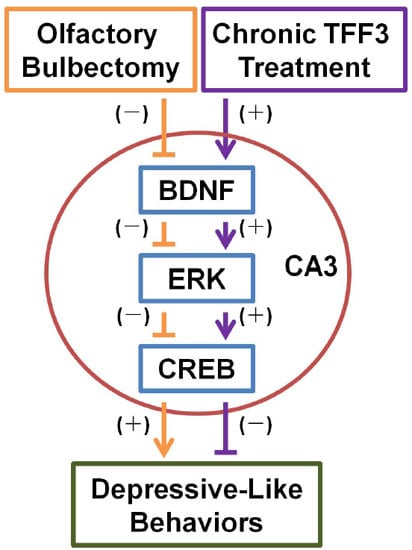
Neuropeptide Trefoil Factor 3 Reverses Depressive-Like Behaviors by Activation of BDNF-ERK-CREB Signaling in Olfactory Bulbectomized Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
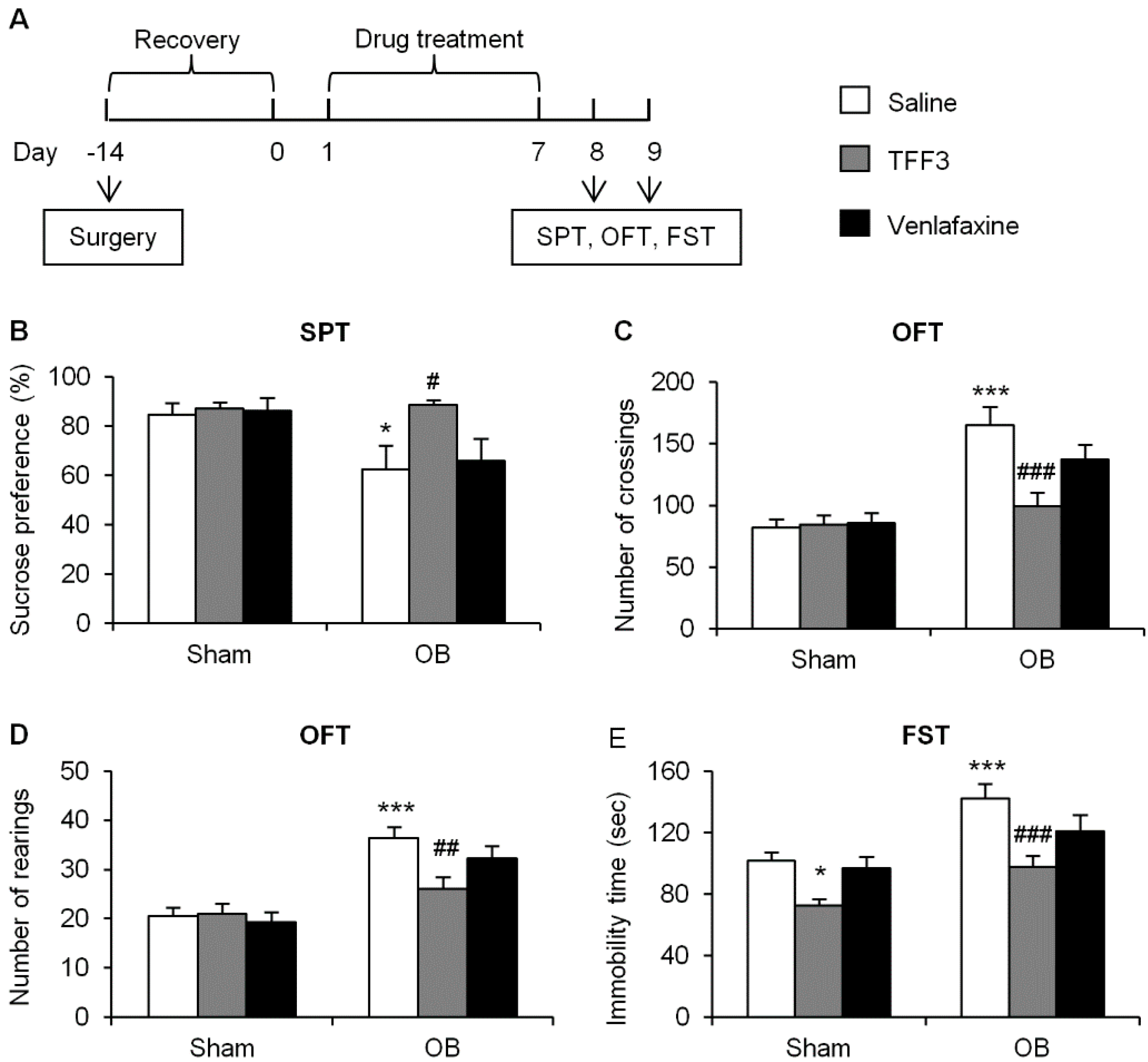
2.1.1. Effects of Chronic TFF3 Administration on Olfactory Bulbectomy (OB)-Induced Depressive-Like Behaviors
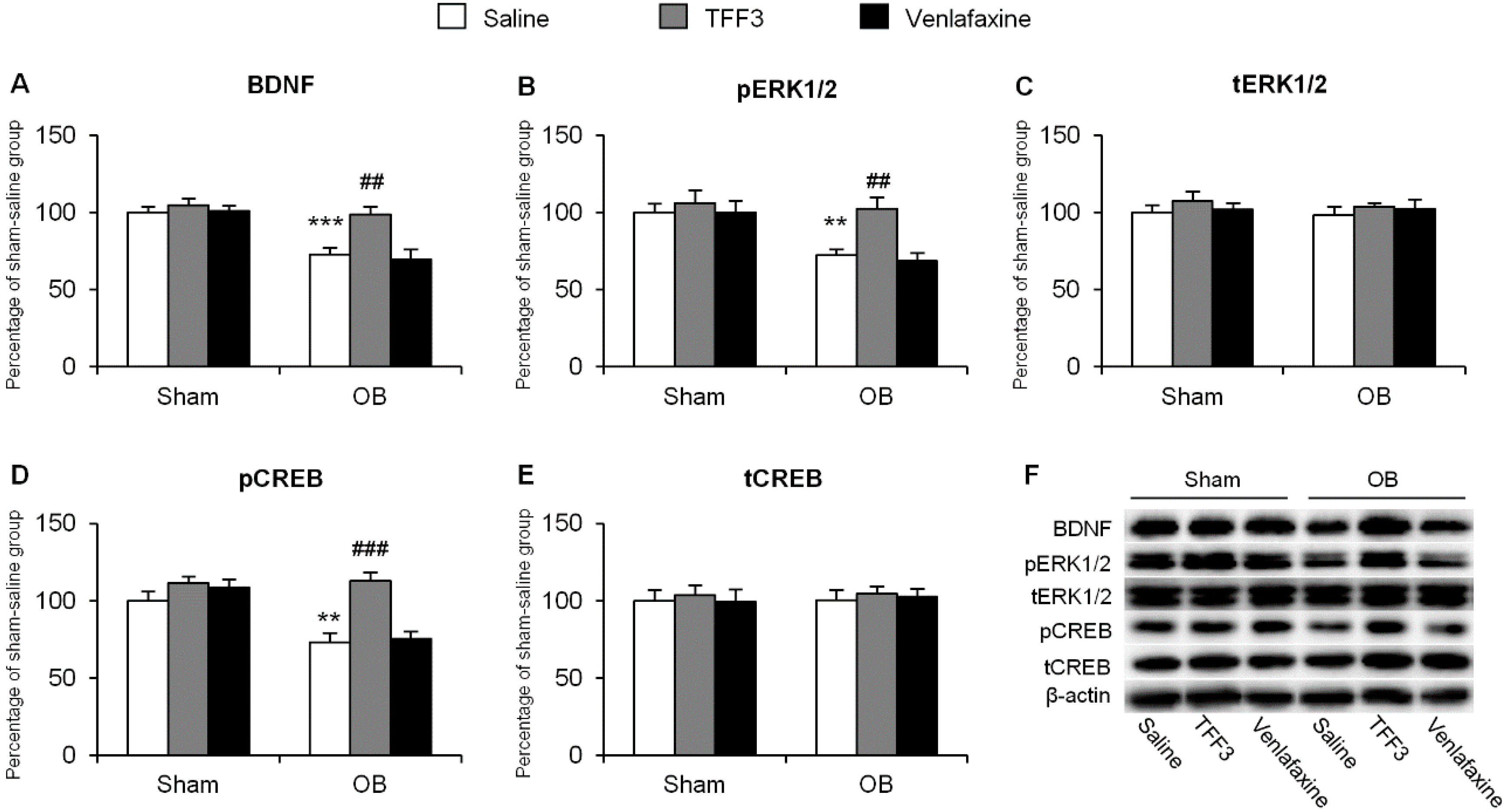
2.1.2. Effects of Chronic TFF3 Administration on BDNF, Phosphorylation of ERK1/2 and CREB in the Hippocampus in the OB Rats

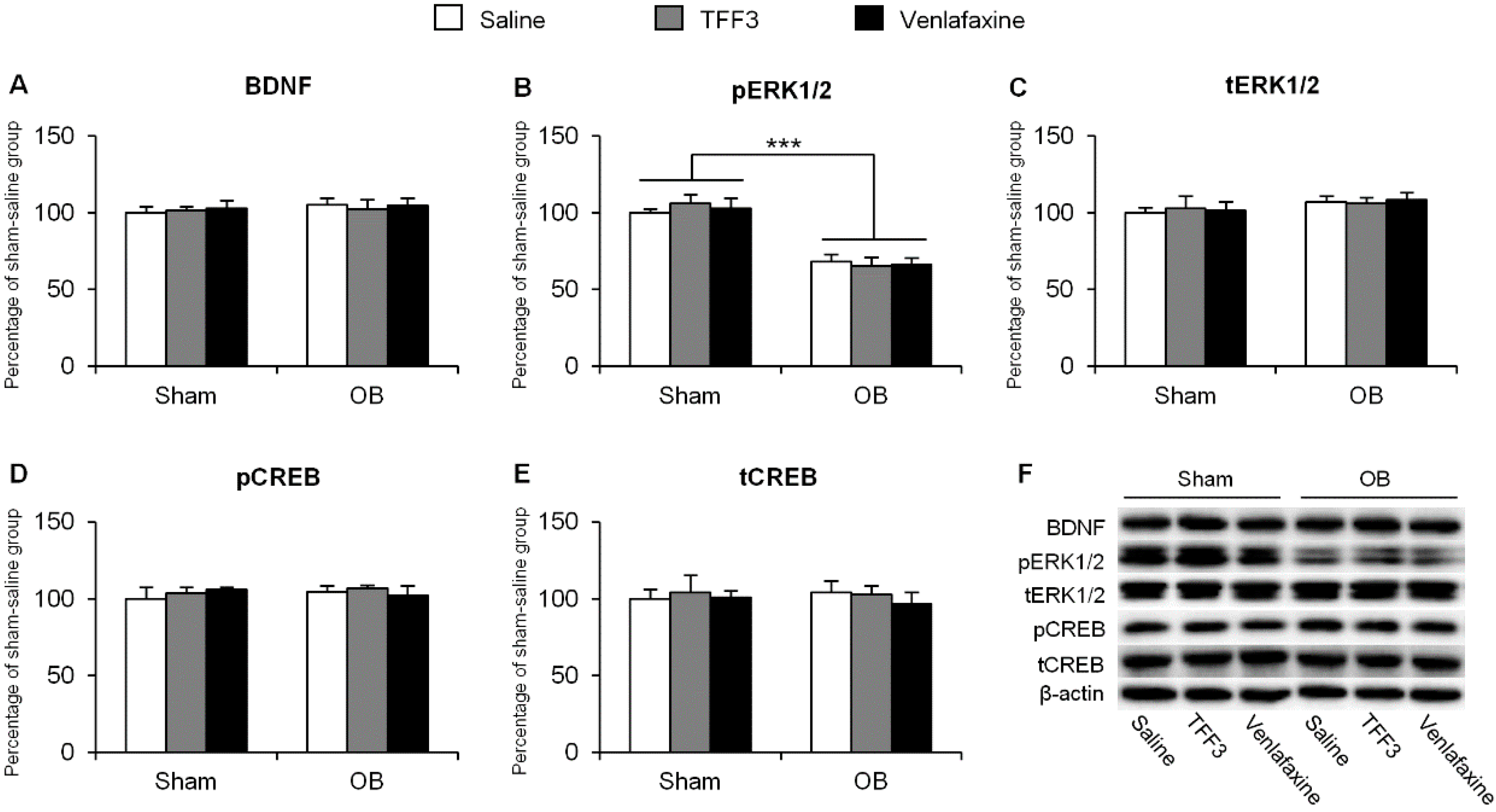
2.1.3. Effects of Tropomyosin-Related Kinase Receptor B (TrkB) Antagonist on Depressive-Like Behaviors in TFF3-Treated OB Rats
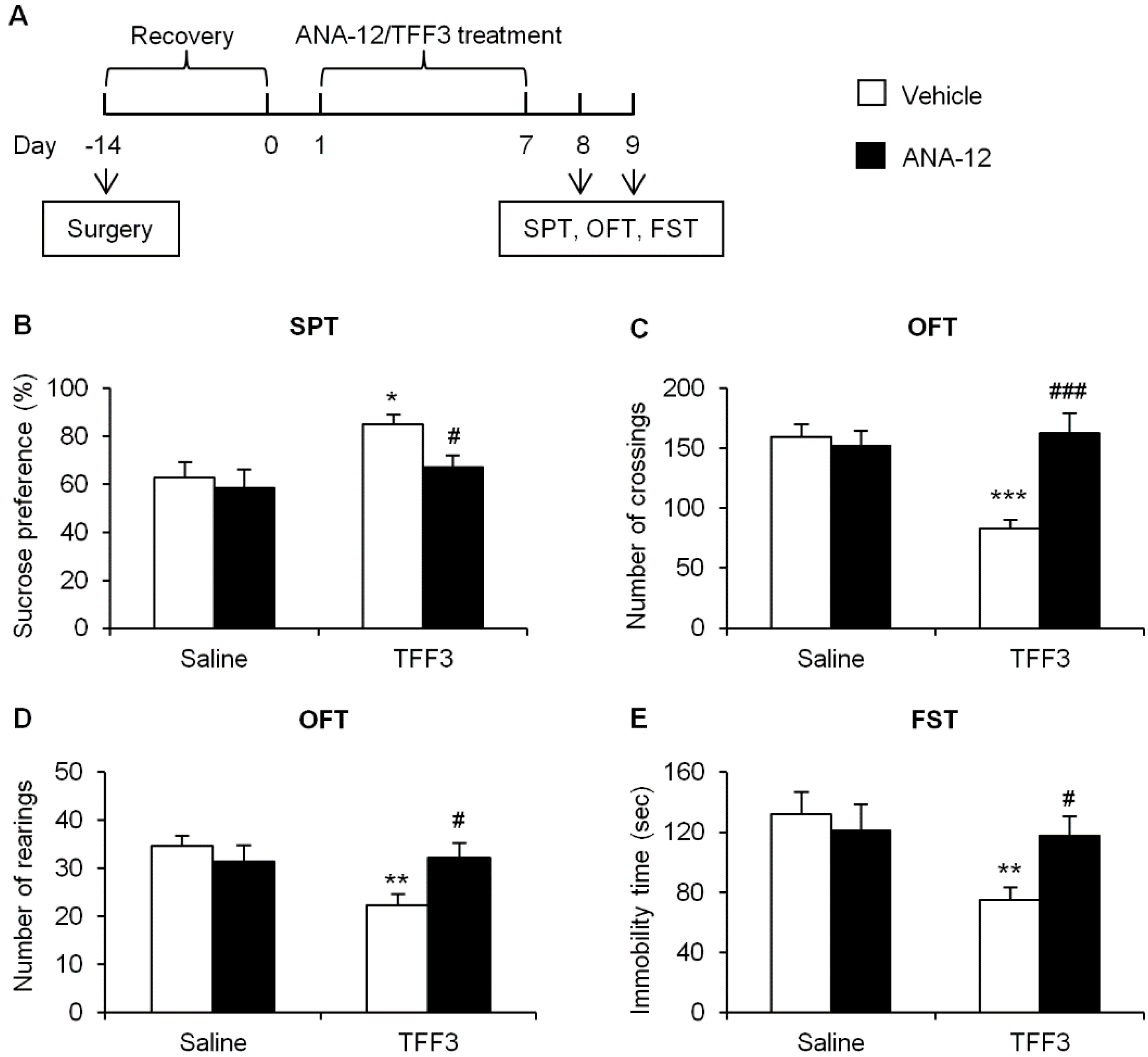
2.1.4. Effects of ERK Phosphorylation Inhibition on Depressive-Like Behaviors in TFF3-Treated OB Rats
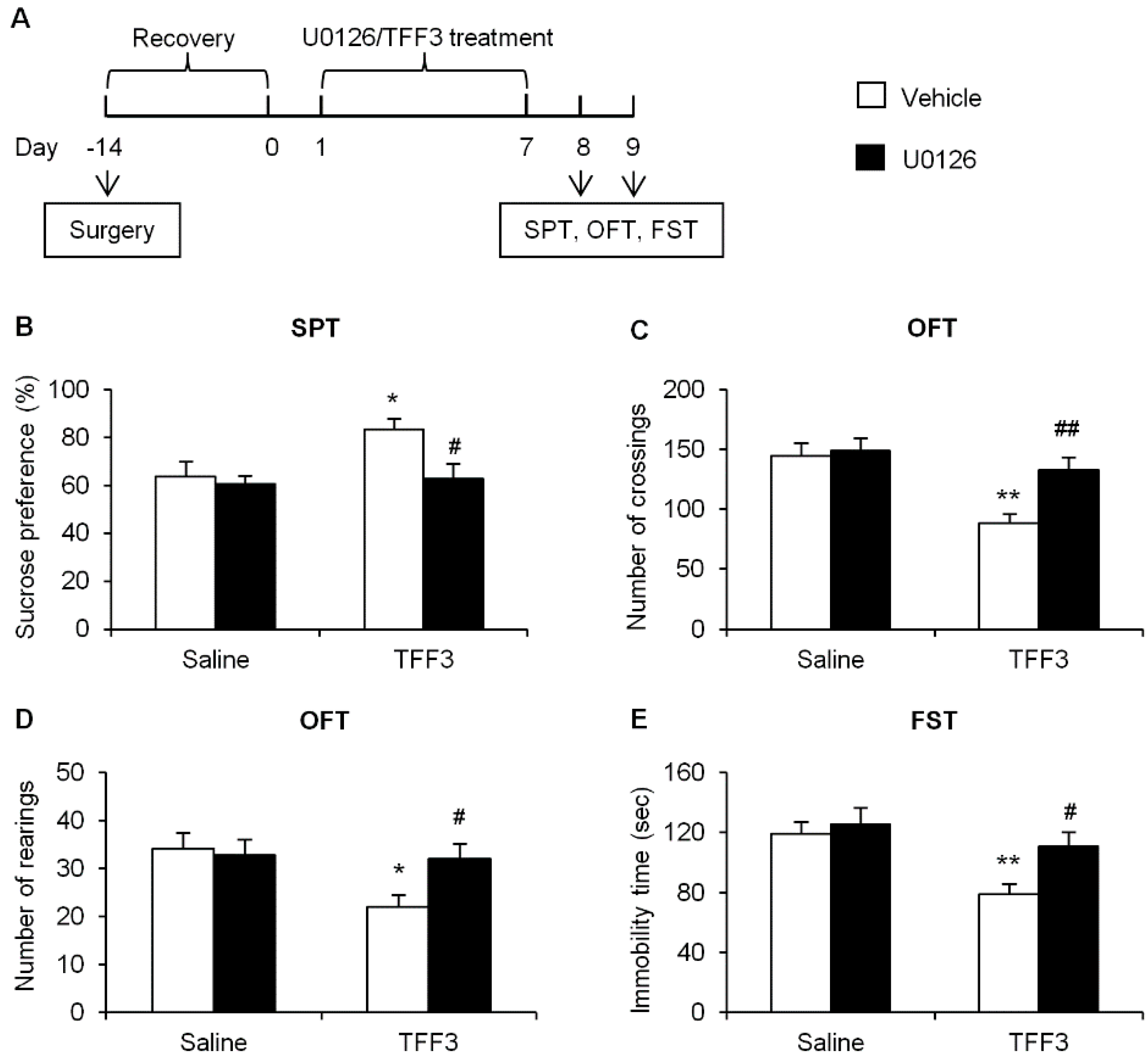
2.2. Discussion
3. Experimental Section
3.1. Animals
3.2. OB Surgery
3.3. Intracerebral Cannula Implantation
3.4. Drugs and Treatment
3.5. Behavioral Tests
3.5.1. Sucrose Preference Test
3.5.2. Open Field Test
3.5.3. Forced Swim Test
3.6. Tissue Sample Preparation
3.7. Western Blot Assays
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Merighi, A.; Salio, C.; Ferrini, F.; Lossi, L. Neuromodulatory function of neuropeptides in the normal CNS. J. Chem. Neuroanat. 2011, 42, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Feany, M.B. Neuropeptide modulation of learning and memory processes. Rev. Neurosci. 1996, 7, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Schank, J.R.; Ryabinin, A.E.; Giardino, W.J.; Ciccocioppo, R.; Heilig, M. Stress-related neuropeptides and addictive behaviors: Beyond the usual suspects. Neuron 2012, 76, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Werner, F.M.; Covenas, R. Classical neurotransmitters and neuropeptides involved in major depression: A review. Int. J. Neurosci. 2010, 120, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Thim, L.; Madsen, F.; Poulsen, S.S. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur. J. Clin. Investig. 2002, 32, 519–527. [Google Scholar] [CrossRef]
- Xiao, P.; Ling, H.; Lan, G.; Liu, J.; Hu, H.; Yang, R. Trefoil factors: Gastrointestinal-specific proteins associated with gastric cancer. Clin. Chim. Acta 2015, 450, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.M.; Poulsom, R.; Wright, N.A. Trefoil peptides. Gut 1999, 44, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.; Jagla, W.; Wiede, A. Molecular medicine of TFF-peptides: From gut to brain. Histol. Histopathol. 2001, 16, 319–334. [Google Scholar] [PubMed]
- Hirota, M.; Awatsuji, H.; Sugihara, Y.; Miyashita, S.; Furukawa, Y.; Hayashi, K. Expression of pS2 gene in rat brain. Biochem. Mol. Biol. Int. 1995, 35, 1079–1084. [Google Scholar] [PubMed]
- Reymond, A.; Marigo, V.; Yaylaoglu, M.B.; Leoni, A.; Ucla, C.; Scamuffa, N.; Caccioppoli, C.; Dermitzakis, E.T.; Lyle, R.; Banfi, S.; et al. Human chromosome 21 gene expression atlas in the mouse. Nature 2002, 420, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Kjellev, S.; Vestergaard, E.M.; Nexo, E.; Thygesen, P.; Eghoj, M.S.; Jeppesen, P.B.; Thim, L.; Pedersen, N.B.; Poulsen, S.S. Pharmacokinetics of trefoil peptides and their stability in gastrointestinal contents. Peptides 2007, 28, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Bignotti, E.; Ravaggi, A.; Tassi, R.A.; Calza, S.; Rossi, E.; Falchetti, M.; Romani, C.; Bandiera, E.; Odicino, F.E.; Pecorelli, S.; et al. Trefoil factor 3: A novel serum marker identified by gene expression profiling in high-grade endometrial carcinomas. Br. J. Cancer 2008, 99, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jin, H.; Holder, D.; Ozer, J.S.; Villarreal, S.; Shughrue, P.; Shi, S.; Figueroa, D.J.; Clouse, H.; Su, M.; et al. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat. Biotechnol. 2010, 28, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.W.; Bartlett, J.W.; Blennow, K.; Fox, N.C.; Shaw, L.M.; Trojanowski, J.Q.; Zetterberg, H.; Schott, J.M. Cerebrospinal fluid markers including trefoil factor 3 are associated with neurodegeneration in amyloid-positive individuals. Transl. Psychiatry 2014, 4, e419. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Schwegler, H.; Chwieralski, C.E.; Laube, G.; Linke, R.; Pohle, W.; Hoffmann, W. Trefoil factor family (TFF) expression in the mouse brain and pituitary: Changes in the developing cerebellum. Peptides 2004, 25, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.G.; Dobrowolny, H.; Trubner, K.; Steiner, J.; Bogerts, B.; Hoffmann, W. Differential regional and cellular distribution of TFF3 peptide in the human brain. Amino Acids 2015, 47, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Jagla, W.; Wiede, A.; Dietzmann, K.; Rutkowski, K.; Hoffmann, W. Co-localization of TFF3 peptide and oxytocin in the human hypothalamus. FASEB J. 2000, 14, 1126–1131. [Google Scholar] [PubMed]
- Nestler, E.J.; Carlezon, W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef]
- Berton, O.; Nestler, E.J. New approaches to antidepressant drug discovery: Beyond monoamines. Nat. Rev. Neurosci. 2006, 7, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.S.; Zhu, W.L.; Liu, J.F.; Luo, Y.X.; Si, J.J.; Wang, S.J.; Xue, Y.X.; Ding, Z.B.; Shi, J.; Lu, L. PI3K/Akt signaling pathway in the basolateral amygdala mediates the rapid antidepressant-like effects of trefoil factor 3. Neuropsychopharmacology 2012, 37, 2671–2683. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P.; Wrynn, A.S.; Leonard, B.E. The olfactory bulbectomized rat as a model of depression: An update. Pharmacol. Ther. 1997, 74, 299–316. [Google Scholar] [CrossRef]
- Song, C.; Leonard, B.E. The olfactory bulbectomised rat as a model of depression. Neurosci. Biobehav. Rev. 2005, 29, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Baus-Loncar, M.; Giraud, A.S. Multiple regulatory pathways for trefoil factor (TFF) genes. Cell. Mol. Life Sci. 2005, 62, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sun, Z.; Zhang, W.; Liu, H.; Shao, D.; Ren, Y.; Wen, Y.; Cao, L.; Wolfram, J.; Yang, Z.; et al. Protective effects of intestinal trefoil factor (ITF) on gastric mucosal epithelium through activation of extracellular signal-regulated kinase 1/2 (ERK1/2). Mol. Cell. Biochem. 2015, 404, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Gourley, S.L.; Wu, F.J.; Kiraly, D.D.; Ploski, J.E.; Kedves, A.T.; Duman, R.S.; Taylor, J.R. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol. Psychiatry 2008, 63, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Karege, F.; Vaudan, G.; Schwald, M.; Perroud, N.; la Harpe, R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res. Mol. Brain Res. 2005, 136, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dowlatshahi, D.; MacQueen, G.M.; Wang, J.F.; Young, L.T. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry 2001, 50, 260–265. [Google Scholar] [CrossRef]
- Wallace, D.L.; Han, M.H.; Graham, D.L.; Green, T.A.; Vialou, V.; Iniguez, S.D.; Cao, J.L.; Kirk, A.; Chakravarty, S.; Kumar, A.; et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat. Neurosci. 2009, 12, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Blendy, J.A. The role of CREB in depression and antidepressant treatment. Biol. Psychiatry 2006, 59, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.L.; Wang, S.J.; Liu, M.M.; Shi, H.S.; Zhang, R.X.; Liu, J.F.; Ding, Z.B.; Lu, L. Glycine site N-methyl-d-aspartate receptor antagonist 7-CTKA produces rapid antidepressant-like effects in male rats. J. Psychiatry Neurosci. 2013, 38, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Friedman, A.K.; Sun, H.; Heller, E.A.; Ku, S.M.; Juarez, B.; Burnham, V.L.; Mazei-Robison, M.S.; Ferguson, D.; Golden, S.A.; et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat. Neurosci. 2014, 17, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Cazorla, M.; Premont, J.; Mann, A.; Girard, N.; Kellendonk, C.; Rognan, D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J. Clin. Investig. 2011, 121, 1846–1857. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hope, B.T.; Dempsey, J.; Liu, S.Y.; Bossert, J.M.; Shaham, Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat. Neurosci. 2005, 8, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Van der Stelt, H.M.; Breuer, M.E.; Olivier, B.; Westenberg, H.G. Permanent deficits in serotonergic functioning of olfactory bulbectomized rats: An in vivo microdialysis study. Biol. Psychiatry 2005, 57, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.E.; Machado, D.G.; Budni, J.; Neis, V.B.; Balen, G.O.; Lopes, M.W.; de Souza, L.F.; Dafre, A.L.; Leal, R.B.; Rodrigues, A.L. Fluoxetine modulates hippocampal cell signaling pathways implicated in neuroplasticity in olfactory bulbectomized mice. Behav. Brain Res. 2013, 237, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Moriguchi, S.; Tagashira, H.; Fukunaga, K. Rivastigmine improves hippocampal neurogenesis and depression-like behaviors via 5-HT1A receptor stimulation in olfactory bulbectomized mice. Neuroscience 2014, 272, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Rinwa, P.; Kumar, A.; Garg, S. Suppression of neuroinflammatory and apoptotic signaling cascade by curcumin alone and in combination with piperine in rat model of olfactory bulbectomy induced depression. PLoS ONE 2013, 8, e61052. [Google Scholar] [CrossRef] [PubMed]
- Rinwa, P.; Kumar, A. Quercetin suppress microglial neuroinflammatory response and induce antidepressent-like effect in olfactory bulbectomized rats. Neuroscience 2013, 255, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xiong, Z.; Yang, J.; Wang, W.; Wang, Y.; Hu, Z.L.; Wang, F.; Chen, J.G. Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br. J. Pharmacol. 2012, 166, 1872–1887. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.T.; Li, J.; Liu, B.B.; Luo, L.; Liu, Q.; Geng, D. BDNF-ERK-CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. J. Psychiatry Neurosci. 2014, 39, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Castren, E.; Rantamaki, T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev. Neurobiol. 2010, 70, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Zhang, R.G.; Xue, F.; Wang, H.N.; Chen, Y.C.; Hu, G.T.; Peng, Y.; Peng, Z.W.; Tan, Q.R. Quetiapine and repetitive transcranial magnetic stimulation ameliorate depression-like behaviors and up-regulate the proliferation of hippocampal-derived neural stem cells in a rat model of depression: The involvement of the BDNF/ERK signal pathway. Pharmacol. Biochem. Behav. 2015, 136, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Bateup, H.; Qi, H.; Takamiya, K.; Huganir, R.L.; Spedding, M.; Roth, B.L.; McEwen, B.S.; Greengard, P. Involvement of AMPA receptor phosphorylation in antidepressant actions with special reference to tianeptine. Eur. J. Neurosci. 2007, 26, 3509–3517. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Shi, H.S.; Luo, Y.X.; Zhang, R.X.; Li, J.L.; Shi, J.; Lu, L.; Zhu, W.L. Neuropeptide trefoil factor 3 attenuates naloxone-precipitated withdrawal in morphine-dependent mice. Psychopharmacology 2014, 231, 4659–4668. [Google Scholar] [CrossRef] [PubMed]
- Nakagawasai, O.; Nemoto, W.; Onogi, H.; Moriya, T.; Lin, J.R.; Odaira, T.; Yaoita, F.; Ogawa, T.; Ohta, K.; Endo, Y.; et al. BE360, a new selective estrogen receptor modulator, produces antidepressant and antidementia effects through the enhancement of hippocampal cell proliferation in olfactory bulbectomized mice. Behav. Brain Res. 2015, 297, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Yu, H.Y.; Kang, D.Y.; Ma, Z.Q.; Qu, R.; Fu, Q.; Ma, S.P. Antidepressant-like effects of salidroside on olfactory bulbectomy-induced pro-inflammatory cytokine production and hyperactivity of HPA axis in rats. Pharmacol. Biochem. Behav. 2014, 124, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Mannari, C.; Origlia, N.; Scatena, A.; del Debbio, A.; Catena, M.; Dell’agnello, G.; Barraco, A.; Giovannini, L.; Dell'osso, L.; Domenici, L.; et al. BDNF level in the rat prefrontal cortex increases following chronic but not acute treatment with duloxetine, a dual acting inhibitor of noradrenaline and serotonin re-uptake. Cell. Mol. Neurobiol. 2008, 28, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [PubMed]
- De Foubert, G.; Carney, S.L.; Robinson, C.S.; Destexhe, E.J.; Tomlinson, R.; Hicks, C.A.; Murray, T.K.; Gaillard, J.P.; Deville, C.; Xhenseval, V.; et al. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience 2004, 128, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Gould, G.G.; Altamirano, A.V.; Javors, M.A.; Frazer, A. A comparison of the chronic treatment effects of venlafaxine and other antidepressants on serotonin and norepinephrine transporters. Biol. Psychiatry 2006, 59, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B.; Entsuah, R.; Benattia, I.; Demitrack, M.; Sloan, D.M.; Thase, M.E. Comprehensive analysis of remission (COMPARE) with venlafaxine vs. SSRIs. Biol. Psychiatry 2008, 63, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, Z.; He, J.; Haimanot, S.; Li, X.; Dyck, L.; Li, X.M. Synergetic effects of quetiapine and venlafaxine in preventing the chronic restraint stress-induced decrease in cell proliferation and BDNF expression in rat hippocampus. Hippocampus 2006, 16, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, O.; Deveci, A.; Taneli, F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: A preliminary study. Prog. Neuropsychopharmacol. Bol. Psychiatry 2005, 29, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Matrisciano, F.; Bonaccorso, S.; Ricciardi, A.; Scaccianoce, S.; Panaccione, I.; Wang, L.; Ruberto, A.; Tatarelli, R.; Nicoletti, F.; Girardi, P.; et al. Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. J. Psychiatr. Res. 2009, 43, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.L.; Shi, H.S.; Wang, S.J.; Wu, P.; Ding, Z.B.; Lu, L. Hippocampal CA3 calcineurin activity participates in depressive-like behavior in rats. J. Neurochem. 2011, 117, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Zhang, J.C.; Wu, J.; Fujita, Y.; Yao, W.; Ren, Q.; Yang, C.; Li, S.X.; Shirayama, Y.; Hashimoto, K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Zhao, L.; Si, J.; Liu, J.; Zhu, W.; Chai, B.; Zhang, Y.; Feng, J.; Ding, Z.; Luo, Y.; et al. Predictable chronic mild stress in adolescence increases resilience in adulthood. Neuropsychopharmacology 2013, 38, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Luo, Y.; Zhang, R.; Shi, H.; Zhu, W.; Shi, J. Neuropeptide Trefoil Factor 3 Reverses Depressive-Like Behaviors by Activation of BDNF-ERK-CREB Signaling in Olfactory Bulbectomized Rats. Int. J. Mol. Sci. 2015, 16, 28386-28400. https://doi.org/10.3390/ijms161226105
Li J, Luo Y, Zhang R, Shi H, Zhu W, Shi J. Neuropeptide Trefoil Factor 3 Reverses Depressive-Like Behaviors by Activation of BDNF-ERK-CREB Signaling in Olfactory Bulbectomized Rats. International Journal of Molecular Sciences. 2015; 16(12):28386-28400. https://doi.org/10.3390/ijms161226105
Chicago/Turabian StyleLi, Jiali, Yixiao Luo, Ruoxi Zhang, Haishui Shi, Weili Zhu, and Jie Shi. 2015. "Neuropeptide Trefoil Factor 3 Reverses Depressive-Like Behaviors by Activation of BDNF-ERK-CREB Signaling in Olfactory Bulbectomized Rats" International Journal of Molecular Sciences 16, no. 12: 28386-28400. https://doi.org/10.3390/ijms161226105
APA StyleLi, J., Luo, Y., Zhang, R., Shi, H., Zhu, W., & Shi, J. (2015). Neuropeptide Trefoil Factor 3 Reverses Depressive-Like Behaviors by Activation of BDNF-ERK-CREB Signaling in Olfactory Bulbectomized Rats. International Journal of Molecular Sciences, 16(12), 28386-28400. https://doi.org/10.3390/ijms161226105