Ultrasound Tissue Characterization of Vulnerable Atherosclerotic Plaque
Abstract
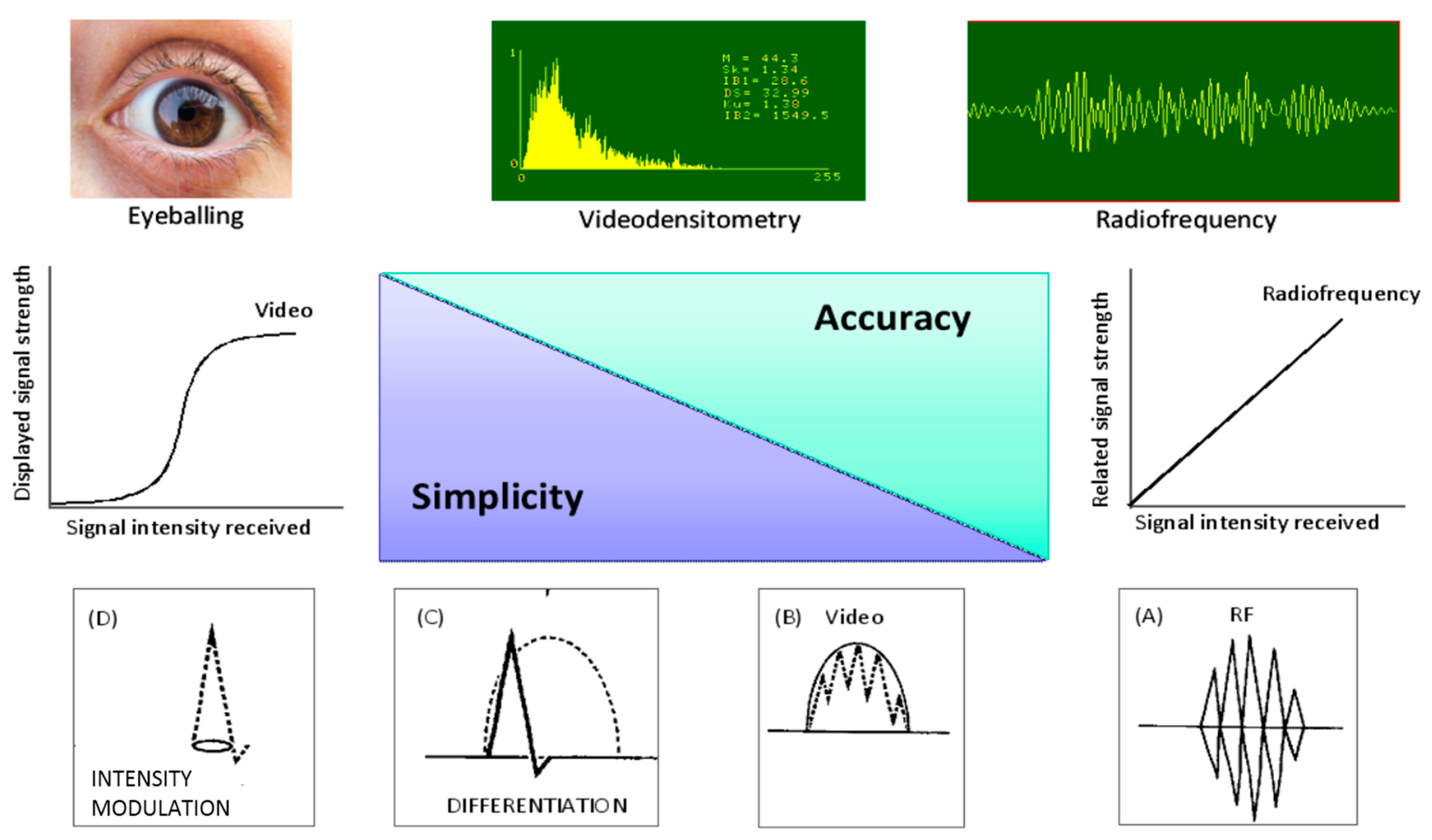
:1. Tissue Characterization of Vulnerable Plaque: From Histology to Ultrasound
| Histology | Ultrasound |
|---|---|
| Outward remodeling | Stenosis > 70% |
| Decreased Fibrous Tissue | Hypoechoic core |
| Increased Lipid-Hemorrhages | Hypoechoic core |
| More necrotic core | Dishomogeneous texture |
| Macrophages—inflammation | Dishomogeneous texture |
| Micro-calcification | Spotty hyper-dense foci |
| Endothelial rupture | Irregular border by CEUS |
| Intimal neovessel formation | Vascularization by CEUS |
2. Tissue Characterization of the Atherosclerotic Plaque: Ex Vivo Studies
3. In Vivo Ultrasonic Tissue Characterization
| Parameter | B-Mode Ultrasound Imaging | ||
|---|---|---|---|
| Vascular | Transesophageal | Intravascular | |
| Ultrasound frequency | 5–15 | 5–10 | 15–20 |
| Signal-to-noise ratio | ++ | ++ | +++ |
| Accuracy | ++ | ++ | +++ |
| Prognostic value | ++ | ++ | +++ |
| Applicability | Bedside | Echo lab | Cath lab |
| Invasiveness | Non-invasive | Semi-invasive | Invasive |
| Main target artery | Carotid (femoral) | Thoracic Aorta | Coronary |
4. Ultrasound Plaque Morphology as an Index of Clinical Instability
5. Clinical Implications
| Type of Plaque | Unstable | Stable |
|---|---|---|
| Visual assessment | Hypo-, Anechoic | Iso-, Hyper-echoic |
| Heterogeneous | Homogeneous | |
| Irregular surface | Regular surface | |
| Videodensitometry | Low median gray level | High median gray level |
| High entropy | Low entropy | |
| Radiofrequency | <13 dB | 14–33 dB |
| CEUS | Neovessel Present | Neovessel Absent |
| Risk | Low-Risk | High-Risk |
|---|---|---|
| Plaque border profile | Smooth | Irregular |
| Echo-density | Iso-, Hyper-echoic | Hypo-, Anechoic |
| Plaque luminal border * | Regular | Irregular |
| Plaque neovascularization * | Absent | Present |
| Spotty calcification | Rare | Frequent |
| Massive calcification | Frequent | Rare |
| Plaque burden | Low (<40% stenosis) | High (>70% stenosis) |
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mor-Avi, V.; Lang, R.M.; Badano, L.P.; Belohlavek, M.; Cardim, N.M.; Derumeaux, G.; Galderisi, M.; Marwick, T.; Nagueh, S.F.; Sengupta, P.P.; et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur. J. Echocardiogr. 2011, 12, 167–205. [Google Scholar] [CrossRef] [PubMed]
- Fleg, J.L.; Stone, G.W.; Fayad, Z.A.; Granada, J.F.; Hatsukami, T.S.; Kolodgie, F.D.; Ohayon, J.; Pettigrew, R.; Sabatine, M.S.; Tearney, G.J.; et al. Detection of high-risk atherosclerotic plaque: Report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc. Imaging 2012, 5, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S.; American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical cardiovascular disease risk: Consensus statement from the American society of echocardiography. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Gallino, A.; Stuber, M.; Crea, F.; Falk, E.; Corti, R.; Lekakis, J.; Schwitter, J.; Camici, P.; Gaemperli, O.; di Valentino, M.; et al. In vivo imaging of atherosclerosis. Atherosclerosis 2012, 224, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Wissler, R.W. Principles of the pathogenesis of atherosclerosis. In Heart Disease: A Textbook of Cardiovascular Medicine; Braunwald, E., Ed.; Saunders: Philadelphia, PA, USA, 1984; pp. 1183–1194. [Google Scholar]
- Picano, E.; Landini, L.; Distante, A.; Sarnelli, R.; Benassi, A.; L’Abbate, A. Different degrees of atherosclerosis detected by backscattered ultrasound: An in vitro study on fixed human aortic walls. J. Clin. Ultrasound 1983, 11, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Landini, L.; Distante, A.; Benassi, A.; Sarnelli, R.; L’Abbate, A. Fibrosis, lipids, and calcium in human atherosclerotic plaque. In vitro differentiation from normal aortic walls by ultrasonic attenuation. Circ. Res. 1985, 56, 556–562. [Google Scholar]
- Picano, E.; Landini, L.; Distante, A.; Salvadori, M.; Lattanzi, F.; Masini, M.; L’Abbate, A. Angle dependence of ultrasonic backscatter in arterial tissues: A study in vitro. Circulation 1985, 72, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Landini, L.; Lattanzi, F.; Mazzarisi, A.; Sarnelli, R.; Distante, A.; Benassi, A.; L’Abbate, A. The use of frequency histograms of ultrasonic backscatter amplitudes for detection of atherosclerosis in vitro. Circulation 1986, 74, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Landini, L.; Lattanzi, F.; Salvadori, M.; Benassi, A.; L’Abbate, A. Time domain echo pattern evaluations from normal and atherosclerotic arterial walls: A study in vitro. Circulation 1988, 77, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Wolverson, M.K.; Bashiti, H.M.; Peterson, G.J. Ultrasonic tissue characterization of atheromatous plaques using a high resolution real time scanner. Ultrasound Med. Biol. 1983, 9, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, B.; Saffitz, J.E.; Miller, J.G.; Sobel, B.E. Quantitative ultrasonic characterization of the nature of atherosclerotic plaques in human aorta. Circ. Res. 1987, 60, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Hiro, T.; Leung, C.Y.; Karimi, H.; Farvid, A.R.; Tobis, J.M. Angle dependence of intravascular ultrasound imaging and its feasibility in tissue characterization of human atherosclerotic tissue. Am. Heart J. 1999, 137, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, N.; Berry, G.J.; Kolz, M.L.; Oshima, A.; Metz, J.A.; Preuss, P.; Brisken, A.F.; Pauliina Moore, M.; Yock, P.G.; Fitzgerald, P.J. Tissue characterization of atherosclerotic plaques by intravascular radiofrequency signal analysis: An in vitro study of human coronary artery. Am. Heart J. 2000, 140, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Reilly, L.M.; Lusby, R.J.; Hughes, L.; Ferrell, L.D.; Stoney, R.J.; Ehrenfeld, W.K. Carotid plaque histology using real time ultrasonography. Clinical and therapeutic implications. Am. J. Surg. 1983, 146, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Gray-Weale, A.C.; Graham, J.C.; Burnett, J.R.; Byrne, K.; Lusby, R.J. Carotid artery atheroma: Comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J. Cardiovasc. Surg. 1988, 29, 676–681. [Google Scholar]
- European Carotid Plaque Study Group. Carotid artery composition—Relation to clinical presentation and ultrasound B-mode imaging. Eur. J. Endovasc. Surg. 1995, 10, 23–32. [Google Scholar]
- Joakimsen, O.; Bшona, K.H.; Stensland-Bugge, E. Reproducibility of ultrasound assessment of carotid plaque occurrence, thickness, and morphology. The Tromsø study. Stroke 1997, 28, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Grønholdt, M.L.; Wiebe, B.M.; Laursen, H.; Nielsen, T.G.; Schroeder, T.V.; Sillesen, H. Lipid-rich carotid artery plaques appear echolucent on ultrasound B-mode images and may be associated with intraplaque hemorrhage. Eur. J. Vasc. Endovasc. Surg. 1997, 14, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, A.M.; Urbani, M.P.; Picano, E.; Paterni, M.; Borgatti, E.; de Fabritiis, A.; Landini, L. In vivo ultrasonic parametric imaging of carotid atherosclerotic plaque by videodensitometric technique. Angiology 1995, 46, 663–672. [Google Scholar] [CrossRef] [PubMed]
- El-Barghouty, N.M.; Levine, T.; Ladva, S.; Flanagan, A.; Nicolaides, A. Histological verification of computerized carotid plaque characterization. Eur. J. Vasc. Endovasc. Surg. 1996, 11, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Baroncini, L.A.V.; Pazin Filho, A.; Murta, O., Jr.; Martins, A.R.; Ramos, S.O.; Cherri, J.; Piccinato, C.E. Ultrasonic tissue characterization of vulnerable carotid plaque: Correlation between videodensitometric method and histological exam. Cardiov. Ultrasound 2006, 4. [Google Scholar] [CrossRef]
- Urbani, M.P.; Picano, E.; Parenti, G.; Mazzarisi, A.; Fiori, L.; Paterni, M.; Pelosi, G.; Landini, L. In vivo radiofrequency-based ultrasonic tissue characterization of the atherosclerotic plaque. Stroke 1993, 24, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Takatsu, H.; Noda, T.; Ito, Y.; Kunishima, A.; Arai, M.; Nishigaki, K.; Takemura, G.; Morita, N.; Minatoguchi, S.; et al. Noninvasive quantitative tissue characterization and two-dimensional color-coded map of human atherosclerotic lesions using ultrasound integrated backscatter. Comparison between histology and integrated backscatter images before and after death. J. Am. Coll. Cardiol. 2001, 38, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Waki, H.; Masuyama, T.; Mori, H.; Maeda, T.; Kitade, K.; Moriyasu, K.; Tsujimoto, M.; Fujimoto, K.; Koshimae, N.; Matsuura, N. Ultrasonic tissue characterization of the atherosclerotic carotid plaque: Histologic correlates of carotid integrated backscatter. Circ. J. 2003, 67, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Gussenhoven, W.J.; Essed, C.E.; Frietman, P.; Mastik, F.; Lancee, C.; Slager, C.; Serruys, P.; Gerritsen, P.; Pieterman, H.; Bom, N. Intravascular echographic assessment of vessel wall characteristics: A correlation with histology. Int. J. Cardiovasc. Imaging 1989, 4, 105–116. [Google Scholar] [CrossRef]
- Coli, S.; Magnoni, M.; Sangiorgi, G.; Marrocco-Trischitta, M.M.; Melisurgo, G.; Mauriello, A.; Spagnoli, L.; Chiesa, R.; Cianflone, D.; Maseri, A. Contrast-enhanced ultrasound imaging of intraplaque neovascularization in carotid arteries: Correlation with histology and plaque echogenicity. J. Am. Coll. Cardiol. 2008, 52, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Faggioli, G.L.; Pini, R.; Mauro, R.; Pasquinelli, G.; Fittipaldi, S.; Freyrie, A.; Serra, C.; Stella, A. Identification of carotid vulnerable plaque in contrast-enhanced ultrasound: Correlation with plaque histology, symptoms and cerebral computed tomography. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Partovi, S.; Loebe, M.; Aschwanden, M.; Baldi, T.; Jäger, K.A.; Feinstein, S.B.; Staub, D. Contrast-enhanced ultrasound for assessing carotid atherosclerosis plaque lesions. Am. J. Radiol. 2012, 198, W13–W19. [Google Scholar]
- Gronholdt, M.L.; Nordestgaard, B.G.; Schroeder, T.V.; Vorstrup, S.; Sillesen, H. Ultrasound echolucent carotid plaques predict future strokes. Circulation 2001, 104, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, E.B.; Bonaa, K.H.; Joakimsen, O. Echo-lucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis. Circulation 2001, 103, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Honda, O.; Sugiyama, S.; Kugiyama, K.; Fukushima, H.; Nakamura, S.; Koide, S.; Kojima, S.; Hirai, N.; Kawano, H.; Soejima, H.; et al. Echolucent carotid plaques predict future coronary events in patients with coronary artery disease. J. Am. Coll. Cardiol. 2004, 43, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Biasi, G.M.; Froio, A.F.; Diethrich, E.B.; Deleo, G.; Galimberti, S.; Mingazzini, P.; Nicolaides, A.N.; Griffin, M.; Raithel, D.; Reid, D.B.; et al. Carotid plaque echolucency increases the risk of stroke in carotid stenting. The Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation 2004, 110, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Peçanha, P.B.; Venneri, L.; Pasanisi, E.; Pratali, L.; Picano, E. The impact of carotid plaque presence and morphology on mortality outcome in cardiological patients. Cardiovasc. Ultrasound 2006, 4. [Google Scholar] [CrossRef]
- Yamada, K.; Kawasaki, M.; Yoshima, S.; Enomoto, Y.; Asano, T.; Minatocuchi, S.; Iwana, T. Prediction of silent ischemic lesions after carotid stenting using integrated backscatter ultrasouns and magnetic resonance imaging. Atherosclerosis 2010, 208, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Katakami, N.; Kaneto, H.; Takahara, M.; Nishio, M.; Kasami, R.; Sakamoto, K.; Umayahara, Y.; Sumitsuji, S.; Ueda, Y.; et al. The utility of ultrasound tissue characterization in the prediction of cardiovascular events in diabetic patients. Atherosclerosis 2013, 230, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Deng, Y.B.; Liu, Y.N.; Bi, X.J.; Sun, J.; Tang, Q.Y.; Deng, Q. Use of carotid plaque neovascularization of contrast-enhanced ultrasound to predict coronary events in patients with coronary artery disease. Radiology 2013, 268, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Fagerberg, B.; Hulthe, J. Non-stenotic echolucent ultrasound-assessed femoral artery plaques are predictive for future cardiovascular events in middle-aged men. Atherosclerosis 2005, 181, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Tzourio, C.; Bertrand, B.; Chauvel, C.; Bousser, M.G.; Amarenco, P. Aortic plaque morphology and vascular events: A follow-up study in patients with ischemic stroke. FAPS Investigators. French Study of Aortic Plaques in Stroke. Circulation 1997, 96, 3838–3841. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Jin, H.Y.; Seo, J.S.; Yang, T.H.; Kim, D.K.; Park, Y.A.; Cho, K.I.; Park, Y.H.; Kim, D.S. Meta-analysis of plaque composition by intravascular ultrasound and its relation to distal emboli after percutaneous coronary interventions. Am. J. Cardiol. 2013, 111, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Mintz, G.S.; Tam, A.; McPherson, J.A.; Iñiguez, A.; Fajadet, J.; Fahy, M.; Weisz, G.; de Bruyne, B.; Serruys, P.W.; et al. Prevalence, distribution, predictors and outcomes of patients with calcified nodules in native coronary arteries: A 3-vessel intravascular ultrasound anaysis from Providing Regional Observations to Study predictors of Events in the Coronary Tree (PROSPECT). Circulation 2012, 126, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, A.; Biasucci, L.M.; Lanza, G.A.; Coli, S.; Silvestri, P.; Cianflone, D.; Liuzzo, G.; Burzotta, F.; Crea, F.; Maseri, A. Inflammation as a possible link between coronary and carotid plaque instability. Circulation 2004, 109, 3158–3163. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimi, P.; Jashari, F.; Johansson, E.; Gronlund, C.; Bajraktari, G.; Wester, P.; Henein, M.Y. Vulnerable plaques in the contralateral carotid arteries in symptomatic patients: A detailed ultrasonic analysis. Atherosclerosis 2014, 235, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Sano, K.; Okubo, M.; Yokoyama, H.; Ito, Y.; Murata, I.; Tsuchiya, K.; Minatoguchi, S.; Zhou, X.; Fujita, H.; et al. Volumetric quantitative analysis of tissue characteristics of coronary plaques after statin therapy using three-dimensional integrated backscatter intravascular ultrasound. J. Am. Coll. Cardiol. 2005, 45, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Komiyama, N.; Courtney, B.K.; Nakayama, T.; Namikawa, S.; Kuriyama, N.; Koizumi, T.; Nameki, M.; Fitzgerald, P.J.; Komuro, I. Plasma low-density lipoprotein reduction and structural effects on coronary atherosclerotic plaques by atorvastatin as clinically assessed with intravascular ultrasound radio-frequency signal analysis: A randomized prospective study. Am. Heart J. 2005, 150, 287. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yoshimura, S.; Kawasaki, M.; Enomoto, Y.; Asano, T.; Minatoguchi, S.; Iwama, T. Effects of atorvastatin on carotid atherosclerotic plaques: A randomized trial with quantitative tissue characterization of carotid atherosclerotic plaques with integrated backscatter. Cerebrovasc. Dis. 2009, 28, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Sugiyama, S.; Kugiyama, K.; Honda, O.; Fukushima, H.; Koga, H.; Horibata, Y.; Hirai, T.; Sakamoto, T.; Yoshimura, M.; et al. Stabilization of carotid atheroma assessed by quantitative ultrasound analysis in non-hypercholesterolemic patients with coronary artery disease. J. Am. Coll. Cardiol. 2005, 46, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Gerasimidis, T.; Moumtzouoglou, A.; Kapelouzou, A.; Sailer, N.; Fotiadis, G.; Vitta, I.; Katinios, A.; Kougias, P.; Bandios, S.; et al. Intensive lipid-lowering therapy ameliorates novel calcification markers and GSM score in patients with carotid stenosis. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, H.; Sakaguchi, M.; Furukado, S.; Hoshi, T.; Abe, Y.; Hougaku, H.; Hori, M.; Kitagawa, K. Statin therapy increases carotid plaque echogenicity in hypercholesterolemic patients. Ultrasound Med. Biol. 2008, 34, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Della-Morte, D.; Moussa, I.; Elkind, M.S.; Sacco, R.L.; Rundek, T. The short-term effects of atorvastatin on carotid plaque morphology assessed by computer-assisted gray scale densitometry: A pilot study. Neurol. Res. 2011, 33, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Ostling, G.; Gonçalves, I.; Wikstrand, J.; Berglund, G.; Nilsson, J.; Hedblad, B. Long-term treatment with low dose metoprolol is associated with increased plaque echogenicity: The β-blocker cholesterol lowering asymptomatic plaque study. Atherosclerosis 2011, 215, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Pellikka, P.A.; Douglas, P.S.; Miller, J.G.; Abraham, T.P.; Baumann, R.; Buxton, D.B.; Byrd, B.F., III; Chen, P.; Cook, N.L.; Gardin, J.M.; et al. American Society of Echocardiography Cardiovascular technology and research summit: A roadmap for 2020. J. Am. Soc. Echocardiogr. 2013, 26, 325–338. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picano, E.; Paterni, M. Ultrasound Tissue Characterization of Vulnerable Atherosclerotic Plaque. Int. J. Mol. Sci. 2015, 16, 10121-10133. https://doi.org/10.3390/ijms160510121
Picano E, Paterni M. Ultrasound Tissue Characterization of Vulnerable Atherosclerotic Plaque. International Journal of Molecular Sciences. 2015; 16(5):10121-10133. https://doi.org/10.3390/ijms160510121
Chicago/Turabian StylePicano, Eugenio, and Marco Paterni. 2015. "Ultrasound Tissue Characterization of Vulnerable Atherosclerotic Plaque" International Journal of Molecular Sciences 16, no. 5: 10121-10133. https://doi.org/10.3390/ijms160510121
APA StylePicano, E., & Paterni, M. (2015). Ultrasound Tissue Characterization of Vulnerable Atherosclerotic Plaque. International Journal of Molecular Sciences, 16(5), 10121-10133. https://doi.org/10.3390/ijms160510121






