In Vitro Proliferation and Anti-Apoptosis of the Papain-Generated Casein and Soy Protein Hydrolysates towards Osteoblastic Cells (hFOB1.19)
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Proliferation of the Six Hydrolysates towards hFOB1.19 Cells
| Hydrolysates | Hydrolysis Times (h) | Other Conditions | DH (%) |
|---|---|---|---|
| CH1 | 1.5 | pH 6.0, 60 °C, papain of 2 kU/g protein | 7.3 |
| CH2 | 3.5 | 10.8 | |
| CH3 | 7.0 | 13.3 | |
| SH1 | 3.0 | 7.5 | |
| SH2 | 5.0 | 10.9 | |
| SH3 | 7.0 | 13.2 |
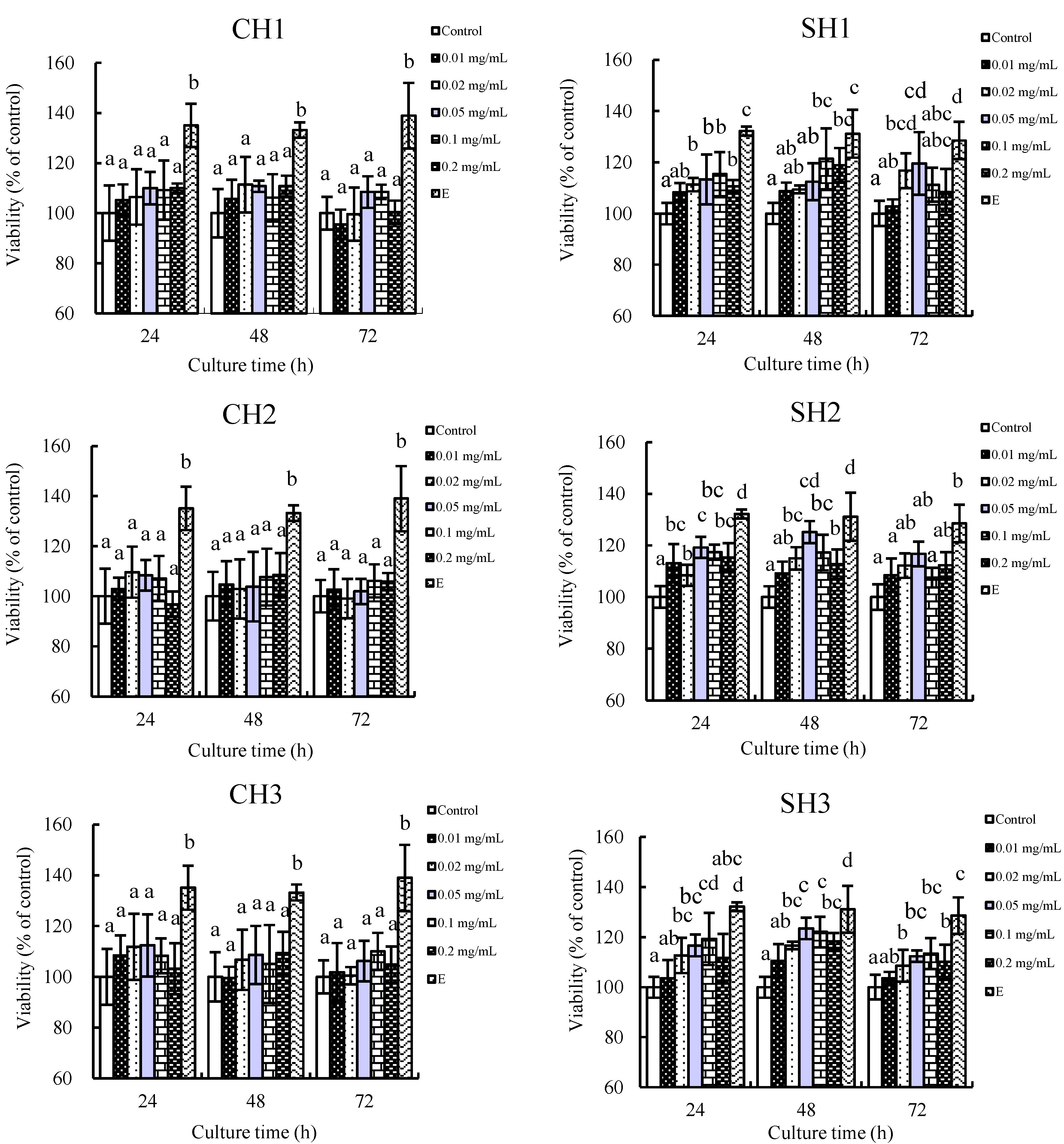
2.2. Effects of the Two Hydrolysates on Cell Cycle Distribution of hFOB1.19 Cells

2.3. Anti-Apoptosis of the Two Hydrolysates towards hFOB1.19 Cells
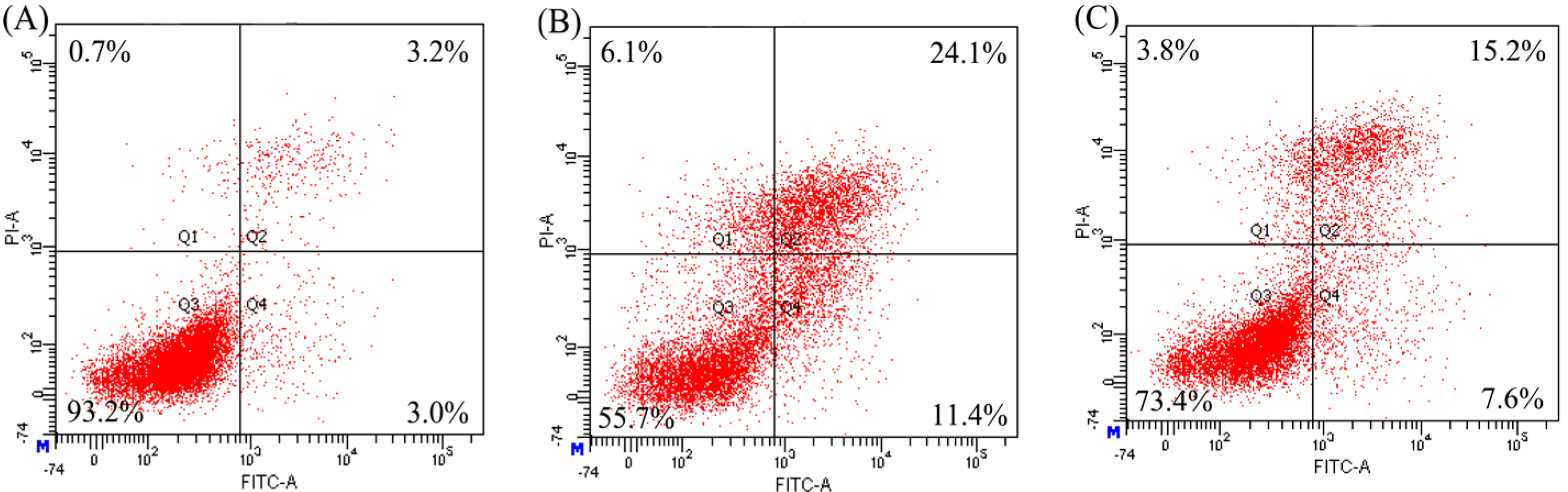
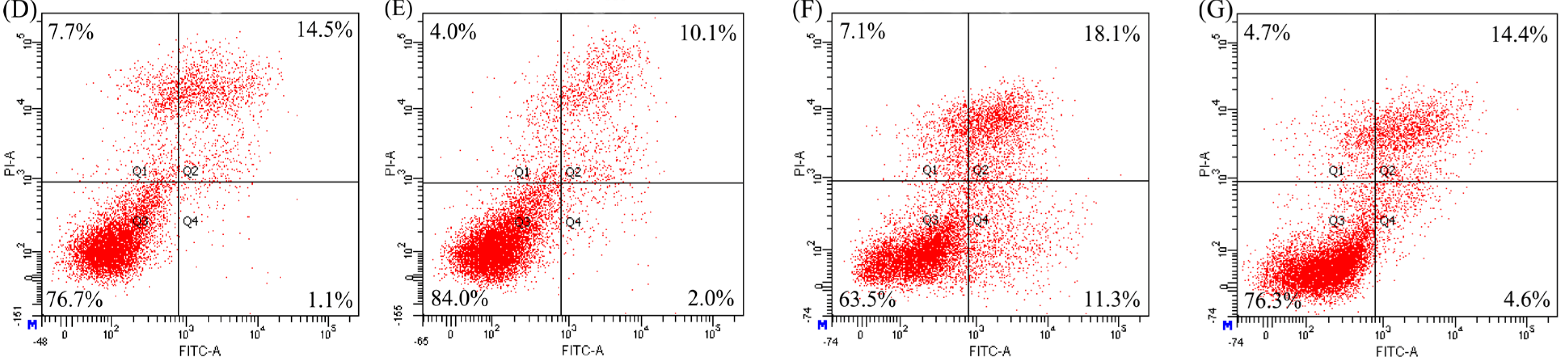
| Modes | Apoptosis Inducers | Percentage of Apoptotic Cells (%) | ||
|---|---|---|---|---|
| Model Group | CH3 Group | SH3 Group | ||
| Apoptotic prevention | EP | 31.6 | 22.6 | 15.1 |
| NaF | 19.5 | 17.7 | 12.4 | |
| Apoptotic reversal | EP | 13.3 | 13.3 | 11.7 |
| NaF | 14.5 | 15.3 | 11.0 | |
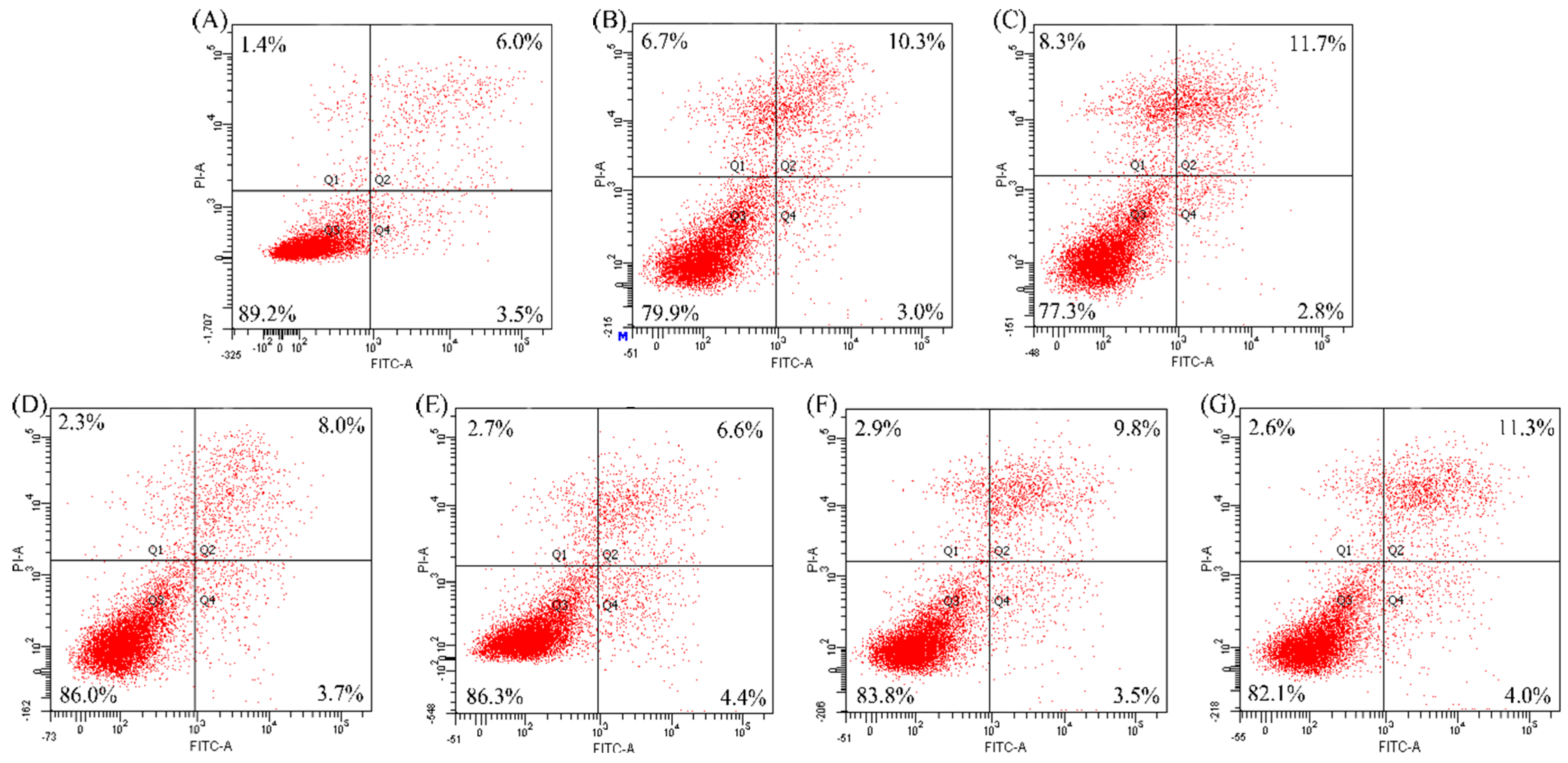
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of the Hydrolysates
3.3. Chemical Analyses
3.4. Cell Culture Conditions and Cell Viability Assaying
3.5. Cell Cycle and Apoptosis Assaying
3.6. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adamson, N.J.; Reynolds, E.C. Characterization of casein phosphopeptides prepared using alcalase: Determination of enzyme specificity. Enzym. Microb. Technol. 1996, 19, 202–207. [Google Scholar] [CrossRef]
- Henderson, J.E.; Kremer, R.; Goltzman, D. Systemic factors in skeletal manifestations of malignancy. In Principles of Bone Biology; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 1079–1092. [Google Scholar]
- Reid, I.R. Osteoporosis treatment: Focus on safety. Eur. J. Int. Med. 2013, 24, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Mardon, J.; Habauzit, V.; Trzeciakiewicz, A.; Davicco, M.J.; Lebecque, P.; Mercier, S.; Tressol, J.C.; Horcajada, M.N.; Demigné, C.; Coxam, V. Influence of high and low protein intakes on age-related bone loss in rats submitted to adequate or restricted energy conditions. Calcif. Tissue Int. 2008, 82, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.S.; Frick, K.K.; Bushinsky, D.A. Mechanism of acid-induced bone resorption. Curr. Opin. Nephrol. Hypertens. 2014, 13, 423–436. [Google Scholar] [CrossRef]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Donida, B.M.; Mrak, E.; Gravaghi, C.; Villa, I.; Cosentino, S.; Zacchi, E.; Perego, S.; Rubinacci, A.; Fiorilli, A.; Tettamanti, G.; et al. Casein phosphopeptides promote calcium uptake and modulate the differentiation pathway in human primary osteoblast-like cells. Peptides 2009, 30, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.M.; Racette, S.B.; van Pelt, R.E.; Peterson, L.R.; Villareal, D.T. Effects of soy protein isolate and moderate exercise on bone turnover and bone mineral density in postmenopausal women. Menopause 2007, 14, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Liu, C.L.; Li, Y.P.; Sun, J.; Wang, P.; Di, K.Q.; Chen, H.; Zhao, Y.Y. Effect of cerium ion on the proliferation, differentiation and mineralization function of primary mouse osteoblasts in vitro. J. Rare Earths 2010, 28, 138–142. [Google Scholar] [CrossRef]
- Arjmanoi, B.H.; Alekel, L.; Hollis, B.W.; Amin, D.; Stacewicz-Sapuntzakis, M.; Guo, P.; Kukreja, S.C. Dietary soybean protein prevents bone loss in an ovariectomised rat model of osteoporosis. J. Nutr. 1996, 126, 161–167. [Google Scholar]
- Budek, A.Z.; Bjornvad, C.R.; Molgaard, C.; Bügel, S.; Vestergaard, M.; Pulkkinend, P.; Michaelsen, K.F.; Sangild, P.T. Effects of casein, whey and soy proteins on volumetric bone density and bone strength in immunocompromised piglets. e-SPEN Eur. J. Clin. Nutr. Metab. 2007, 2, 57–62. [Google Scholar] [CrossRef]
- Canalis, E.; Pash, J.; Varghese, J.S. Skeletal growth factors. Crit. Rev. Eukaryot. Gene Expr. 1993, 3, 155–166. [Google Scholar] [PubMed]
- Rizzoli, R.; Bonjour, J.P. Dietary protein and bone health. J. Bone Miner. Res. 2004, 19, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.M.; Kilpatrick, J.I.; van Es, M.H.; Weafer, P.P.; Prendergast, P.J.; Jarvis, S.P. Bone cell elasticity and morphology changes during the cell cycle. J. Biomech. 2011, 44, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Tsuruoka, N.; Yamato, R.; Sakai, Y.; Yoshitake, Y.; Yonekura, H. Promotion by collagen tripeptide of type I collagen gene expression in human osteoblastic cells and fracture healing of rat femur. Biosci. Biotechnol. Biochem. 2007, 71, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhao, X.H. In vitro responses of hFOB1.19 cells towards chum salmon (Oncorhynchus keta) skin gelatin hydrolysates in cell proliferation, cycle progression and apoptosis. J. Funct. Foods 2013, 5, 279–288. [Google Scholar] [CrossRef]
- Chen, X.X.; Deng, Y.F.; Zhou, Z.L.; Tao, Q.S.; Zhu, J.; Li, X.L.; Chen, J.L.; Hou, J.F. 17β-estradiol combined with testosterone promotes chicken osteoblast proliferation and differentiation by accelerating the cell cycle and inhibiting apoptosis in vitro. Vet. Res. Commun. 2010, 34, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.C. Apoptosis, programmed cell death and the hypersensitive response. Eur. J. Plant Pathol. 1998, 104, 117–124. [Google Scholar] [CrossRef]
- Bellido, T.; Plotkin, L.I. Novel actions of bisphosphonates in bone: Preservation of osteoblast and osteocyte viability. Bone 2011, 49, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Ferreri, M.; Wang, Z.; Su, Y.; Han, B.; Su, J. Sodium fluoride affects proliferation and apoptosis through insulin-like growth factor I receptor in primary cultured mouse osteoblasts. Biol. Trace Elem. Res. 2011, 144, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.S.; Zhao, S.; Bellido, T.; Plotkin, L.I.; Jimenez, F.; Bonewald, L.F. CD40 ligand blocks apoptosis induced by tumor necrosis factor α, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology 2003, 144, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Pagel, C.N.; Niese, M.R.; Abraham, L.A.; Chinni, C.; Song, S.J.; Pike, R.N.; Mackie, E.J. Inhibition of osteoblast apoptosis by thrombin. Bone 2003, 33, 733–743. [Google Scholar] [CrossRef]
- Grey, A.; Zhu, Q.; Watson, M.; Callon, K.; Cornish, J. Lactoferrin potently inhibits osteoblast apoptosis, via an LRP1-independent pathway. Mol. Cell. Endocrinol. 2006, 251, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Zhang, Z.F.; Pei, X.R.; Han, X.L.; Wang, J.B.; Wang, L.L.; Long, Z.; Shen, X.Y.; Li, Y. Immunomodulatory effects of marine oligopeptide preparation from chum salmon (Oncorhynchus keta) in mice. Food Chem. 2009, 113, 464–470. [Google Scholar]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I.; Hernández-Ledesma, B. Antihypertensive peptides from food proteins: A review. Food Funct. 2012, 3, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhao, X.H. Utilization of chum salmon (Oncorhynchus keta) skin gelatin hydrolysates to attenuate hydrogen peroxide-induced oxidative injury in rat hepatocyte BRL cell model. J. Aquat. Food Prod. Technol. 2014. [Google Scholar] [CrossRef]
- Jiang, S.J.; Zhao, X.H. Transglutaminase-induced cross-linking and glucosamine conjugation in soybean protein isolates and its impacts on some functional properties of the products. Eur. Food Res. Technol. 2010, 231, 679–689. [Google Scholar] [CrossRef]
- Eckert, R.L.; Katzenellenbogen, B.S. Effect of estrogens and antiestrogen receptor dynamics and the induction of progesterone receptor in MCF-7 human breast cancer cells. Cancer Res. 1982, 42, 139–144. [Google Scholar] [PubMed]
- Kunst, T. Handbook of Food Enzymology; Whitaker, J.R., Voragen, A.G.J., Wong, D.W.S., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; Chapter 17; pp. 221–236. [Google Scholar]
- Zhou, P.; Regenstein, J.M. Determination of total protein content in gelatin solutions with the lowry or biuret assay. J. Food Sci. 2006, 71, 474–479. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk protein. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Mu, X.M.; Jiang, Z.Z.; Zhang, L.Y. Effect of triptolide on estradiol release from cultured rat granulose cells. Endocr. J. 2012, 59, 473–481. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.-W.; Zhao, X.-H. In Vitro Proliferation and Anti-Apoptosis of the Papain-Generated Casein and Soy Protein Hydrolysates towards Osteoblastic Cells (hFOB1.19). Int. J. Mol. Sci. 2015, 16, 13908-13920. https://doi.org/10.3390/ijms160613908
Pan X-W, Zhao X-H. In Vitro Proliferation and Anti-Apoptosis of the Papain-Generated Casein and Soy Protein Hydrolysates towards Osteoblastic Cells (hFOB1.19). International Journal of Molecular Sciences. 2015; 16(6):13908-13920. https://doi.org/10.3390/ijms160613908
Chicago/Turabian StylePan, Xiao-Wen, and Xin-Huai Zhao. 2015. "In Vitro Proliferation and Anti-Apoptosis of the Papain-Generated Casein and Soy Protein Hydrolysates towards Osteoblastic Cells (hFOB1.19)" International Journal of Molecular Sciences 16, no. 6: 13908-13920. https://doi.org/10.3390/ijms160613908
APA StylePan, X.-W., & Zhao, X.-H. (2015). In Vitro Proliferation and Anti-Apoptosis of the Papain-Generated Casein and Soy Protein Hydrolysates towards Osteoblastic Cells (hFOB1.19). International Journal of Molecular Sciences, 16(6), 13908-13920. https://doi.org/10.3390/ijms160613908






