Abstract
Polar and but not lateral flagellin proteins from Aeromonas hydrophila strain AH-1 (serotype O11) were found to be glycosylated. Top-down mass spectrometry studies of purified polar flagellins suggested the presence of a 403 Da glycan of mass. Bottom-up mass spectrometry studies showed the polar flagellin peptides to be modified with 403 Da glycans in O-linkage. The MS fragmentation pattern of this putative glycan was similar to that of pseudaminic acid derivative. Mutants lacking the biosynthesis of pseudaminic acid (pseB and pseI homologues) were unable to produce polar flagella but no changes were observed in lateral flagella by post-transcriptional regulation of the flagellin. Complementation was achieved by reintroduction of the wild-type pseB and pseI. We compared two pathogenic features (adhesion to eukaryotic cells and biofilm production) between the wild-type strain and two kinds of mutants: mutants lacking polar flagella glycosylation and lacking the O11-antigen lipopolysaccharide (LPS) but with unaltered polar flagella glycosylation. Results suggest that polar flagella glycosylation is extremely important for A. hydrophila AH-1 adhesion to Hep-2 cells and biofilm formation. In addition, we show the importance of the polar flagella glycosylation for immune stimulation of IL-8 production via toll-“like” receptor 5 (TLR5).
1. Introduction
Mesophilic Aeromonas spp. strains are important pathogens of humans and lower vertebrates, including amphibians, reptiles, and fish [1]. Infections produced by these strains in humans can be classified into two major groups: noninvasive disease such as gastroenteritis, and systemic illnesses [2]. Strains from Aeromonas hydrophila, A. veronii biovar veronii, or sobria are described as virulent for humans [3] and fish [4]; these strains are serologically related by their O-antigen lipopolysaccharide (LPS) (serotype O11). This has a known chemical structure containing O-polysaccharide chains of homogeneous chain length [5]. In addition, strains express a crystalline surface array protein with a molecular weight of ca. 52,000, which forms the S layer that lies peripheral to the cell wall [6]. The strains from this serotype are the most frequently isolated from septicemia caused by mesophilic Aeromonas spp. [2].
Flagella motility in Aeromonas represents an important advantage, allowing bacteria to move towards favorable conditions or avoid unfavorable environments, and it allows it to successfully compete with other microorganisms [7]. The mesophilic Aeromonas spp. constitutively express a single polar flagellum, and approximately 60% express numerous lateral flagella when grown in viscous environments or on surfaces [8,9]. Several studies have shown that both the polar and lateral flagella systems of the mesophilic Aeromonas spp. are involved in adherence to both biotic and abiotic surfaces, as well as in the biofilm formation [10].
One of the most common protein post-translational modifications is glycosylation, which for many years was thought to be a solely eukaryotic mechanism. More recently, many different protein glycosylation systems have been largely identified in all forms of life including prokaryotes. Carbohydrates are covalently attached to serine or threonine residues (O-glycosylation), or to asparagine residues (N-glycosylation). A recent review gives an overview of O-glycosylation in bacterial systems [11]. The available bacterial genomic information and bioinformatic analysis, together with functional analysis, has allowed the identification of many genes that participate in flagellin glycosylation, and also the definition of glycosylation pathways. These studies showed the large diversity in each bacterial species in the number of O-glycosylation genes involved and their location [12,13,14,15]. However, the diversity of structure and composition of glycans which modify flagellins from Gram-negative bacteria is restricted to certain species, or strains, as described in a recent review [16].
In the current study we show that polar but not lateral flagellins of A. hydrophila strain AH-1 from serotype O11 [16] are modified at multiple sites with putative glycans, which we propose to be pseudaminic acid-like moieties. We also show the requirement of glycosylation for polar flagella production. The O-antigen LPS or the flagella have been described as important molecules for bacterial adherence or biofilm formation in Aeromonas [17]. We recently characterized the O11 antigen LPS from the same strain A. hydrophila AH-1, and obtained mutants lacking this LPS structure [18]. This allowed us to evaluate the importance of both flagellin and LPS in adhesion to Hep-2 cells and biofilm formation. In addition, the importance of polar flagella glycosylation for immune stimulation of IL-8 production via toll-“like” receptor 5 (TLR5) was also evaluated.
2. Results
The A. hydrohila AH-1 strain belongs to serotype O11 and is able to produce an S-layer [19]. This strain is motile in liquid medium (swimming) through expression of a polar flagellum and exhibits swarming behaviors in semisolid media through expression of lateral flagella (Figure 1).
DNA probes from polar flagella region 2 of A. hydrophila AH-3 [20] allowed identification of a clone from a cosmid genomic library of A. hydrophila AH-1. This clone allowed the DNA sequence of complete region 2 [21] of AH-1 to be obtained. This DNA sequence also allowed the isolation of a non-polar flagellated mutant AH-1ΔFlaB-J; this mutant was unable to swim, but able to swarm, suggesting expression of lateral flagella only. Subsequently, this mutant was used to isolate lateral flagella on this strain. DNA probes from the lateral flagella cluster of A. hydrophila AH-3 [22] allowed identification of a clone from the same cosmid genomic library of A. hydrophila AH-1 with the partial lateral flagella cluster of this strain. The partial DNA sequence of the AH-1 lateral flagella cluster allowed us to identify two lateral flagellins (LafA1 and A2) in this strain. This compares with a single lateral flagellin observed in strain AH-3 [22].
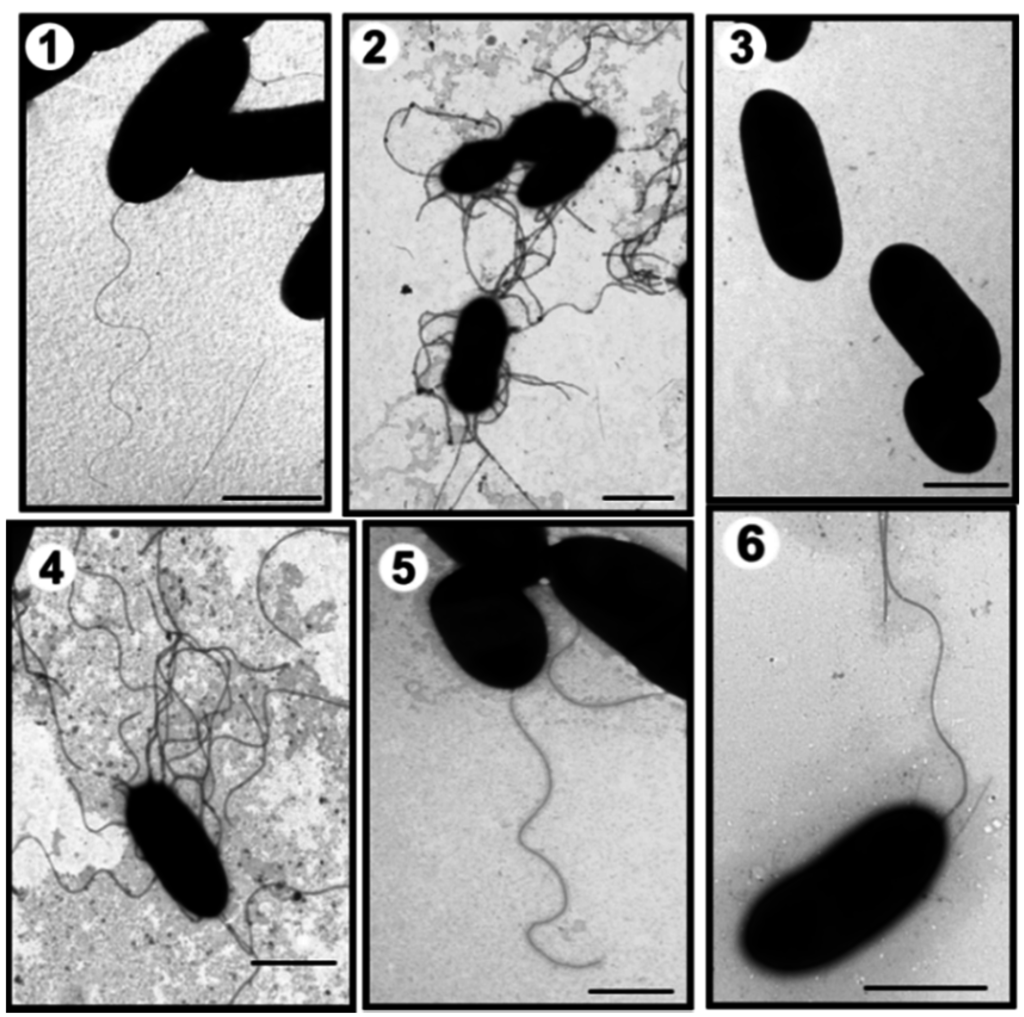
Figure 1.
Transmission electron microscopy of A. hydrophila strains. AH-1 wild type grown in liquid media (TSB) (1) or in solid media (TSA) (2); AH-1ΔpseI mutant grown in TSB (3) or in TSA (4); AH-1ΔpseI mutant + pBAD-pseI grown in TSB (5), and AH-1ΔrmlB mutant grown in TSB (6). Bar = 1 μm.
2.1. Mass Spectrometry Analyses of Wild-Type Lateral and Polar Flagellins
Both polar and lateral flagellins were purified (Figure 2) and their intact mass profiles analyzed using LC–MS.
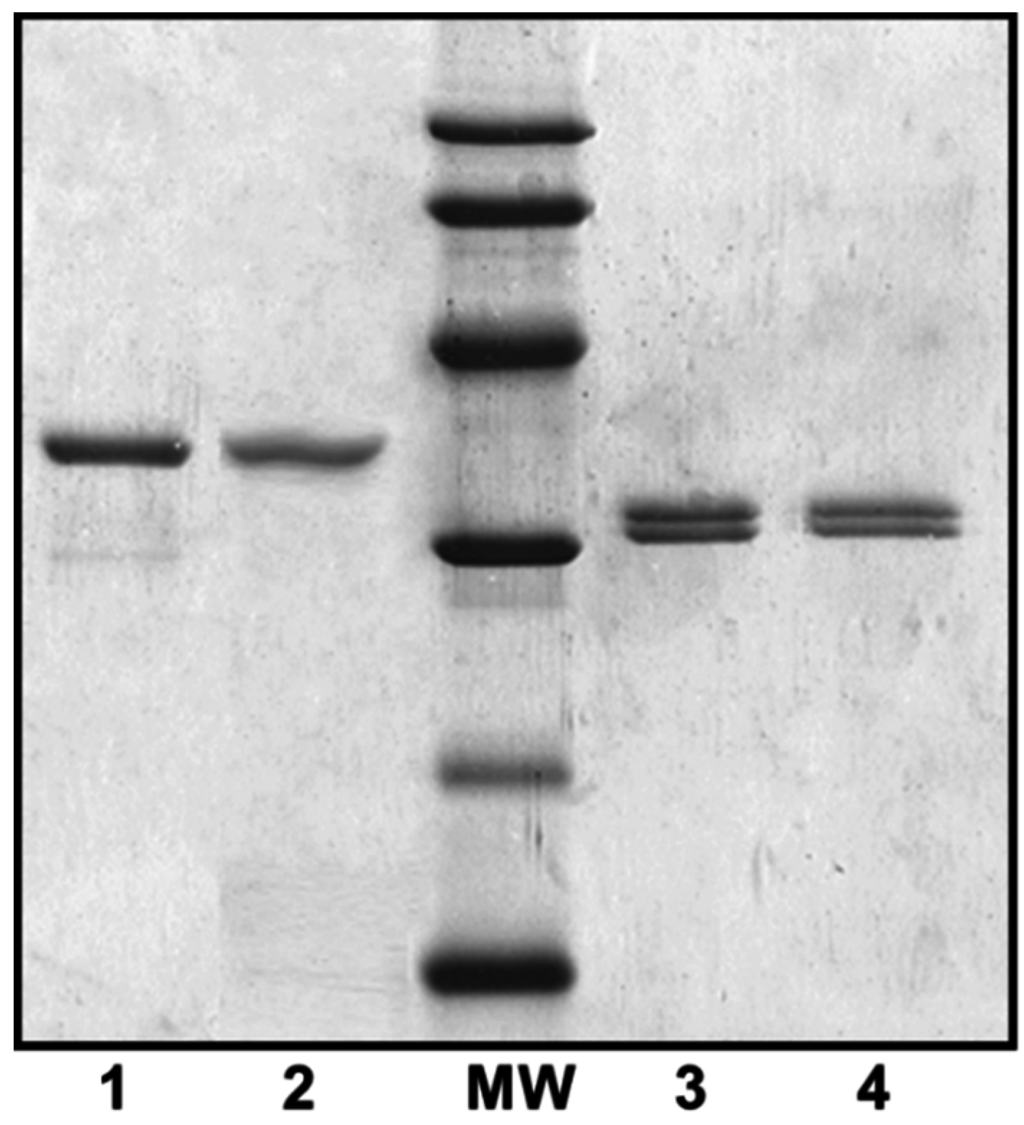
Figure 2.
Purified polar and lateral flagellins from several A. hydrophila strains. 1, A. hydrophila AH-1 (wild-type) polar flagellin; 2, A. hydrophila AH-1∆rmlB mutant (no O-antigen LPS produced) polar flagellin; MW = molecular weight standard (14, 20, 30, 45, 60, and 94 kDa); 3, A. hydrophila AH-1ΔflaB-J (unable to produce polar flagellum) lateral flagellin; and 4, A. hydrophila AH-1∆rmlB mutant lateral flagellin.
The MS spectrum of a purified lateral flagellin showed a multiply charged ion envelope, as shown in Figure 3a, typical of protein. The spectrum was deconvoluted (Figure 3b) and showed two protein masses of 30,402 and 30,267 Da.
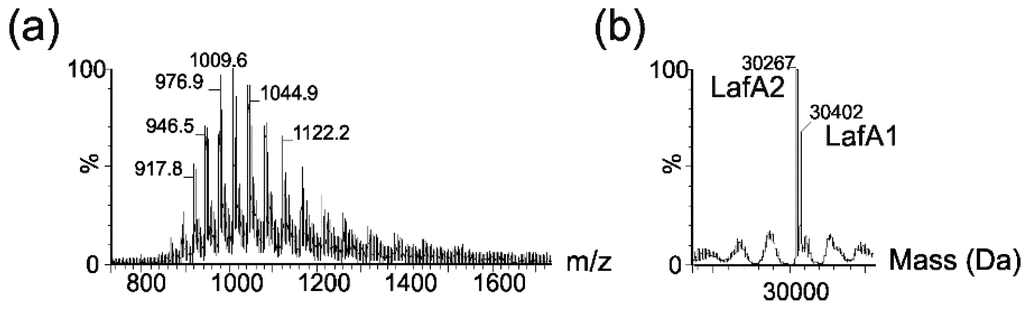
Figure 3.
Electrospray mass spectrometry of intact lateral flagellin proteins from Aeromonas hydrophila AH-1. (a) Electrospray mass spectrum of intact lateral flagellin proteins showing a complex envelope of multiply charged protein ions; (b) The reconstructed molecular mass profile of the lateral flagellin resolves two masses at 30,267 and 30,402 Da, closely corresponding to the predicted mass of lateral flagellin proteins LafA1 and Laf A2.
These masses corresponded almost exactly to the predicted masses of the lateral flagellin proteins, LafA1 (30,401 Da) and LafA2 (30,297 Da). In addition, nLC-MS/MS analyses of tryptic digests of polar flagellins showed 42% and 45% sequence coverage for LafA1 and LafA2, respectively. Manual inspection of the MS/MS data showed no evidence of glycan-related oxonium ions in peptide spectra. Taken together, these data strongly suggested that the flagellins were not post-translationally modified with carbohydrates, as had been reported with lateral flagellins of A. hydrophila AH-3 [23].
LC-MS analysis of purified polar flagellins produced a characteristic multiply charged protein ion envelope (Figure 4a). When deconvoluted, a major mass of 34,947 Da was observed (Figure 4b).
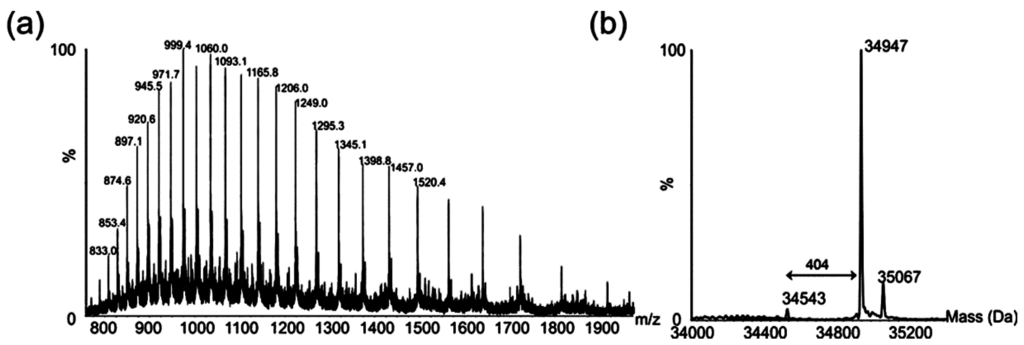
Figure 4.
Electrospray mass spectrometry of intact polar flagellin proteins from Aeromonas hydrophila AH-1. (a) Electrospray mass spectrum of intact polar flagellin proteins showing a complex envelope of multiply charged protein ions; (b) The reconstructed molecular mass profile of these polar flagellins resolves three masses at 34,543, 34,947, and 35,067 Da.
The masses of the polar flagellin proteins predicted from the translated gene sequences are 31,413 and 31,361 Da, and neither mass was observed in the deconvoluted mass spectrum. The observed difference between the observed masses, highlighted in Figure 4b, suggests a mass difference between flagellins of 404 Da.
To further investigate the nature of the protein modification, a tandem MS experiment was carried out on one multiply charged protein ion (m/z = 999.4). The resulting MS/MS spectrum was dominated by an intense ion at m/z = 404.4. An MS3 experiment on the ion observed at m/z = 404.4 produced an MS3 which showed consecutive losses of water and a methyl group from the parent ion (Figure 5).
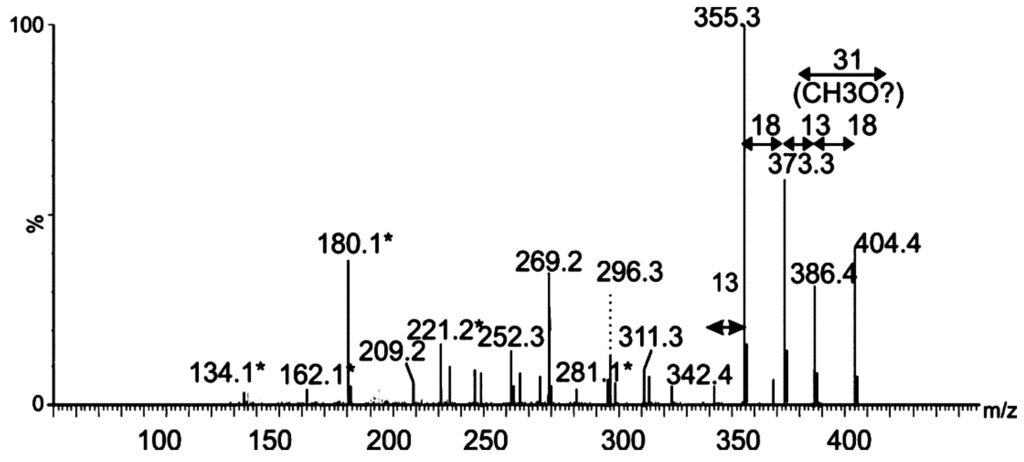
Figure 5.
Mass spectrometry analysis of putative glycan oxonium ion. Tandem mass spectrum of singly charged ion at m/z 404 following feCID of intact polar flagellin protein showed consecutive losses of water and methyl groups from the parent ion. Subsequent losses from this ion gave rise to a fragmentation pattern similar to that observed with nonulosonic acid sugars. Daughter ions marked with an asterisk denote those fragment ions found in MS/MS spectra of pseudaminic acid. Figure legend: = 376 Da sugar; * = fragment ions common to pseudaminic acid.
The MS/MS spectrum did not contain any recognizable peptide-related ions, and the fragmentation pattern strongly suggested that this moiety was a glycan. Of note, many sugar fragment ions, observed at m/z = 134.1, 162.1, 180.13, 221.19, 281.1 (denoted with an asterisk in Figure 5), were also observed in the MS/MS spectrum of the flagellin-modifying sugar Pse5Ac7Ac9Ac, found in A. caviae [24]. Other fragment ions, observed at m/z = 342.4, 355.4 and 373.2, were a single m/z unit different from fragment ions observed in the MS/MS spectrum of Pse5Ac7Ac9Ac (Figure 4). From this data, the top-ranked plausible elemental formula was C19H32O9. These data, combined with the accurate mass analyses, suggest the base sugar is a pseudaminic acid-like sugar, with putative additions of two methyl groups and two molecules of water, and an unknown mass of 25 Da. Detailed structural analyses using NMR will be required to confirm this suggestion and the complete structure of this putative sugar moiety.
2.2. Tandem Mass Spectrometry Analyses of Proteolytic Digests of Polar Flagellin Proteins
nLC-MS/MS analyses of tryptic digests of polar flagellin preparation showed peptide sequences corresponding to FlaA and FlaB proteins giving 35% and 36% sequence coverage, respectively (Figure 6).
De novo sequencing of tryptic peptides showed many MS/MS spectra of high m/z peptide precursor ions, with very weak peptide type y or b fragment ions. Inspection of the protein sequence showed that the central variable region of the flagellin sequence was lacking tryptic cleavage sites, resulting in tryptic peptides likely too large to be readily sequenced by nLC-MS/MS. To overcome this challenge, the flagellin protein was treated with proteinase K for 15 min–16 h and the resulting peptides analyzed by nLC-MS/MS. From these data, a total of five non-overlapping glycopeptides were de novo sequenced, as indicated in Figure 6. All of the glycopeptides were observed to be modified with one or more glycan moieties of 403 Da, with the spectra dominated by an intense glycan oxonium ion at m/z = 404. This can be seen in Figure 6, which shows the MS/MS spectrum of the polar flagellin peptide 167SISGIAK173. Glycan fragment ions were observed in the low m/z region of the glycopeptide spectrum, indicated with an asterisk in Figure 6. In addition to the glycan oxonium ion at m/z = 404, glycan-related fragment ions were identical to those ions observed in the top-down fragmentation of the putative glycan. In all but one case, the glycopeptides corresponded to identical regions of FlaA and FlaB (except SISGIAK). The absence of asparagine residues on all sequenced glycopeptides suggests that the glycan is attached via O-linkage to serine or threonine residues.
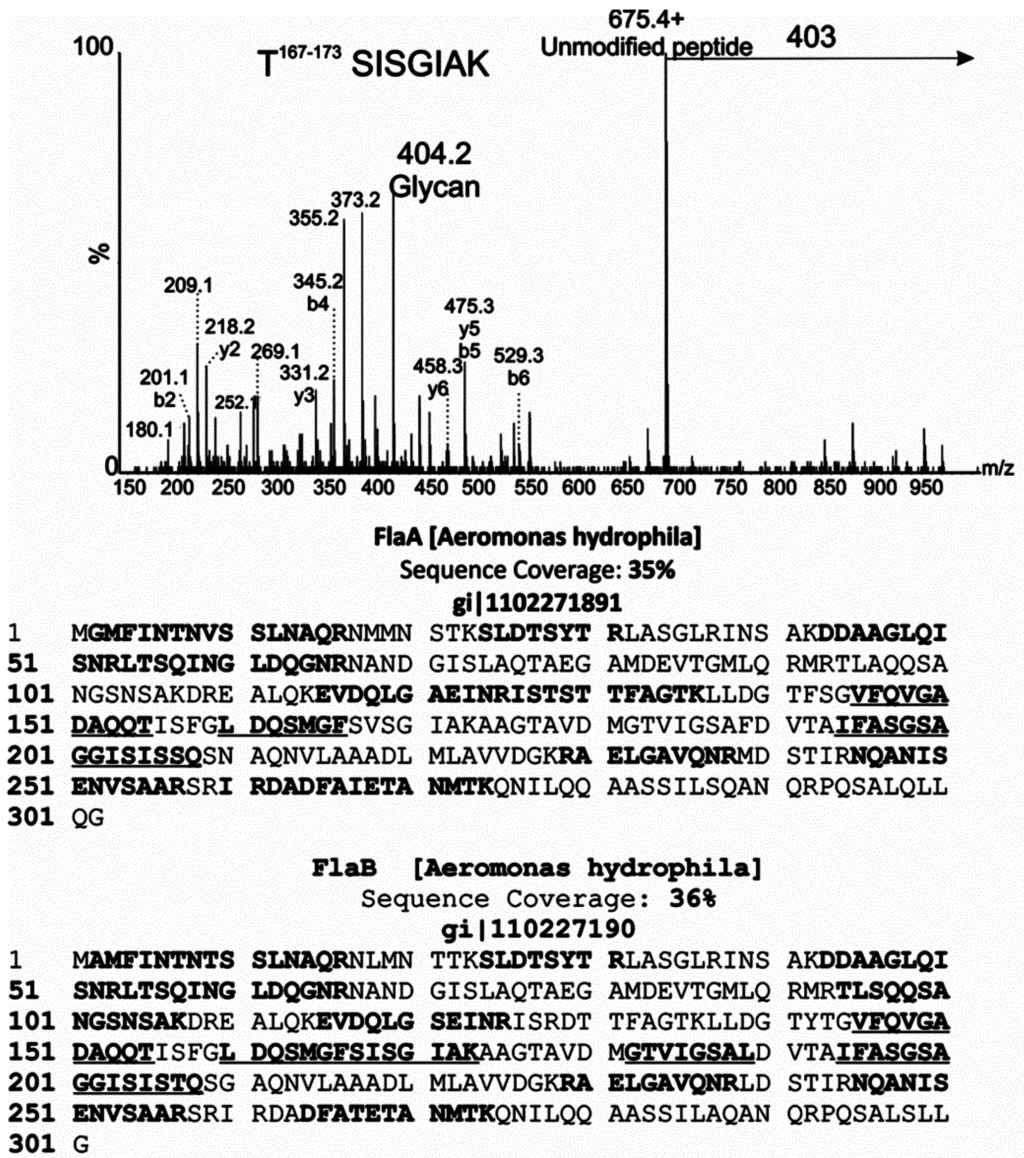
Figure 6.
MS/MS analysis of glycopeptides from polar flagellar protein of Aeromonas hydrophila AH-1. nLC-MS/MS spectrum of the doubly protonated glycopeptide ion at m/z = 539.5. The MS/MS spectrum was dominated by an ion at m/z = 675.4, corresponding to the unmodified peptide 167SISGIAK173. A neutral loss of 403 Da was observed from the glycopeptide precursor, and a corresponding glycan oxonium ion observed at m/z = 404. Peptide type y and b ions are indicated, and were weak in comparison to glycan-related fragment ions, which are indicated by an asterisk. Peptide sequence coverage of lateral flagellins FlaA and B is shown below. In each case, bold indicates an identified non-modified peptide. Bold and underline indicates peptides that were de novo sequenced and modified with 403 Da glycan moiety.
2.3. Putative Pseudaminic Acid Biosynthetic Mutants
Due to the suggestion of a putative pseudaminic acid-like glycosylation of AH-1 polar flagella, genes in the biosynthetic pathway of this sugar were mutated. Using oligonucleotides 5′-TCCAGAAGGTTATCGCACT-3′ and 5′-GATGCTGGGAGCTATTACG-3′ with genomic DNA from strain AH-1, an internal DNA fragment (500 bp) corresponding to a pseB homologue was amplified [25]. Genome walking allowed the completion of the DNA sequence of pseB-C homologues in strain AH-1. The same approach using oligonucleotides 5′-CCTATACCGCTGACACC AT-3′ and 5′-TCACCACTTTTTCCTGACC-3′ was used to amplify an internal DNA fragment (679 bp) of pseI homologue [25], and completely sequence gene pseG-I homologues in this strain. Subsequently, in frame mutants AH-1∆pseB and AH-1∆pseI were constructed. Using TEM, these mutants were shown to be unable to produce polar flagellum but lateral flagella was unaffected under induced conditions by TEM (Figure 1 data shown for AH-1∆pseI). The introduction of the Aeromonas wild-type corresponding genes recovered the production of polar flagella in the mutants (data not shown).
2.4. Adhesion to HEp-2 Cells and Biofilm Formation
The role of polar flagella glycosylation, lateral flagella, and O11-antigen LPS in the adherence to eukaryotic cells was investigated. The adhesion of several mutants to cultured monolayers of HEp-2 cells was observed. The AH-1ΔrmlB mutant lacking O11-antigen LPS [18], but with expression of either polar or lateral flagella under induced conditions by TEM (Figure 1), showed no changes in flagellin molecular weight (Figure 2). Bacterial motility, when compared to wild type, was also unaffected, as was bacterial adherence to HEp-2 cells (Table 1).
By contract, mutants ∆pseB or I that lacked expression of flagella (polar and lateral for their grown in broth) showed a minimal adhesion values with a 86% reduction compared with the wild type. The same mutants expressed lateral flagella when grown in solid media, and showed an increase in their rate of adherence to Hep-2 cells (Table 1). The AH-1∆rmlB mutant lacking the O11-antigen LPS showed comparatively less reduction in adhesion (29%) compared with the reduced adhesion observed in the flagella mutants. In all the cases, complementation of mutants with the wild-type gene/s showed recovery of the wild-type adhesion values (Table 1). The results obtained in previous adhesion studies prompted us to study the biofilm production from the wild type and different mutant strains in a microtiter assay (Table 2).

Table 1.
Adhesion of different A. hydrophila AH-1 serotype O11 strains to HEp-2 cells as described in Experimental Section. Values presented are mean ± SD from three independent experiments carried out in duplicate or triplicate (n = 6 or n = 9).
| Strain and Main Characteristics | Mean No. of Bacteria | % Reduction |
|---|---|---|
| HEp-2 Cell ± SD | in Adhesion a | |
| AH-1; wild-type serotype O11 | 21.4 ± 3.6 | – |
| AH-1ΔpseB (flagella−) (O11+; flagella polar−/lateral−, grown in TSB) | 2.7 ± 0.9 | 87 * |
| AH-1∆pseB (flagella−) (O11+; flagella polar−/lateral+, grown in TSA) | 7.5 ± 1.8 | 65 * |
| AH-1∆pseI (flagella−) (O11+; flagella polar−/lateral−, grown in TSB) | 2.9 ± 0.5 | 86 * |
| flagella polar−/lateral−, grown in TSA | 8.1 ± 1.1 | 63 * |
| AH-1∆pseB + pBAD-pseB (flagella−) (O11+; flagella polar+/lateral−, grown in TSB) | 19.8 ± 2.4 | <8 |
| flagella polar+/lateral−, grown in TSB | 20.7 ± 2.0 | <8 |
| AH-1∆rmlB (O11− mutant) (O11−; flagella+) | 15.4 ± 2.6 | 29 * |
| AH-1∆rmlB + pBAD-rmlB (O11+; flagella+) | 20.4 ± 3.2 | <8 |
a The level of adhesion of strain AH-1 was used as 100% value. Student’s t-test, p = 0.001 for adhesion values; * Values statistically different from the wild-type strain AH-1 level of adhesion. No significate differences were observed between the AH-1 level of adhesion when grown in liquid medium (polar flagella+, lateral flagella−) or solid medium (polar flagella+, lateral flagella+); −, lack of the corresponding structure; +, presence of the corresponding structure.
The wild-type strain grown on liquid media (no lateral flagella produced) showed biofilm formation ability with an average OD570 value of 1.43. Mutants lacking flagella grown in liquid or solid media (+ or − lateral flagella) are unable to form biofilms, with values <0.1, out of the range of detection for the assay (Table 2). The AH-1ΔrmlB mutant lacking the O11-antigen LPS showed an approximately 50% reduction in the ability to produce biofilm (0.78 average value versus 1.43 for wild type). In all cases, biofilm formations of the mutants were fully rescued by the introduction of the wild-type genes (Table 2). Mutants’ strains with only the plasmid vector alone show no differences in both studies.

Table 2.
Biofilm values of several A. hydrophila AH-1 serotype O11 strains using the method of Pratt and Kolter as indicated in the Experimental Section. Values presented are mean ± SD from three independent experiments carried out in triplicate (n = 9).
| Strain and Characteristics | Value (OD570) |
|---|---|
| AH-1, wild-type serotype O11 | 1.43 ± 0.15 |
| AH-1∆pseB (flagella−) (O11+; flagella polar−/lateral−, grown in TSB) | <0.1 |
| AH-1∆pseB (flagella−) (O11+; flagella polar−/lateral+, grown in TSA) | <0.1 |
| AH-1ΔpseI (flagella−) (O11+; flagella polar−/lateral−, grown in TSB) | <0.1 |
| AH-1∆pseI (flagella−) (O11+; flagella polar−/lateral+, grown in TSA) | <0.1 |
| AH-1∆pseB + pBAD-pseB (flagella−) (O11+; flagella polar+/lateral−, grown in TSB) | 1.37 ± 0.11 |
| AH-1∆pseI + pBAD-pseI (flagella−) (O11+; flagella polar+/lateral−, grown in TSB) | 1.39 ± 0.18 |
| AH-1∆rmlB (O11−; flagella+) | 0.78 ± 0.13 |
| AH-1∆rmlB + pBAD-rmlB | 1.41 ± 0.17 |
2.5. IL-8 Immune Stimulation
When we stimulated HEK293-null cells (control) with purified A. hydrophila AH-1 polar flagellins, no IL-8 production was observed (Figure 7).
The degree of TLR5 activation was studied through the production of IL-8 using the HEK293 cell line, which was stably transfected with TLR5. HEK293-hTLR5 cells stimulated with purified A. hydrophila AH-1polar flagellins (wild type) showed varying levels of IL-8 production in agreement with the amount of flagellin used (Figure 7). However, HEK293-hTLR5 cells were stimulated with identical amounts of A. hydrophila AH-1 polar flagellin FlaB unglycosylated monomer obtained in E. coli, and the amount of IL-8 production was reduced (60%).
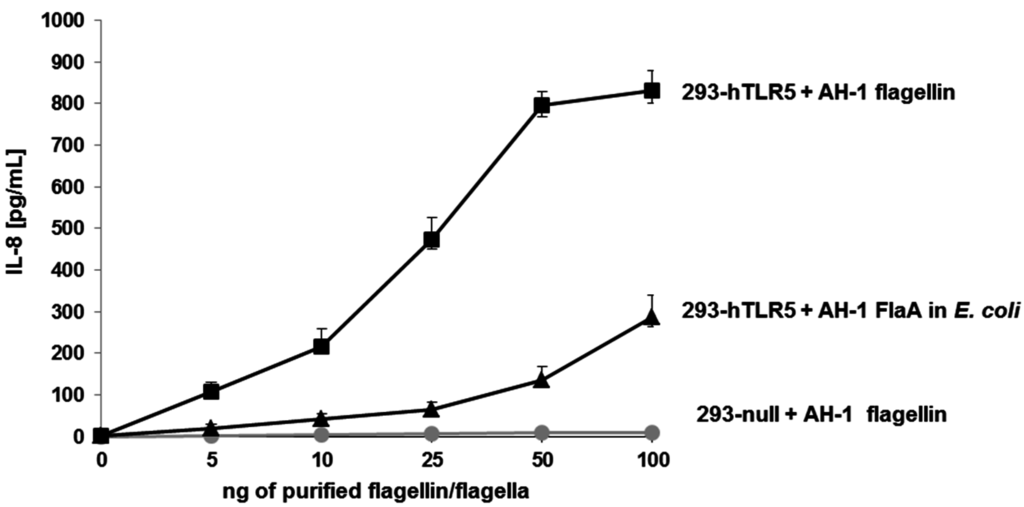
Figure 7.
IL-8 production of HEK293-null and HEK293-hTLR5 cells. HEK293-null and HEK293-hTLR5 cells were stimulated with five different increased amounts (from 5 to 100 ng) of purified polar flagella from A. hydrophila AH-1 (wild-type) strain. HEK293-hTLR5 cells were also stimulated with A. hydrophila AH-1 polar flagellin FlaB obtained in E. coli (non-glycosylated). Data shown are means ± SD of three independent experiments.
3. Discussion
In the current study, we demonstrated that A. hydrophila strain AH-1 (serotype O11) flagella, the constitutively expressed polar flagellum but not the inducible peritrichous lateral flagella, showed modification with a putative O-linked glycan moiety. As previously published, strain AH-3 (serotype O34) showed both flagella glycosylated. Both serotypes are among the four dominant serotypes (O11, O16, O18, and O34) that are associated with gastroenteritis and septicemia in clinical studies [26]. Mass spectrometry fragmentation of the putative glycan provides some evidence that the sugar contains similarities to previously characterized pseudaminic acid-like sugars. The mass spectrometry data and putative elemental formula suggest the presence of two methyl groups and two molecules of water, and an unknown mass of 25 Da.
The altered or incomplete flagellin glycosylation resulted in altered motility phenotypes. This was also observed with Clostridium difficile, where the abolition of flagellin glycosylation resulted in the loss of motility [27]. Studies of A. hydrophila strain AH-3 showed that deletion mutants of pseudaminic acid biosynthesis abolish polar and lateral flagellum formation [23]. However, deletion mutants of pseudaminic acid biosynthesis in strain AH-1 (serotype O11) abolish polar flagellum formation but not lateral flagella. Because the presence of the putative pseudaminic acid seems to be a requirement for flagellin export and flagella formation in Aeromonas [23], this point is in agreement with our results that lateral flagella in this strain AH-1 are not glycosylated.
AH-1ΔrmlB mutant is unable to produce O11-antigen LPS [18]; however, it is able to produce either polar or lateral flagella under induced conditions by TEM studies. No interaction between the flagella O-glycosylation and O-antigen LPS biosynthetic pathway was found in A. hydrophila. This is in contrast with studies of Pseudomonas aeruginosa, which suggested involvement of the O-antigen biosynthesis in O-glycosylation [28]. In addition, O-antigen LPS and flagella have been described as important molecules for bacterial adherence or biofilm formation in Aeromonas [17].
Results from the current study suggest that flagella is a more important adhesion factor than O-antigen LPS, as shown by adhesion to Hep-2 cells. Furthermore, the polar flagellum seems to be a determinant factor, with the lateral flagella unable to fully compensate for lack of expression of polar flagellin. This is the first study to show that polar flagellum glycosylation in Aeromonas is a determinant factor in adherence to eukaryotic cells. The results obtained in biofilm formation studies are more marked, with polar flagellum a requirement for biofilm formation, with mutants deficient in polar flagellin expression unable to form biofilms. Then, if there is any compensation from the lateral flagella to the loss of polar flagellum, it is unable to achieve the degree for biofilm formation. The O-antigen LPS mutant, able to produce either polar or lateral flagellin, was observed to form biofilms, but in a reduced capacity.
Toll-like receptors (TLRs) are major components of innate immunity. TLR5 is involved in recognizing bacterial flagellin and, after binding, it triggers the induction of pro-inflammatory cytokines such as IL-8 by the myeloid differentiation primary response gene 88 (MyD88)-dependent signaling pathway [29]. The results obtained indicate that A. hydrophila AH-1 polar flagellin glycosylation is important for and quantitatively related to immune stimulation of IL-8 production via TLR5. A clear reduction in IL-8 production was observed when polar non-glycosylated flagellin monomers (FlaB) were expressed in E. coli. Fish infected with pathogenic bacteria showed a significant enhanced IL-8 expression in the blood and intestine [30]. It is tempting to speculate the possible role of A. hydrophila AH-1 polar flagella glycosylation in cell invasion as observed for different A. hydrophila wild-type strains [17].
This study supports the existence of a complex mechanism of flagella glycosylation in different A. hydrophila strains. We show for the first time the presence of inducible lateral flagella, either with lateral flagellins glycosylated or not, in different A. hydrophila strains. We also show that different A. hydrophila strains used different glycans for polar flagellin glycosylation. Furthermore, we continue to shed light on the biological role of flagellum glycosylation, showing their implication in flagellum production, motility, adhesion to eukaryotic cells, biofilm formation, and TLR5 activation.
4. Experimental Section
4.1. Bacterial Strains, Plasmids, and Growth Conditions
E. coli strains were grown on Luria-Bertani (LB) Miller broth and LB Miller agar at 37 °C, while Aeromonas strains were grown either in tryptic soy broth (TSB) or agar (TSA) at 30 °C. When indicated, kanamycin (50 μg/mL), rifampicin (100 μg/mL), spectinomycin (50 μg/mL), and chloramphenicol (25 μg/mL) were added to the media.
The bacterial strains and plasmids used in this study are listed in Table 3.

Table 3.
Bacterial strains and plasmids used.
| Strain or Plasmid | Relevant Characteristics | Reference or Source |
|---|---|---|
| E. coli Strains | ||
| DH5α | F− end A hsdR17 (rK− mK+) supE44 thi-1 recA1 gyr-A96 Ф80lacZM15 | [31] |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac (F− proABlacIqZ_M15 Tn10) | Stratagene |
| BL21(λD3) | F− ompT hsdSB (rB− mB−) gal dcm(λD3) | Novagen |
| A. hydrophila Strains | ||
| AH-1 | O11, Wild type | [19] |
| AH-Rif R | AH-1, spontaneous rifampicin resistant mutant, Rif R | [19] |
| AH-1ΔflaB-J | AH-1 mutant in frame unable produce polar flagellum but able to produce lateral flagella | This study |
| AH-1ΔrmlB | AH-1 mutant in frame unable produce O11-antigen LPS | [18] |
| AH-1ΔpseB | AH-1 pseB mutant in frame with pDM4 | This study |
| AH-1ΔpseI | AH-1 pseI mutant in frame with pDM4 | This study |
| Plasmids | ||
| pRK2073 | Helper plasmid,,Spc R | [32] |
| pBAD33 | arabinose inducible expression vector, Cm R | [33] |
| pBAD-pseB | pBAD33 with AH-1 pseB | This study |
| pBAD-pseI | pBAD33 with AH-1 pseI | This study |
| pDM4 | pir dependent with sacAB genes, oriR6K, Cm R | [34] |
| pDM4-flaB-J | pDM4 with AH-1 flaB-J fragment, Cm R | This study |
| pDM4-pseB | pDM4 with AH-1 pseB fragment, Cm R | This study |
| pDM4-pseI | pDM4 with AH-1 pseI fragment, Cm R | This study |
| pET-30 Xa/LIC | IPTG inducible expression vector Km R | Novagen |
| pET-30-FlaB-AH1 | pET-30 Xa/LIC with A. hydrophila AH-1 flaB | This study |
R, resistance.
4.2. DNA Techniques
Standard procedures [35,36,37,38,39] were used as previously described [21,22].
4.3. Construction of Defined Mutants
The chromosomal in-frame flaB-J, pseB, pseI and rmlB deletion mutants A. hydrophila AH-1∆flaB-J, AH-1∆pseB, and AH-1ΔpseI, respectively, were constructed by allelic exchange as described by Milton et al. [34]. Briefly, upstream (fragment AB) and downstream (fragment CD) of flaB-J, pseB, and pseI were independently amplified using two sets of asymmetric PCRs. Primer pairs A-FlaB (5′-CGGGATCCAACAGTCTGCCAATGGTTC-3′) and B-FlaB (5′-CCCATCCACTAAACTTAAACAGTTAG CCTGAGCCAAAATG-3′) and C-FlaJ (5′-TGTTTAAGTTTAGTGGATGGGAGACA ACAGCTAGGGGAGTT-3′) and D-FlaJ (5′-CGGGATCCAACGTTTCACAAGCAAGA-3′) amplify DNA fragments of 581 (AB) and 637 (CD) bp for flaB-J in-frame deletion. Primer pairs A-PseB (5′-GAAGATCTGAGGACAAACAACGGATG-3′) and B-PseB (5′-CCCATCCACTAAACTTAAACATGTCTTGACCAGCATCTT-3′) and C-PseB (5′-TGTTTAAGTTTAGTGGATGGGATGAACCAGCAGGTGTTGT-3′) and D-PseB (5′-GAAGATCTAAGCTGAAGACCGTCATGT-3′) amplify DNA fragments of 759 (AB) and 574 (CD) bp for pseB in-frame deletion. Primer pairs A-PseI (5′-CGGGATCCAATGCTGGATGATGAGCAA-3′) and B-PseI (5′-CCCATCCACTAAACTTAAACAGTCAGCGGTATAGGTTTGCA-3′) and C-PseI (5′-TGTTTAAGTTTAGTGGATGGGAGACGAGGCAAAGCAGTC-3′) and D-PseI (5′-CGGGATCCTTTAACTGGCCTGGCTCTA-3′) amplify DNA fragments of 764 (AB) and 630 (CD) bp for pseI in-frame deletion. We use plasmid pDM4 [22] to obtain A. hydrophila AH-1ΔflaB-J, AH-1ΔpseB, and AH-1ΔpseI mutants as previously described for other mutants [40].
4.4. Plasmid Constructions
Plasmids pBAD-pseB and pBAD-pseI containing the complete pseB and pseI of A. hydrophila AH-1 under the arabinose promoter (pBAD) on pBAD33 [33] were obtained. Oligonucleotides 5′-GCTCTAGATGGAATAAAACTGGCATCA-3′ and 5′-CCAAGCTTGACCTTGGGTCAGATAATCA-3′ generated a band of 1198 bp containing the pseB; Oligonucleotides 5′-TCCCCCGGGTTCACTTTTCACGCCTAT-3′ and 5′-GCTCTAGACTAATGCTAAAGCGACAACG-3′ generated a band of 1880 bp containing the pseI. The construction of the pBAD-pseB and pBAD-pseI plasmids was done as previously described for other genes [40]. The plasmids were introduced independently into the E. coli DH5α [31].
4.5. Flagella Purification
A. hydrophila AH-1 was grown in TSB for the polar flagellum purification and in TSA for the isolation of lateral flagella, and the flagella purified as previously described [40]. Purified flagella were analyzed by SDS-PAGE or by glycosylation chemical studies.
4.6. Motility Assays (Swarming and Swimming)
The assays were performed as previously described [21,22].
4.7. Transmission Electron Microscopy (TEM)
TEM was performed on Formvar-coated grids and negative stained with a 2% solution of uranyl acetate pH 4.1.
4.8. Electrospray Liquid Chromatography Mass Spectrometry Analysis of Intact Flagellins
Mass spectrometry studies of intact flagellin proteins were carried out using 50 μL aliquots of protein-containing sample as described previously, with some modification [23]. The purified flagellin preparations were injected onto a protein microtrap (Michrom Bioresources Inc., Auburn, CA, USA) connected to a gradient HPLC pump (Agilent 1100 HPLC, Agilent Technologies, Santa Clara, CA, USA). Solvent A was 0.1% formic acid in HPLC-grade water (Fisher, Waltham, MA, USA) and solvent B was 0.1% formic acid in acetonitrile. An HPLC gradient of 5%–60% solvent B (1 mL/min) over 60 min was used to resolve the protein mixture. A pre-column splitter was used to direct 35 μL/min of the HPLC column eluate into the electrospray interface of the QTOF2 (Waters, Milford, MA, USA), allowing real-time monitoring of ion elution profiles. Intact masses of proteins were calculated by spectral deconvolution, using MaxEnt (Waters, Beverly, MA, USA). For each protein, front-end collision-induced dissociation (feCID) was performed by increasing the cone voltage from 45 to 85 V and glycan-associated oxonium ions were observed. Tandem MS of prominent ions were also performed. In addition, multiply charged protein ions were selected for MS/MS. The collision energy was increased from X to Y incrementally and labile-, protein-, and glycan-associated fragment ions were observed.
4.9. Solution Enzymatic Digests and Bottom-Up Mass Spectrometry Analysis of Glycopeptides
In preliminary experiments, tryptic digests of intact proteins were performed, with 50 to 200 μg of pure flagellin prepartion in solution digested with trypsin (Promega, Madison, WI, USA) at a ratio of 30:1 (protein/enzyme, w/w) in 50 mM ammonium bicarbonate at 37 °C overnight. Protein digests were analyzed by nano-liquid chromatography MS/MS (nLC-MS/MS) using either a Q-TOF Ultima hybrid quadrupole time-of-flight MS (Waters) or an LTQ XL orbitrap MS (Thermo Fisher Scientific, Ottawa, ON, Canada) coupled to a nanoAcquity ultrahigh-pressure liquid chromatography system (Waters). MS/MS spectra were acquired automatically on doubly, triply, and quadruply charged ions in collision-induced dissociation (CID) mode for initial glycopeptide identification. Peak lists were automatically generated by proteinlynx software (Waters) with the following parameters: smoothing—four channels, two smooths, Savitzky-Golay mode; centroid—minimum peak width at half height of four channels, centroid top 80%. Tryptic peptides were analyzed by nano-LC-MS/MS, and spectra were searched against the National Centre for Biotechnology nonredundant database and an in-house database of sequenced flagellin proteins using mascot 2.0.6 (Matrix Science, London, UK). A peptide score of 30 and above for a top-ranked hit was taken as positive identification, with each MS/MS spectrum verified by manual inspection. MS/MS spectra not identified by MASCOT were de novo sequenced. Proteinase K digests were analyzed up nanoliquid chromatography MS/MS, using a Q-TOF Ultima, coupled to a nanoAcquity ultrahigh-pressure liquid chromatography system (Waters, Milford, MA, USA). The column setup and gradients did not deviate from those reported in our other studies [23]. MS/MS spectra were acquired automatically on doubly, triply, and quadruply charged ions in collision-induced dissociation (CID) mode for initial glycopeptide identification. High resolution multi-stage mass spectrometry studies of glycan moieties were performed in high resolution (100,000) mode on the LTQ XL Orbitrap Mass spectrometer (Fisher Scientific, Waltham, MA, USA). All proteinaseK MS/MS spectra were de novo sequenced, no database searching was employed in peptide sequence assignment.
4.10. Adherence Assay to HEp-2 Cell
The adherence assay was conducted in triplicate as previously described [40].
4.11. Biofilm Formation
Quantitative biofilm formation was performed in a microtiter plate as described previously [40], by adapting the method of Pratt and Kotler [41].
4.12. Purification of A. hydrophila AH-1 His6-FlaB
For flaB overexpression the pET-30 Xa/LIC vector (Novagen, Nottingham, UK) and AccuPrime (Invitrogene, Madrid, Spain). High-fidelity polymerase was used. The A. hydrophila AH-1 flaB was amplified from genomic DNA using primers PET-A3flaB-for 5′-GGTATTGAGGGTCGCATGCTTGCTGTGTGTTTACC-3′ and PET-A3flaB-rev 5′-AGAGGAGAGTTAGAGCCATTCCTTTCTTCCCAAAGC-3′, and the PCR product ligated into pET-30 Xa/LIC (Novagen), and electroporated into E. coli BL21(λDE3). The His6-FlaB protein was overexpressed and cell lysates obtained as previously reported for other proteins [42].
4.13. Interleukin-8 (IL-8) Assay with Human Embryonic Kidney Cells
IL-8 concentration using HEK293-hTLR5 and HEK293-null cells was performed as previously described [40].
4.14. Statistical Analysis
Results are expressed together with the standard deviations (SD) from several experiments and Student’s t-test was used to compare mean values. Differences were considered significant when p-values were <0.05.
Acknowledgments
This work was supported by Plan Nacional de I + D + i (Ministerio de Economía y Competitividad and Ministerio de Sanidad, Spain) and from Generalitat de Catalunya (Centre de Referència en Biotecnologia). We thank Maite Polo for her technical assistance and the Servicios Científico-Técnicos from University of Barcelona.
Author Contributions
All the authors equally contributed.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Janda, J.M.; Kokka, R.P. The pathogenicity of Aeromonas strains relative to genospecies and phenospecies identification. FEMS Microbiol. Lett. 1991, 90, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Janda, J.M.; Brenden, R. Importance of Aeromonas sobria in Aeromonas bacteremia. J. Infect. Dis. 1987, 155, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Guthertz, L.S.; Kokka, R.P.; Shimada, T. Aeromonas species in septicemia: Laboratory characteristics and clinical observations. Clin. Infect. Dis. 1994, 19, 77–83. [Google Scholar] [PubMed]
- Kokka, R.P.; Janda, J.M. Isolation and identification of autoagglutinating serogroup O:11 Aeromonas strains in the clinical laboratory. J. Clin. Microbiol. 1990, 28, 1297–1299. [Google Scholar] [PubMed]
- Shaw, D.H.; Squires, M. O-Antigen structure in a virulent strain of Aeromonas hydrophila. FEMS Microbiol. Lett. 1984, 24, 277–280. [Google Scholar] [CrossRef]
- Murray, R.G.E.; Dooley, J.S.G.; Whippey, P.W.; Trust, T.J. Structure of an S-layer on a pathogenic strain of Aeromonas hydrophila. J. Bacteriol. 1988, 170, 2625–2636. [Google Scholar] [PubMed]
- Frenchel, T. Microbial behavior in a heterogeneous world. Science 2002, 296, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Kirov, S.M.; Tassel, B.C.; Semmler, A.B.T.; O’Donovan, L.A.; Rabaan, A.A.; Shaw, J.G. Lateral flagella and swarming motility in Aeromonas species. J. Bacteriol. 2002, 184, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Sakazaki, R.; Suzuki, K. Peritrichous flagella in mesophilic strains of Aeromonas. Jpn. J. Med. Sci. Biol. 1985, 38, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Kirov, S.M.; Castrisios, M.; Shaw, J.G. Aeromonas flagella (polar and lateral) are enterocyte adhesins that contribute to biofilm formation on surfaces. Infect. Immun. 2004, 72, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Iwashkiw, J.A.; Vozza, N.F.; Kinsella, R.L.; Feldman, M.F. Pour some sugar on it: The expanding world of bacterial protein O-linked glycosylation. Mol. Microbiol. 2013, 89, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Schirm, M.; Soo, E.C.; Aubry, A.J.; Austin, J.; Thibault, P.; Logan, S.M. Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori. Mol. Microbiol. 2003, 48, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Goon, S.; Kelly, J.F.; Logan, S.M.; Ewing, C.P.; Guerry, P. Pseudaminic acid, the major modification on Campylobacter flagellin, is synthesized via the Cj1293 gene. Mol. Microbiol. 2003, 50, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Faridmoayer, A.; Fentabil, M.A.; Mills, D.C.; Klassen, J.S.; Feldman, M.F. Functional characterization of bacterial oligosaccharyltransferases involved in O-linked protein glycosylation. J. Bacteriol. 2007, 189, 8088–8098. [Google Scholar] [CrossRef] [PubMed]
- Iwashkiw, J.A.; Seper, A.; Weber, B.S.; Scott, N.E.; Vinogradov, E.; Stratilo, C.; Reiz, B.; Cordwell, S.J.; Whittal, R.; Schild, S.; et al. Identification of a general O-linked protein glycosylation system in Acinetobacter baumannii and its role in virulence and biofilm formation. PLoS Pathog. 2012, 8, e1002758. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Tomás, J.M. Gram-negative flagella glycosylation. Int. J. Mol. Sci. 2014, 15, 2840–2857. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Rubires, X.; Aguilar, A.; Tomás, J.M. The role of flagella and motility on the adherence and invasion to fish cell lines by Aeromonas hydrophila strains serogroup O:34. FEMS Microbiol. Lett. 1997, 151, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Canals, R.; Knirel, Y.A.; Tomás, J.M. Molecular and chemical analysis of the lipopolysaccharide from Aeromonas hydrophila strain AH-1 (serotype O11). Mar. Drugs 2015, 13, 2233–2249. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.B.; Srinivasa Rao, P.S.; Lee, H.C.; Vilches, S.; Merino, S.; Tomás, J.M.; Leung, K.Y. A Type III secretion system is required for Aeromonas hydrophila AH-1 pathogenesis. Infect. Immun. 2004, 72, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Jiménez, N.; Molero, R.; Bouamama, L.; Regué, M.; Tomás, J.M. A UDP-HexNAc: Polyprenol-P GalNAc-1-P transferase (WecP) representing a new subgroup of this enzyme family. J. Bacteriol. 2011, 193, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Canals, R.; Ramirez, S.; Vilches, S.; Horsburgh, G.; Shaw, J.G.; Tomás, J.M.; Merino, S. Polar flagella biogenesis in Aeromonas hydrophila. J. Bacteriol. 2006, 188, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Canals, R.; Altarriba, M.; Vilches, S.; Horsburgh, G.; Shaw, J.G.; Tomás, J.M.; Merino, S. Analysis of the lateral flagella gene system of Aeromonas hydrophila AH-3. J. Bacteriol. 2006, 188, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Wilhelms, M.; Fulton, K.M.; Twine, S.M.; Tomás, J.M.; Merino, S. Differential glycosylation of polar and lateral flagellins in Aeromonas hydrophila AH-3. J. Biol. Chem. 2012, 287, 27851–27862. [Google Scholar] [CrossRef] [PubMed]
- Schirm, M.; Schoenhofen, I.C.; Logan, S.M.; Waldron, K.C.; Thibault, P. Identification of Unusual Bacterial Glycosylation by Tandem Mass Spectrometry Analyses of Intact Proteins. Anal. Chem. 2005, 77, 7774–7782. [Google Scholar] [CrossRef] [PubMed]
- Schoenhofen, I.C.; Vinogradov, E.; Whitfield, D.M.; Brisson, J.R.; Logan, S.M. The CMP-legionaminic acid pathway in Campylobacter: Biosynthesis involving novel GDP-linked precursors. Glycobiology 2009, 19, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Kokka, R.P.; Janda, J.M.; Oshiro, L.S.; Altwegg, M.; Shimada, T.; Sakazaki, R.; Brenner, D.J. Biochemical and genetic characterization of autoagglutinating phenotypes of Aeromonas. species associated with invasive and noninvasive disease. J. Infect. Dis. 1991, 163, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Twine, S.M.; Reid, C.W.; Aubry, A.; McMullin, D.R.; Fulton, K.M.; Austin, J.; Logan, S.M. Motility and flagellar glycosylation in Clostridium difficile. J. Bacteriol. 2009, 191, 7050–7062. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Matewish, M.J.; McNally, D.J.; Ishiyama, N.; Anderson, E.M.; Brewer, D.; Brisson, J.R.; Berghuis, A.M.; Lam, J.S. Flagellin glycosylation in Pseudomonas aeruginosa PAK requires the O-antigen biosynthesis enzyme WbpO. J. Biol. Chem. 2008, 283, 3507–3518. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.C. Regulation of Salmonella flagellin-induced interleukin-8 in intestinal epithelial cells by muramyl dipeptide. Cell Immunol. 2012, 278, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Swain, B.; Maiti, N.K.; Routray, P.; Samanta, M. Inductive expression of toll-like receptor 5 (TLR5) and associated downstream signaling molecules following ligand exposure and bacterial infection in the Indian major carp, mrigal (Cirrhinus mrigala). Fish. Shellfish Immunol. 2012, 32, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Nogueras, M.M.; Merino, S.; Aguilar, A.; Benedi, V.J.; Tomás, J.M. Cloning, sequencing, and role in serum susceptibility of porin II from mesophilic Aeromonas hydrophila. Infect. Immun. 2000, 68, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [PubMed]
- Milton, D.L.; O’Toole, R.; Horstedt, P.; Wolf-Watz, H. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 1996, 178, 1310–1319. [Google Scholar] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Studholme, D.J.; Dixon, R. Domain architectures of sigma54-dependent transcriptional activators. J. Bacteriol. 2003, 185, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Münch, R.; Hiller, K.; Grote, A.; Scheer, M.; Klein, J.; Schobert, M.; Jahn, D. Virtual Footprint and PRODORIC: An integrative framework for regulon prediction in prokaryotes. Bioinformatics 2005, 21, 4187–4189. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Wilhelms, M.; Tomás, J.M. Role of Aeromonas hydrophila flagella glycosylation in adhesion to Hep-2 cells, biofilm formation and immune stimulation. Int. J. Mol. Sci. 2014, 15, 21935–21946. [Google Scholar] [CrossRef] [PubMed]
- Pratt, L.A.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Role of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, N.; Vilches, S.; Lacasta, A.; Regué, M.; Merino, S.; Tomás, J.M. A bifunctional enzyme in a single gene catalyzes the incorporation of GlcN into the Aeromonas core LPS. J. Biol. Chem. 2009, 284, 32995–33005. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).