Antiproliferative, Antibacterial and Antifungal Activity of the Lichen Xanthoria parietina and Its Secondary Metabolite Parietin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antimicrobial Activity of Acetone Extract from Xanthoria parietina and Parietin
| Microrganism | MIC | MBC | |||||
|---|---|---|---|---|---|---|---|
| AE | Parietin | CTAX | PENG | TET | AE | Parietin | |
| S. aureus ATCC 13709 | 7.8 ± 0.1 | 7.8 ± 0.2 | 2 ± 0.1 | 0.03 ± 0 | 2 ± 0.1 | 62.5 ± 0.6 | 62.5 ± 0.8 |
| S. aureus CI | 15.6 ± 0.3 | 15.6 ± 0.4 | R | R | R | >100 | >100 |
| E. faecalis ATCC 14428 | 15.6 ± 0.1 | 7.8 ± 0.3 | R | 8 ± 0.2 | 2 ± 0.1 | >100 | 62.5 ± 0.7 |
| E. faecalis CI | 31.3 ± 0.2 | 15.6 ± 0.1 | R | R | R | >100 | >100 |
| P. vulgaris ATCC 12454 | 15.6 ± 0.3 | 15.6 ± 0.2 | 2 ± 0.1 | 4 ± 0.3 | R | R | R |
| P. vulgaris CI | 31.3 ± 0.1 | 31.3 ± 0.3 | 32 ± 0.3 | R | R | R | R |
| P. mirabilis ATCC 7002 | 15.6 ± 0.1 | 15.6 ± 0.1 | 0.03 ± 0 | 4 ± 0.2 | 32 ± 0.6 | 62.5 ± 0.7 | 62.5 ± 0.4 |
| P. mirabilis CI | 15.6 ± 0.1 | 31.3 ± 0.2 | 32 ± 0.6 | R | R | R | R |
| S. typhi ATCC 19430 | 15.6 ± 0.2 | 31.3 ± 0.4 | 0.5 ± 0.1 | 4 ± 0.2 | 1 ± 0.3 | 62.5 ± 0.5 | >100 |
| S. typhi CI | 31.3 ± 0.2 | 62.5 ± 0.2 | 1 ± 0.1 | 2 ± 0.1 | 1 ± 0.1 | >100 | >100 |
| E. cloacae ATCC 10699 | 31.3 ± 0.0 | 31.3 ± 0.3 | R | 4 ± 0.4 | R | >100 | >100 |
| E. cloacae CI | 62.5 ± 0.1 | 62.5 ± 0.2 | R | R | R | >100 | >100 |
| E. aerogenes ATCC 13048 | 31.3 ± 0.2 | 15.6 ± 0.1 | R | 4 ± 0.1 | R | R | R |
| E. aerogenes CI | 62.5 ± 0.5 | 31.3 ± 0.2 | R | R | R | R | R |
| P. aeruginosa ATCC 27853 | 31.3 ± 0.1 | 31.3 ± 0.3 | 16 ± 0.3 | R | 32 ± 0.1 | R | R |
| P. aeruginosa CI | 62.5 ± 0.2 | 62.5 ± 0.1 | 32 ± 0.4 | R | R | R | R |
| K. pneumoniae ATCC 27736 | 62.5 ± 0.3 | 31.3 ± 0.2 | 0.1 ± 0.0 | R | 16 ± 0.1 | R | R |
| K. pneumoniae CI | >100 | 62.5 ± 0.7 | 32 ± 0.4 | R | R | R | R |
2.2. Antifungal Activity of Acetone Extract from Xanthoria parietina and Parietin
| Microrganism | MIC | MFC | |||
|---|---|---|---|---|---|
| AE | Parietin | KCON | AE | Parietin | |
| Rhyzoctonia solani | 62.5 ± 0.3 | 31.3 ± 0.5 | 0.2 ± 0.0 | – | – |
| Botridis cinerea | >100 | 62.5 ± 0.4 | 0.2 ± 0.1 | – | – |
| Candida albicans CI | >100 | 62.5 ± 0.7 | 0.4 ± 0.1 | – | – |
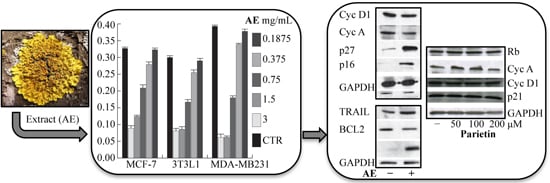
2.3. Anticancer Action of Acetone Extract from Xanthoria parietina and Parietin
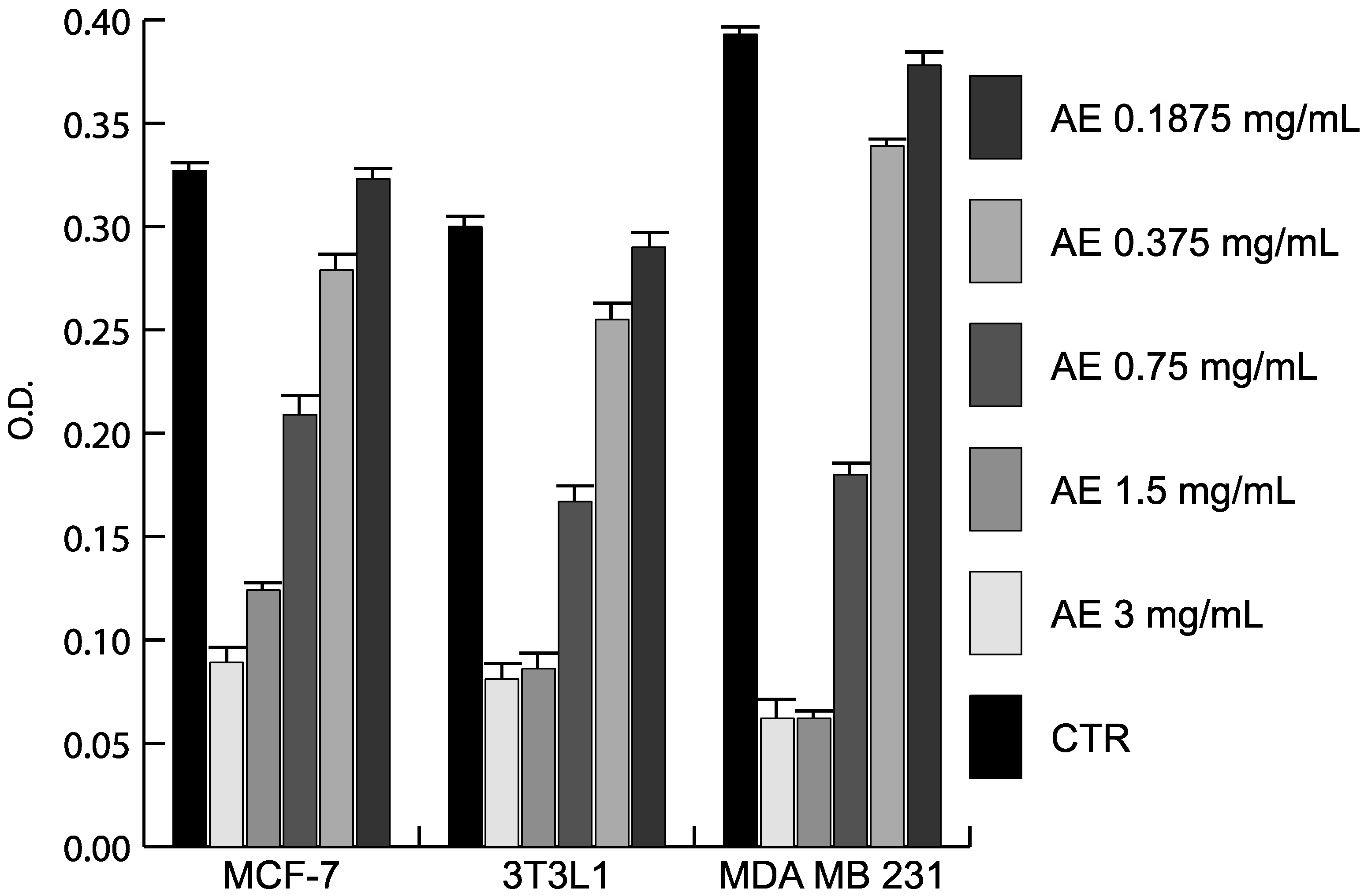
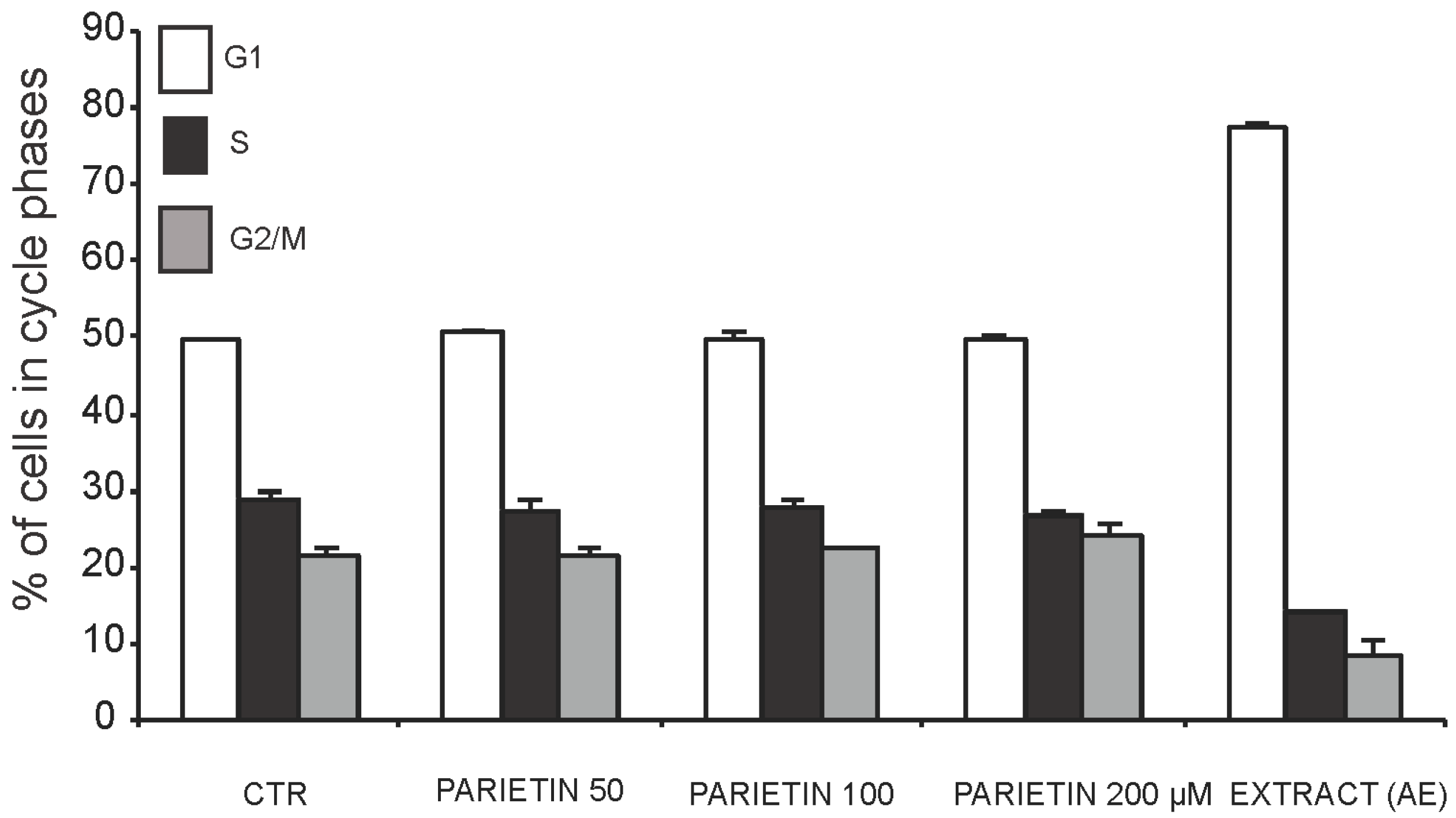
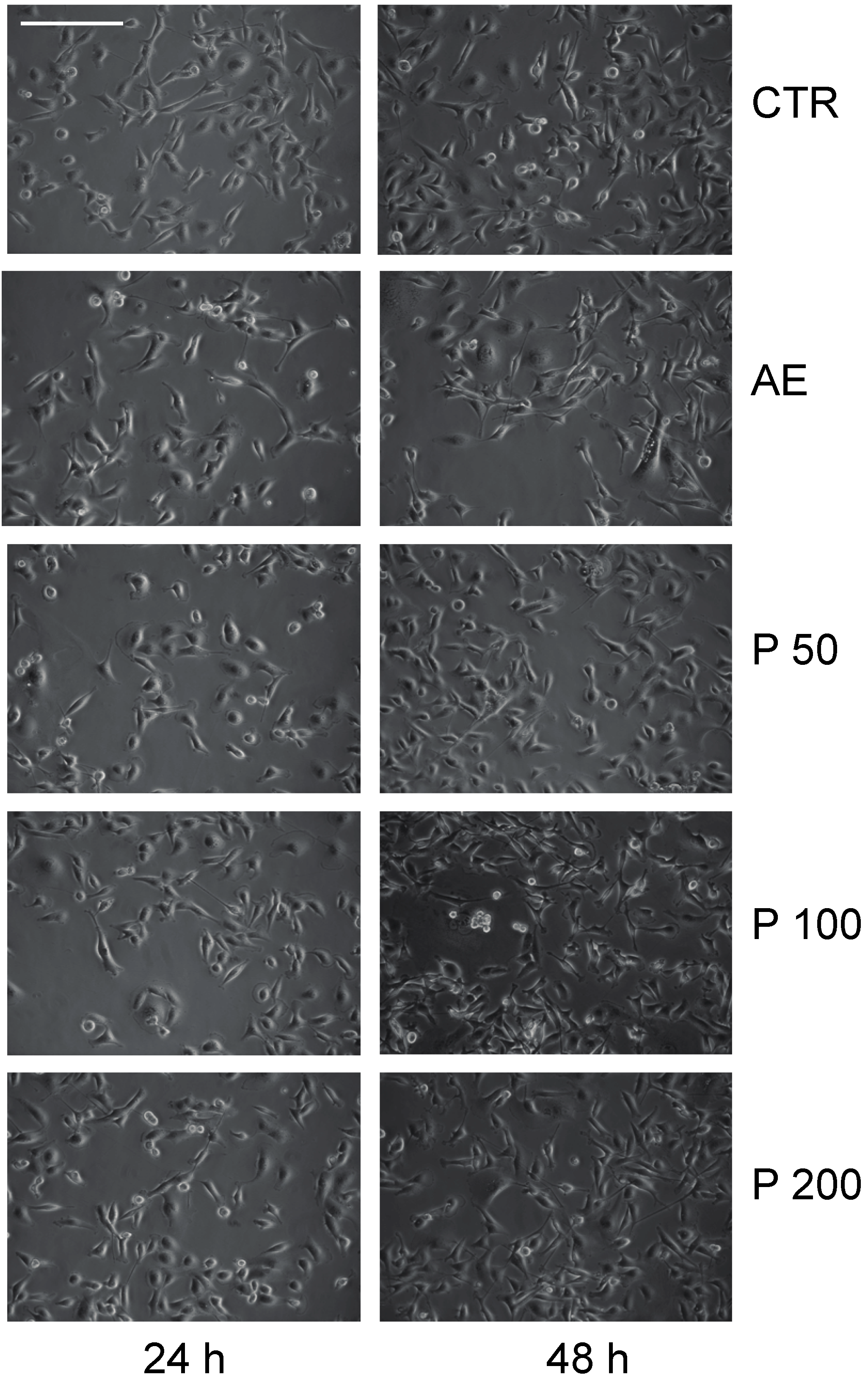
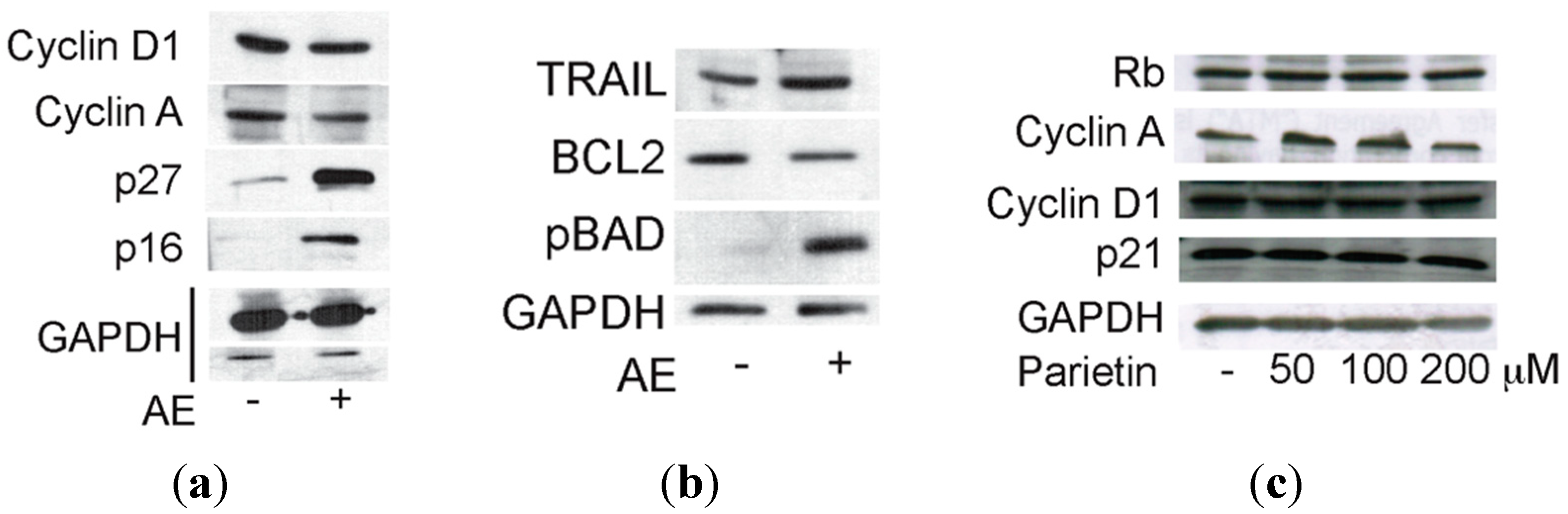
2.4. Discussion
3. Experimental Section
3.1. Plant Material and Extraction Procedures
3.2. Microorganisms and Media
3.3. Minimal Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Determination
3.4. Antifungal Activity
3.5. Cancer Cell Lines and Materials
3.6. Crystal Violet Assays
3.7. Cell Cycle Analysis
3.8. Western Blot Analyses
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Abbreviations
| Bcl-2 | B Cell Lymphoma 2 |
| BAD | Bcl-2-associated agonist of cell death |
| HPLC | high-performance liquid chromatography |
| MBC | Minimum bactericidal concentration |
| MIC | Minimal inhibitory concentration |
| MS | mass spectrometry |
| NMR | nuclear magnetic resonance |
| TRAIL | TNF-related apoptosis-inducing ligand |
Conflicts of Interest
References
- Nash, T.H. Lichen Biology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Dayan, F.E.; Romagini, J.G. Structural diversity of lichen metabolites and their potential for use. Adv. Microb. Toxin Res. Biotechnol. Explor. 2002, 151–169. [Google Scholar] [CrossRef]
- Lawrey, J.D. Biological role of lichen substances. Byrologist 1986, 89, 111–122. [Google Scholar] [CrossRef]
- Boustie, J.; Grube, M. Lichens—A promising source of bioactive secondary metabolites. Plant Genet. Resour. 2005, 3, 273–278. [Google Scholar] [CrossRef]
- Shrestha, G.; St. Clair, L.L. Lichens: A promising source of antibiotic and anticancer drugs. Phytochem. Rev. 2013, 12, 229–244. [Google Scholar] [CrossRef]
- Saklani, A.; Upreti, D.K. Folk uses of some lichens of Sikkim. J. Ethnopharmacol. 1992, 37, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Honegger, R.; Zippler, U.; Scherrer, S.; Dyer, P.S. Genetic diversity in Xanthoria parietina (L.) Th. Fr. (lichen-forming ascomycete) from worldwide locations. Lichenologist 2004, 36, 381–390. [Google Scholar] [CrossRef]
- Gauslaa, Y.; McEvoy, M. Seasonal changes in solar radiation drive acclimation of the sun-screening compound parietin in the lichen Xanthoria parietina. Basic Appl. Ecol. 2005, 6, 75–82. [Google Scholar] [CrossRef]
- Müller, K. Pharmaceutically relevant metabolites from lichens. Appl. Microbiol. Biot. 2002, 56, 9–16. [Google Scholar]
- Backorova, M.; Backor, M.; Mikes, J.; Jendzelovsky, R.; Fedorocko, P. Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol. In Vitro 2011, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kosanic, M.; Manojlovic, N.; Jankovic, S.; Stanojkovic, T.; Rankovic, B. Evernia. prunastri and Pseudoevernia. furfuraceae lichens and their major metabolites as antioxidant, antimicrobial and anticancer agents. Food Chem. Toxicol. 2013, 53, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Manojlovic, N.T.; Solujic, S.; Sukdolak, S.; Krstic, L.J. Isolation and antimicrobial activity of anthraquinones from some species of the lichen genus Xanthoria. J. Serb. Chem. Soc. 2000, 65, 555–560. [Google Scholar]
- Manojlovic, N.T.; Solujic, S.; Sukdolak, S.; Milosev, M. Antifungal activity of Rubia tinctorum, Rhamnus frangula and Caloplaca cerina. Fitoterapia 2005, 76, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Tamokou, J.D.D.; Tala, M.F.; Wabo, H.K.; Kuiate, J.R.; Tane, P. Antimicrobial activities of methanol extract and compounds from stem bark of Vismia rubescens. J. Ethnopharmacol. 2009, 124, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, P.D.; Porter, N. Lichen-forming fungi: Potential sources of novel metabolites. Trend Biotechnol. 1991, 9, 409–414. [Google Scholar] [CrossRef]
- Cocchietto, M.; Skert, N.; Nimis, P.L.; Sava, G. A review on usnic acid, an interesting natural compound. Naturwisseneschaften 2002, 89, 137–146. [Google Scholar] [CrossRef]
- Pompilio, A.; Pomponio, S.; di Vincenzo, V.; Crocetta, V.; Nicoletti, M.; Piovano, M.; Garbarino, J.A.; di Bonaventura, G. Antimicrobial and antibiofilm activity of secondary metabolites of lichens against methicillin-resistant Staphylococcus aureus strains from cystic fibrosis patients. Future Microbiol. 2013, 8, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Celenza, G.; Segatore, B.; Setacci, D.; Bellio, P.; Brisdelli, F.; Piovano, M.; Garbarino, J.A.; Nicoletti, M.; Perilli, M.; Amicosante, G. In vitro antimicrobial activity of pannarin alone and in combination with antibiotics against methicillin-resistant Staphylococcus aureus clinical isolates. Phytomedicine 2012, 19, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Schmeda-Hirschmann, G.; Tapia, A.; Lima, B.; Pertino, M.; Sortino, M.; Zacchino, S.; de Arias, A.R.; Feresin, G.F. A new antifungal and antiprotozoal depside from the andean lichen Protousnea poeppigii. Phytother. Res. 2007, 22, 349–355. [Google Scholar] [CrossRef]
- Goel, M.; Dureja, P.; Rani, A.; Uniyal, P.L.; Laatsch, H. Isolation, characterization and antifungal activity of major constituents of the Himalayan lichen Parmelia reticulata Tayl. J. Agric. Food Chem. 2011, 59, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Kopperman, H.I. l-usnic acid: Tumor inhibitor isolated from lichens. Experientia 1975, 31, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Piovano, M.; Lombardo, L.; Vanella, L.; Cardile, V.; Garbarino, J. Pannarin inhibits cell growth and induces cell death in human prostate carcinoma DU-145 cells. Anti-Cancer Drugs 2006, 17, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Piovano, M.; Lombardo, L.; Garbarino, J.; Cardile, V. Lichen metabolites prevent UV light and nitric oxide-mediated plasmid DNA damage and induce apoptosis in human melanoma cells. Life Sci. 2008, 83, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Backorova, M.; Jendzelovsky, R.; Kello, M.; Backor, M.; Mikes, J.; Fedorocko, P. Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines. Toxicol. In Vitro 2012, 26, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, D.; Ceccarelli, D.; Tiezzi, A.; Pisani, T.; Munzi, S.; Gaggi, C.; Loppi, S. Antiproliferative activity of lichen extracts on murine myeloma cells. Biologia 2009, 64, 59–62. [Google Scholar] [CrossRef]
- Honegger, R. The impact of different long-term storage conditions on the viability of lichen-forming ascomycetes and their green algal photobiont, Trebouxia. spp. Plant Biol. 2003, 5, 324–330. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S. Phytochemical investigation of the Australian lichens Ramalina glaucescens and Xanthoria parietina. Nat. Prod. Commun. 2009, 4, 959–964. [Google Scholar] [PubMed]
- Ieven, M.; Dirk, A.; Vanden Berghe, V.; Francis, M.; Vlietinck, A.; Lammens, E. Screening of higher plants for biological activities. I. Antimicrobial activity. Planta Med. 1979, 36, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ericcson, H.M.; Sherris, J.C. Antibiotic sensitivity testing: Report of an international collaborative study. Acta Pathol. Microbiol. Scand. 1971, 217, 1–90. [Google Scholar]
- Bontempo, P.; Carafa, V.; Grassi, R.; Basile, A.; Tenore, G.C.; Formisano, C.; Rigano, D.; Altucci, L. Antioxidant, antimicrobial and anti-proliferative activities of Solanum tuberosum L. var. Vitelotte. Food Chem. Toxicol. 2013, 55, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Conte, B.; Rigano, D.; Senatore, F.; Sorbo, S. Antibacterial and antifungal properties of acetonic extract of Feijoa sellowiana fruits and its effect on Helicobacter pylori growth. J. Med. Food 2010, 13, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, A.; Dell’Aversana, C.; Bugge, A.; Sarno, R.; Valente, S.; Rotili, D.; Manzo, F.; Teti, D.; Mandrup, S.; Ciana, P.; et al. HDACs class II-selective inhibition alters nuclear receptor-dependent differentiation. J. Mol. Endocrinol. 2010, 45, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.; Franci, G.; Dell’Aversana, C.; Ricciardiello, F.; Petraglia, F.; Carissimo, A.; Perone, L.; Maruotti, G.M.; Savarese, M.; Martinelli, P.; et al. MePR: A novel human mesenchymal progenitor model with characteristics of pluripotency. Stem Cells Dev. 2013, 22, 2368–2383. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, A.; Clarke, N.; Voltz, E.; Germain, E.; Ambrosino, C.; Bontempo, P.; Alvarez, R.; Schiavone, E.M.; Ferrara, F.; Bresciani, F.; et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat. Med. 2005, 11, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, A.; Pereira, R.; Khanwalkar, H.; Matarese, F.; García-Rodríguez, J.; Miceli, M.; Logie, C.; Kedinger, V.; Ferrara, F.; Stunnenberg, H.G.; et al. Death receptor pathway activation and increase of ROS production by the triple epigenetic inhibitor UVI5008. Mol. Cancer Ther. 2011, 10, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
- Lepore, I.; Dell’Aversana, C.; Pilyugin, M.; Conte, M.; Nebbioso, A.; de Bellis, F.; Tambaro, F.P.; Izzo, T.; Garcia-Manero, G.; Ferrara, F.; et al. HDAC inhibitors repress BARD1 isoform expression in acute myeloid leukemia cells via activation of miR-19a and/or b. PLoS ONE 2013, 8, e83018. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Casalino, L.; Petraglia, F.; Miceli, M.; Menafra, R.; Radic, B.; Tarallo, V.; Vitale, M.; Scarfò, M.; Pocsfalvi, G.; et al. The class I-specific HDAC inhibitor MS-275 modulates the differentiation potential of mouse embryonic stem cells. Biol. Open 2013, 2, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basile, A.; Rigano, D.; Loppi, S.; Di Santi, A.; Nebbioso, A.; Sorbo, S.; Conte, B.; Paoli, L.; De Ruberto, F.; Molinari, A.M.; et al. Antiproliferative, Antibacterial and Antifungal Activity of the Lichen Xanthoria parietina and Its Secondary Metabolite Parietin. Int. J. Mol. Sci. 2015, 16, 7861-7875. https://doi.org/10.3390/ijms16047861
Basile A, Rigano D, Loppi S, Di Santi A, Nebbioso A, Sorbo S, Conte B, Paoli L, De Ruberto F, Molinari AM, et al. Antiproliferative, Antibacterial and Antifungal Activity of the Lichen Xanthoria parietina and Its Secondary Metabolite Parietin. International Journal of Molecular Sciences. 2015; 16(4):7861-7875. https://doi.org/10.3390/ijms16047861
Chicago/Turabian StyleBasile, Adriana, Daniela Rigano, Stefano Loppi, Annalisa Di Santi, Angela Nebbioso, Sergio Sorbo, Barbara Conte, Luca Paoli, Francesca De Ruberto, Anna Maria Molinari, and et al. 2015. "Antiproliferative, Antibacterial and Antifungal Activity of the Lichen Xanthoria parietina and Its Secondary Metabolite Parietin" International Journal of Molecular Sciences 16, no. 4: 7861-7875. https://doi.org/10.3390/ijms16047861
APA StyleBasile, A., Rigano, D., Loppi, S., Di Santi, A., Nebbioso, A., Sorbo, S., Conte, B., Paoli, L., De Ruberto, F., Molinari, A. M., Altucci, L., & Bontempo, P. (2015). Antiproliferative, Antibacterial and Antifungal Activity of the Lichen Xanthoria parietina and Its Secondary Metabolite Parietin. International Journal of Molecular Sciences, 16(4), 7861-7875. https://doi.org/10.3390/ijms16047861