Human Anti-Oxidation Protein A1M—A Potential Kidney Protection Agent in Peptide Receptor Radionuclide Therapy
Abstract
:1. Peptide Receptor Radionuclide Therapy (PRRT)
1.1. Background
1.2. Diagnostic Assessment
1.3. Dose-Limiting Organs
2. Dosimetry
| 90Y-DOTATOC (Gy/GBq) | 177Lu-DOTATATE (Gy/GBq) | |
|---|---|---|
| Absorbed dose per unit administered activity | 2.84 ± 0.64 | 0.88 ± 0.19 |
| 2.44 (1.12–4.5) * | 0.62 (0.45–17.74) * | |
| 2.73 ± 1.41 | 0.9 ± 0.3 |
2.1. PRRT—Side Effects and Protective Measures
2.2. Kidney Activity Distributions
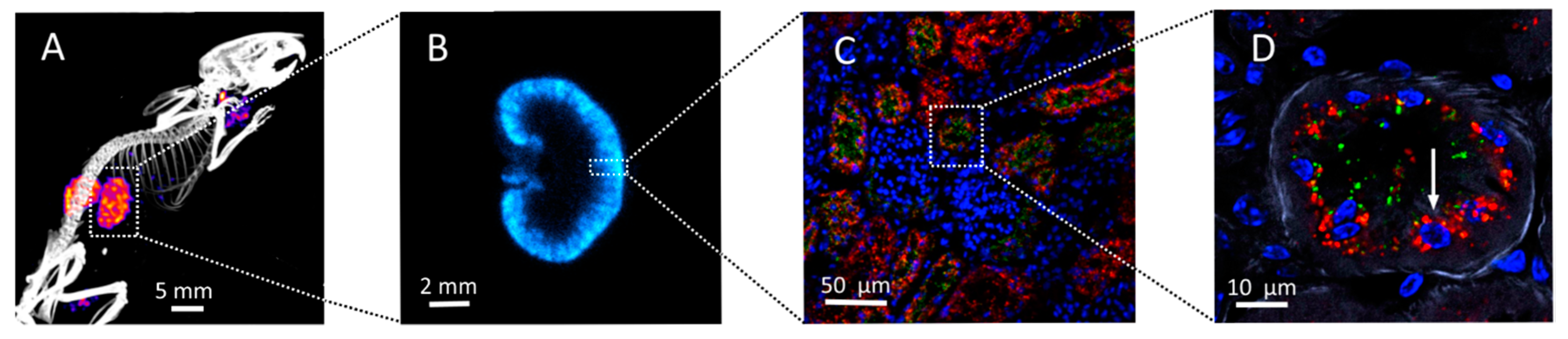
3. Oxidative Stress
3.1. Oxidation and Antioxidation
3.2. Radiation and Oxidative Stress
3.3. α1-Microglobulin (A1M)
3.3.1. Structure, Expression, and Distribution
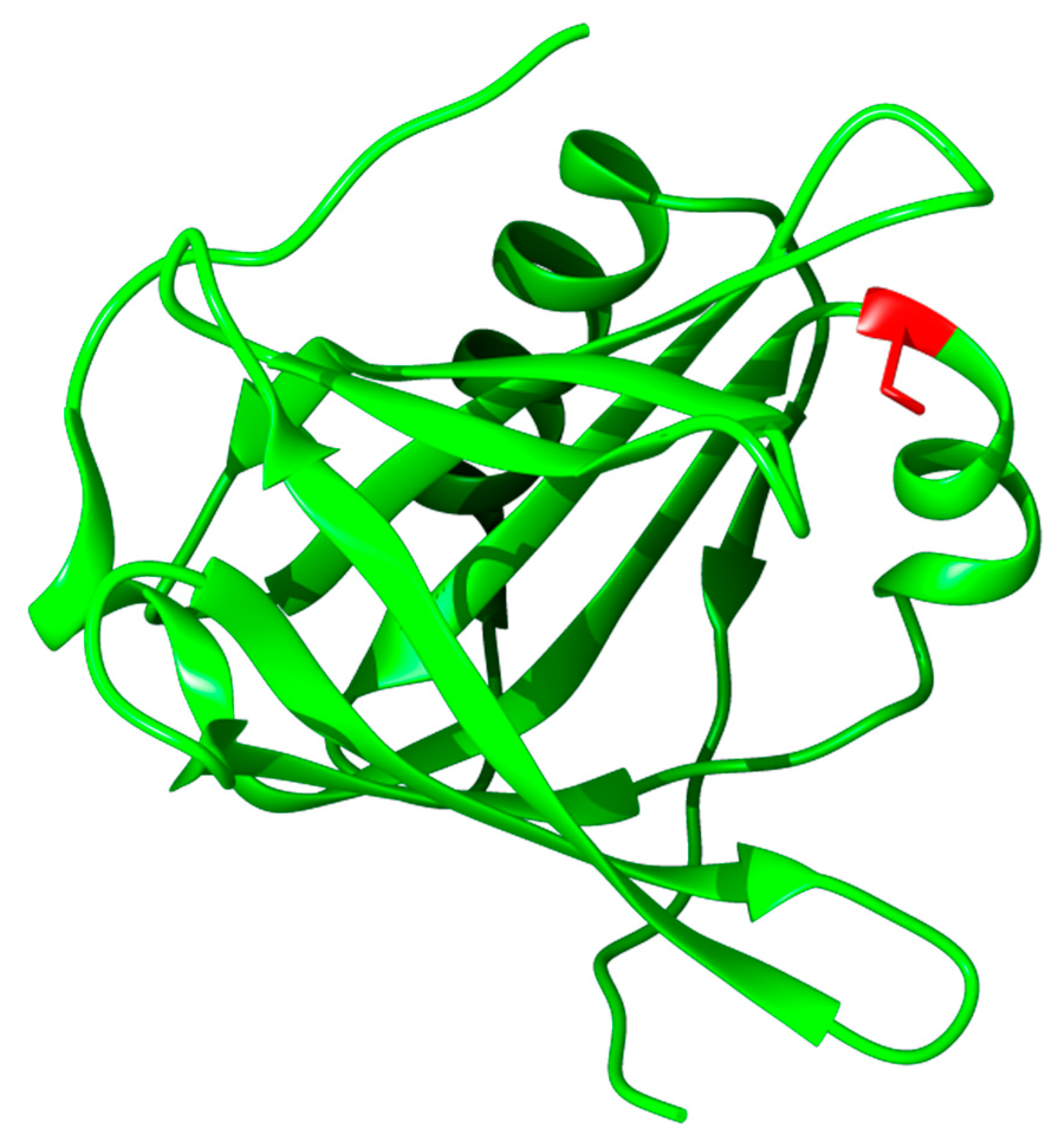
3.3.2. Biochemical Properties, Physiological Function, and Therapeutic Applications
4. A1M in PRRT
4.1. A1M Protects against Radiation-Induced Tissue Damage
4.2. Infused A1M Is Localized to Kidneys in Vivo
4.3. Protection Hypothesis and Proof-of-Concept Experiments
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taal, B.G.; Visser, O. Epidemiology of neuroendocrine tumours. Neuroendocrinology 2004, 80, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003, 97, 934–959. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Horsch, D.; O’Dorisio, M.S.; O’Dorisio, T.M.; Howe, J.R.; Cremonesi, M.; Kwekkeboom, D.J.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Valkema, R.; de Jong, M.; Bakker, W.H.; Breeman, W.A.; Kooij, P.P.; Lugtenburg, P.J.; de Jong, F.H.; Christiansen, A.; Kam, B.L.; de Herder, W.W.; et al. Phase I study of peptide receptor radionuclide therapy with (In-DTPA) octreotide: The rotterdam experience. Semin. Nucl. Med. 2002, 32, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; Krenning, E.P.; Lebtahi, R.; Komminoth, P.; Kos-Kudla, B.; de Herder, W.W.; Plockinger, U.; Mallorca Consensus Conference Participants; European Neuroendocrine Tumor Society. Enets consensus guidelines for the standards of care in neuroendocrine tumors: Peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology 2009, 90, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Kam, B.L.; Teunissen, J.J.; Krenning, E.P.; de Herder, W.W.; Khan, S.; van Vliet, E.I.; Kwekkeboom, D.J. Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, S103–S112. [Google Scholar] [CrossRef] [PubMed]
- Forrer, F.; Waldherr, C.; Maecke, H.R.; Mueller-Brand, J. Targeted radionuclide therapy with 90Y-DOTATOC in patients with neuroendocrine tumors. Anticancer Res. 2006, 26, 703–707. [Google Scholar] [PubMed]
- Maecke, H.R.; Reubi, J.C. Somatostatin receptors as targets for nuclear medicine imaging and radionuclide treatment. J. Nucl. Med. 2011, 52, 841–844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reubi, J.C.; Schar, J.C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Macke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog (177Lu-DOTA 0,Tyr3)octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Advanced Accelerator Applications. Available online: http://www.adacap.com/netter-1/ (accessed on 1 October 2015).
- Rindi, G. The enets guidelines: The new TNM classification system. Tumori 2010, 96, 806–809. [Google Scholar] [PubMed]
- Gains, J.E.; Bomanji, J.B.; Fersht, N.L.; Sullivan, T.; D’Souza, D.; Sullivan, K.P.; Aldridge, M.; Waddington, W.; Gaze, M.N. 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma. J. Nucl. Med. 2011, 52, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, M.; Bodei, L.; de Cicco, C.; Grana, C.M.; Cremonesi, M.; Botteri, E.; Baio, S.M.; Arico, D.; Sansovini, M.; Paganelli, G. Peptide receptor radionuclide therapy with (90)Y-DOTATOC in recurrent meningioma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Iten, F.; Muller, B.; Schindler, C.; Rochlitz, C.; Oertli, D.; Macke, H.R.; Muller-Brand, J.; Walter, M.A. Response to ((90)Yttrium-DOTA)-TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer: A phase ii clinical trial. Clin. Cancer Res. 2007, 13, 6696–6702. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Handkiewicz-Junak, D.; Grana, C.; Mazzetta, C.; Rocca, P.; Bartolomei, M.; Lopera-Sierra, M.; Cremonesi, M.; Chinol, M.; Macke, H.R.; et al. Receptor radionuclide therapy with 90Y-DOTATOC in patients with medullary thyroid carcinomas. Cancer Biother. Radiopharm. 2004, 19, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Iten, F.; Muller, B.; Schindler, C.; Rasch, H.; Rochlitz, C.; Oertli, D.; Maecke, H.R.; Muller-Brand, J.; Walter, M.A. ((90)Yttrium-DOTA)-TOC response is associated with survival benefit in iodine-refractory thyroid cancer long-term results of a phase 2 clinical trial. Cancer 2009, 115, 2052–2062. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, J.J.M.; Kwekkeboom, D.J.; Kooij, P.P.M.; Bakker, W.H.; Krenning, E.P. Peptide receptor radionuclide therapy for non-radioiodine-avid differentiated thyroid carcinoma. J. Nucl. Med. 2005, 46, 107s–114s. [Google Scholar] [PubMed]
- Sundin, A.; Vullierme, M.P.; Kaltsas, G.; Plockinger, U.; Mallorca Consensus Conference Participants; European Neuroendocrine Tumor Society. Enets consensus guidelines for the standards of care in neuroendocrine tumors: Radiological examinations. Neuroendocrinology 2009, 90, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Bombardieri, E.; Ambrosini, V.; Aktolun, C.; Baum, R.P.; Bishof-Delaloye, A.; del Vecchio, S.; Maffioli, L.; Mortelmans, L.; Oyen, W.; Pepe, G.; et al. 111In-pentetreotide scintigraphy: Procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, I.; Henze, M.; Engelbrecht, S.; Eisenhut, M.; Runz, A.; Schafer, M.; Schilling, T.; Haufe, S.; Herrmann, T.; Haberkorn, U. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (octreoscan) spect in patients with neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Cremonesi, M.; Ferrari, M.; Pacifici, M.; Grana, C.M.; Bartolomei, M.; Baio, S.M.; Sansovini, M.; Paganelli, G. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: The role of associated risk factors. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Valkema, R.; Pauwels, S.A.; Kvols, L.K.; Kwekkeboom, D.J.; Jamar, F.; de Jong, M.; Barone, R.; Walrand, S.; Kooij, P.P.; Bakker, W.H.; et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0), Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J. Nucl. Med. 2005, 46 (Suppl. 1), 83S–91S. [Google Scholar] [PubMed]
- Ljungberg, M.; Sjogreen-Gleisner, K. The accuracy of absorbed dose estimates in tumours determined by quantitative spect: A monte carlo study. Acta Oncol. 2011, 50, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Konijnenberg, M.; Melis, M.; Valkema, R.; Krenning, E.; de Jong, M. Radiation dose distribution in human kidneys by octreotides in peptide receptor radionuclide therapy. J. Nucl. Med. 2007, 48, 134–142. [Google Scholar] [PubMed]
- Sgouros, G.; Hobbs, R.F. Dosimetry for radiopharmaceutical therapy. Semin. Nucl. Med. 2014, 44, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Sjogreen, K.; Ljungberg, M.; Wingardh, K.; Minarik, D.; Strand, S.E. The lundadose method for planar image activity quantification and absorbed-dose assessment in radionuclide therapy. Cancer Biother. Radiopharm. 2005, 20, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.A.; Thomas, S.R.; Stubbs, J.B.; Stabin, M.G.; Hays, M.T.; Koral, K.F.; Robertson, J.S.; Howell, R.W.; Wessels, B.W.; Fisher, D.R.; et al. Mird pamphlet No. 16: Techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J. Nucl. Med. 1999, 40, 37S–61S. [Google Scholar] [PubMed]
- Foster, D.M. Developing and testing integrated multicompartment models to describe a single-input multiple-output study using the saam II software system. Adv. Exp. Med. Biol. 1998, 445, 59–78. [Google Scholar] [PubMed]
- Bouchet, L.G.; Bolch, W.E.; Blanco, H.P.; Wessels, B.W.; Siegel, J.A.; Rajon, D.A.; Clairand, I.; Sgouros, G. Mird pamphlet No 19: Absorbed fractions and radionuclide S values for six age-dependent multiregion models of the kidney. J. Nucl. Med. 2003, 44, 1113–1147. [Google Scholar] [PubMed]
- Rolleman, E.J.; Valkema, R.; de Jong, M.; Kooij, P.P.; Krenning, E.P. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lapa, C.; Werner, R.A.; Bluemel, C.; Lueckerath, K.; Muegge, D.O.; Strate, A.; Haenscheid, H.; Schirbel, A.; Allen-Auerbach, M.S.; Bundschuh, R.A.; et al. Prediction of clinically relevant hyperkalemia in patients treated with peptide receptor radionuclide therapy. EJNMMI Res. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- De Keizer, B.; van Aken, M.O.; Feelders, R.A.; de Herder, W.W.; Kam, B.L.; van Essen, M.; Krenning, E.P.; Kwekkeboom, D.J. Hormonal crises following receptor radionuclide therapy with the radiolabeled somatostatin analogue (177Lu-DOTA0,Tyr3)octreotate. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Valkema, R.; Krenning, E.P.; de Jong, M. Reduction of renal uptake of radiolabeled octreotate by amifostine coadministration. J. Nucl. Med. 2012, 53, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Ahlstedt, J.; Tran, T.A.; Strand, F.; Holmqvist, B.; Strand, S.E.; Gram, M.; Åkerström, B. Biodistribution and pharmacokinetics of recombinant α1-microglobulin and its potential use in radioprotection of kidneys. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 333–347. [Google Scholar] [PubMed]
- Halliwell, B.; Gutteridge, J.M. The definition and measurement of antioxidants in biological systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Buehler, P.W.; D’Agnillo, F. Toxicological consequences of extracellular hemoglobin: Biochemical and physiological perspectives. Antioxid. Redox Signal. 2010, 12, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.M.; Basak, A. Human catalase: Looking for complete identity. Protein Cell 2010, 1, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; Bocedi, A.; Visca, P.; Altruda, F.; Tolosano, E.; Beringhelli, T.; Fasano, M. Hemoglobin and heme scavenging. IUBMB Life 2005, 57, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Tenhunen, R.; Marver, H.S.; Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; de Toledo, S.M.; Little, J.B. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene 2003, 22, 7050–7057. [Google Scholar] [CrossRef] [PubMed]
- Lyng, F.M.; Seymour, C.B.; Mothersill, C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br. J. Cancer 2000, 83, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Lyng, F.M.; Seymour, C.B.; Mothersill, C. Initiation of apoptosis in cells exposed to medium from the progeny of irradiated cells: A possible mechanism for bystander-induced genomic instability? Radiat. Res. 2002, 157, 365–370. [Google Scholar] [CrossRef]
- Ekström, B.; Berggård, I. Human α1-microglobulin. Purification procedure, chemical and physiochemical properties. J. Biol. Chem. 1977, 252, 8048–8057. [Google Scholar] [PubMed]
- Åkerström, B.; Gram, M. A1M, an extravascular tissue cleaning and housekeeping protein. Free Radic. Biol. Med. 2014, 74, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Flower, D.R. The lipocalin protein family: Structure and function. Biochem.J. 1996, 318, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Åkerström, B.; Lögdberg, L. An intriguing member of the lipocalin protein family: α1-Microglobulin. Trends Biochem. Sci. 1990, 15, 240–243. [Google Scholar] [CrossRef]
- Breustedt, D.A.; Schonfeld, D.L.; Skerra, A. Comparative ligand-binding analysis of ten human lipocalins. Biochim. Biophys. Acta 2006, 1764, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Meining, W.; Skerra, A. The crystal structure of human α1-microglobulin reveals a potential haem-binding site. Biochem. J. 2012, 445, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tejler, L.; Eriksson, S.; Grubb, A.; Astedt, B. Production of protein HC by human fetal liver explants. Biochim. Biophys. Acta 1978, 542, 506–514. [Google Scholar] [CrossRef]
- Kaumeyer, J.F.; Polazzi, J.O.; Kotick, M.P. The mRNA for a proteinase inhibitor related to the HI-30 domain of inter-α-trypsin inhibitor also encodes α-1-microglobulin (protein HC). Nucleic Acids Res. 1986, 14, 7839–7850. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Bratt, T.; Altieri, M.; Kastern, W.; Åkerström, B. Rat α1-microglobulin: Co-expression in liver with the light chain of inter-α-trypsin inhibitor. Biochim. Biophys. Acta 1992, 1130, 63–67. [Google Scholar] [CrossRef]
- Fries, E.; Blom, A.M. Bikunin—Not just a plasma proteinase inhibitor. Int. J. Biochem. Cell Biol. 2000, 32, 125–137. [Google Scholar] [CrossRef]
- Larsson, J.; Wingårdh, K.; Berggård, T.; Davies, J.R.; Lögdberg, L.; Strand, S.E.; Åkerström, B. Distribution of iodine 125-labeled α1-microglobulin in rats after intravenous injection. J. Lab. Clin. Med. 2001, 137, 165–175. [Google Scholar] [CrossRef] [PubMed]
- DeMars, D.D.; Katzmann, J.A.; Kimlinger, T.K.; Calore, J.D.; Tracy, R.P. Simultaneous measurement of total and IGA-conjugated α1-microglobulin by a combined immunoenzyme/immunoradiometric assay technique. Clin. Chem. 1989, 35, 766–772. [Google Scholar] [PubMed]
- Berggård, T.; Thelin, N.; Falkenberg, C.; Enghild, J.J.; Åkerström, B. Prothrombin, albumin and immunoglobulin a form covalent complexes with α1-microglobulin in human plasma. Eur. J. Biochem. FEBS 1997, 245, 676–683. [Google Scholar] [CrossRef]
- Nordberg, J.; Allhorn, M.; Winqvist, I.; Åkerström, B.; Olsson, M.L. Quantitative and qualitative evaluation of plasma and urine α1-microglobulin in healthy donors and patients with different haemolytic disorders and haemochromatosis. Clin. Chim. Acta Int. J. Clin. Chem. 2007, 386, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Allhorn, M.; Berggård, T.; Nordberg, J.; Olsson, M.L.; Åkerström, B. Processing of the lipocalin α1-microglobulin by hemoglobin induces heme-binding and heme-degradation properties. Blood 2002, 99, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Siebel, J.F.; Kosinsky, R.L.; Åkerström, B.; Knipp, M. Insertion of heme b into the structure of the cys34-carbamidomethylated human lipocalin α1-microglobulin: Formation of a ((heme)2(α1-microglobulin))3 complex. ChemBioChem 2012, 13, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Allhorn, M.; Klapyta, A.; Åkerström, B. Redox properties of the lipocalin α1-microglobulin: Reduction of cytochrome c, hemoglobin, and free iron. Free Radic. Biol. Med. 2005, 38, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.G.; Olofsson, T.; Tapper, H.; Åkerström, B. The lipocalin α1-microglobulin protects erythroid K562 cells against oxidative damage induced by heme and reactive oxygen species. Free Radic. Res. 2008, 42, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Rutardottir, S.; Nilsson, E.J.; Pallon, J.; Gram, M.; Åkerström, B. The cysteine 34 residue of A1M/α1-microglobulin is essential for protection of irradiated cell cultures and reduction of carbonyl groups. Free Radic. Res. 2013, 47, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Åkerström, B.; Maghzal, G.J.; Winterbourn, C.C.; Kettle, A.J. The lipocalin α1-microglobulin has radical scavenging activity. J. Biol. Chem. 2007, 282, 31493–31503. [Google Scholar] [CrossRef] [PubMed]
- Berggård, T.; Cohen, A.; Persson, P.; Lindqvist, A.; Cedervall, T.; Silow, M.; Thogersen, I.B.; Jönsson, J.A.; Enghild, J.J.; Åkerström, B. α1-Microglobulin chromophores are located to three lysine residues semiburied in the lipocalin pocket and associated with a novel lipophilic compound. Protein Sci. 1999, 8, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Campagnoli, M.; Perani, E.; Romano, A.; Labo, S.; Monzani, E.; Minchiotti, L.; Galliano, M. Human α1-microglobulin is covalently bound to kynurenine-derived chromophores. J. Biol. Chem. 2004, 279, 51033–51041. [Google Scholar] [CrossRef] [PubMed]
- May, K.; Rosenlöf, L.; Olsson, M.G.; Centlow, M.; Mörgelin, M.; Larsson, I.; Cederlund, M.; Rutardottir, S.; Siegmund, W.; Schneider, H.; et al. Perfusion of human placenta with hemoglobin introduces preeclampsia-like injuries that are prevented by α1-microglobulin. Placenta 2011, 32, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.G.; Allhorn, M.; Larsson, J.; Cederlund, M.; Lundqvist, K.; Schmidtchen, A.; Sorensen, O.E.; Mörgelin, M.; Åkerström, B. Up-regulation of A1M/α1-microglobulin in skin by heme and reactive oxygen species gives protection from oxidative damage. PLoS ONE 2011, 6, e27505. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.G.; Rosenlöf, L.W.; Kotarsky, H.; Olofsson, T.; Leanderson, T.; Mörgelin, M.; Fellman, V.; Åkerström, B. The radical-binding lipocalin A1M binds to a complex I subunit and protects mitochondrial structure and function. Antioxid. Redox Signal. 2013, 18, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.G.; Allhorn, M.; Olofsson, T.; Åkerström, B. Up-regulation of α1-microglobulin by hemoglobin and reactive oxygen species in hepatoma and blood cell lines. Free Radic.Biol. Med. 2007, 42, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.G.; Centlow, M.; Rutardottir, S.; Stenfors, I.; Larsson, J.; Hosseini-Maaf, B.; Olsson, M.L.; Hansson, S.R.; Åkerström, B. Increased levels of cell-free hemoglobin, oxidation markers, and the antioxidative heme scavenger α1-microglobulin in preeclampsia. Free Radic. Biol. Med. 2010, 48, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Wester-Rosenlöf, L.; Casslen, V.; Axelsson, J.; Edström-Hägerwall, A.; Gram, M.; Holmqvist, M.; Johansson, M.E.; Larsson, I.; Ley, D.; Marsal, K.; et al. A1M/α1-microglobulin protects from heme-induced placental and renal damage in a pregnant sheep model of preeclampsia. PLoS ONE 2014, 9, e86353. [Google Scholar] [CrossRef] [PubMed]
- Nääv, A.; Erlandsson, L.; Axelsson, J.; Larsson, I.; Johansson, M.; Wester-Rosenlöf, L.; Mörgelin, M.; Casslen, V.; Gram, M.; Åkerström, B.; et al. A1M ameliorates preeclampsia-like symptoms in placenta and kidney induced by cell-free fetal hemoglobin in rabbit. PLoS ONE 2015, 10, e0125499. [Google Scholar] [CrossRef] [PubMed]
- Sverrisson, K.; Axelsson, J.; Rippe, A.; Gram, M.; Åkerström, B.; Hansson, S.R.; Rippe, B. Extracellular fetal hemoglobin induces increases in glomerular permeability: Inhibition with α1-microglobulin and tempol. Am. J. Physiol. Ren. Physiol. 2014, 306, F442–F448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Olsson, M.G.; Nilsson, E.J.; Rutardottir, S.; Paczesny, J.; Pallon, J.; Åkerström, B. Bystander cell death and stress response is inhibited by the radical scavenger α1-microglobulin in irradiated cell cultures. Radiat. Res. 2010, 174, 590–600. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahlstedt, J.; Tran, T.A.; Strand, S.-E.; Gram, M.; Åkerström, B. Human Anti-Oxidation Protein A1M—A Potential Kidney Protection Agent in Peptide Receptor Radionuclide Therapy. Int. J. Mol. Sci. 2015, 16, 30309-30320. https://doi.org/10.3390/ijms161226234
Ahlstedt J, Tran TA, Strand S-E, Gram M, Åkerström B. Human Anti-Oxidation Protein A1M—A Potential Kidney Protection Agent in Peptide Receptor Radionuclide Therapy. International Journal of Molecular Sciences. 2015; 16(12):30309-30320. https://doi.org/10.3390/ijms161226234
Chicago/Turabian StyleAhlstedt, Jonas, Thuy A. Tran, Sven-Erik Strand, Magnus Gram, and Bo Åkerström. 2015. "Human Anti-Oxidation Protein A1M—A Potential Kidney Protection Agent in Peptide Receptor Radionuclide Therapy" International Journal of Molecular Sciences 16, no. 12: 30309-30320. https://doi.org/10.3390/ijms161226234
APA StyleAhlstedt, J., Tran, T. A., Strand, S.-E., Gram, M., & Åkerström, B. (2015). Human Anti-Oxidation Protein A1M—A Potential Kidney Protection Agent in Peptide Receptor Radionuclide Therapy. International Journal of Molecular Sciences, 16(12), 30309-30320. https://doi.org/10.3390/ijms161226234







