DR, HSP, Ifn, IFN β, Ige, IgG, IgG1, IgG2a, Igm, Ikb, Ikk (family), IL12 (complex), IL12 (family), Immunoglobulin, Interferon α, mediator, MHC Class I (complex), MHC Class II (complex), NACA, NPEPPS, OGDH, PI3K p85, PLA2, PLC γ, PRKRA, Rac, Tlr, TXLNA | 7 | 8 | Cancer, hematological disease, immunological disease |
| 24 | ACPP, ACTR2, ARHGAP1, CBR1, CBR3, CDC42, CDC42EP4, Cg, chemokine, CLDN11, COL15A1, collagen, DHCR7, Endothelin, EPHA3, FCGR1A/2A/3A, FSH, GNRH, GNRH2, GNRHR, HSD3B1, Hsd3b4 (includes others), IKK (complex), LIMK2, Metalloprotease, MTORC1, MYO5B, NPC1L1, PAPPA, PEPD, PHKA2, RAB11A, Tnf (family), TNFRSF6B, UCN2 | 4 | 8 | Endocrine system development and function, lipid metabolism, small molecule biochemistry |
| ID | Molecules in Network | Score | Focus Molecules | Top Diseases and Functions |
|---|---|---|---|---|
| 1 | 60S ribosomal subunit, AARS, AHSA1, AIMP1, C11orf58, DARS, EEF1A1, EEF1B2, EEF1D, EEF1G, EPRS, ERK1/2, GARS, HARS, KARS, MARS, OLA1, PDE6H, PDGF (family), Pki, QARS, RPL8, RPL10, RPL18, RPL21, RPL23, RPL27, RPL30, RPL32, RPL10A, RPL13A, RPLP0, RPLP1, RPS3A, VARS | 48 | 31 | Protein synthesis, gene expression, RNA post-transcriptional modification |
| 2 | ANXA3, APEX1, BTF3, Cbp/p300, CDH17, CTNNB1, CYB5R3, ESD, FEN1, GANAB, GOLPH3, GST, GSTO1, HDLBP, HIST2H2AC, Holo RNA polymerase II, LMNA, NACA, OAT, PCNA, PGAM1, PRKCSH, PRKDC, RUVBL1, SERBP1, SLC38A2, SSRP1, SUPT16H, TAGLN2, TCF/LEF, thymidine kinase, TMPO, XRCC5, XRCC6, YBX3 | 45 | 30 | DNA replication, recombination, and repair, cellular response to therapeutics, cell morphology |
| 3 | DDOST, DDX17, FUBP1, FUS, hnRNP H, HNRNPA1, HNRNPDL, HNRNPF, HNRNPH1, HNRNPK, HNRNPL, HNRNPR, HNRNPU, IGF2BP3, Karyopherin β, KHSRP, Mapk, MATR3, NONO, PCBP1, PCBP2, PDGF-AA, PSPC1, PTBP1, PUF60, RAN, RAN-GTP, RANGAP1, RBM14, SF3A3, SFPQ, SYNCRIP, TNPO1, Transportin, YBX1 | 43 | 29 | RNA post-transcriptional modification, protein synthesis, DNA replication, recombination, and repair |
| 4 | 14-3-3 (β, ε, ζ), CALU, CAPZA1, CAPZB, caspase, CLTC, DLAT, FLNA, GNB2L1, HMBS, HNRNPM, Hsp27, Hsp90, HSP90AA1, HSP90AB1, HSPA8, NCL, NPM1, NUDT21, NUMA1, p85 (pik3r), PCMT1, PKM, PLEC, RPL12, RPL22, SFMBT2, SPTBN1, TRIM28, tubulin (complex), UBXN1, VPS35, YWHAB, YWHAE, YWHAH | 43 | 29 | Cancer, gastrointestinal disease, hepatic system disease |
| 5 | 14-3-3(β, γ, θ, η, ζ), AChR, ACTR3, ALDOC, ALYREF, ATP5A1, Calmodulin, DDX39B, DYNC1H1, EPCAM, F1 ATPase, FABP5, GTPase, IARS2, IMMT, IQGAP1, KIF5B, LRPPRC, MARCKS, mediator, MYH9, Pde, RPL3, RPL4, RPL7, RPLP2, RTN4, SARNP, SLC25A3, TPD52L2, TPI1, TUFM, YWHAG, YWHAQ, ZFC3H1 | 40 | 28 | Metabolic disease, molecular transport, RNA trafficking |
| 6 | 14-3-3(η, θ, ζ), Cytokeratin, DSP, EIF3, EIF2S2, EIF3A, EIF3B, EIF3C, EIF3E, EIF3F, EIF3I, EIF3M, EIF4A, EIF4A1, EIF4A3, EIF4B, EIF4F, Eif4g, EIF4G1, EIF4G2, EIF4H, GPI, KRT1, KRT2, KRT8, KRT9, KRT14, KRT18, KRT19, p70 S6k, PABPC1, PI3K (complex), PKP2, PNN, YWHAZ | 38 | 27 | Gene expression, protein synthesis, cellular assembly and organization |
| 7 | 3-hydroxyacyl-CoA dehydrogenase, ACAA1, ACAA2, ACAT1, ACAT2, acetyl-CoA C-acetyltransferase, acetyl-CoA C-acyltransferase, CD99, CNBP, DDX21, DHX9, EDC4, FBL, GLUD1, GSR, H2AFY, HADH, HADHA, HDGF, HNRNPA3, HSD17B10, LOC102724594/U2AF1, NFkB (complex), OTUB1, PDLIM1, peptidase, PPARα-RXRα, RBM39, RCC2, SRSF1, Tap, TOP1, U2af, U2AF2, UBE2 | 36 | 26 | Renal damage, renal tubule injury, endocrine system development and function |
| 8 | Adaptor protein 1, α tubulin, Ap1 γ, AP1B1, AP1G1, β Tubulin, CCT4, CCT5, CCT8, CCT6A, CNN3, CS, DPYSL2, Dynein, EHD1, ERP29, ETFB, FH, Integrin α 5 β 1, LCP1, malate dehydrogenase, MAPK1, MDH1, MDH2, TKT, TUBA1B, TUBB6, TUBB8, TUBB2B, TUBB4B, tubulin (family), Vdac, VDAC1, VDAC2, WARS | 36 | 26 | Cancer, hematological disease, immunological disease |
| 9 | adenosine-tetraphosphatase, ATP synthase, ATP5B, ATP5H, collagen, Collagen α1, Collagen type III, Cytoplasmic Dynein, DCTN2, DHX15, EFTUD2, FKBP4, HNMT, HNRNPA2B1, MAPRE1, P38 MAPK, PA2G4, peroxidase (miscellaneous), PGK1, PPIA, PRDX1, PRDX2, PRDX6, PRPF8, PRPF19, RRBP1, SERPINH1, SF3B1, SF3B2, SND1, snRNP, SNRPD1, STIP1, TARS, VIL1 | 36 | 26 | RNA post-transcriptional modification, free radical scavenging, small molecule biochemistry |
| 10 | ACLY, ALDH1A1, ANXA5, CK1, CYB5B, DHCR7, DNAJA2, ECHS1, FASN, Focal adhesion kinase, HMGCS1, HSP, Hsp70, HSPA4, HSPA9, HSPB1, HSPE1, HSPH1, IDI1, JINK1/2, MHC Class II (complex), PGD, Pias, POR, PPA1, PSAP, SLC25A6, Srebp, ST13, TOMM22, TOMM40, UBA1, UBE2L3, Ubiquitin, UGDH | 36 | 26 | Cell cycle, endocrine system development and function, lipid metabolism |
| 11 | ADK, Akt, ARHGDIA, atypical protein kinase C, CADM1, CAPRIN1, CPNE1, EEF2, Fascin, GDI2, HN1, ILF2, ILF3, LRRC47, Mcm, MCM2, MCM3, MCM4, MCM6, N-Cadherin, Pak, PLIN3, PPP2R1B, Rab5, Rab11, RAB11A, RAB2A, RAB7A, RDX, Rho gdi, RNH1, RPA, SEC13, TWF2, VAT1 | 34 | 25 | DNA replication, recombination, and repair, cell signaling, post-translational modification |
| 12 | ADH5, API5, Arp2/3, ARPC2, ARPC5, CLIC1, CNN2, DDX1, Eif2, ERK, GPIIB-IIIA, HNRNPH3, IGF2BP1, Ku, LAMB1, Laminin1, LAP3, Profilin, Ribosomal 40s subunit, Rnr, RPS2, RPS7, RPS8, RPS10, RPS12, RPS14, RPS15, RPS24, RPS27A, RPS4X, RPSA, RTCB, Talin, TLN1, VCL | 34 | 25 | Developmental disorder, hematological disease, hereditary disorder |
| 13 | Arf, ARF1, ARHGAP1, B2M, B2m-Mhc1a, CALR, CANX, CCAR2, CCDC47, Cd1, CD1D-CANX-CALR-ERp57, COP I, COPA, COPB1, COPE, COPG1, DNAJ, DNAJA1, DNAJC8, HLA-B27, Hsp22/Hsp40/Hsp90, HSP90B1, HSPA5, HYOU1, Jnk, LMAN1, MHC Class I (complex), P4HB, PDIA3, PDIA4, PDIA6, PRDX4, RAB1B, RPN1, TXNDC5 | 34 | 25 | Post-translational modification, protein folding, developmental disorder |
| 14 | ACO2, Aconitase, AHCY, AKR1C3, AKR1C1/AKR1C2, Aldose Reductase, CACYBP, chymotrypsin, COMT, ENO1, ERP44, ETF1, Filamin, FLNB, FLNC, G6PD, GADD45, GCN1L1, glutathione peroxidase, Lamin b, LONP1, LRRC59, ME1, N-cor, PARK7, PEBP1, PFKP, Pkc(s), PTGES3, Rar, RPL7A, SEPT2, T3-TR-RXR, TALDO1, thyroid hormone receptor | 30 | 23 | Nucleic acid metabolism, small molecule biochemistry, cellular movement |
| 15 | 14-3-3, APC/APC2, ATIC, CBX3, CHD4, CLIC4, CSE1L, GART, H3F3A/H3F3B, HIST1H1C, Histone H1, Histone h3, Histone h4, IDH1, IL-2R, Importin α, Importin β, IPO5, IPO7, KPNA2, KPNA3, KPNB1, Mucin, NuRD, NUTF2, PAICS, PHGDH, PTMA, RANBP1, SAE1, SNRPD3, SSB, TIP60, Vegf, WDR1 | 30 | 23 | Molecular transport, protein trafficking, amino acid metabolism |
| 16 | ACTA1, ACTG1, Actin, ACTN4, ACTR2, aldo, α actin, α Actinin, BASP1, Cadherin, CAP1, CFL1, CFL2, CKB, Cofilin, CORO1C, COTL1, DBN1, F Actin, G-Actin, LMO7, MYH10, MYL12A, Myosin2, NAPA, NWASP, PAFAH1B2, PDCD6IP, Pka, PLS3, TMOD3, TPM3, TPM4, Tropomyosin, TWF1 | 30 | 23 | Cellular assembly and organization, cellular function and maintenance, developmental disorder |
| 17 | 19S proteasome, 20s proteasome, 26s Proteasome, ATP5C1, ATPase, CCT3, CCT7, Cyclin E, Immunoproteasome Pa28/20s, LRP, MHC CLASS I (family), NSFL1C, Proteasome PA700/20s, PSMA, PSMA1, PSMA2, PSMA4, PSMA7, PSMB1, PSMC4, PSMD, PSMD1, PSMD2, PSMD3, PSME1, PSME2, Rac, SLC3A2, SOD1, SQSTM1, TUBB, UBE2N, USP5, VAPA, VCP | 30 | 23 | Developmental disorder, hereditary disorder, inflammatory disease |
| 18 | ACTN1, α catenin, ANXA2, ATP6V1A, C1QBP, CAPNS1, Caveolin, CDHE/CDHN, CSRP1, CTNNA1, CTNND1, CTNNα-CTNNβ-CTNNδ, CYC1, Cyclin B, Cytochrome bc1, cytochrome C, cytochrome-c oxidase, DNM1L, Dynamin, EPPK1, ETFA, HSPD1, Il8r, JUP, MDK, Mitochondrial complex 1, PARP, PHB, PHB2, PRDX3, Ras homolog, SPTAN1, STOML2, UQCRC1, ZYX | 28 | 22 | Cellular movement, cell morphology, cellular function and maintenance |
| 19 | ALDOA, AP2B1, APOA1, ATP2A2, calpain, CAPN1, CAPN2, Casein, creatine kinase, DBI, DPP3, Fibrinogen, GC, Growth hormone, HBD, HDL, hemoglobin, HIST1H2BL, Ldh (complex), LDHA, LDHB, LDL, NAMPT, Nos, Nr1h, PF4, Pro-inflammatory Cytokine, QDPR, SAA, SERPINA1, Sod, SRC (family), TFRC, TGM2, VLDL-cholesterol | 22 | 19 | Cellular function and maintenance, carbohydrate metabolism, free radical scavenging |
| 20 | Alp, APLP2, BCAT1, CDH1, Collagen type I, Collagen type IV, Collagen(s), CTSB, CTTN, elastase, F11R, Fgf, Fgfr, Fibrin, FN1, GLG1, GOT, GOT1, GOT2, GPD2, Integrin, Laminin, LMNB1, MAT2A, Mmp, Notch, PDGF BB, Rap1, RPN2, Secretase γ, SERPINA3, SERPINB1, TAGLN, Tgf β, trypsin | 19 | 17 | Amino acid metabolism, small molecule biochemistry, dermatological diseases and conditions |
| 21 | ACTL8, AGPAT2, ANP32E, BROX, CDV3, CEP78, CHMP6, CHMP7, CHMP1B, CHMP2A, CHMP4A, CHMP4B, CMPK1, GMPS, HEBP1, HIST1H2AE, HSDL2, NAP1L2, NAP1L4, NARS, NME3, NME4, NME7, NME1-NME2, NPM3, PNPO, QPRT, RNPEP, TCEAL1, TSN, TTLL4, TUBAL3, UBC, USP54, ZBTB18 | 17 | 16 | Infectious disease, cell morphology, cellular assembly and organization |
| 22 | ATP1A1, ATP6V1B2, Calcineurin A, CCT2, Ck2, Dishevelled, EIF2S1, EIF5A, Gsk3, ITPR, MAP2K1/2, MIR124, Mlc, Pdgf (complex), PFN1, phosphatase, PICALM, Pkg, PP1 protein complex group, PP1-C, PP1/PP2A, PP2A, PPP1CA, Ppp2c, PPP2R4, PPP2R1A, PRKAA, Rb, RCN1, Rock, SET, SHMT2, Spectrin, STARD10, XPO1 | 16 | 15 | Molecular transport, RNA trafficking, hereditary disorder |
| 23 | ACKR1, ACSL3, AFP, ALDH18A1, AP4S1, APLNR, CCND1, CKAP4, DPY30, E2f, EMR2, EMR3, ENOPH1, FPR3, FSD1, γ tubulin, Gpcr, GPR15, GPR35, GPR137B, GPRC5C, Metalloprotease, MFSD1, miR-491-5p (and other miRNAs w/seed GUGGGGA), NAP1L1, NDC1, NUP155, PXN, RAB10, RXFP2, SSR4, STAT, STX2, TTLL4, UBC | 9 | 10 | Cell-to-cell signaling and interaction, cellular assembly and organization, cellular compromise |
| 24 | AHNAK, BCR (complex), Complement component 1, CXADR, DDX6, ENaC, EZR, Fcer1, GTF2I, HINT1, HLA-DR, Iga, Ige, IgG1, Igg3, Igm, Ikb, IMPDH2, JAK, KHDRBS1, MEF2, MIRLET7, NFAT (complex), Nfat (family), NFkB (family), PI3K (family), PI3K p85, PLC γ, Ptk, Raf, Ras, Rsk, Sapk, SYK/ZAP, TEC | 7 | 9 | Cancer, organismal injury and abnormalities, infectious disease |
| 25 | Adaptor protein 2, ADCY, ADRB, Ap1, BSG, Calcineurin protein(s), CaMKII, CDK1, Cg, Clathrin, Creb, DDX5, estrogen receptor, FSH, G protein, GFPT1, GML, GSTP1, IKK (complex), Insulin, Lh, MCTS1, MTORC1, NADPH oxidase, NMDA Receptor, Pdgfr, Pka catalytic subunit, PLC, Proinsulin, RNA polymerase II, Shc, SKP1, Sos, TCF, UCHL1 | 7 | 9 | Neurological disease, psychological disorders, skeletal and muscular disorders |
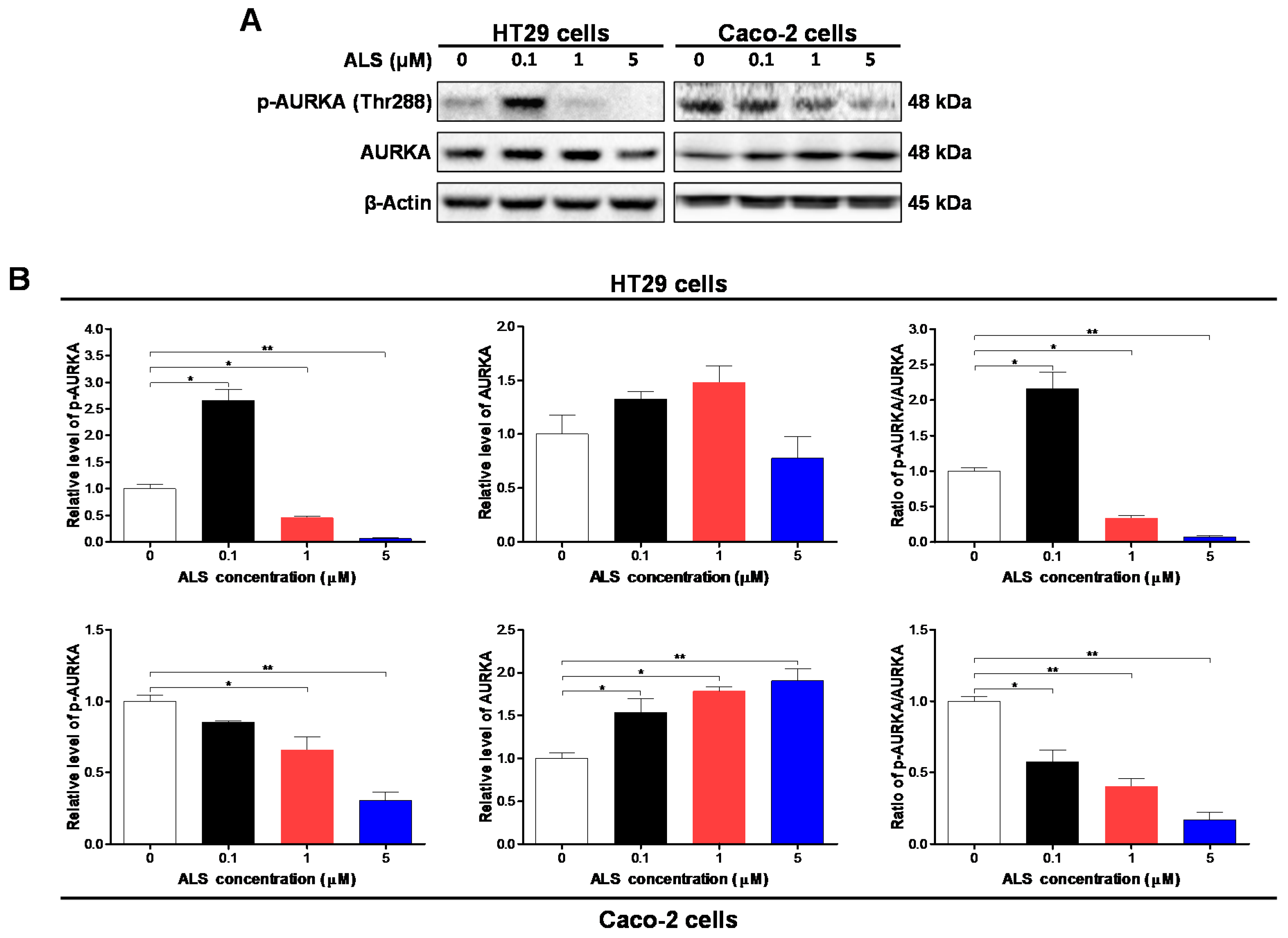
2.3. ALS Decreases the Phosphorylation Level of Aurora Kinase A (AURKA) in HT29 and Caco-2 Cells
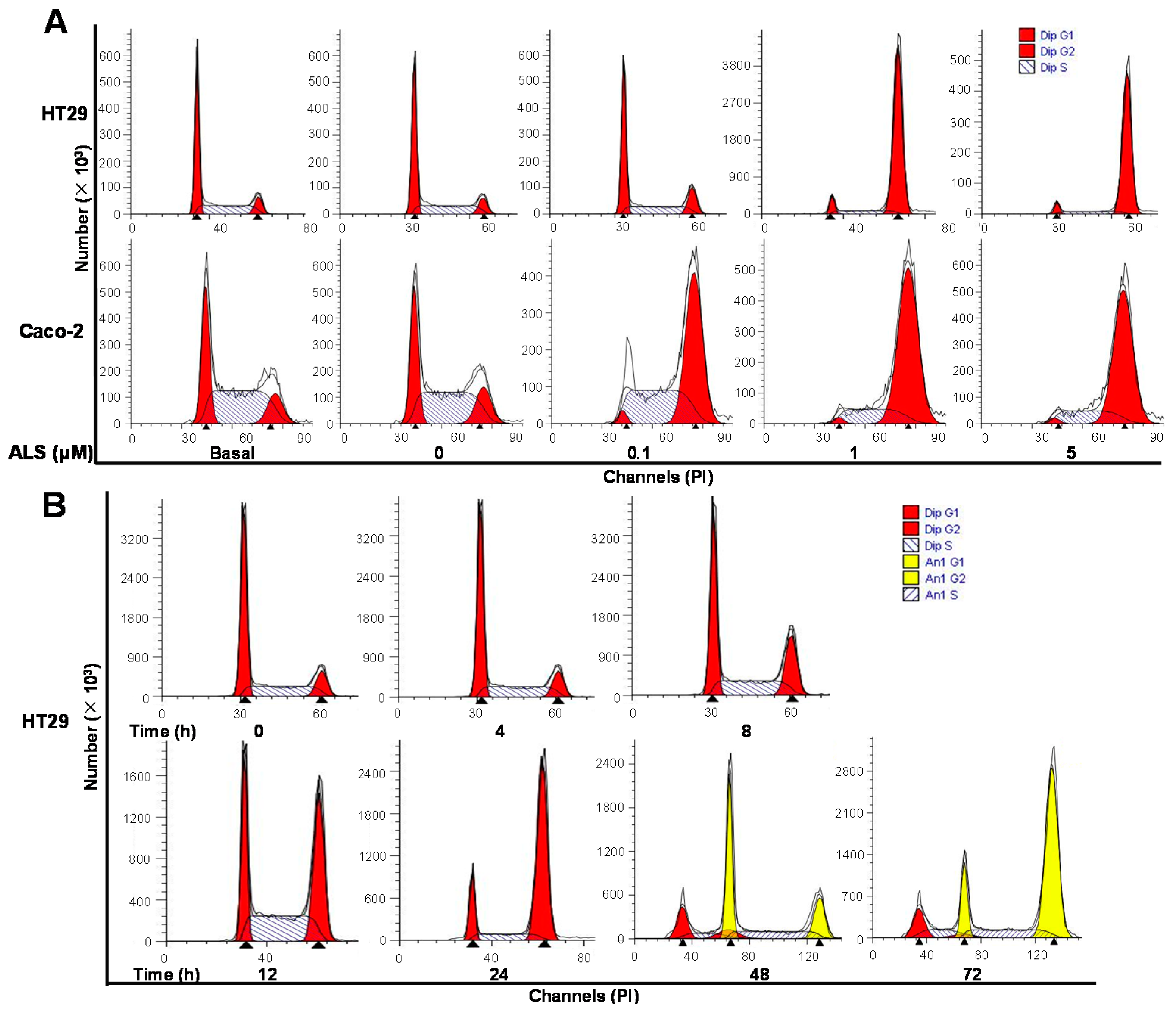
2.4. ALS Modulates the Cell Cycle Distribution of HT29 and Caco-2 Cells
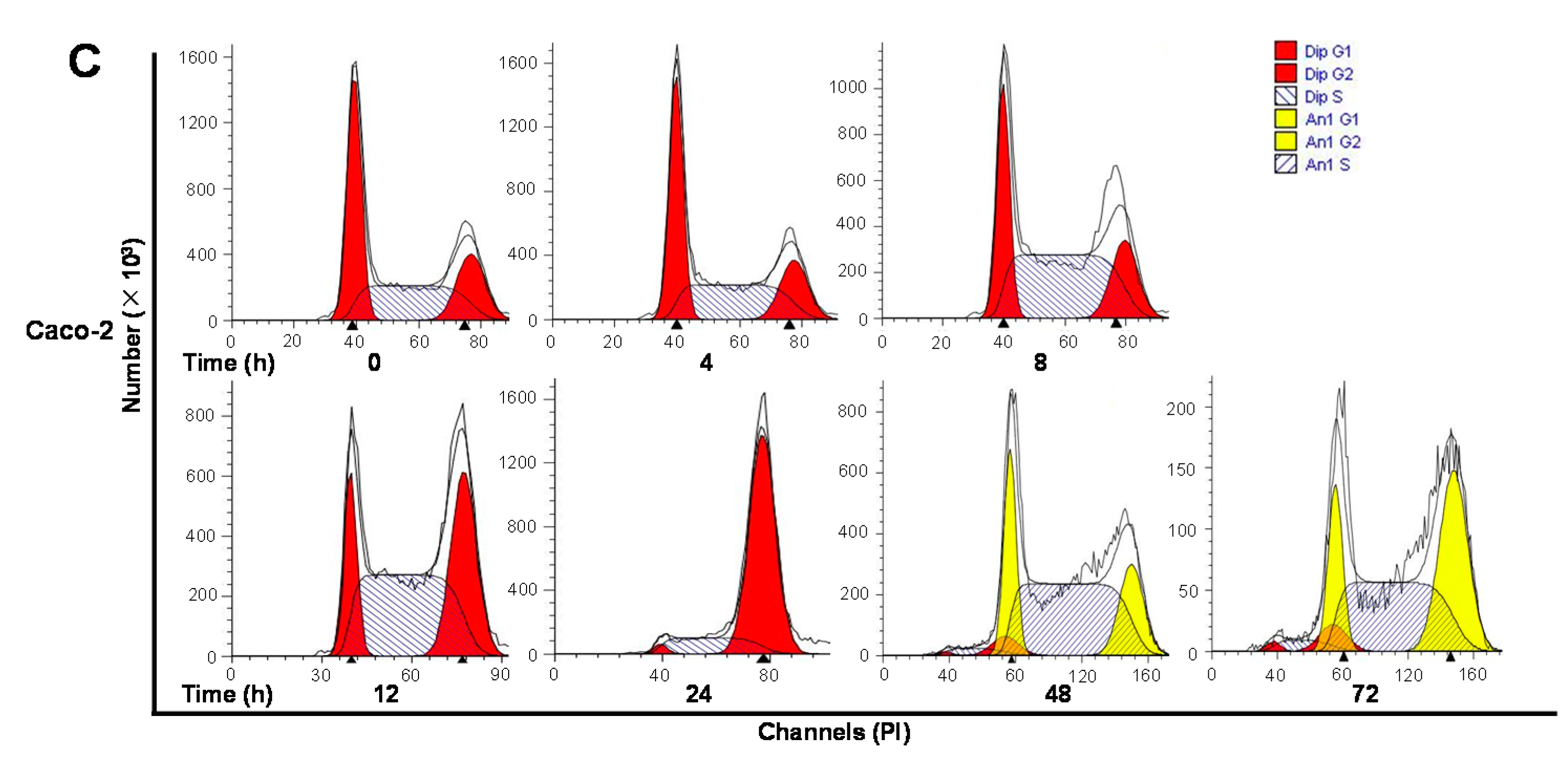
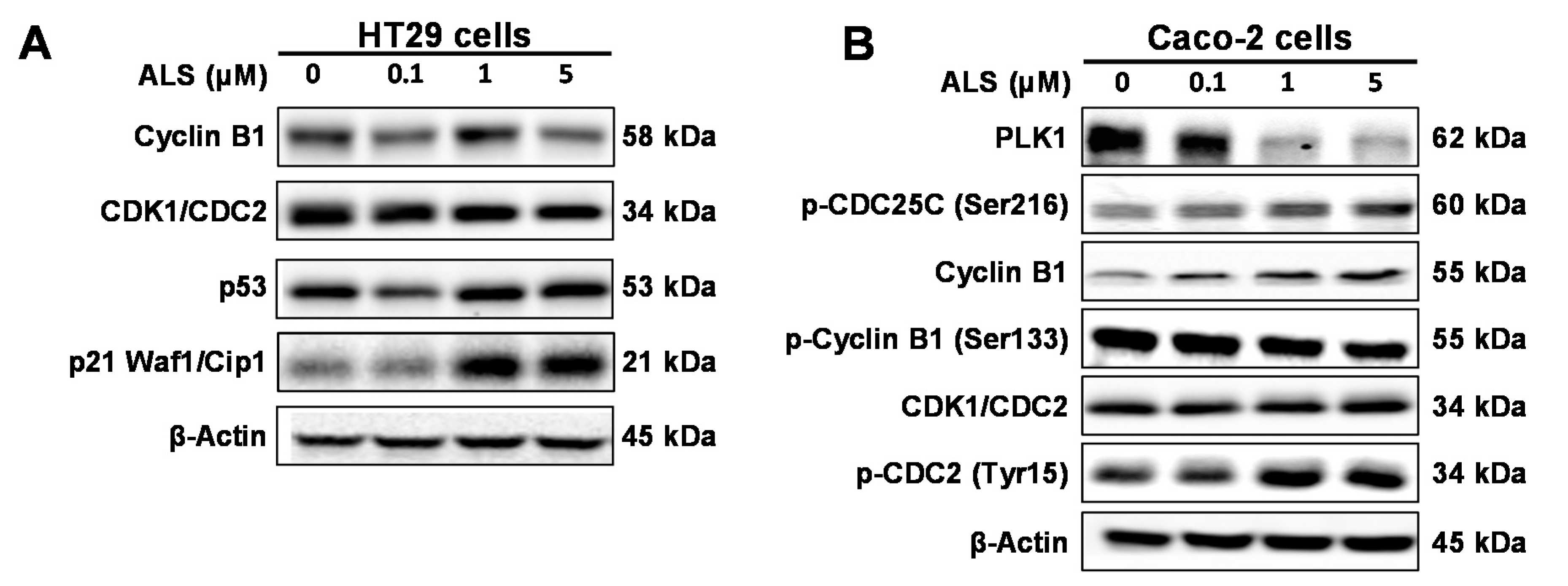
2.5. ALS Differentially Alters Key Regulators of Cell Cycle in HT29 and Caco-2 Cells
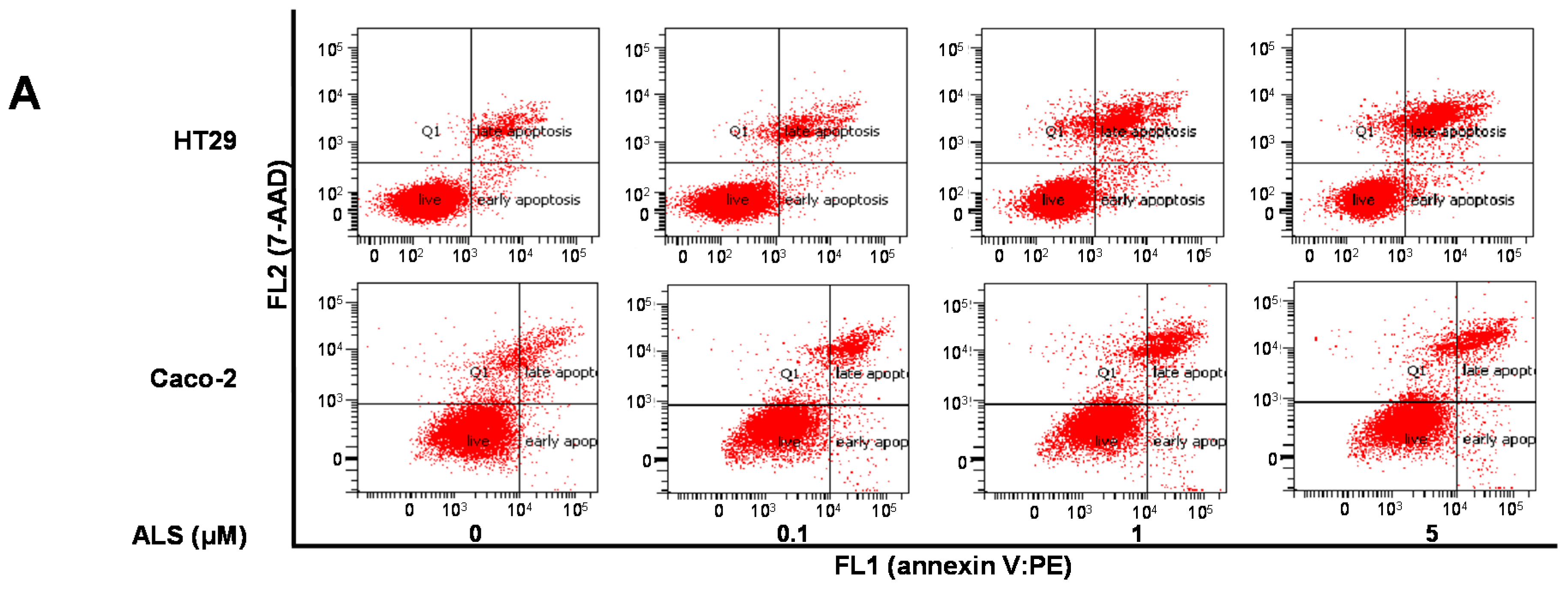
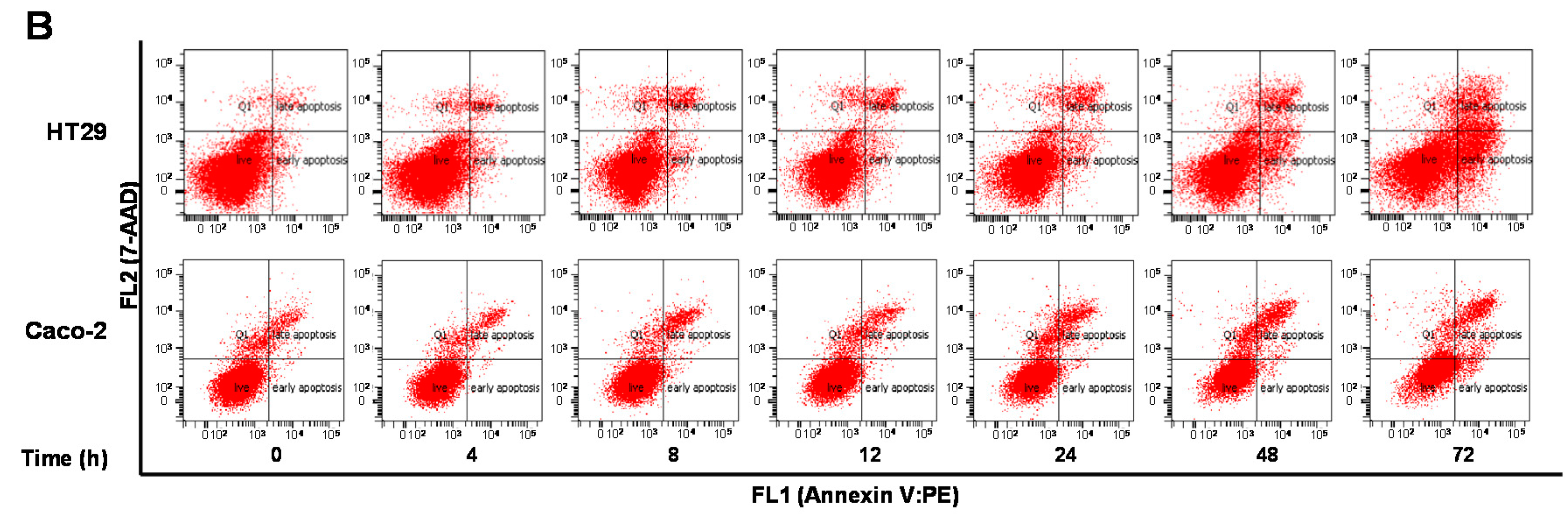
2.6. ALS Differentially Induces Cell Death in HT29 and Caco-2 Cells

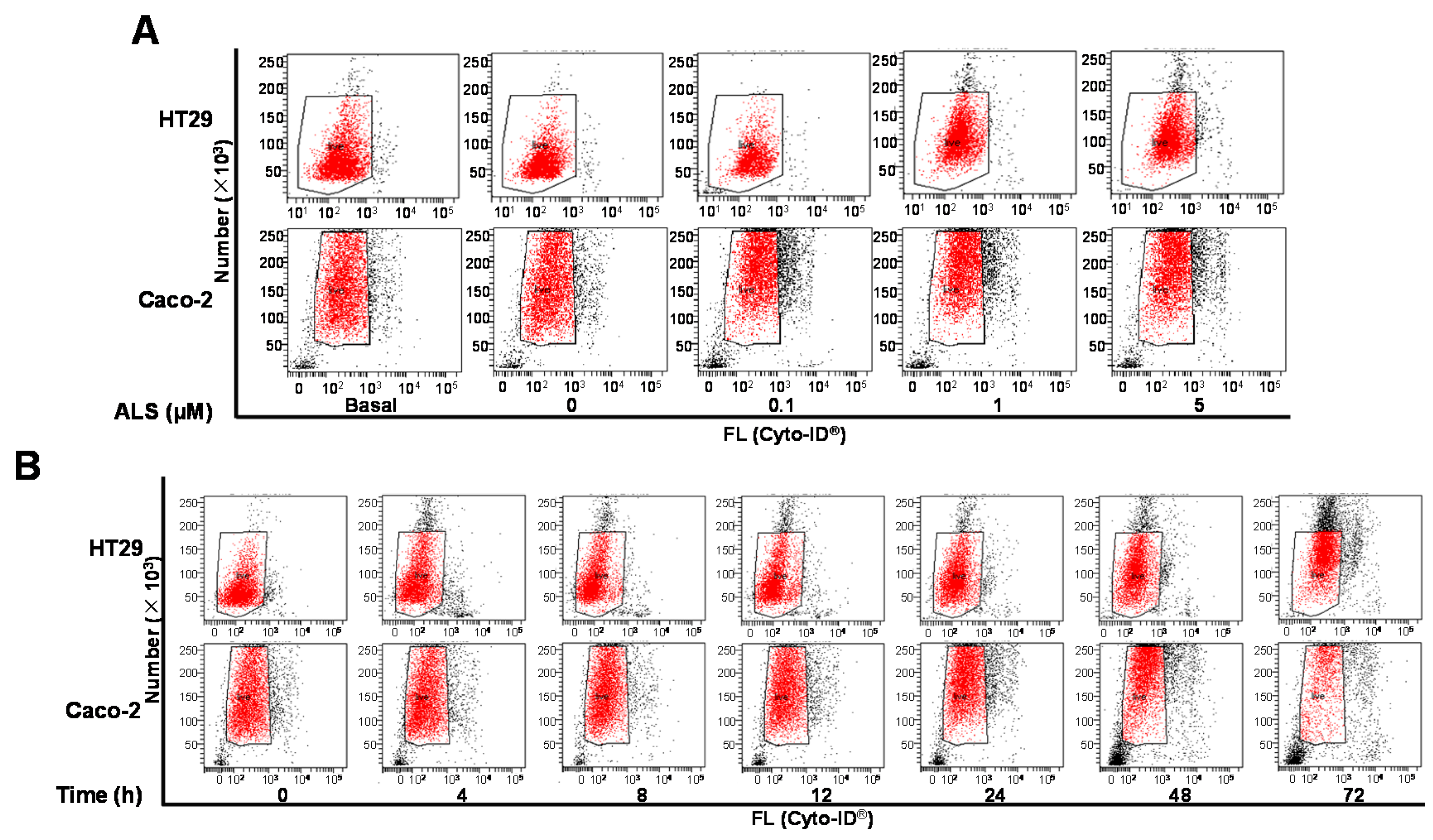
2.7. ALS Induces Autophagy of HT29 and Caco-2 Cells
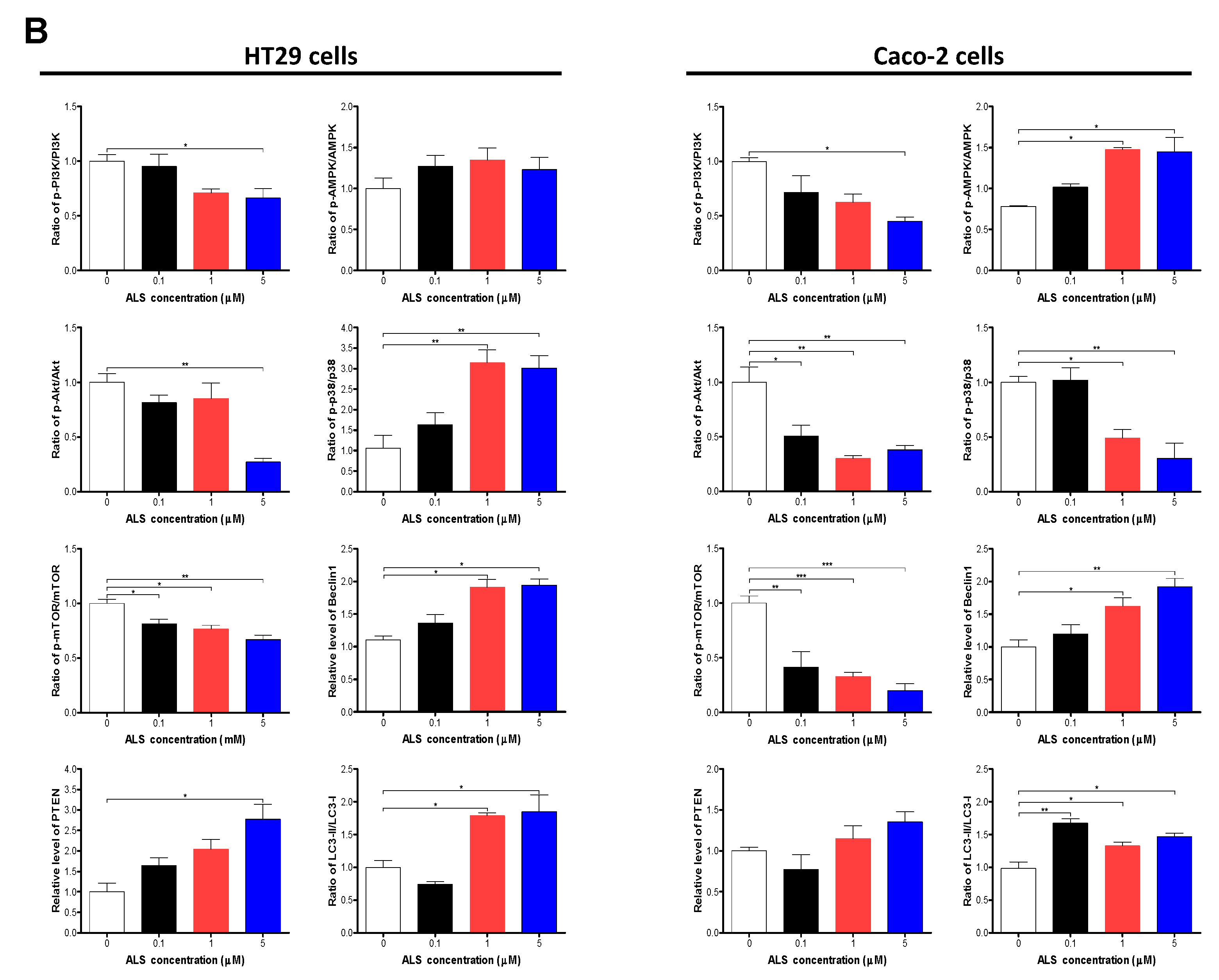
2.8. ALS Regulates PI3K/Akt/mTOR Axis, AMPK, and p38 MAPK Signaling Pathways in HT29 and Caco-2 Cells

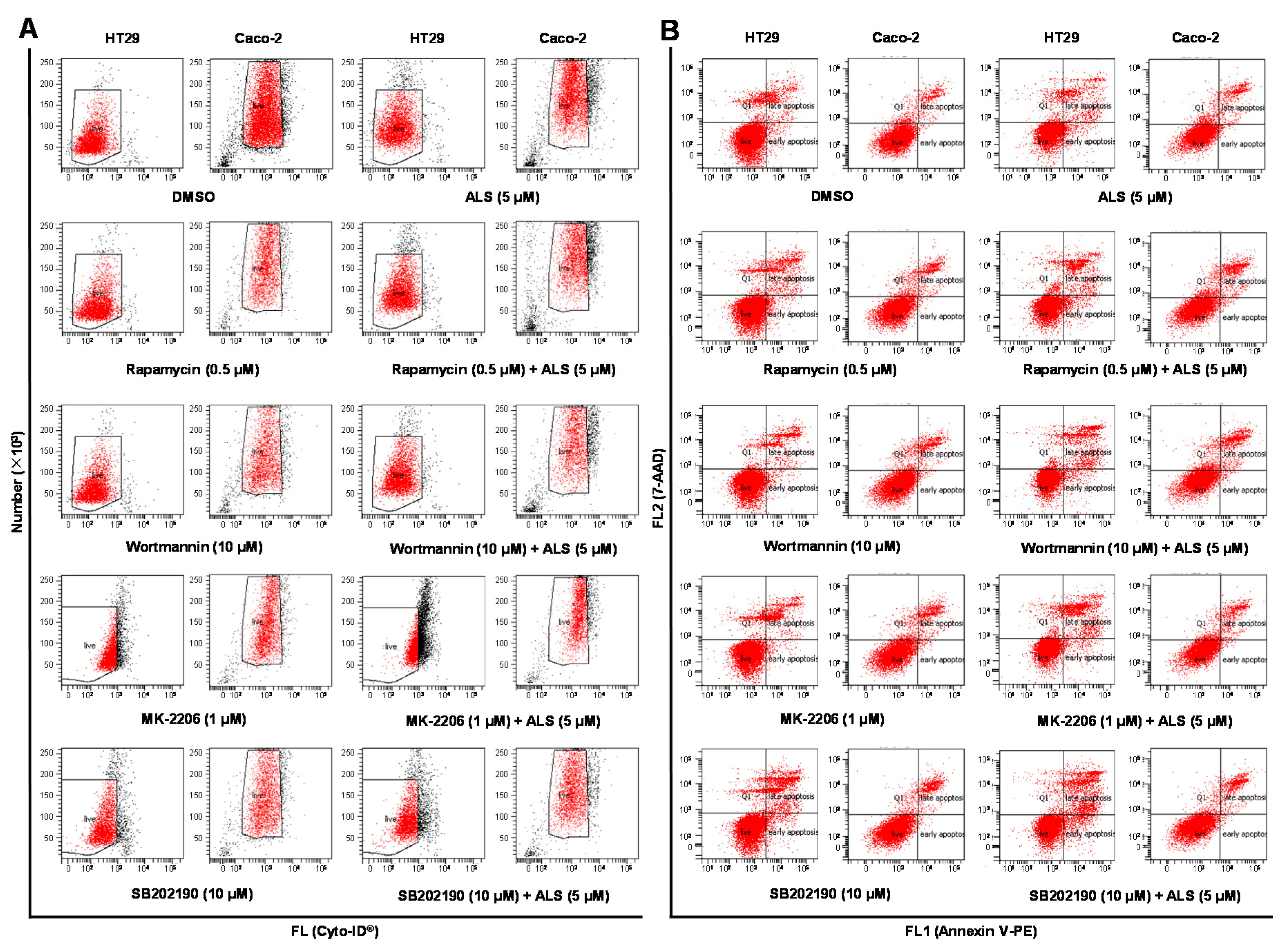
2.9. There Is a Crosstalk between ALS-Induced Apoptosis and Autophagy in HT29 and Caco-2 Cells
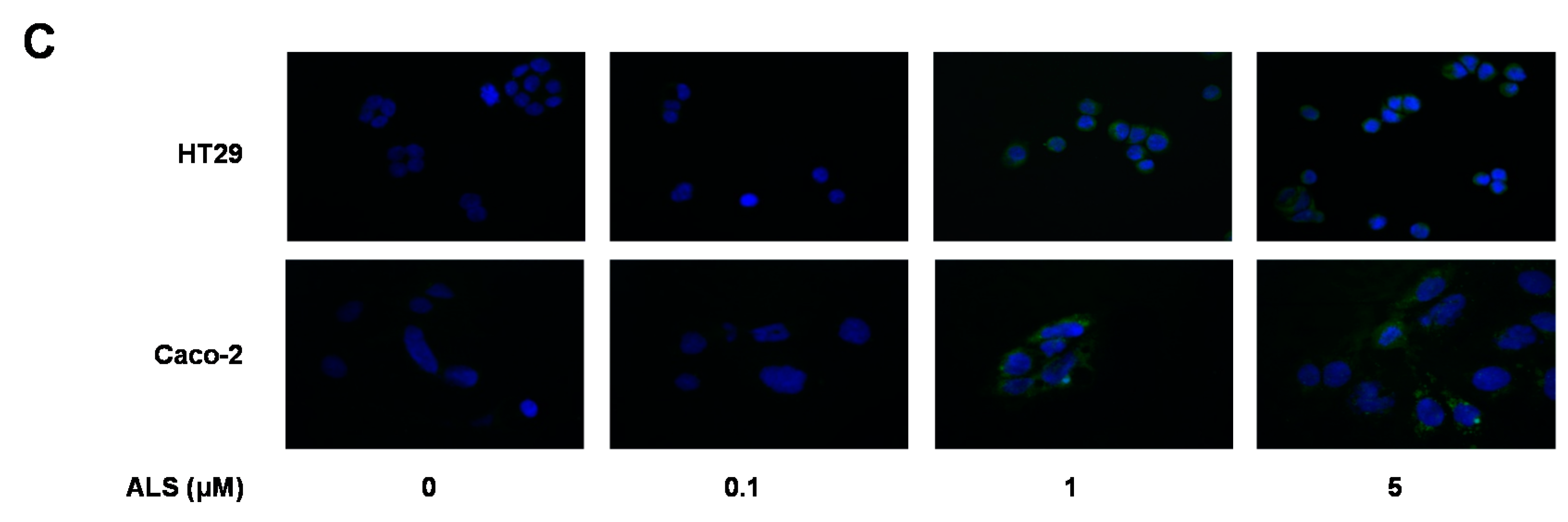
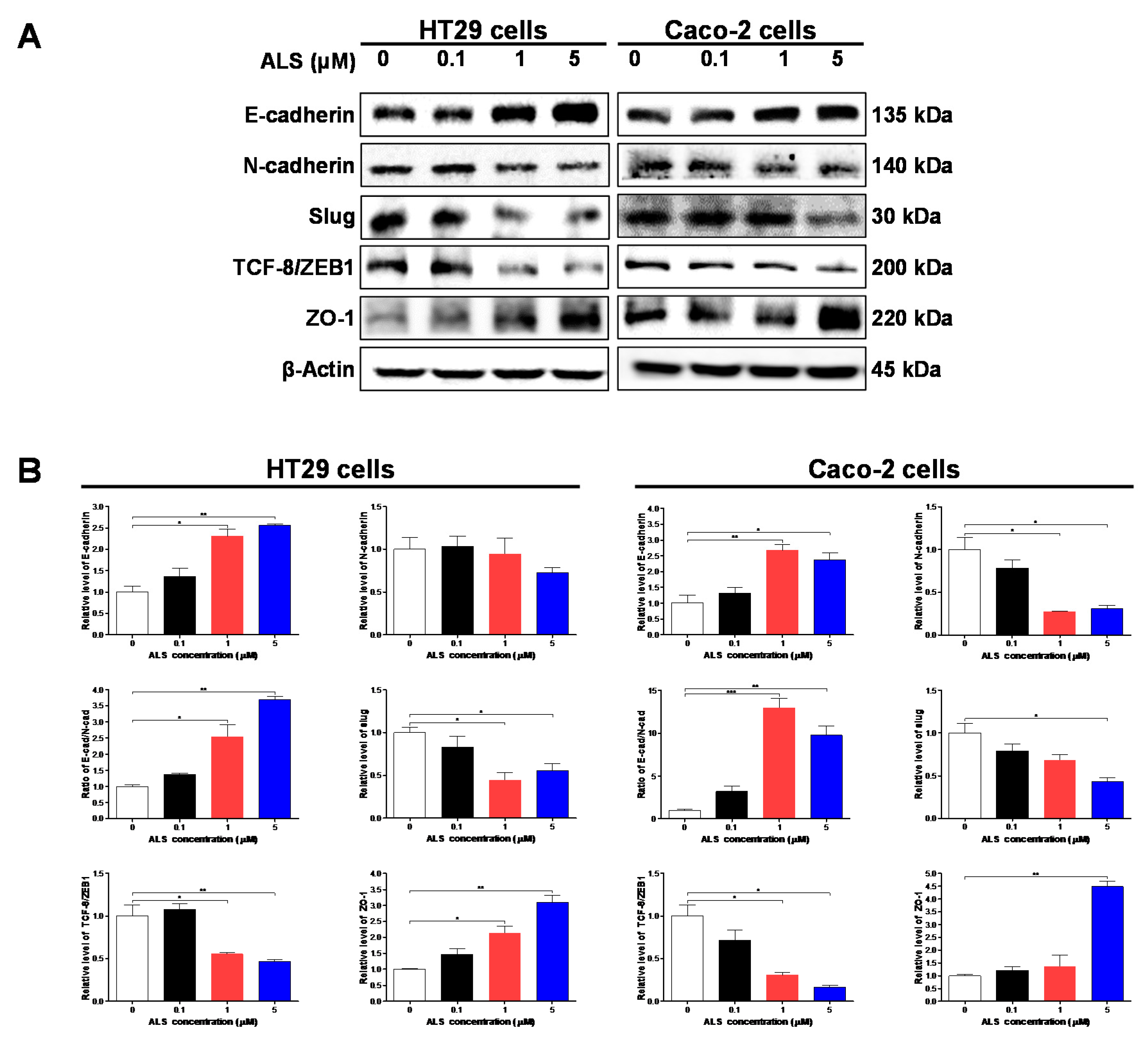
2.10. ALS Suppresses EMT in HT29 and Caco-2 Cells
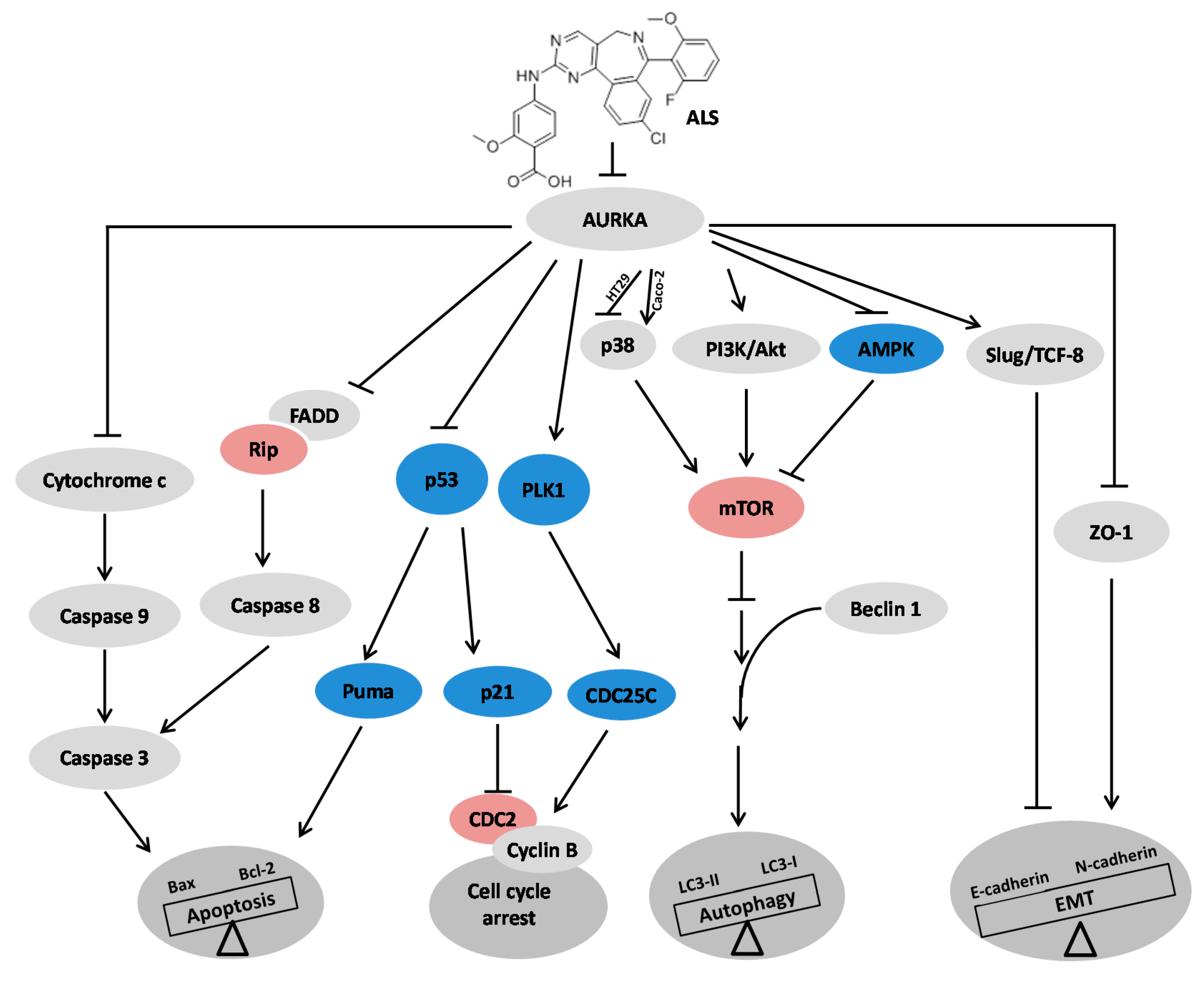
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Lines and Cell Culture
4.3. Proteomic Response to ALS Treatment Analyzed by Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC)-Based Approach
4.3.1. Digestion and Desalting SILAC Protein Samples
4.3.2. LC-MS/MS and Statistical Analysis
4.3.3. Pathway and Network Analysis
4.4. Cell Viability Assay
4.5. Cell Cycle Distribution Analysis
4.6. Quantification of Cellular Apoptosis
4.7. Quantification of Cellular Autophagy
4.8. Simultaneous Determination of Apoptosis and Autophagy Using Flow Cytometry
4.9. Confocal Fluorescence Microscopy
4.10. Western Blotting Analysis
4.11. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Desantis, C.; Jemal, A. Colorectal cancer statistics, 2014. Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Zeng, H.; Zhang, S.; He, J. Annual report on status of cancer in China, 2011. Chin. J. Cancer Res. 2015, 27, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Society, A.C. Cancer facts and figures. Available online: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf (accessed on 16 May 2013).
- Chu, E. An update on the current and emerging targeted agents in metastatic colorectal cancer. Clin. Colorectal Cancer 2012, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.; Morelli, F.; Cinieri, S.; Santini, D.; Silvestris, N.; Fazio, N.; Orlando, L.; Tonini, G.; Colucci, G.; Maiello, E. Adjuvant colon cancer chemotherapy: Where we are and where we’ll go. Cancer Treat. Rev. 2010, 36 (Suppl. 3), S34–S41. [Google Scholar] [CrossRef]
- Troiani, T.; Martinelli, E.; Orditura, M.; de Vita, F.; Ciardiello, F.; Morgillo, F. Beyond bevacizumab: New anti-VEGF strategies in colorectal cancer. Expert Opin. Investig. Drugs 2012, 21, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A. Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2001, 2, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Bolanos-Garcia, V.M. Aurora kinases. Int. J. Biochem. Cell Biol. 2005, 37, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Gautschi, O.; Heighway, J.; Mack, P.C.; Purnell, P.R.; Lara, P.N., Jr.; Gandara, D.R. Aurora kinases as anticancer drug targets. Clin. Cancer Res. 2008, 14, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Dotan, E.; Meropol, N.J.; Zhu, F.; Zambito, F.; Bove, B.; Cai, K.Q.; Godwin, A.K.; Golemis, E.A.; Astsaturov, I.; Cohen, S.J. Relationship of increased aurora kinase A gene copy number, prognosis and response to chemotherapy in patients with metastatic colorectal cancer. Br. J. Cancer 2012, 106, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Nosho, K.; Shima, K.; Irahara, N.; Kure, S.; Toyoda, S.; Kirkner, G.J.; Goel, A.; Fuchs, C.S.; Ogino, S. Aurora-A expression is independently associated with chromosomal instability in colorectal cancer. Neoplasia 2009, 11, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Goos, J.A.; Coupe, V.M.; Diosdado, B.; Delis-Van Diemen, P.M.; Karga, C.; Belien, J.A.; Carvalho, B.; van den Tol, M.P.; Verheul, H.M.; Geldof, A.A.; et al. Aurora kinase A (AURKA) expression in colorectal cancer liver metastasis is associated with poor prognosis. Br. J. Cancer 2013, 109, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Cammareri, P.; Scopelliti, A.; Todaro, M.; Eterno, V.; Francescangeli, F.; Moyer, M.P.; Agrusa, A.; Dieli, F.; Zeuner, A.; Stassi, G. Aurora-a is essential for the tumorigenic capacity and chemoresistance of colorectal cancer stem cells. Cancer Res. 2010, 70, 4655–4665. [Google Scholar] [CrossRef] [PubMed]
- Boss, D.S.; Beijnen, J.H.; Schellens, J.H. Clinical experience with aurora kinase inhibitors: A review. Oncologist 2009, 14, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; O’Donnell, J.P.; Salazar, C.R.; van Brocklyn, J.R.; Barnett, K.D.; Pearl, D.K.; deCarvalho, A.C.; Ecsedy, J.A.; Brown, S.L.; Mikkelsen, T.; et al. The selective Aurora-A kinase inhibitor MLN8237 (alisertib) potently inhibits proliferation of glioblastoma neurosphere tumor stem-like cells and potentiates the effects of temozolomide and ionizing radiation. Cancer Chemother. Pharmacol. 2014, 73, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Singh, K.; Mir, M.C.; Parker, Y.; Lindner, D.; Dreicer, R.; Ecsedy, J.A.; Zhang, Z.; Teh, B.T.; Almasan, A.; et al. The investigational Aurora kinase A inhibitor MLN8237 induces defects in cell viability and cell-cycle progression in malignant bladder cancer cells in vitro and in vivo. Clin. Cancer Res. 2013, 19, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Sehdev, V.; Peng, D.; Soutto, M.; Washington, M.K.; Revetta, F.; Ecsedy, J.; Zaika, A.; Rau, T.T.; Schneider-Stock, R.; Belkhiri, A.; et al. The Aurora kinase A inhibitor MLN8237 enhances cisplatin-induced cell death in esophageal adenocarcinoma cells. Mol. Cancer Ther. 2012, 11, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.X.; Zhou, Z.W.; Yang, Y.X.; He, Z.X.; Zhang, X.; Wang, D.; Yang, T.; Wang, N.J.; Zhao, R.J.; Zhou, S.F. Inhibition of mitotic Aurora kinase A by alisertib induces apoptosis and autophagy of human gastric cancer AGS and NCI-N78 cells. Drug Des. Dev. Ther. 2015, 9, 487–508. [Google Scholar]
- Ding, Y.H.; Zhou, Z.W.; Ha, C.F.; Zhang, X.Y.; Pan, S.T.; He, Z.X.; Edelman, J.L.; Wang, D.; Yang, Y.X.; Zhang, X.; et al. Alisertib, an Aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer cells. Drug Des. Dev. Ther. 2015, 9, 425–464. [Google Scholar]
- Wang, F.; Li, H.; Yan, X.G.; Zhou, Z.W.; Yi, Z.G.; He, Z.X.; Pan, S.T.; Yang, Y.X.; Wang, Z.Z.; Zhang, X.; et al. Alisertib induces cell cycle arrest and autophagy and suppresses epithelial-to-mesenchymal transition involving PI3K/Akt/mTOR and sirtuin 1-mediated signaling pathways in human pancreatic cancer cells. Drug Des. Dev. Ther. 2015, 9, 575–601. [Google Scholar]
- Niu, N.K.; Wang, Z.L.; Pan, S.T.; Ding, H.Q.; Au, G.H.; He, Z.X.; Zhou, Z.W.; Xiao, G.; Yang, Y.X.; Zhang, X.; et al. Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway. Drug Des. Dev. Ther. 2015, 9, 1555–1584. [Google Scholar]
- Gorgun, G.; Calabrese, E.; Hideshima, T.; Ecsedy, J.; Perrone, G.; Mani, M.; Ikeda, H.; Bianchi, G.; Hu, Y.; Cirstea, D.; et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood 2010, 115, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Klionsky, D.J. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu. Rev. Biochem. 2000, 69, 303–342. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Heras-Sandoval, D.; Perez-Rojas, J.M.; Hernandez-Damian, J.; Pedraza-Chaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014, 26, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Lozy, F.; Karantza, V. Autophagy and cancer cell metabolism. Semin. Cell Dev. Biol. 2012, 23, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Mester, J.; Eng, C. When overgrowth bumps into cancer: The PTEN-opathies. Am. J. Med. Genet. Part C Semin. Med. Genet. 2013, 163C, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Fogg, V.C.; Margolis, B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006, 22, 207–235. [Google Scholar] [CrossRef] [PubMed]
- Heerboth, S.; Housman, G.; Leary, M.; Longacre, M.; Byler, S.; Lapinska, K.; Willbanks, A.; Sarkar, S. EMT and tumor metastasis. Clin. Transl. Med. 2015, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Derksen, P.W.; Liu, X.; Saridin, F.; van der Gulden, H.; Zevenhoven, J.; Evers, B.; van Beijnum, J.R.; Griffioen, A.W.; Vink, J.; Krimpenfort, P.; et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 2006, 10, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, J.T.; Henry, M.D. Epithelial-to-mesenchymal transition in prostate cancer: Paradigm or puzzle? Nat. Rev. Urol. 2011, 8, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Polette, M.; Mestdagt, M.; Bindels, S.; Nawrocki-Raby, B.; Hunziker, W.; Foidart, J.M.; Birembaut, P.; Gilles, C. β-catenin and ZO-1: Shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs 2007, 185, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- D'Assoro, A.B.; Liu, T.; Quatraro, C.; Amato, A.; Opyrchal, M.; Leontovich, A.; Ikeda, Y.; Ohmine, S.; Lingle, W.; Suman, V.; et al. The mitotic kinase Aurora—A promotes distant metastases by inducing epithelial-to-mesenchymal transition in ERα+ breast cancer cells. Oncogene 2014, 33, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Chang, P.C.; Cheng, Y.W.; Tang, F.M.; Lin, Y.S. Suppression of the STK15 oncogenic activity requires a transactivation-independent p53 function. EMBO J. 2002, 21, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Macurek, L.; Lindqvist, A.; Medema, R.H. Aurora-A and hBora join the game of Polo. Cancer Res. 2009, 69, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef]
- Chow, J.P.; Siu, W.Y.; Ho, H.T.; Ma, K.H.; Ho, C.C.; Poon, R.Y. Differential contribution of inhibitory phosphorylation of CDC2 and CDK2 for unperturbed cell cycle control and DNA integrity checkpoints. J. Biol. Chem. 2003, 278, 40815–40828. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Singh, R.P.; Agarwal, C.; Siriwardana, S.; Sclafani, R.A.; Agarwal, R. Resveratrol causes CDC2-tyr15 phosphorylation via ATM/ATR-Chk1/2-CDC25C pathway as a central mechanism for S phase arrest in human ovarian carcinoma Ovcar-3 cells. Carcinogenesis 2005, 26, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Eckerdt, F.; Bereiter-Hahn, J.; Kurunci-Csacsko, E.; Kaufmann, M.; Strebhardt, K. Cooperative phosphorylation including the activity of polo-like kinase 1 regulates the subcellular localization of cyclin B1. Oncogene 2002, 21, 8282–8292. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Yang, Y.X.; Liu, Q.L.; Pan, S.T.; He, Z.X.; Zhang, X.; Yang, T.; Chen, X.W.; Wang, D.; Qiu, J.X.; et al. The investigational Aurora kinase A inhibitor alisertib (MLN8237) induces cell cycle G2/M arrest, apoptosis, and autophagy via p38 MAPK and Akt/mTOR signaling pathways in human breast cancer cells. Drug Des. Dev. Ther. 2015, 9, 1627–1652. [Google Scholar]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Chinnaiyan, A.M.; O’Rourke, K.; Tewari, M.; Dixit, V.M. FADD, a novel death domain-containing protein, interacts with the death domain of fas and initiates apoptosis. Cell 1995, 81, 505–512. [Google Scholar] [CrossRef]
- Jung, C.H.; Ro, S.H.; Cao, J.; Otto, N.M.; Kim, D.H. mTOR regulation of autophagy. FEBS Lett. 2010, 584, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Alers, S.; Loffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Yue, Z.; Talloczy, Z.; Hagemann, T.; Cho, W.; Messing, A.; Sulzer, D.L.; Goldman, J.E. Autophagy induced by Alexander disease-mutant GFAP accumulation is regulated by p38/MAPK and mTOR signaling pathways. Hum. Mol. Genet. 2008, 17, 1540–1555. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Kongara, S.; Beaudoin, B.; Karp, C.M.; Bray, K.; Degenhardt, K.; Chen, G.; Jin, S.; White, E. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007, 21, 1367–1381. [Google Scholar] [CrossRef] [PubMed]
- Lum, J.J.; Bauer, D.E.; Kong, M.; Harris, M.H.; Li, C.; Lindsten, T.; Thompson, C.B. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005, 120, 237–248. [Google Scholar] [CrossRef] [PubMed]
- El-Khattouti, A.; Selimovic, D.; Haikel, Y.; Hassan, M. Crosstalk between apoptosis and autophagy: Molecular mechanisms and therapeutic strategies in cancer. J. Cell Death 2013, 6, 37–55. [Google Scholar] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu. Rev. Cell Dev. Biol. 2011, 27, 347–376. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Hulit, J.; Suyama, K.; Chung, S.; Keren, R.; Agiostratidou, G.; Shan, W.; Dong, X.; Williams, T.M.; Lisanti, M.P.; Knudsen, K.; et al. N-cadherin signaling potentiates mammary tumor metastasis via enhanced extracellular signal-regulated kinase activation. Cancer Res. 2007, 67, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Lehembre, F.; Yilmaz, M.; Wicki, A.; Schomber, T.; Strittmatter, K.; Ziegler, D.; Kren, A.; Went, P.; Derksen, P.W.; Berns, A.; et al. NCAM-induced focal adhesion assembly: A functional switch upon loss of E-cadherin. EMBO J. 2008, 27, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Aigner, K.; Dampier, B.; Descovich, L.; Mikula, M.; Sultan, A.; Schreiber, M.; Mikulits, W.; Brabletz, T.; Strand, D.; Obrist, P.; et al. The transcription factor ZEB1 (dEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene 2007, 26, 6979–6988. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Oren-Giladi, P.; Kaidar-Person, O.; Shaked, Y.; Geiger, T. Proteomics of microparticles with SILAC Quantification (PROMIS-Quan): A novel proteomic method for plasma biomarker quantification. Mol. Cell. Proteom. 2015, 14, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.W.; Li, X.X.; He, Z.X.; Pan, S.T.; Yang, Y.; Zhang, X.; Chow, K.; Yang, T.; Qiu, J.X.; Zhou, Q.; et al. Induction of apoptosis and autophagy via sirtuin1- and PI3K/Akt/mTOR-mediated pathways by plumbagin in human prostate cancer cells. Drug Des. Dev. Ther. 2015, 9, 1511–1554. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, B.-J.; Zhou, Z.-W.; Zhu, D.-J.; Ju, Y.-L.; Wu, J.-H.; Ouyang, M.-Z.; Chen, X.-W.; Zhou, S.-F. Alisertib Induces Cell Cycle Arrest, Apoptosis, Autophagy and Suppresses EMT in HT29 and Caco-2 Cells. Int. J. Mol. Sci. 2016, 17, 41. https://doi.org/10.3390/ijms17010041
Ren B-J, Zhou Z-W, Zhu D-J, Ju Y-L, Wu J-H, Ouyang M-Z, Chen X-W, Zhou S-F. Alisertib Induces Cell Cycle Arrest, Apoptosis, Autophagy and Suppresses EMT in HT29 and Caco-2 Cells. International Journal of Molecular Sciences. 2016; 17(1):41. https://doi.org/10.3390/ijms17010041
Chicago/Turabian StyleRen, Bao-Jun, Zhi-Wei Zhou, Da-Jian Zhu, Yong-Le Ju, Jin-Hao Wu, Man-Zhao Ouyang, Xiao-Wu Chen, and Shu-Feng Zhou. 2016. "Alisertib Induces Cell Cycle Arrest, Apoptosis, Autophagy and Suppresses EMT in HT29 and Caco-2 Cells" International Journal of Molecular Sciences 17, no. 1: 41. https://doi.org/10.3390/ijms17010041
APA StyleRen, B.-J., Zhou, Z.-W., Zhu, D.-J., Ju, Y.-L., Wu, J.-H., Ouyang, M.-Z., Chen, X.-W., & Zhou, S.-F. (2016). Alisertib Induces Cell Cycle Arrest, Apoptosis, Autophagy and Suppresses EMT in HT29 and Caco-2 Cells. International Journal of Molecular Sciences, 17(1), 41. https://doi.org/10.3390/ijms17010041