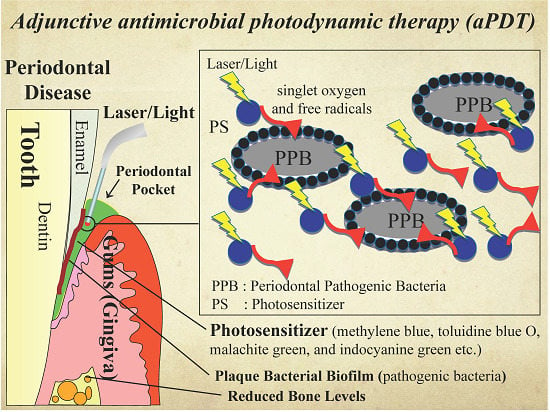
Adjunctive Application of Antimicrobial Photodynamic Therapy in Nonsurgical Periodontal Treatment: A Review of Literature
Abstract
:1. Introduction
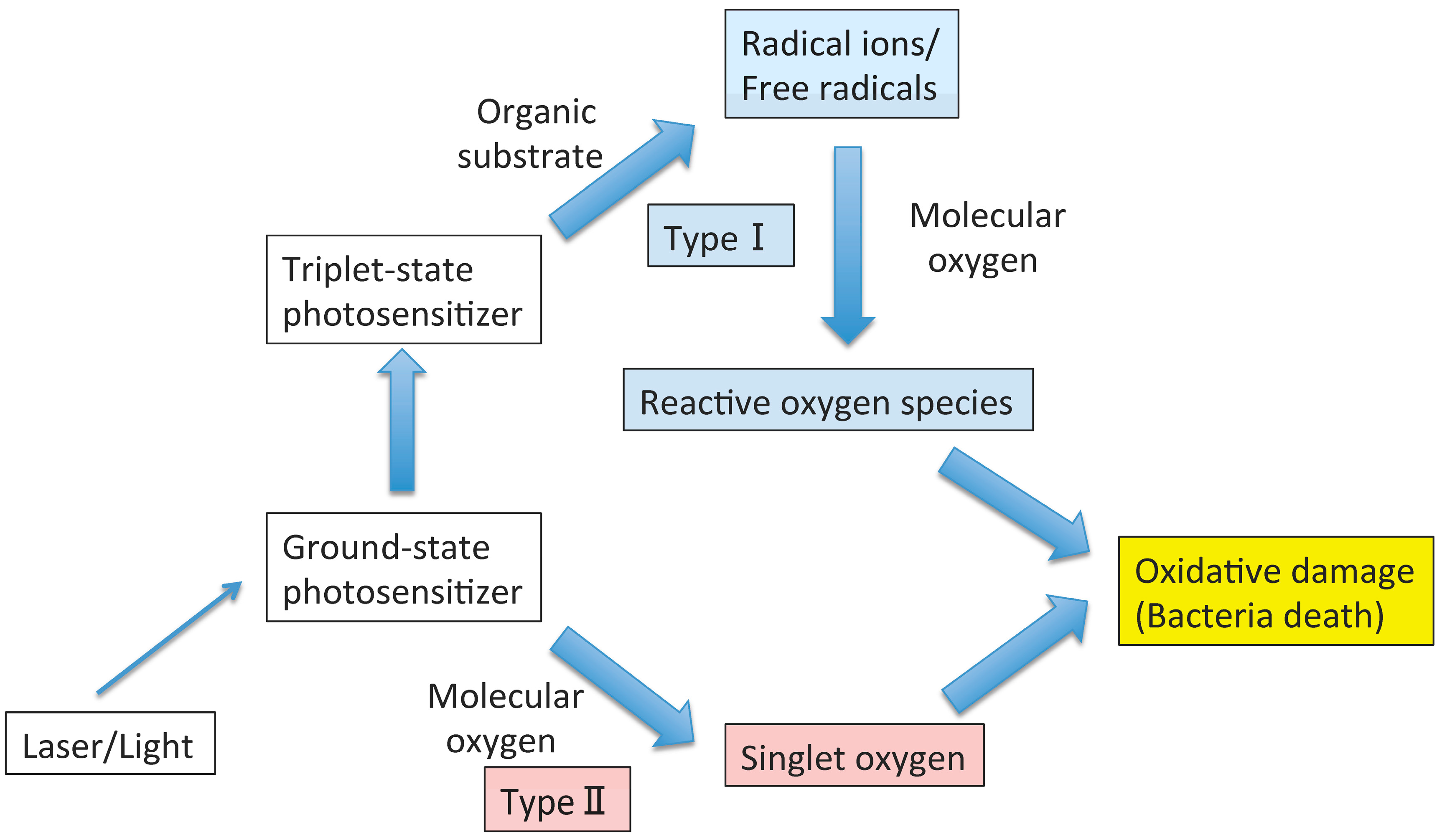
2. Antimicrobial PDT
| Author and Year (Ref.) | Photosensitizer | Samples/Bacterial Strain | Conclusion |
|---|---|---|---|
| Bhatti et al., 2002 [15] | Toluidine blue O | Planktonic culture/Porphyromonas gingivalis | Disruption of membrane functions associated with a decrease in membrane fluidity may contribute to the bactericidal effect of light-activated toluidine blue |
| Bhatti et al., 1997 [16] | Toluidine blue O | Planktonic culture/Porphyromonas gingivalis | In the presence of toluidine blue O, a light dose-dependent increase in bacterial killing was attained (100% killing at 4.4 J) |
| Chan et al., 2003 [17] | Methylene blue | Planktonic culture/Actinobacillus actinomycetemcomitans, Fusobacterium nucleatum, Porphyromonas gingivalis, Prevotella intermedia, and Streptococcus sanguinis | Using a diode laser of appropriate power and wavelength to deliver 60 s of irradiation could be a useful adjunct therapy with mechanical debridement for the prevention of re-colonization of subgingival lesions by pathogenic microorganisms |
| Matevski et al., 2003 [18] | Toluidine blue O | Planktonic culture/Porphyromonas gingivalis | The data indicated that aPDT using a conventional light source was at least as effective as laser-mediated treatment in vitro |
| Souko et al., 1998 [19] | A conjugate between poly-l-lysine and the photosensitizer chlorin e6 | Planktonic culture/Porphyromonas gingivalis and Actinomyces viscosus | The cationic pL-ce6 conjugate may have applications in PDT of periodontal disease |
| Wilson et al., 1993 [20] | Toluidine blue O; Methylene blue | Planktonic culture/Porphyromonas gingivalis, Fusobacterium nucleatum, and Actinobacillus actinomycetemcomitans | Low doses of light (22 J/cm2) were effective to kill bacteria in vivo, and the technique may be useful to eliminate periodontopathogenic bacteria from diseased sites |
| Nagahara et al., 2013 [21] | Indocyanine green-loaded nanospheres | Planktonic culture/Porphyromonas gingivalis | ICG-Nano/c with low-level diode laser (0.5 W; 805 nm) irradiation might be useful as a potential photodynamic periodontal therapy |
| Topaloglu et al., 2013 [22] | Indocyanine green | Planktonic culture/Staphylococcus aureus and Pseudomonas aeruginosa | The combination of ICG and 809-nm laser light was an effective antibacterial method to destroy antibiotic-resistant strains of Gram-positive and -negative bacteria |
| Klepac-Ceraj et al., 2011 [23] | Methylene blue-loaded polymeric nanoparticles | Planktonic culture, plaque scraping, and biofilm/human dental plaque bacteria | Cationic methylene blue-loaded poly lactic-co-glycolic acid nanoparticles showed the potential to be used as carriers of methylene blue for photodynamic periodontal therapy Systems |
| Voos et al., 2014 [24] | Safranine O | Planktonic culture and biofilms/Streptococcus gordonii, Streptococcus mutans, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, and Porphyromonas gingivalis | Oral pathogenic species in planktonic solution were suppressed significantly by antimicrobial photodynamic periodontal therapy with safranin O. Particularly for bacteria in a 24-h ex vivo biofilm, this method was more effective than treatment with 0.2% CHX. Both antibacterial treatments did not show any significant effect on the biofilm cultured for 72 h |
| Sarkar et al., 1993 [25] | Toluidine blue O | Plaque scraping/human dental plaque bacteria | The use of low-power lasers, in conjunction with appropriate photosensitizers, may be a useful adjunct therapy to mechanical debridement for treating inflammatory periodontal diseases if similar effectiveness against subgingival plaque bacteria can be achieved in vivo |
| Dobson et al., 1992 [26] | Methylene blue; Toluidine blue O; Phthalocyanine; Hematoporphyrin HCl; Hematoporphyrin ester | Biofilms/Streptococcus sanguinis, Porphyromonas gingivalis, Fusobacterium nucleatum, and Actinobacillus actinomycetemcomitans | Lethal photosensitization may be effective in eliminating periodontopathogenic bacteria from dental plaque |
| Wood et al., 1999 [27] | Phthalocyanine | Biofilms/Human dental plaque bacteria | Confocal scanning laser microscopy of the biofilms showed that the photosensitizer was taken up into the biomass of the biofilm, and that significant cell death was caused by PDT |
3. PDT as a Non-Antibiotic Antimicrobial Therapy
4. Periodontitis
5. Periodontitis and Laser Treatment
6. PDT as a Light Sterilization Therapy for Periodontitis
| Author and Year (Ref.) | Treatment Arms | Results |
|---|---|---|
| Smiley et al., 2015 [14] | Test: SRP + aPDT; Control: SRP | aPDT with a diode laser adjunctive to SRP had a beneficial effect with a moderate level of certainty |
| Sgolastra et al., 2013 [12] | Test: SRP + aPDT; Control: SRP | The use of adjunctive aPDT with conventional SRP provided short-term benefits |
| Sgolastra et al., 2013 [79] | Test 1: SRP + aPDT; Test 2: aPDT; Control: SRP | The use of aPDT adjunctive to conventional treatment provided short-term benefits. There was no evidence of effectiveness for the use of aPDT as an alternative to SRP |
| Azarpazhooh et al., 2010 [43] | Test 1: SRP + aPDT; Test 2: aPDT; Control: SRP | aPDT as an independent treatment or an adjunct therapy to SRP was not superior to SRP |
| Atieh et al., 2010 [80] | Test: SRP + aPDT; Control: SRP | The use of aPDT in conjunction with SRP was associated with significant clinical parameter improvements |
7. Animal Studies of the Effects of aPDT on Periodontitis
8. Effects of PDT on the Host
9. Effect of aPDT on Periodontitis with an Unusual Host Response
10. Conclusions
Author Contributions
Conflicts of Interest
References
- Kharkwal, G.B.; Sharma, S.K.; Huang, Y.Y.; Dai, T.; Hamblin, M.R. Photodynamic therapy for infections: Clinical applications. Lasers Surg. Med. 2011, 43, 755–767. [Google Scholar] [PubMed]
- Wilson, J.J.; Jones, H.; Burock, M.; Smith, D.; Fraker, D.L.; Metz, J.; Glatstein, E.; Hahn, S.M. Patterns of recurrence in patients treated with photodynamic therapy for intraperitoneal carcinomatosis and sarcomatosis. Int. J. Oncol. 2004, 24, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Kusuzaki, K.; Matsumine, A.; Satonaka, H.; Shintani, K.; Nakamura, T.; Uchida, A. Methylene blue in place of acridine orange as a photosensitizer in photodynamic therapy of osteosarcoma. In Vivo 2008, 22, 297–303. [Google Scholar] [PubMed]
- Huang, Y.Y.; Tanaka, M.; Vecchio, D.; Garcia-Diaz, M.; Chang, J.; Morimoto, Y.; Hamblin, M.R. Photodynamic therapy induces an immune response against a bacterial pathogen. Expert Rev. Clin. Immunol. 2012, 8, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T. A new strategy to destroy antibiotic resistant microorganisms: Antimicrobial photodynamic treatment. Mini Rev. Med. Chem. 2009, 9, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, F.; Martinelli, M.; Cocchi, A.; Arbia, D.; Fantetti, L.; Roncucci, G. In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob. Agents Chemother. 2010, 54, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Diamond, I.; Granelli, S.G.; McDonagh, A.F.; Nielsen, S.; Wilson, C.B.; Jaenicke, R. Photodynamic therapy of malignant tumours. Lancet 1972, 2, 1175–1177. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Merchat, M.; Bertolini, G.; Giacomini, P.; Villanueva, A.; Jori, G. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B 1996, 32, 153–157. [Google Scholar] [CrossRef]
- Merchat, M.; Spikes, J.D.; Bertoloni, G.; Jori, G. Studies on the mechanism of bacteria photosensitization by meso-substituted cationic porphyrins. J. Photochem. Photobiol. B 1996, 35, 149–157. [Google Scholar] [CrossRef]
- Wilson, M.; Burns, T.; Pratten, J.; Pearson, G.J. Bacteria in supragingival plaque samples can be killed by low-power laser light in the presence of a photosensitizer. J. Appl. Bacteriol. 1995, 78, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Graziani, F.; Gatto, R.; Monaco, A. Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2013, 40, 514–526. [Google Scholar] [CrossRef]
- Passanezi, E.; Damante, C.A.; de Rezende, M.L.; Greghi, S.L. Lasers in periodontal therapy. Periodontology 2000 2015, 67, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.; MacRobert, A.; Henderson, B.; Wilson, M. Exposure of porphyromonas gingivalis to red light in the presence of the light-activated antimicrobial agent toluidine blue decreases membrane fluidity. Curr. Microbiol. 2002, 45, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.; MacRobert, A.; Meghji, S.; Henderson, B.; Wilson, M. Effect of dosimetric and physiological factors on the lethal photosensitization of porphyromonas gingivalis in vitro. Photochem. Photobiol. 1997, 65, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Lai, C.H. Bactericidal effects of different laser wavelengths on periodontopathic germs in photodynamic therapy. Lasers Med. Sci. 2003, 18, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Matevski, D.; Weersink, R.; Tenenbaum, H.C.; Wilson, B.; Ellen, R.P.; Lepine, G. Lethal photosensitization of periodontal pathogens by a red-filtered xenon lamp in vitro. J. Periodontal Res. 2003, 38, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Soukos, N.S.; Ximenez-Fyvie, L.A.; Hamblin, M.R.; Socransky, S.S.; Hasan, T. Targeted antimicrobial photochemotherapy. Antimicrob. Agents Chemother. 1998, 42, 2595–2601. [Google Scholar] [PubMed]
- Wilson, M.; Dobson, J.; Sarkar, S. Sensitization of periodontopathogenic bacteria to killing by light from a low-power laser. Oral Microbiol. Immunol. 1993, 8, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, A.; Mitani, A.; Fukuda, M.; Yamamoto, H.; Tahara, K.; Morita, I.; Ting, C.C.; Watanabe, T.; Fujimura, T.; Osawa, K.; et al. Antimicrobial photodynamic therapy using a diode laser with a potential new photosensitizer, indocyanine green-loaded nanospheres, may be effective for the clearance of porphyromonas gingivalis. J. Periodontal Res. 2013, 48, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Topaloglu, N.; Gulsoy, M.; Yuksel, S. Antimicrobial photodynamic therapy of resistant bacterial strains by indocyanine green and 809-nm diode laser. Photomed. Laser Surg. 2013, 31, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Klepac-Ceraj, V.; Patel, N.; Song, X.; Holewa, C.; Patel, C.; Kent, R.; Amiji, M.M.; Soukos, N.S. Photodynamic effects of methylene blue-loaded polymeric nanoparticles on dental plaque bacteria. Lasers Surg. Med. 2011, 43, 600–606. [Google Scholar] [PubMed]
- Voos, A.C.; Kranz, S.; Tonndorf-Martini, S.; Voelpel, A.; Sigusch, H.; Staudte, H.; Albrecht, V.; Sigusch, B.W. Photodynamic antimicrobial effect of safranine O on an ex vivo periodontal biofilm. Lasers Surg. Med. 2014, 46, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Wilson, M. Lethal photosensitization of bacteria in subgingival plaque from patients with chronic periodontitis. J. Periodontal Res. 1993, 28, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.; Wilson, M. Sensitization of oral bacteria in biofilms to killing by light from a low-power laser. Arch. Oral Biol. 1992, 37, 883–887. [Google Scholar] [CrossRef]
- Wood, S.; Nattress, B.; Kirkham, J.; Shore, R.; Brookes, S.; Griffiths, J.; Robinson, C. An in vitro study of the use of photodynamic therapy for the treatment of natural oral plaque biofilms formed in vivo. J. Photochem. Photobiol. B 1999, 50, 1–7. [Google Scholar] [CrossRef]
- Qin, Y.; Luan, X.; Bi, L.; He, G.; Bai, X.; Zhou, C.; Zhang, Z. Toluidine blue-mediated photoinactivation of periodontal pathogens from supragingival plaques. Lasers Med. Sci. 2008, 23, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Soukos, N.S.; Mulholland, S.E.; Socransky, S.S.; Doukas, A.G. Photodestruction of human dental plaque bacteria: Enhancement of the photodynamic effect by photomechanical waves in an oral biofilm model. Lasers Surg. Med. 2003, 33, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Soukos, N.S.; Socransky, S.S.; Mulholland, S.E.; Lee, S.; Doukas, A.G. Photomechanical drug delivery into bacterial biofilms. Pharm. Res. 2000, 17, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Guggenheim, B.; Schmidlin, P.R. Efficacy of gasiform ozone and photodynamic therapy on a multispecies oral biofilm in vitro. Eur. J. Oral Sci. 2007, 115, 77–80. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.F.; Hope, C.K.; Wilson, M. Oral bacteria in multi-species biofilms can be killed by red light in the presence of toluidine blue. Lasers Surg. Med. 2002, 31, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Zeina, B.; Greenman, J.; Purcell, W.M.; Das, B. Killing of cutaneous microbial species by photodynamic therapy. Br. J. Dermatol. 2001, 144, 274–278. [Google Scholar] [CrossRef]
- Malik, Z.; Ladan, H.; Nitzan, Y. Photodynamic inactivation of gram-negative bacteria: Problems and possible solutions. J. Photochem. Photobiol. B 1992, 14, 262–266. [Google Scholar] [CrossRef]
- Soukos, N.S.; Som, S.; Abernethy, A.D.; Ruggiero, K.; Dunham, J.; Lee, C.; Doukas, A.G.; Goodson, J.M. Phototargeting oral black-pigmented bacteria. Antimicrob. Agents Chemother. 2005, 49, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Soukos, N.S.; Goodson, J.M. Photodynamic therapy in the control of oral biofilms. Periodontology 2000 2011, 55, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Zaura-Arite, E.; van Marle, J.; ten Cate, J.M. Conofocal microscopy study of undisturbed and chlorhexidine-treated dental biofilm. J. Dent. Res. 2001, 80, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L.; Patel, R. The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther. 2007, 82, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Cerveny, K.E.; DePaola, A.; Duckworth, D.H.; Gulig, P.A. Phage therapy of local and systemic disease caused by vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 2002, 70, 6251–6262. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, U.S.; Tran, L.T.; Sole, N.; Rovaldi, C.; Akiyama, A.; Friden, P.M.; Forstner, J.F.; Rothstein, D.M. P-113d, an antimicrobial peptide active against pseudomonas aeruginosa, retains activity in the presence of sputum from cystic fibrosis patients. Antimicrob. Agents Chemother. 2001, 45, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.; Carvalho, C.M.; Faustino, M.A.; Neves, M.G.; Tome, J.P.; Tome, A.C.; Cavaleiro, J.A.; Cunha, A.; Gomes, N.C.; Alves, E.; et al. Antimicrobial photodynamic therapy: Study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Mroz, P.; Dai, T.; Huang, L.; Morimoto, Y.; Kinoshita, M.; Yoshihara, Y.; Shinomiya, N.; Seki, S.; Nemoto, K.; et al. Linezolid and vancomycin decrease the therapeutic effect of methylene blue-photodynamic therapy in a mouse model of mrsa bacterial arthritis. Photochem. Photobiol. 2013, 89, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Azarpazhooh, A.; Shah, P.S.; Tenenbaum, H.C.; Goldberg, M.B. The effect of photodynamic therapy for periodontitis: A systematic review and meta-analysis. J. Periodontol. 2010, 81, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Fukuda, M.; Mitani, A.; Ting, C.C.; Osawa, K.; Nagahara, A.; Satoh, S.; Fujimura, T.; Takahashi, S.; Iwamura, Y.; et al. Nd:Yag laser irradiation of the tooth root surface inhibits demineralization and root surface softening caused by minocycline application. Photomed. Laser Surg. 2013, 31, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, P.A.; Edwards, C.A.; de Boever, J.A.; Loesche, W.J. Ultrastructural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J. Periodontol. 1988, 59, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Giuliana, G.; Ammatuna, P.; Pizzo, G.; Capone, F.; D’Angelo, M. Occurrence of invading bacteria in radicular dentin of periodontally diseased teeth: Microbiological findings. J. Clin. Periodontol. 1997, 24, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Grossi, S.G.; Zambon, J.J.; Ho, A.W.; Koch, G.; Dunford, R.G.; Machtei, E.E.; Norderyd, O.M.; Genco, R.J. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J. Periodontol. 1994, 65, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Galler, C. Periodontal summary score. A new and relevant periodontal assessment guide. J. Ont. Dent. Assoc. 2000, 1, 21–28. [Google Scholar]
- Kuo, L.C.; Polson, A.M.; Kang, T. Associations between periodontal diseases and systemic diseases: A review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health 2008, 122, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Barone, A.; Covani, U.; Ciaglia, R.N.; Romanos, G.E. Effects of CO2 laser treatment on fibroblast attachment to root surfaces. A scanning electron microscopy analysis. J. Periodontol. 2002, 73, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, D.W.; Cobb, C.M.; MacNeill, S.; Rapley, J.W.; Killoy, W.J. Determination of energy density threshold for laser ablation of bacteria. An in vitro study. J. Clin. Periodontol. 1997, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Aoki, A.; Takasaki, A.A.; Mizutani, K.; Sasaki, K.M.; Izumi, Y. Application of lasers in periodontics: True innovation or myth? Periodontology 2000 2009, 50, 90–126. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, A.; Yamaguchi, T.; Nishikata, J.; Okuda, K.; Suda, S.; Orima, K.; Kobayashi, T.; Yamazaki, K.; Yoshikawa, E.; Yoshie, H. Effects of Nd:YAG and CO2 laser treatment and ultrasonic scaling on periodontal pockets of chronic periodontitis patients. J. Periodontol. 2003, 74, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Ben Hatit, Y.; Blum, R.; Severin, C.; Maquin, M.; Jabro, M.H. The effects of a pulsed Nd:YAG laser on subgingival bacterial flora and on cementum: An in vivo study. J. Clin. Laser Med. Surg. 1996, 14, 137–143. [Google Scholar] [PubMed]
- Gold, S.I.; Vilardi, M.A. Pulsed laser beam effects on gingiva. J. Clin. Periodontol. 1994, 21, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Dukic, W.; Bago, I.; Aurer, A.; Roguljic, M. Clinical effectiveness of diode laser therapy as an adjunct to non-surgical periodontal treatment: A randomized clinical study. J. Periodontol. 2013, 84, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Kamma, J.J.; Vasdekis, V.G.; Romanos, G.E. The effect of diode laser (980 nm) treatment on aggressive periodontitis: Evaluation of microbial and clinical parameters. Photomed. Laser Surg. 2009, 27, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Slot, D.E.; Jorritsma, K.H.; Cobb, C.M.; van der Weijden, F.A. The effect of the thermal diode laser (wavelength 808–980 nm) in non-surgical periodontal therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2014, 41, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.M.; Pereira, A.N.; Fujihara, N.A.; Nogueira, F.N.; Eduardo, C.P. Effect of low-power laser irradiation on protein synthesis and ultrastructure of human gingival fibroblasts. Lasers Surg. Med. 2004, 34, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.N.; Eduardo Cde, P.; Matson, E.; Marques, M.M. Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg. Med. 2002, 31, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.C.; de Sousa, G.R.; de Barros Silveira, L.; Prates, R.A.; Pinotti, M.; Ribeiro, M.S. Clinical study of the gingiva healing after gingivectomy and low-level laser therapy. Photomed. Laser Surg. 2006, 24, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Dogan, G.E.; Demir, T.; Orbak, R. Effect of low-level laser on guided tissue regeneration performed with equine bone and membrane in the treatment of intrabony defects: A clinical study. Photomed. Laser Surg. 2014, 32, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Folwaczny, M.; Aggstaller, H.; Mehl, A.; Hickel, R. Removal of bacterial endotoxin from root surface with ER:YAG laser. Am. J. Dent. 2003, 16, 3–5. [Google Scholar] [PubMed]
- Schwarz, F.; Bieling, K.; Venghaus, S.; Sculean, A.; Jepsen, S.; Becker, J. Influence of fluorescence-controlled ER:YAG laser radiation, the vector system and hand instruments on periodontally diseased root surfaces in vivo. J. Clin. Periodontol. 2006, 33, 200–208. [Google Scholar] [CrossRef]
- Watanabe, H.; Ishikawa, I.; Suzuki, M.; Hasegawa, K. Clinical assessments of the erbium:YAG laser for soft tissue surgery and scaling. J. Clin. Laser Med. Surg. 1996, 14, 67–75. [Google Scholar] [PubMed]
- Bonito, A.J.; Lux, L.; Lohr, K.N. Impact of local adjuncts to scaling and root planing in periodontal disease therapy: A systematic review. J. Periodontol. 2005, 76, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.M.; Theodoro, L.H.; Bosco, A.F.; Nagata, M.J.; Bonfante, S.; Garcia, V.G. Treatment of experimental periodontal disease by photodynamic therapy in rats with diabetes. J. Periodontol. 2008, 79, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.M.; Theodoro, L.H.; Bosco, A.F.; Nagata, M.J.; Oshiiwa, M.; Garcia, V.G. Influence of photodynamic therapy on the development of ligature-induced periodontitis in rats. J. Periodontol. 2007, 78, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Dehn, C.; Krause, F.; Jepsen, S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. J. Clin. Periodontol. 2008, 35, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Meisel, P.; Kocher, T. Photodynamic therapy for periodontal diseases: State of the art. J. Photochem. Photobiol. B 2005, 79, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M. Photolysis of oral bacteria and its potential use in the treatment of caries and periodontal disease. J. Appl. Bacteriol. 1993, 75, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.L.; Luan, X.L.; Bi, L.J.; Sheng, Y.Q.; Zhou, C.N.; Zhang, Z.G. Comparison of toluidine blue-mediated photodynamic therapy and conventional scaling treatment for periodontitis in rats. J. Periodontal Res. 2008, 43, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T. Anti-microbial photodynamic therapy: Useful in the future? Lasers Med. Sci. 2007, 22, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, N.; Nikolidakis, D.; Chondros, P.; Becker, J.; Schwarz, F.; Rossler, R.; Sculean, A. Photodynamic therapy as an adjunct to non-surgical periodontal treatment: A randomized, controlled clinical trial. J. Periodontol. 2008, 79, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Chondros, P.; Nikolidakis, D.; Christodoulides, N.; Rossler, R.; Gutknecht, N.; Sculean, A. Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: A randomized controlled clinical trial. Lasers Med. Sci. 2009, 24, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Lulic, M.; Leiggener Gorog, I.; Salvi, G.E.; Ramseier, C.A.; Mattheos, N.; Lang, N.P. One-year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: A proof-of-principle randomized-controlled clinical trial. J. Clin. Periodontol. 2009, 36, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Betsy, J.; Prasanth, C.S.; Baiju, K.V.; Prasanthila, J.; Subhash, N. Efficacy of antimicrobial photodynamic therapy in the management of chronic periodontitis: A randomized controlled clinical trial. J. Clin. Periodontol. 2014, 41, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Petrucci, A.; Gatto, R.; Marzo, G.; Monaco, A. Photodynamic therapy in the treatment of chronic periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2013, 28, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A. Photodynamic therapy as an adjunctive treatment for chronic periodontitis: A meta-analysis. Lasers Med. Sci. 2010, 25, 605–613. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.M.; Theodoro, L.H.; Bosco, A.F.; Nagata, M.J.; Oshiiwa, M.; Garcia, V.G. In vivo effect of photodynamic therapy on periodontal bone loss in dental furcations. J. Periodontol. 2008, 79, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.G.; Longo, M.; Fernandes, L.A.; Gualberto, E.C., Jr.; Santinoni Cdos, S.; Bosco, A.F.; Nagata, M.J.; Theodoro, L.H. Treatment of experimental periodontitis in rats using repeated adjunctive antimicrobial photodynamic therapy. Lasers Med. Sci. 2013, 28, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.G.; Gualberto Junior, E.C.; Fernandes, L.A.; Bosco, A.F.; Hitomi Nagata, M.J.; Casatti, C.A.; Ervolino, E.; Theodoro, L.H. Adjunctive antimicrobial photodynamic treatment of experimentally induced periodontitis in rats with ovariectomy. J. Periodontol. 2013, 84, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.G.; Longo, M.; Gualberto Junior, E.C.; Bosco, A.F.; Nagata, M.J.; Ervolino, E.; Theodoro, L.H. Effect of the concentration of phenothiazine photosensitizers in antimicrobial photodynamic therapy on bone loss and the immune inflammatory response of induced periodontitis in rats. J. Periodontal Res. 2014, 49, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Sigusch, B.W.; Pfitzner, A.; Albrecht, V.; Glockmann, E. Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model. J. Periodontol. 2005, 76, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.R.; Novaes, A.B., Jr.; Garlet, G.P.; de Souza, R.F.; Taba, M., Jr.; Sato, S.; de Souza, S.L.; Palioto, D.B.; Grisi, M.F.; Feres, M. The effect of a single episode of antimicrobial photodynamic therapy in the treatment of experimental periodontitis. Microbiological profile and cytokine pattern in the dog mandible. Lasers Med. Sci. 2011, 26, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Prates, R.A.; Yamada, A.M.; Suzuki, L.C.; Franca, C.M.; Cai, S.; Mayer, M.P.; Ribeiro, A.C.; Ribeiro, M.S. Histomorphometric and microbiological assessment of photodynamic therapy as an adjuvant treatment for periodontitis: A short-term evaluation of inflammatory periodontal conditions and bacterial reduction in a rat model. Photomed. Laser Surg. 2011, 29, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Soukos, N.S.; Wilson, M.; Burns, T.; Speight, P.M. Photodynamic effects of toluidine blue on human oral keratinocytes and fibroblasts and streptococcus sanguis evaluated in vitro. Lasers Surg. Med. 1996, 18, 253–259. [Google Scholar] [CrossRef]
- Houreld, N.; Abrahamse, H. In vitro exposure of wounded diabetic fibroblast cells to a helium-neon laser at 5 and 16 J/cm2. Photomed. Laser Surg. 2007, 25, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Mroz, P.; Dai, T.; Huang, L.; Morimoto, Y.; Kinoshita, M.; Yoshihara, Y.; Nemoto, K.; Shinomiya, N.; Seki, S.; et al. Photodynamic therapy can induce a protective innate immune response against murine bacterial arthritis via neutrophil accumulation. PLoS ONE 2012, 7, e39823. [Google Scholar] [CrossRef] [PubMed]
- Braham, P.; Herron, C.; Street, C.; Darveau, R. Antimicrobial photodynamic therapy may promote periodontal healing through multiple mechanisms. J. Periodontol. 2009, 80, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Seguier, S.; Souza, S.L.; Sverzut, A.C.; Simioni, A.R.; Primo, F.L.; Bodineau, A.; Correa, V.M.; Coulomb, B.; Tedesco, A.C. Impact of photodynamic therapy on inflammatory cells during human chronic periodontitis. J. Photochem. Photobiol. B 2010, 101, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Mitani, A.; Fukuda, M.; Mogi, M.; Osawa, K.; Takahashi, S.; Aino, M.; Iwamura, Y.; Miyajima, S.; Yamamoto, H.; et al. Irradiation with a low-level diode laser induces the developmental endothelial locus-1 gene and reduces proinflammatory cytokines in epithelial cells. Lasers Med. Sci. 2014, 29, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Novaes, A.B., Jr.; Schwartz-Filho, H.O.; de Oliveira, R.R.; Feres, M.; Sato, S.; Figueiredo, L.C. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: Microbiological profile. Lasers Med. Sci. 2012, 27, 389–395. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.R.; Schwartz-Filho, H.O.; Novaes, A.B.; Garlet, G.P.; de Souza, R.F.; Taba, M.; Scombatti de Souza, S.L.; Ribeiro, F.J. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: Cytokine profile in gingival crevicular fluid, preliminary results. J. Periodontol. 2009, 80, 98–105. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.R.; Schwartz-Filho, H.O.; Novaes, A.B., Jr.; Taba, M., Jr. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: A preliminary randomized controlled clinical study. J. Periodontol. 2007, 78, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.L.; Novaes, A.B., Jr.; Grisi, M.F.; Taba, M., Jr.; Souza, S.L.; Palioto, D.B.; de Oliveira, P.G.; Casati, M.Z.; Casarin, R.C.; Messora, M.R. Antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: A split-mouth randomized controlled trial. J. Periodontol. 2015, 86, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Vohra, F.; Akram, Z.; Safi, S.H.; Devi Vaithilingam, R.; Ghanem, A.; Sergis, K.; Javed, F. Role of antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: A systematic review. Photodiagnosis Photodyn. Ther. 2015. [Google Scholar] [CrossRef] [PubMed]
- Noro Filho, G.A.; Casarin, R.C.; Casati, M.Z.; Giovani, E.M. PDT in non-surgical treatment of periodontitis in HIV patients: A split-mouth, randomized clinical trial. Lasers Surg. Med. 2012, 44, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Soga, Y.; Saito, T.; Nishimura, F.; Ishimaru, F.; Mineshiba, J.; Mineshiba, F.; Takaya, H.; Sato, H.; Kudo, C.; Kokeguchi, S.; et al. Appearance of multidrug-resistant opportunistic bacteria on the gingiva during leukemia treatment. J. Periodontol. 2008, 79, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Romano, F.; Torta, I.; Cirillo, D.; Caposio, P.; Romagnoli, R. Debridement and local application of tetracycline-loaded fibres in the management of persistent periodontitis: Results after 12 months. J. Clin. Periodontol. 2004, 31, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Bondan, E.F.; Lallo, M.A.; Baz, E.I.; Sinhorini, I.L.; Graca, D.L. Ultrastructural study of the remyelinating process following local ethidium bromide injection in the brainstem of dexamethasone-immunosuppressed rats (in Portuguese). Arq. Neuropsiquiatr. 2004, 62, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, E.S.; Melhado, R.M.; Theodoro, L.H.; Garcia, V.G. A histologic assessment of the influence of low-intensity laser therapy on wound healing in steroid-treated animals. Photomed. Laser Surg. 2004, 22, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Tenius, F.P.; Biondo-Simões, M.L.P.; Ioshii, S.O. Effects of chronic use of dexamethasone on cutaneous wound healing in rats. An. Bras. Dermatol. 2007, 82, 141–149. [Google Scholar]
- Bottura, P.E.; Milanezi, J.; Fernandes, L.A.; Caldas, H.C.; Abbud-Filho, M.; Garcia, V.G.; Baptista, M.A. Nonsurgical periodontal therapy combined with laser and photodynamic therapies for periodontal disease in immunosuppressed rats. Transplant. Proc. 2011, 43, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.A.; de Almeida, J.M.; Theodoro, L.H.; Bosco, A.F.; Nagata, M.J.; Martins, T.M.; Okamoto, T.; Garcia, V.G. Treatment of experimental periodontal disease by photodynamic therapy in immunosuppressed rats. J. Clin. Periodontol. 2009, 36, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.A.; Martins, T.M.; de Almeida, J.M.; Theodoro, L.H.; Garcia, V.G. Radiographic assessment of photodynamic therapy as an adjunctive treatment on induced periodontitis in immunosuppressed rats. J. Appl. Oral Sci. 2010, 18, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.G.; Fernandes, L.A.; de Almeida, J.M.; Bosco, A.F.; Nagata, M.J.; Martins, T.M.; Okamoto, T.; Theodoro, L.H. Comparison between laser therapy and non-surgical therapy for periodontitis in rats treated with dexamethasone. Lasers Med. Sci. 2010, 25, 197–206. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikuchi, T.; Mogi, M.; Okabe, I.; Okada, K.; Goto, H.; Sasaki, Y.; Fujimura, T.; Fukuda, M.; Mitani, A. Adjunctive Application of Antimicrobial Photodynamic Therapy in Nonsurgical Periodontal Treatment: A Review of Literature. Int. J. Mol. Sci. 2015, 16, 24111-24126. https://doi.org/10.3390/ijms161024111
Kikuchi T, Mogi M, Okabe I, Okada K, Goto H, Sasaki Y, Fujimura T, Fukuda M, Mitani A. Adjunctive Application of Antimicrobial Photodynamic Therapy in Nonsurgical Periodontal Treatment: A Review of Literature. International Journal of Molecular Sciences. 2015; 16(10):24111-24126. https://doi.org/10.3390/ijms161024111
Chicago/Turabian StyleKikuchi, Takeshi, Makio Mogi, Iichiro Okabe, Kosuke Okada, Hisashi Goto, Yasuyuki Sasaki, Takeki Fujimura, Mitsuo Fukuda, and Akio Mitani. 2015. "Adjunctive Application of Antimicrobial Photodynamic Therapy in Nonsurgical Periodontal Treatment: A Review of Literature" International Journal of Molecular Sciences 16, no. 10: 24111-24126. https://doi.org/10.3390/ijms161024111
APA StyleKikuchi, T., Mogi, M., Okabe, I., Okada, K., Goto, H., Sasaki, Y., Fujimura, T., Fukuda, M., & Mitani, A. (2015). Adjunctive Application of Antimicrobial Photodynamic Therapy in Nonsurgical Periodontal Treatment: A Review of Literature. International Journal of Molecular Sciences, 16(10), 24111-24126. https://doi.org/10.3390/ijms161024111