Silkworm Thorn Stem Extract Targets RSK2 and Suppresses Solar UV-Induced Cyclooxygenase-2 Expression
Abstract
:1. Introduction
2. Results
2.1. SWE Inhibits sUV-Induced COX-2 Expression through Transcriptional Suppression
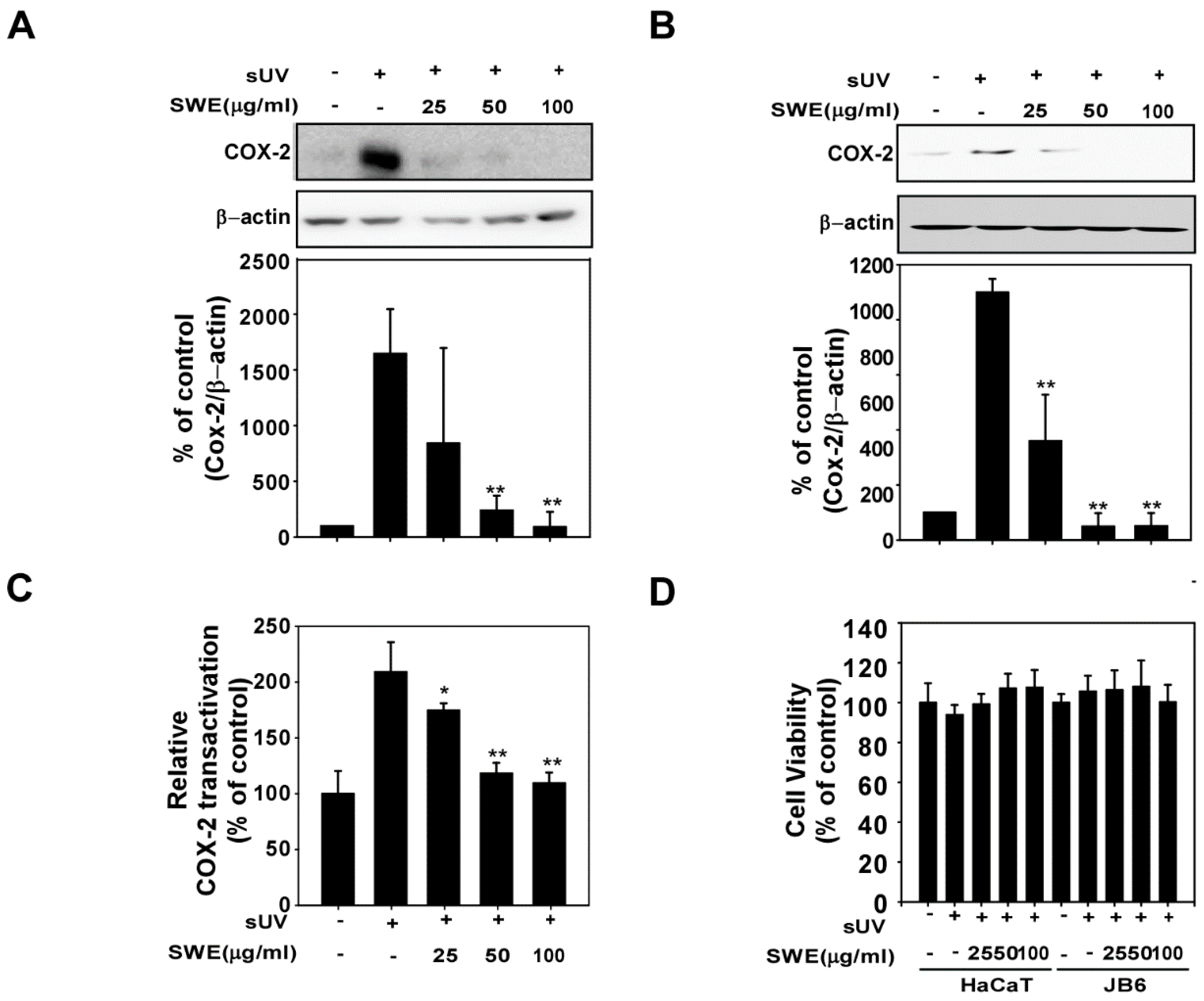
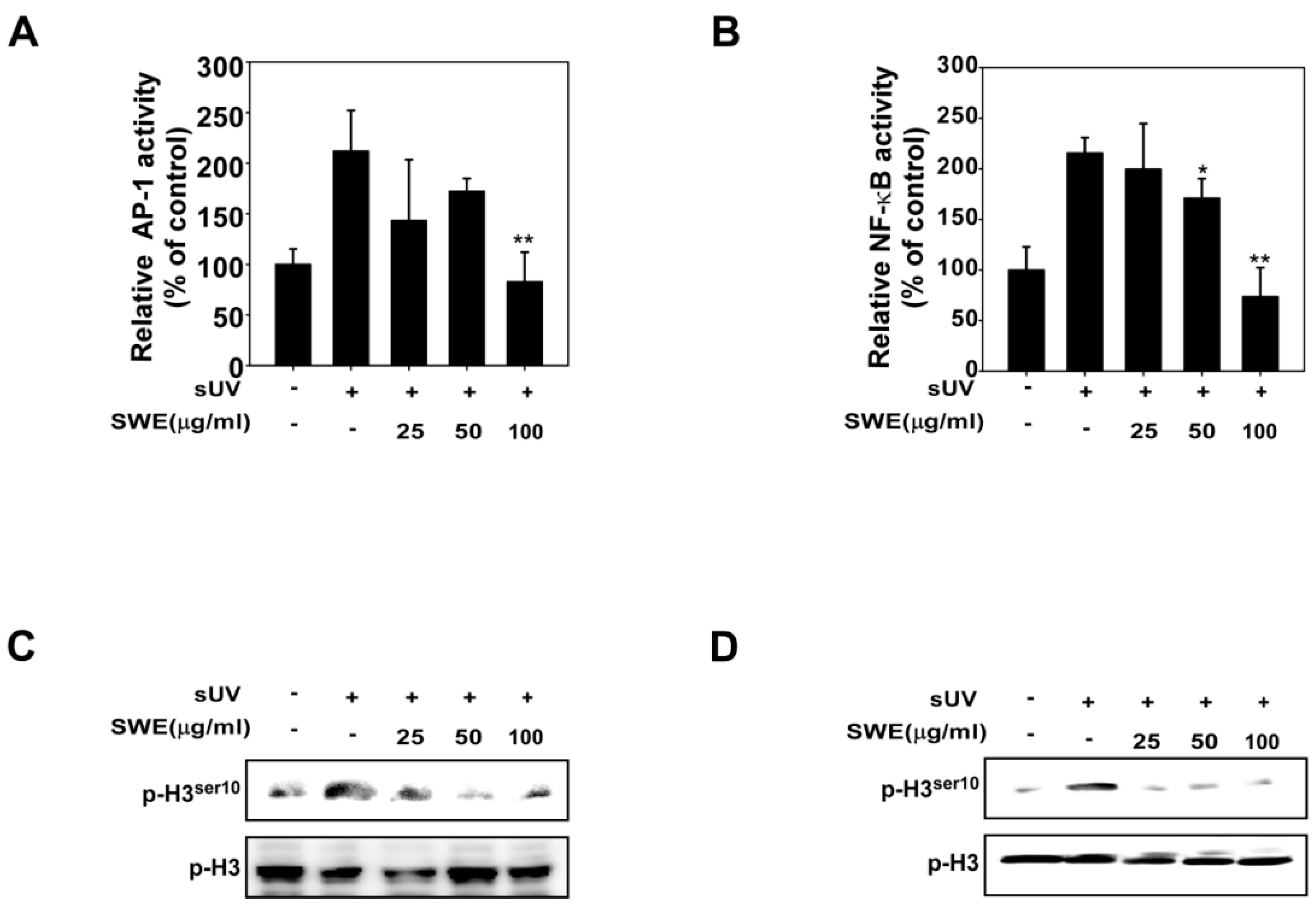
2.2. SWE Inhibits sUV Induced NF-κB/AP-1 Transactivation and Histone H3 Phosphorylation
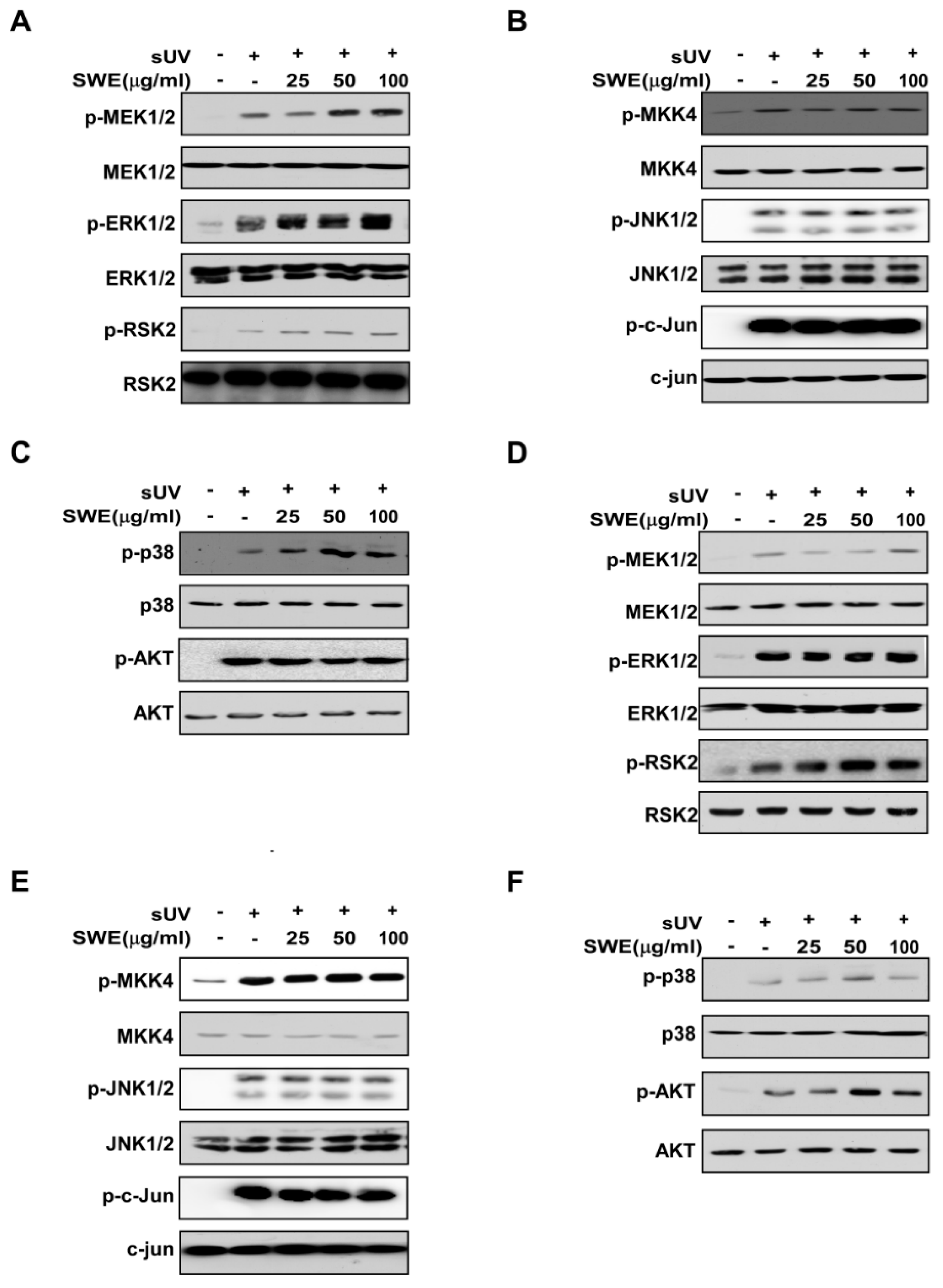
2.3. SWE Does Not Inhibit sUV-Induced MAPK or Akt Signaling Activity
2.4. SWE Inhibits RSK2 Activity though Direct Binding

3. Discussion
4. Experimental Section
4.1. Materials
4.2. Cell Culture and sUV Radiation
4.3. Western Blot Analysis
4.4. Luciferase Assays for cox-2 Promoter Activity and Induced Nuclear Factor-κB (NF-κB) and Activator Protein (AP)-1 Transcriptional Activity
4.5. Cell Viability
4.6. In Vitro RSK2 Kinase Assay
4.7. SWE Pull-down Assay
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wright, F.; Weller, R.B. Risks and benefits of UV radiation in older people: More of a friend than a foe? Maturitas 2015, 81, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B 2001, 63, 8–18. [Google Scholar] [CrossRef]
- Shin, D.J.; Kim, J.E.; Lim, T.G.; Jeong, E.H.; Park, G.; Kang, N.J.; Park, J.S.; Yeom, M.H.; Oh, D.K.; Bode, A.M.; et al. 20-O-beta-D-glucopyranosyl-20(S)-protopanaxadiol suppresses UV-Induced MMP-1 expression through AMPK-mediated mTOR inhibition as a downstream of the PKA-LKB1 pathway. J. Cell. Biochem. 2014, 115, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Song, N.R.; Kim, J.E.; Park, J.S.; Kim, J.R.; Kang, H.; Lee, E.; Kang, Y.G.; Son, J.E.; Seo, S.G.; Heo, Y.S.; et al. Licochalcone A, a polyphenol present in licorice, suppresses UV-induced COX-2 expression by targeting PI3K, MEK1, and B-Raf. Int. J. Mol. Sci. 2015, 16, 4453–4470. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, S.E.; Wang, S.Q. Skin cancer: Role of ultraviolet radiation in carcinogenesis. Rev. Environ. Health 2014, 29, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Lee, K.W.; Kim, J.E.; Jung, S.K.; Kang, N.J.; Hwang, M.K.; Heo, Y.S.; Bode, A.M.; Dong, Z.; Lee, H.J. Delphinidin suppresses ultraviolet B-induced cyclooxygenases-2 expression through inhibition of MAPKK4 and PI-3 kinase. Carcinogenesis 2009, 30, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dong, Q.; Ding, K. Structure elucidation and immunomodulatory activity in vitro of a xylan from roots of Cudrania tricuspidata. Food Chem. 2014, 152, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, K.; Dong, Q.; Fang, J.; Ding, K. Separation, purification and structure elucidation of a polysaccharide from root of Cudrania tricuspidata. Front. Chem. China 2008, 3, 209–212. [Google Scholar] [CrossRef]
- Seo, W.G.; Pae, H.O.; Oh, G.S.; Chai, K.Y.; Yun, Y.G.; Chung, H.T.; Jang, K.K.; Kwon, T.O. Ethyl acetate extract of the stem bark of Cudrania tricuspidata induces apoptosis in human leukemia HL-60 cells. Am. J. Chin. Med. 2001, 29, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ha, H.; Lee, J.K.; Seo, C.S.; Lee, N.H.; Jung, D.Y.; Park, S.J.; Shin, H.K. The fruits of Cudrania tricuspidata suppress development of atopic dermatitis in NC/Nga mice. Phytother. Res. 2012, 26, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, Y.; Kim, J.; Sohn, E.; Kim, C.S.; Lee, Y.M.; Jo, K.; Shin, S.; Song, Y.; Kim, J.H.; et al. Inhibitory Activities of Cudrania tricuspidata Leaves on Pancreatic Lipase In Vitro and Lipolysis In Vivo. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Yang, G.; Lee, K.; Lee, M.; Ham, I.; Choi, H.Y. Inhibition of lipopolysaccharide-induced nitric oxide and prostaglandin E2 production by chloroform fraction of Cudrania tricuspidata in RAW 264.7 macrophages. BMC Complement. Altern. Med. 2012, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Sharma, K. COX-2 signaling and cancer: New players in old arena. Curr. Drug Targets 2014, 15, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.A. COX-2 inhibition: What we learned—A controversial update on safety data. Pain Med. 2013, 14, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Fosslien, E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann. Clin. Lab. Sci. 2000, 30, 3–21. [Google Scholar] [PubMed]
- Chun, K.S.; Langenbach, R. A proposed COX-2 and PGE(2) receptor interaction in UV-exposed mouse skin. Mol. Carcinog. 2007, 46, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Kim, E.K.; Lee, S.H.; Park, K.C.; Kim, K.H.; Eun, H.C.; Chung, J.H. Enhanced expression of cylooxygenase-2 by UV in aged human skin in vivo. Mech. Ageing Dev. 2003, 124, 903–910. [Google Scholar] [CrossRef]
- Surowiak, P.; Gansukh, T.; Donizy, P.; Halon, A.; Rybak, Z. Increase in cyclooxygenase-2 (COX-2) expression in keratinocytes and dermal fibroblasts in photoaged skin. J. Cosmet. Dermatol. 2014, 13, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Keum, Y.S.; Kim, H.G.; Bode, A.M.; Surh, Y.J.; Dong, Z. UVB-induced COX-2 expression requires histone H3 phosphorylation at Ser10 and Ser28. Oncogene 2013, 32, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kwon, J.Y.; Seo, S.K.; Son, J.E.; Jung, S.K.; Min, S.Y.; Hwang, M.K.; Heo, Y.S.; Lee, K.W.; Lee, H.J. Cyanidin suppresses ultraviolet B-induced COX-2 expression in epidermal cells by targeting MKK4, MEK1, and Raf-1. Biochem. Pharmacol. 2010, 79, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Yao, K.; Pugliese, A.; Malakhova, M.L.; Bode, A.M.; Dong, Z. A regulatory mechanism for RSK2 NH2-terminal kinase activity. Cancer Res. 2009, 69, 4398–4406. [Google Scholar] [CrossRef] [PubMed]
- Anjum, R.; Blenis, J. The RSK family of kinases: Emerging roles in cellular signalling. Nat. Rev. Mol. Cell Biol. 2008, 9, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Lee, M.H.; Lee, C.J.; Yao, K.; Lee, H.S.; Bode, A.M.; Dong, Z. RSK2 as a key regulator in human skin cancer. Carcinogenesis 2012, 33, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Arul, N.; Cho, Y.Y. A Rising Cancer Prevention Target of RSK2 in Human Skin Cancer. Front. Oncol. 2013, 3, 201. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Han, H.J.; Hong, S.S.; Hwang, J.H.; Hwang, B.Y.; Yoo, H.S.; Jin, Y.R.; Lee, J.J.; Yu, J.Y.; Lee, K.H.; et al. Cudratricusxanthone A isolated from the root bark of Cudrania tricuspidata inhibits the proliferation of vascular smooth muscle cells through the suppression of PDGF-receptor beta tyrosine kinase. Biol. Pharm. Bull. 2007, 30, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Son, J.E.; Lee, E.; Seo, S.G.; Lee, J.; Kim, J.E.; Kim, J.; Lee, K.W.; Lee, H.J. Eupatilin, a major flavonoid of Artemisia, attenuates aortic smooth muscle cell proliferation and migration by inhibiting PI3K, MKK3/6, and MKK4 activities. Planta Med. 2013, 79, 1009–1016. [Google Scholar] [PubMed]
- Seo, W.G.; Pae, H.O.; Oh, G.S.; Chai, K.Y.; Yun, Y.G.; Kwon, T.O.; Chung, H.T. Inhibitory effect of ethyl acetate fraction from Cudrania tricuspidata on the expression of nitric oxide synthase gene in RAW 264.7 macrophages stimulated with interferon-gamma and lipopolysaccharide. Gen. Pharmacol. Vasc. Syst. 2000, 35, 21–28. [Google Scholar] [CrossRef]
- Nam, S.; Jang, H.W.; Shibamoto, T. Antioxidant activities of extracts from teas prepared from medicinal plants, Morus alba L., Camellia sinensis L., and Cudrania tricuspidata, and their volatile components. J. Agric. Food Chem. 2012, 60, 9097–9105. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.G.; Yun, H.J.; Lee, S.I.; Yoo, W.H. Ethyl acetate fraction from Cudrania tricuspidata inhibits IL-1beta-stimulated osteoclast differentiation through downregulation of MAPKs, c-Fos and NFATc1. Korean J. Intern. Med. 2010, 25, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Kim, C.J.; Song, K.S.; Kim, H.M.; Koshino, H.; Uramoto, M.; Yoo, I.D. Cytotoxic benzyl dihydroflavonols from Cudrania tricuspidata. Phytochemistry 1996, 41, 213–216. [Google Scholar] [PubMed]
- Lim, T.G.; Kim, J.E.; Jung, S.K.; Li, Y.; Bode, A.M.; Park, J.S.; Yeom, M.H.; Dong, Z.; Lee, K.W. MLK3 is a direct target of biochanin A, which plays a role in solar UV-induced COX-2 expression in human keratinocytes. Biochem. Pharmacol. 2013, 86, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.J.; In, H.; Seok, K.Y.; Ki, C.J.; Han, L.K.; Ho, H.T. Quantification of quercetin and kaempferol contents in different parts of Cudrania tricuspidata and their processed foods. Korean J. Hortic. Sci. Technol. 2009, 27, 489–496. [Google Scholar]
- Li, B.; Wang, M.; Tan, Y.N.; Tong, M.M.; Zhai, Y.J. Simultaneous determination of four flavones in root and stem of Cudrania tricuspidata and C. cochinchinensis by HPLC-DAD. China J. Chin. Mater. Medica 2013, 38, 167–170. [Google Scholar]
- Lee, K.M.; Lee, K.W.; Jung, S.K.; Lee, E.J.; Heo, Y.S.; Bode, A.M.; Lubet, R.A.; Lee, H.J.; Dong, Z. Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity. Biochem. Pharmacol. 2010, 80, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Chen, H.; Liu, K.; Langfald, A.; Yang, G.; Zhang, Y.; Yu, D.H.; Kim, M.O.; Lee, M.H.; Li, H.; et al. Kaempferol targets RSK2 and MSK1 to suppress UV radiation-induced skin cancer. Cancer Prev. Res. 2014, 7, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, H.; Demircan, B.; Karagoz, Y. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. 2007, 59, 247–258. [Google Scholar] [PubMed]
- Cerella, C.; Sobolewski, C.; Dicato, M.; Diederich, M. Targeting COX-2 expression by natural compounds: A promising alternative strategy to synthetic COX-2 inhibitors for cancer chemoprevention and therapy. Biochem. Pharmacol. 2010, 80, 1801–1815. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-E.; Lee, K.W. Silkworm Thorn Stem Extract Targets RSK2 and Suppresses Solar UV-Induced Cyclooxygenase-2 Expression. Int. J. Mol. Sci. 2015, 16, 25096-25107. https://doi.org/10.3390/ijms161025096
Kim J-E, Lee KW. Silkworm Thorn Stem Extract Targets RSK2 and Suppresses Solar UV-Induced Cyclooxygenase-2 Expression. International Journal of Molecular Sciences. 2015; 16(10):25096-25107. https://doi.org/10.3390/ijms161025096
Chicago/Turabian StyleKim, Jong-Eun, and Ki Won Lee. 2015. "Silkworm Thorn Stem Extract Targets RSK2 and Suppresses Solar UV-Induced Cyclooxygenase-2 Expression" International Journal of Molecular Sciences 16, no. 10: 25096-25107. https://doi.org/10.3390/ijms161025096
APA StyleKim, J.-E., & Lee, K. W. (2015). Silkworm Thorn Stem Extract Targets RSK2 and Suppresses Solar UV-Induced Cyclooxygenase-2 Expression. International Journal of Molecular Sciences, 16(10), 25096-25107. https://doi.org/10.3390/ijms161025096





