Transcriptomic Response to Nitric Oxide Treatment in Larix olgensis Henry
Abstract
:1. Introduction
2. Results and Discussion
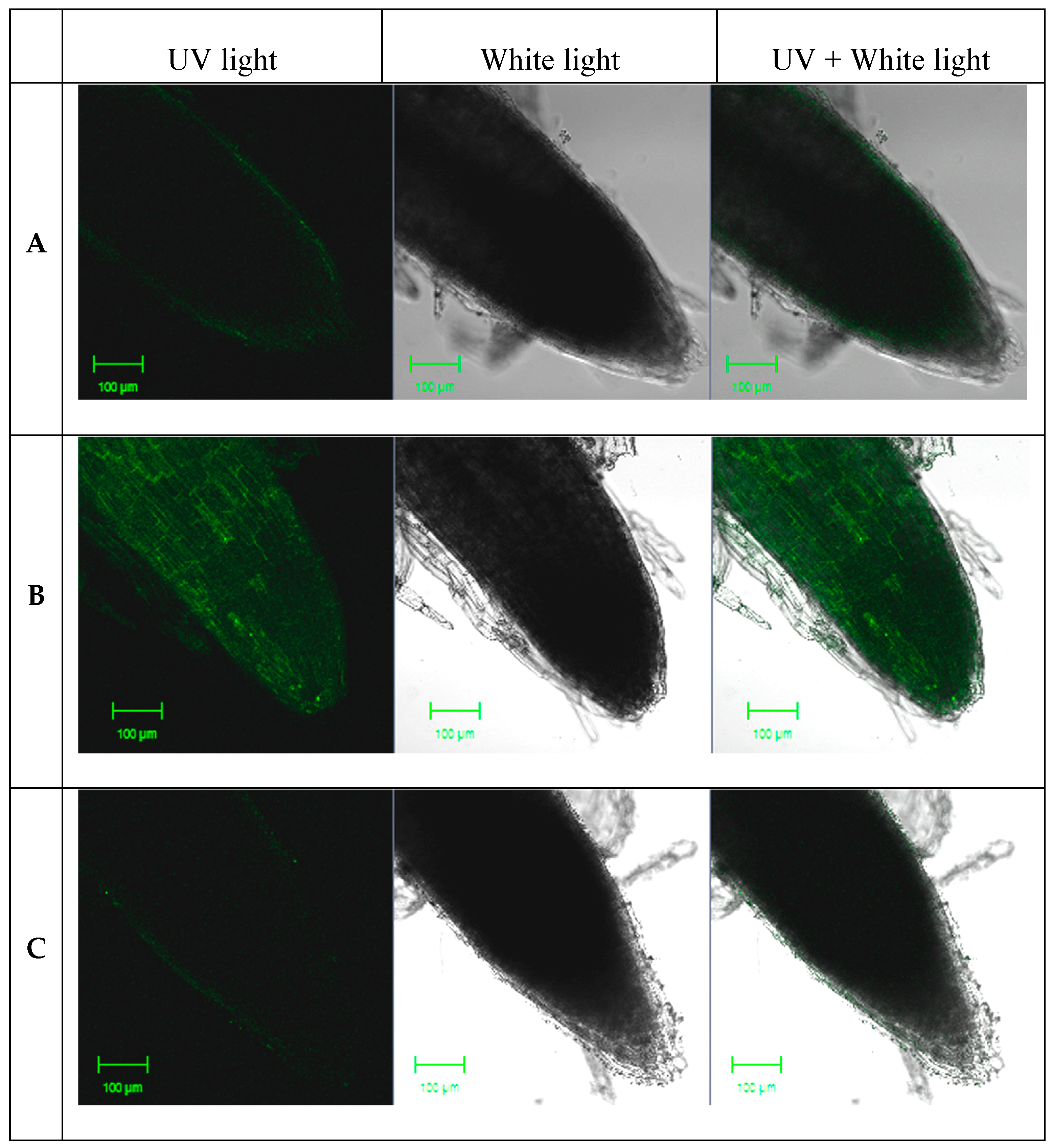
2.1. NO Production in Response to Sodium Nitroprusside (SNP)
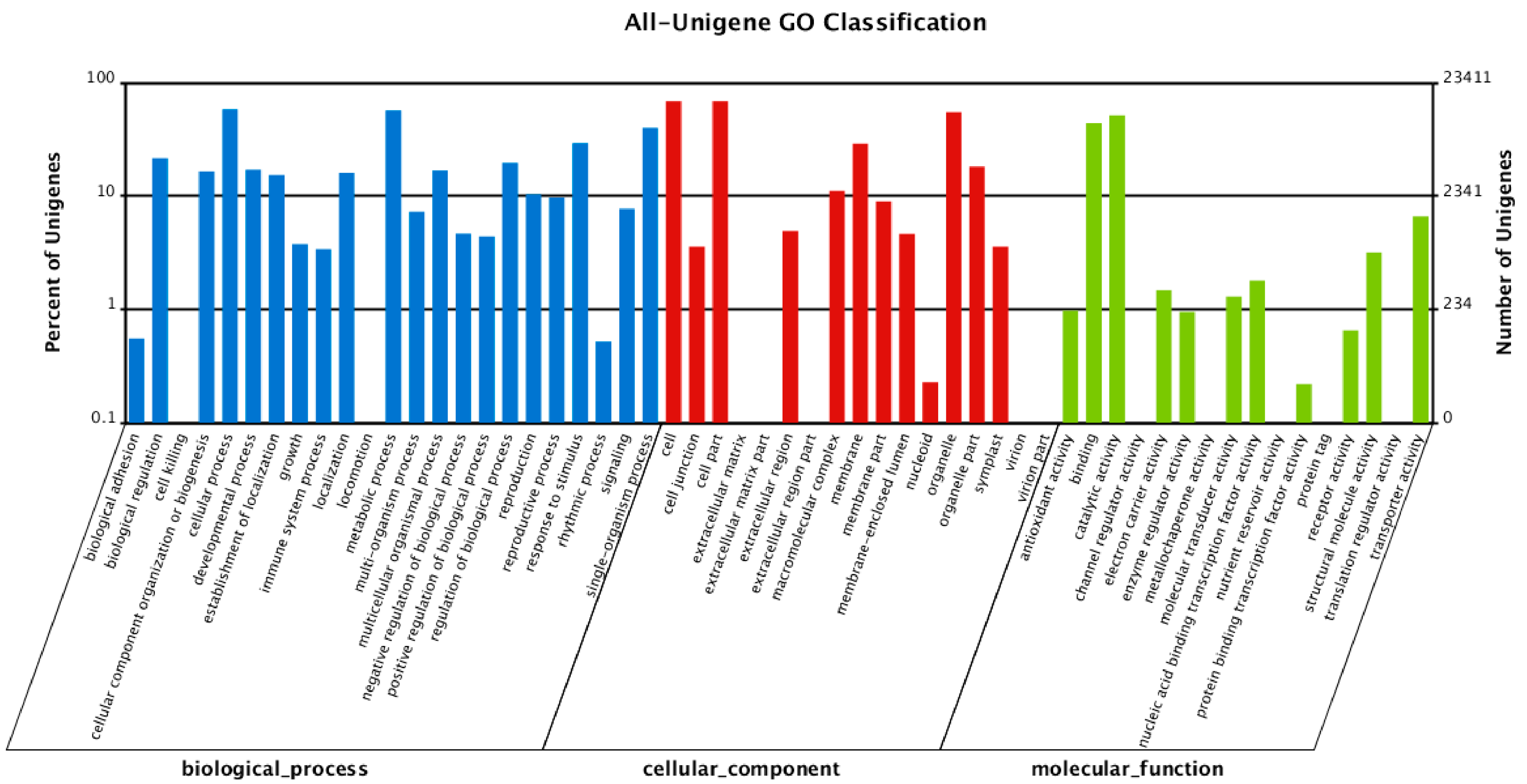
2.2. Functional Annotation of Unigenes
2.3. Investigation of Differentially Expressed Transcripts under SNP Treatment

| Unigene | Pathways (Control vs. SNP) | Nr-ID |
|---|---|---|
| Unigene32649_All | Cyanoamino acid metabolism, Tryptophan metabolism, aminobenzoate degradation, styrene degradation, Nitrogen metabolism | gi|348690846|gb|EGZ30660.1| |
| Unigene32533_All | Carbon metabolism, biosynthesis of amino acids, glycolysis/gluconeogenesis, carbon fixation in photosynthetic organisms, HIF-1 signaling pathway, Alzheimer‘s disease | gi|226495473|ref|NP_001147336.1| |
| CL6269.Contig1_All | RNA transport, Legionellosis | gi|301111276|ref|XP_002904717.1| |
| Unigene32730_All | Ribosome | gi|323451608|gb|EGB07485.1| |
| CL5964.Contig2_All | Mineral absorption | gi|357161316|ref|XP_003579051.1| |
| Unigene32753_All | Spliceosome | gi|145324176|ref|NP_001077677.1| |
| Unigene26640_All | Plant-pathogen interaction | gi|2224913|gb|AAB61709.1| |
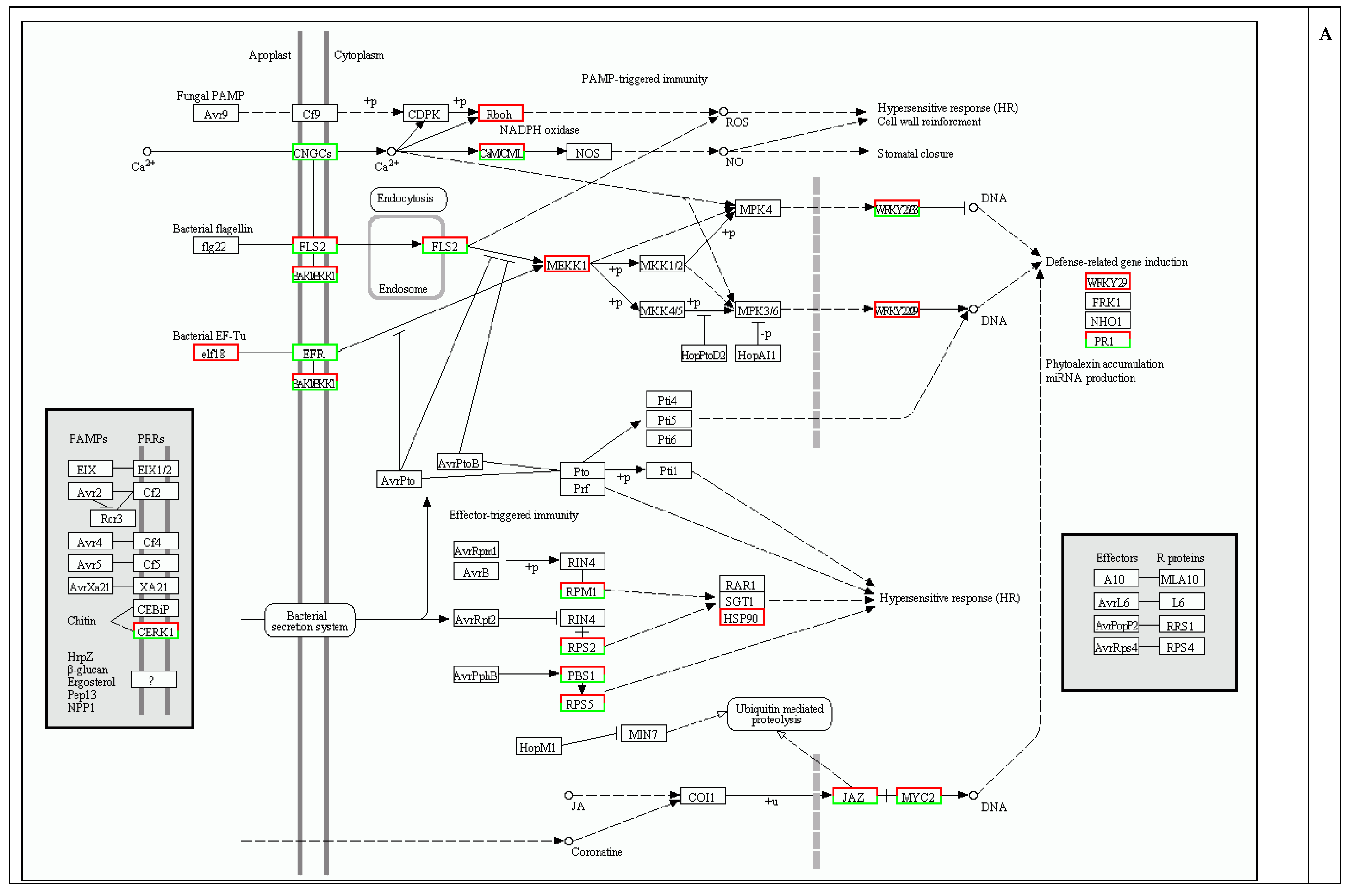
2.4. Protection against Pathogen Infection

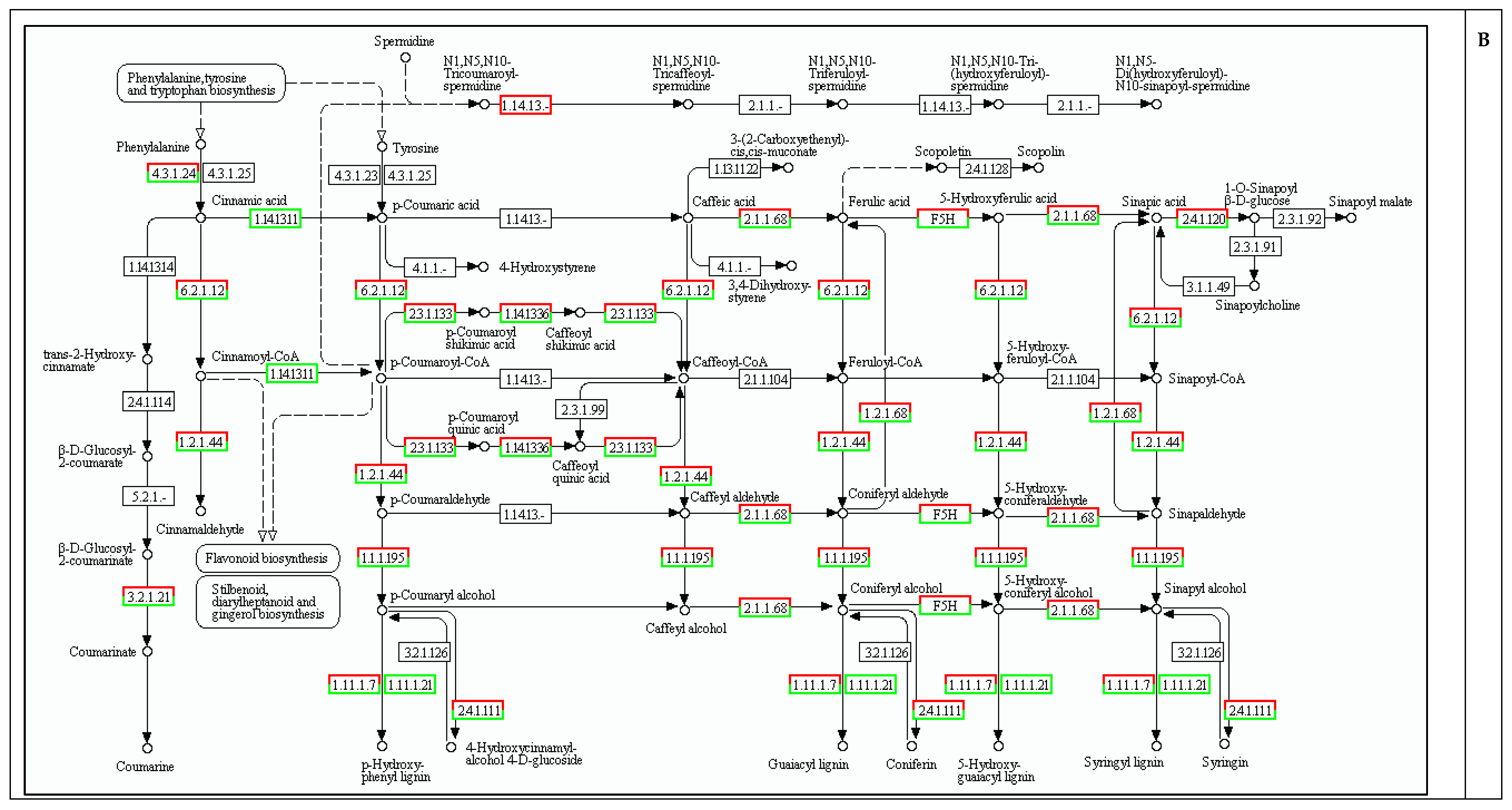
2.5. Cell Wall Biosynthesis
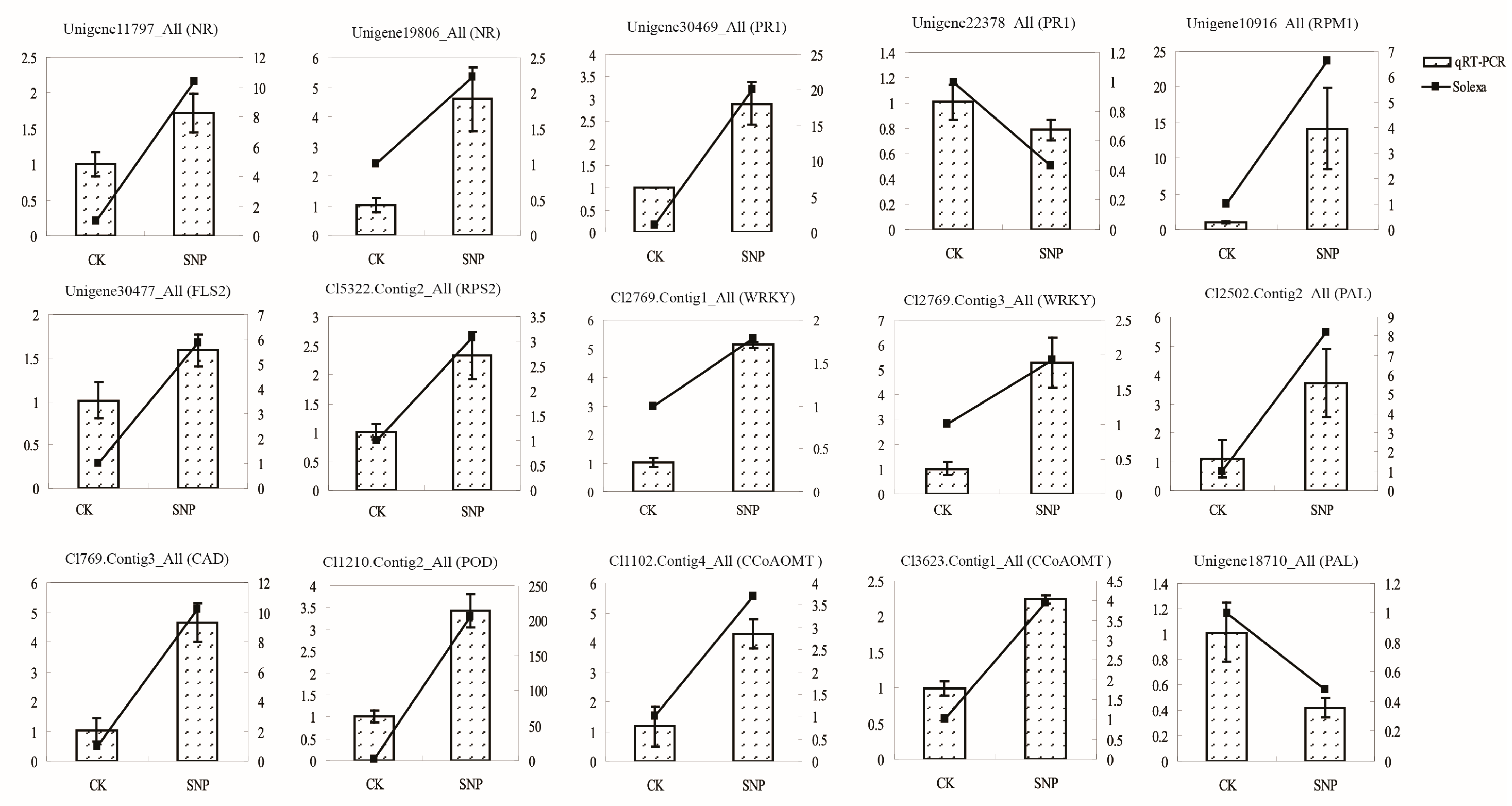
2.6. Validation of Transcriptomic Data by QRT-PCR
3. Experimental Section
3.1. Plant Materials and Treatments
3.2. RNA Extraction, cDNA Library Preparation, and Illumina Sequencing
3.3. De Novo Assembly and Annotation
| Parameters | Control | Sodium Nitroprusside |
|---|---|---|
| Total number of reads | 51,426,920 | 54,580,304 |
| Total nucleotides (bp) | 4,628,422,800 | 4,912,227,360 |
| GC percentage | 46.11% | 46.54% |
| Q20 percentage | 98.15% | 98.28% |
| Total number of contigs | 101,853 | 95,211 |
| Length of all contigs (bp) | 39,753,573 | 38,513,317 |
| Contigs N50 (bp) | 872 | 910 |
| Mean length (bp) of contigs | 390 | 405 |
| Total number of unigenes | 65,207 | 58,080 |
| Length of all unigenes (bp) | 38,628,763 | 38,445,887 |
| Mean length (bp) of contigs | 592 | 662 |
| Unigenes N50 (bp) | 958 | 1117 |
3.4. qRT-PCR Validation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, J.; Baldwin, I.T. Herbivory-induced signalling in plants: Perception and action. Plant Cell Environ. 2009, 32, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.W.; Sun, Z.H.; Zhou, Y.H.; Shi, K.; Li, X.; Zhang, G.Q.; Xia, X.J.; Chen, Z.X.; Yu, J.Q. The role of hydrogen peroxide and nitric oxide in the induction of plant-encoded RNA-dependent RNA polymerase 1 in the basal defense against Tobacco mosaic virus. PLoS ONE 2013, 8, e76090. [Google Scholar] [CrossRef] [PubMed]
- Delledonne, M. NO news is good news for plants. Curr. Opin. Plant Biol. 2005, 8, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.M.; Li, Y.Z. Verticillium dahliae toxins-induced nitric oxide production in Arabidopsis is major dependent on nitrate reductase. BMB Rep. 2008, 41, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.C.; Badger, M.R.; Jones, R.L. Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 2004, 16, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.T.; Li, R.J.; Cai, W.; Liu, W.; Wang, C.L.; Lu, Y.T. Increasing nitric oxide content in Arabidopsis thaliana by expressing rat neuronal nitric oxide synthase resulted in enhanced stress tolerance. Plant Cell Physiol. 2012, 53, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Park, H.C.; Koo, S.C.; Lee, J.H.; Park, C.Y.; Choi, M.S.; Kang, C.H.; Baek, D.; Cheong, Y.H.; Yun, D.J.; et al. Constitutive expression of mammalian nitric oxide synthase in tobacco plants triggers disease resistance to pathogens. Mol. Cells 2012, 34, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Besson-Bard, A.; Wendehenne, D. NO contributes to cadmium toxicity in Arabidopsis thaliana by mediating an iron deprivation response. Plant Signal. Behav. 2009, 4, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; von Rad, U.; Durner, J. Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta 2002, 215, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Parani, M.; Rudrabhatla, S.; Myers, R.; Weirich, H.; Smith, B.; Leaman, D.W.; Goldman, S.L. Microarray analysis of nitric oxide responsive transcripts in Arabidopsis. Plant Biotechnol. J. 2004, 2, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Polverari, A.; Molesini, B.; Pezzotti, M.; Buonaurio, R.; Marte, M.; Delledonne, M. Nitric oxide-mediated transcriptional changes in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2003, 16, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Vidotto, M.; Grapputo, A.; Boscari, E.; Barbisan, F.; Coppe, A.; Grandi, G.; Kumar, A.; Congiu, L. Transcriptome sequencing and de novo annotation of the critically endangered Adriatic sturgeon. BMC Genom. 2013, 14, 407. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.Y.; Liu, S.K.; Gao, X.Y.; Jiang, Y.L.; Perera, D.; Wang, X.L.; Li, C.; Sun, L.Y.; Zhang, J.R.; Kaltenboeck, L.; et al. Male-biased genes in catfish as revealed by RNA-Seq analysis of the testis transcriptome. PLoS ONE 2013, 8, e68452. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.A.; Jakoby, M.; Werber, M.; Martin, C.; Weisshaar, B.; Bailey, P.C. The basic helix-loop-helix transcription factor family in plants: A genome-wide study of protein structure and functional diversity. Mol. Biol. Evol. 2003, 20, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Yang, H.; Ma, L.; Sun, N.; Yu, H.; Liu, T.; Gao, Y.; Gu, H.; Chen, Z.; Wada, M.; et al. A genome-wide analysis of blue-light regulation of Arabidopsis transcription factor gene expression during seedling development. Plant Physiol. 2003, 133, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Knowles, D.G.; McLysaght, A. Recent de novo origin of human protein-coding genes. Genome Res. 2009, 19, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.X.; Xu, C.Y.; Ren, J.; Ma, L.Y.; Duan, J.; Jiang, L.N. Newly transplanted Larix olgensis henry stock with greater root biomass has higher early nitrogen flux rate. Soil Sci. Plant Nutr. 2013, 59, 740–749. [Google Scholar] [CrossRef]
- Blokhina, N.I.; Bondarenko, O.V.; Snezhkova, S.A. Fossil wood of Quercus primorica sp nov (Fagaceae) from the pliocene of southern primorye. Paleontol. J. 2005, 39, 664–670. [Google Scholar]
- Han, H.; Sun, X.; Xie, Y.; Feng, J.; Zhang, S. Transcriptome and proteome profiling of adventitious root development in hybrid larch (Larix kaempferi × Larix olgensis). BMC Plant Biol. 2014, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Men, L.; Yan, S.; Liu, G. De novo characterization of Larix gmelinii (Rupr.) Rupr. transcriptome and analysis of its gene expression induced by jasmonates. BMC Genom. 2013, 14, 548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.G.; Han, S.Y.; Li, X.M.; Qi, L.W. Transcriptome profiling and in silico analysis of somatic embryos in Japanese larch (Larix leptolepis). Plant Cell Rep. 2012, 31, 1637–1657. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Sun, F.; Li, L.; Liu, K.; Zhan, Y. Genome-scale transcriptome analysis in response to nitric oxide in birch cells: Implications of the triterpene biosynthetic pathway. PLoS ONE 2014, 9, e116157. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, J.; Zipfel, C. Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 2012, 15, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.C.; Sell, S.; Huang, X.; Scherf, M.; Werner, T.; Durner, J.; Lindermayr, C. Nitric oxide-responsive genes and promoters in Arabidopsis thaliana: A bioinformatics approach. J. Exp. Bot. 2008, 59, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Klessig, D.F. MAPK cascades in plant defense signaling. Trends Plant Sci. 2001, 6, 520–527. [Google Scholar] [CrossRef]
- Tena, G.; Asai, T.; Chiu, W.L.; Sheen, J. Plant mitogen-activated protein kinase signaling cascades. Curr. Opin. Plant Biol. 2001, 4, 392–400. [Google Scholar] [CrossRef]
- Ligterink, W.; Kroj, T.; zur Nieden, U.; Hirt, H.; Scheel, D. Receptor-mediated activation of a map kinase in pathogen defense of plants. Science 1997, 276, 2054–2057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Klessig, D.F. Resistance gene N-mediated de novo synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection. Proc. Natl. Acad. Sci. USA 1998, 95, 7433–7438. [Google Scholar] [CrossRef] [PubMed]
- Nuhse, T.S.; Peck, S.C.; Hirt, H.; Boller, T. Microbial elicitors induce activation and dual phosphorylation of the Arabidopsis thaliana MAPK 6. J. Biol. Chem. 2000, 275, 7521–7526. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Wendehenne, D.; Klessig, D.F. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 1998, 95, 10328–10333. [Google Scholar] [CrossRef] [PubMed]
- Feechan, A.; Kwon, E.; Yun, B.W.; Wang, Y.; Pallas, J.A.; Loake, G.J. A central role for S-nitrosothiols in plant disease resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 8054–8059. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.W.; Feechan, A.; Yin, M.; Saidi, N.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.G.; Kwon, E.; Spoel, S.H.; et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Axtell, M.J.; Staskawicz, B.J. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 2003, 112, 369–377. [Google Scholar] [CrossRef]
- Ade, J.; DeYoung, B.J.; Golstein, C.; Innes, R.W. Indirect activation of a plant nucleotide binding site-leucine-rich repeat protein by a bacterial protease. Proc. Natl. Acad. Sci. USA 2007, 104, 2531–2536. [Google Scholar] [CrossRef] [PubMed]
- Cherkis, K.A.; Temple, B.R.S.; Chung, E.H.; Sondek, J.; Dangl, J.L. AvrRpm1 missense mutations weakly activate RPS2-mediated immune response in Arabidopsis thaliana. PLoS ONE 2012, 7, e42633. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Robatzek, S.; Somssich, I.E. A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence-related processes. Plant J. 2001, 28, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Rizhsky, L.; Liang, H.; Mittler, R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 2002, 130, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Reinstadler, A.; Lipka, V.; Lippok, B.; Somssich, I.E. Synthetic plant promoters containing defined regulatory elements provide novel insights into pathogen- and wound-induced signaling. Plant Cell 2002, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Kigawa, T.; Inoue, M.; Tateno, M.; Yamasaki, T.; Yabuki, T.; Aoki, M.; Seki, E.; Matsuda, T.; Tomo, Y.; et al. Solution structure of an Arabidopsis WRKY DNA binding domain. Plant Cell 2005, 17, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Maleck, K.; Levine, A.; Eulgem, T.; Morgan, A.; Schmid, J.; Lawton, K.A.; Dangl, J.L.; Dietrich, R.A. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat. Genet. 2000, 26, 403–410. [Google Scholar] [PubMed]
- Chen, W.; Provart, N.J.; Glazebrook, J.; Katagiri, F.; Chang, H.S.; Eulgem, T.; Mauch, F.; Luan, S.; Zou, G.; Whitham, S.A.; et al. Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 2002, 14, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Y.; Abu Qamar, S.; Chen, Z.X.; Mengiste, T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006, 48, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Reuber, T.L.; Ausubel, F.M. Isolation of Arabidopsis genes that differentiate between resistance responses mediated by the RPS2 and RPM1 disease resistance genes. Plant Cell 1996, 8, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Gill, W.M.; Pinkard, E.A.; Mohammed, C.L. Anatomical and histochemical defence responses induced in juvenile leaves of Eucalyptus globulus and Eucalyptus nitens by mycosphaerella infection. For. Pathol. 2007, 37, 361–373. [Google Scholar] [CrossRef]
- Tronchet, M.; Balague, C.; Kroj, T.; Jouanin, L.; Roby, D. Cinnamyl alcohol dehydrogenases-C and D, key enzymes in lignin biosynthesis, play an essential role in disease resistance in Arabidopsis. Mol. Plant Pathol. 2010, 11, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Schenk, P.M.; Kazan, K.; Wilson, I.; Anderson, J.P.; Richmond, T.; Somerville, S.C.; Manners, J.M. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc. Natl. Acad. Sci. USA 2000, 97, 11655–11660. [Google Scholar] [CrossRef] [PubMed]
- Adomas, A.; Heller, G.; Li, G.; Olson, A.; Chu, T.M.; Osborne, J.; Craig, D.; van Zyl, L.; Wolfinger, R.; Sederoff, R.; et al. Transcript profiling of a conifer pathosystem: Response of Pinus sylvestris root tissues to pathogen (Heterobasidion annosum) invasion. Tree Physiol. 2007, 27, 1441–1458. [Google Scholar] [CrossRef] [PubMed]
- Ralph, S.G.; Yueh, H.; Friedmann, M.; Aeschliman, D.; Zeznik, J.A.; Nelson, C.C.; Butterfield, Y.S.N.; Kirkpatrick, R.; Liu, J.; Jones, S.J.M.; et al. Conifer defence against insects: Microarray gene expression profiling of Sitka spruce (Picea sitchensis) induced by mechanical wounding or feeding by spruce budworms (Choristoneura occidentalis) or white pine weevils (Pissodes strobi) reveals large-scale changes of the host transcriptome. Plant Cell Environ. 2006, 29, 1545–1570. [Google Scholar] [PubMed]
- Gabaldon, C.; Gomez Ros, L.V.; Pedreno, M.A.; Ros Barcelo, A. Nitric oxide production by the differentiating xylem of Zinnia elegans. New Phytol. 2005, 165, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bagniewska-Zadworna, A.; Arasimowicz-Jelonek, M.; Smolinski, D.J.; Stelmasik, A. New insights into pioneer root xylem development: Evidence obtained from Populus trichocarpa plants grown under field conditions. Ann. Bot. 2014, 113, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz, M.; Floryszak-Wieczorek, J.; Milczarek, G.; Jelonek, T. Nitric oxide, induced by wounding, mediates redox regulation in pelargonium leaves. Plant Biol. 2009, 11, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Pirttila, A.M.; Halonen, M.; Hohtola, A. Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol. Biotechnol. 2001, 19, 201–203. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Koonin, E.V.; Lipman, D.J. A genomic perspective on protein families. Science 1997, 278, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L.; et al. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Yang, J.; Li, C. Transcriptomic Response to Nitric Oxide Treatment in Larix olgensis Henry. Int. J. Mol. Sci. 2015, 16, 28582-28597. https://doi.org/10.3390/ijms161226117
Hu X, Yang J, Li C. Transcriptomic Response to Nitric Oxide Treatment in Larix olgensis Henry. International Journal of Molecular Sciences. 2015; 16(12):28582-28597. https://doi.org/10.3390/ijms161226117
Chicago/Turabian StyleHu, Xiaoqing, Jingli Yang, and Chenghao Li. 2015. "Transcriptomic Response to Nitric Oxide Treatment in Larix olgensis Henry" International Journal of Molecular Sciences 16, no. 12: 28582-28597. https://doi.org/10.3390/ijms161226117
APA StyleHu, X., Yang, J., & Li, C. (2015). Transcriptomic Response to Nitric Oxide Treatment in Larix olgensis Henry. International Journal of Molecular Sciences, 16(12), 28582-28597. https://doi.org/10.3390/ijms161226117





