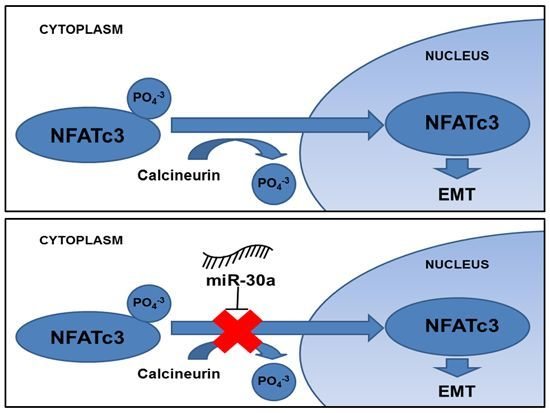
MiR-30a Inhibits the Epithelial—Mesenchymal Transition of Podocytes through Downregulation of NFATc3
Abstract
:1. Introduction
2. Results
2.1. miR-30a Is Downregulated in Podocyte Injury
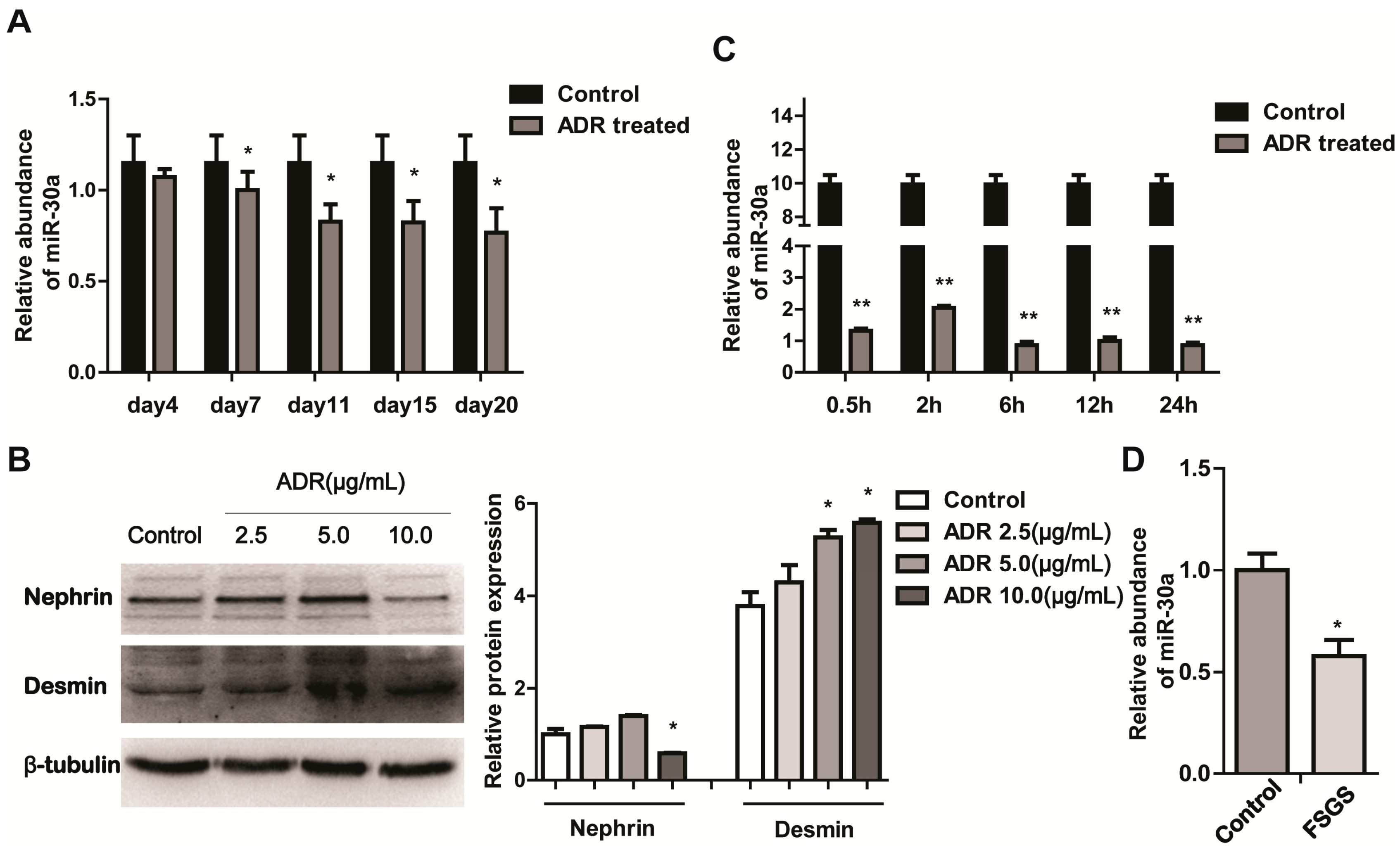
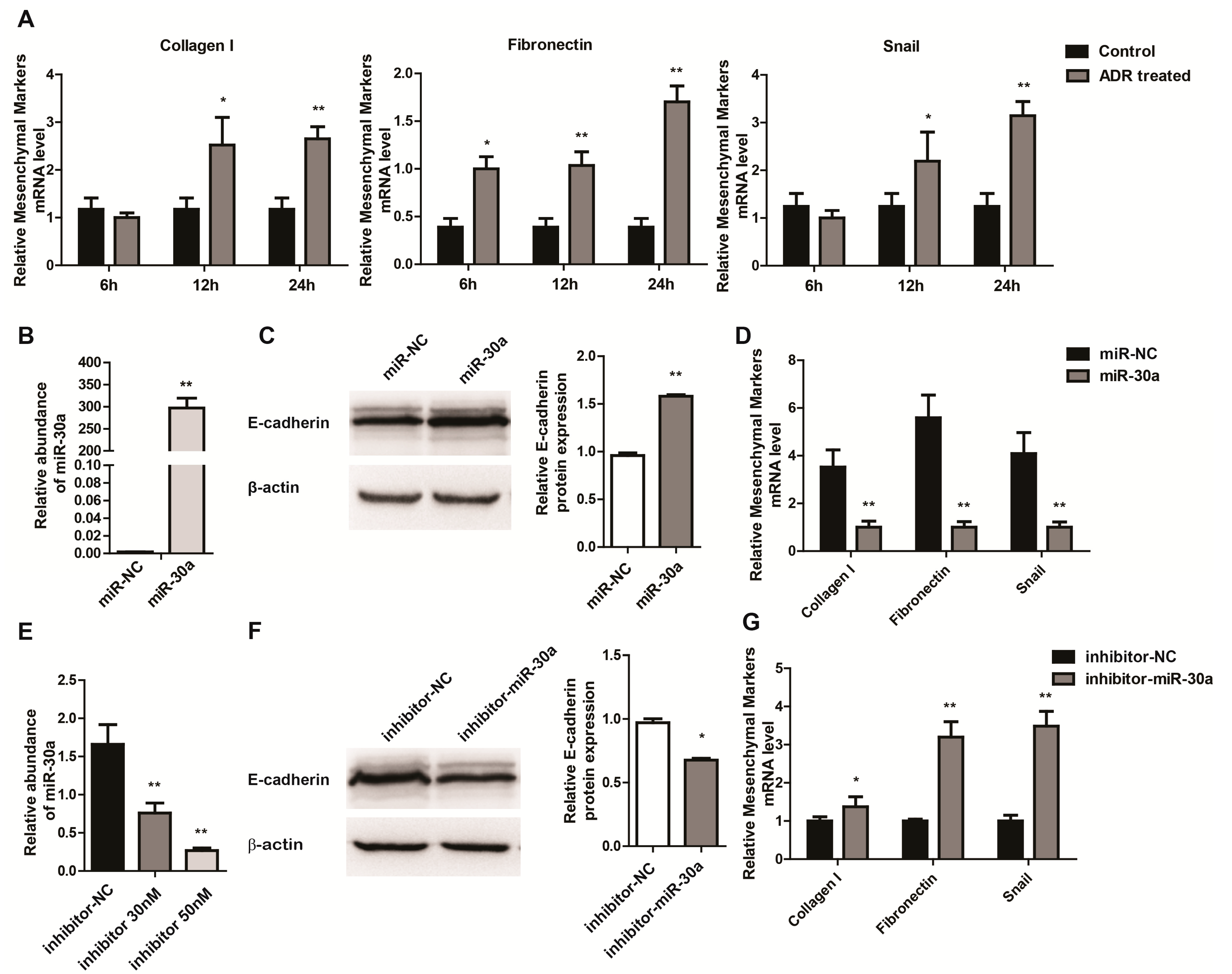
2.2. miR-30a Inhibits Epithelial-to-Mesenchymal Transition in Podocytes
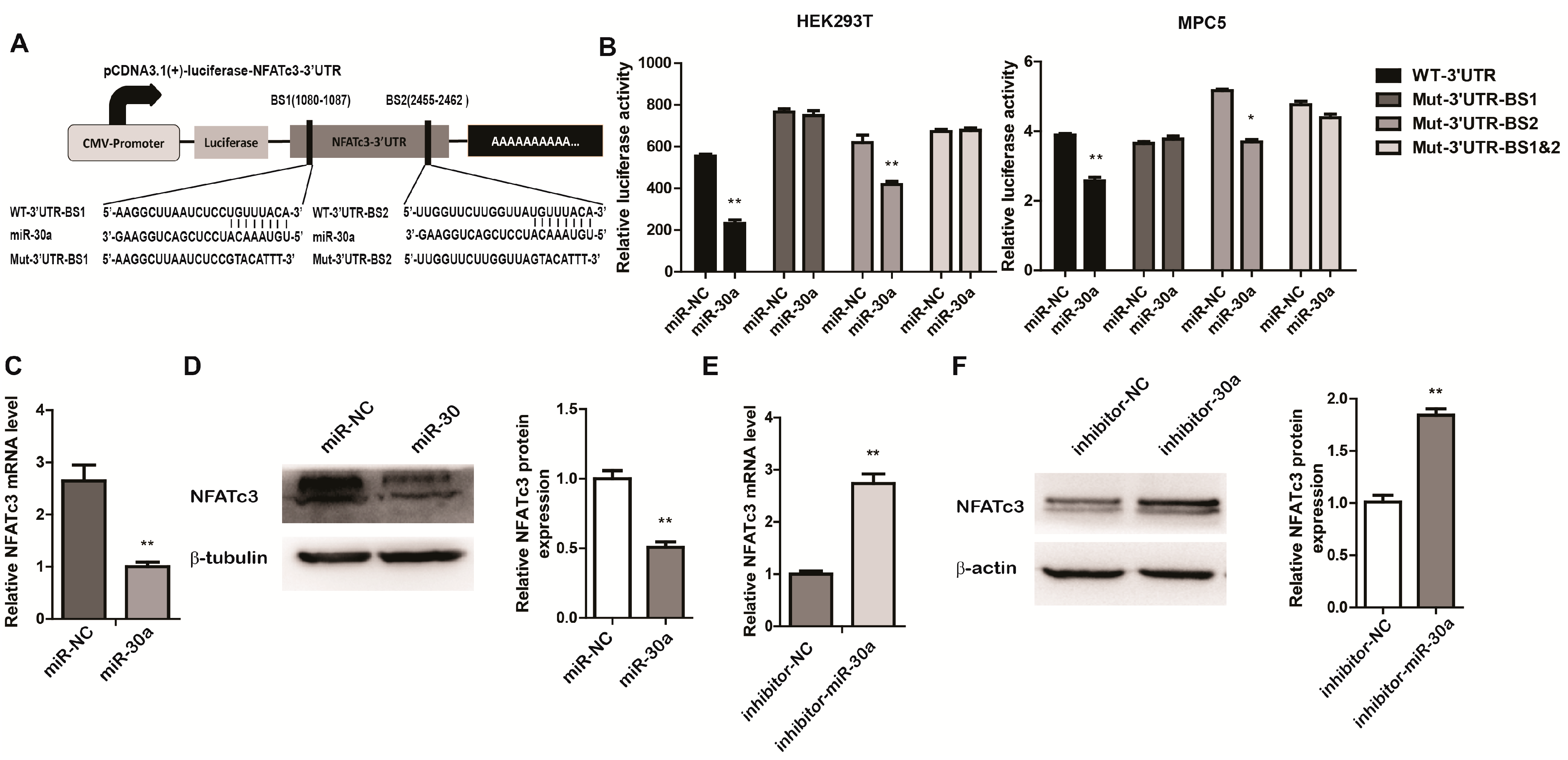
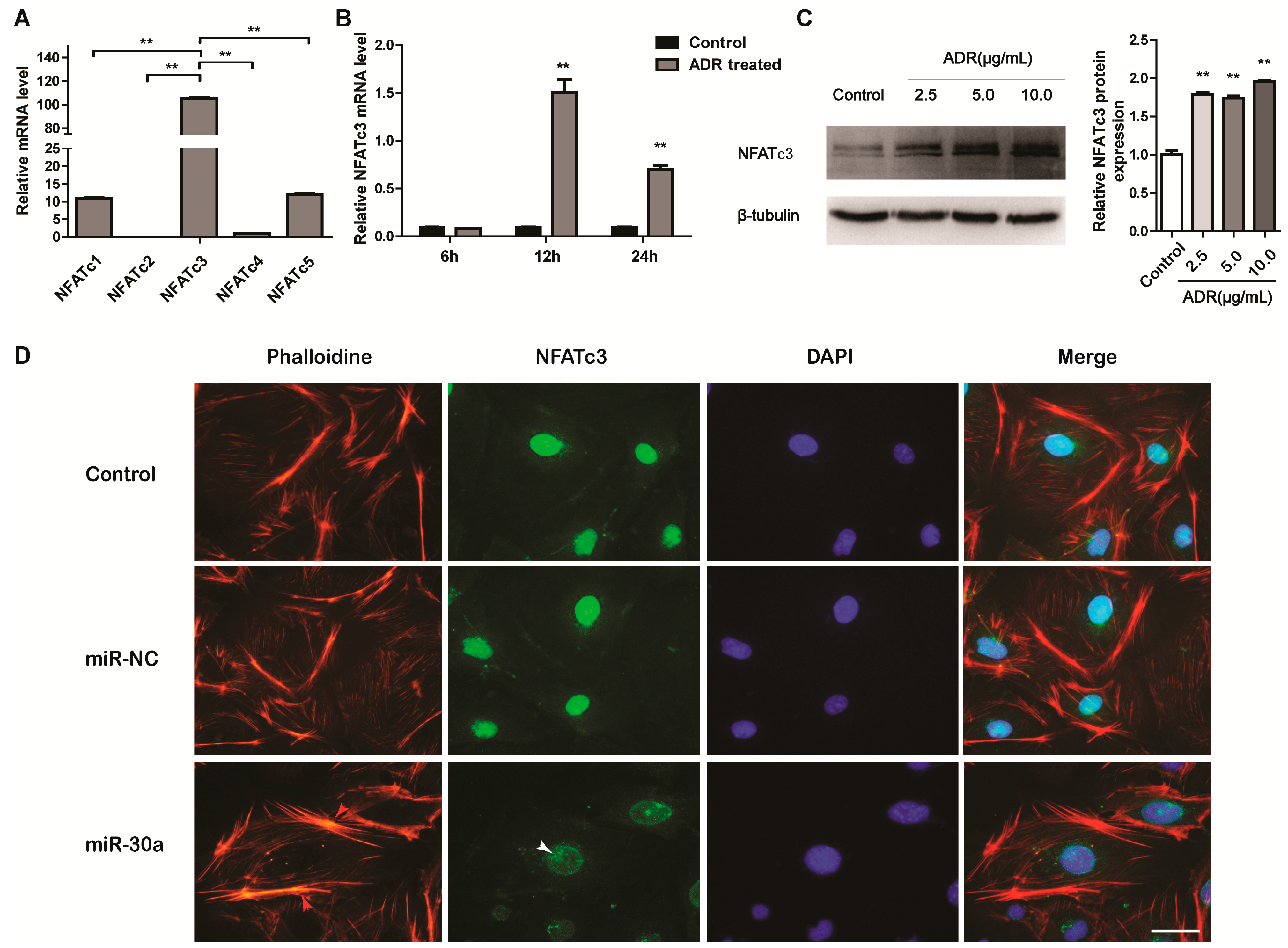
2.3. miR-30a Targets the 3ʹUTR of NFATc3 and Inhibits the Expression of NFATc3
2.4. NFATc3 Is Upregulated in Podocyte Injury
3. Discussion
4. Experimental Section
4.1. Human Samples
4.2. Animals and Treatment
4.3. Cell Culture and Transfection
4.4. Constructs and Luciferase Assays
| Name | Primer Sequences | Product Size |
|---|---|---|
| WT-NFATc3-3ʹUTR | Sense: TTTGCCCACCACGGACTG | 2450 bp |
| Antisense: TGAGGAGGAGCCTGGACTG | ||
| Mut-NFATc3-3ʹUTR-BS1 | Sense: GTTGAGAAGGCTTAATCTCCGTACATTTGCCCACAATGATTCTAT | - |
| Antisense: ACAGAATCATTGTGGGCAAATGTACGGAGATTAAGCCTTCTCAAC | ||
| Mut-NFATc3-3ʹUTR-BS2 | Sense: AAGAAATTGGTTCTTGGTTAGTACATTTGCACTTGGGATTGTG | - |
| Antisense: CACAATCCCAAGTGCAAATGTACTAACCAAGAACCAATTTCTT |
4.5. RNA Extraction and Real Time PCR Analysis
| Gene | Gene Bank Association Number | Sense | Anti-Sense | Product Size |
|---|---|---|---|---|
| NFATc1 | NM_198429 | GCCTCGAACCCTATCGAGTG | AGTTATGGCCAGACAGCACC | 121 bp |
| NFATc2 | NM_010899 | CGAGCTGGACTTTTCCATCCT | TCCAGGACATCATCCGGGTA | 120 bp |
| NFATc3 | NM_010901 | ACGACGAGCTCGACTTCAAA | TGCAGCAGTCCATGATGTGG | 181 bp |
| NFATc4 | NM_023699 | ACCTCCTGAGGGCTACAATG | CTCACTCACTTCCTCCAGGGT | 145 bp |
| NFATc5 | NM_018823 | CAGCCAAAAGGGAACTGGAG | GAAAGCCTTGCTGTGTTCTG | 173 bp |
| Snail | NM_011427 | AGCCCAACTATAGCGAGCTG | CCAGGAGAGAGTCCCAGATG | 150 bp |
| Collagen I | NM_007742 | AGCACGTCTGGTTTGGAGAG | GACATTAGGCGCAGGAAGGT | 112 bp |
| Fibnectin | NM_010233 | CCCCAACTGGTTACCCTTCC | TGTCCGCCTAAAGCCATGTT | 198 bp |
| 18s | NR_003278 | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | 151 bp |
4.6. Protein Extraction and Western Blot Analysis
4.7. Immunofluorescence Staining and F-Actin Cytoskeleton Staining
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Pavenstadt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef]
- Holthofer, H. Molecular architecture of the glomerular slit diaphragm: Lessons learnt for a better understanding of disease pathogenesis. Nephrol. Dial. Transplant. 2007, 22, 2124–2128. [Google Scholar] [CrossRef]
- Itoh, M.; Nakadate, K.; Horibata, Y.; Matsusaka, T.; Xu, J.; Hunziker, W.; Sugimoto, H. The structural and functional organization of the podocyte filtration slits is regulated by Tjp1/ZO-1. PLoS ONE 2014, 9, e106621. [Google Scholar] [CrossRef]
- Li, X.; He, J.C. An update: The role of nephrin inside and outside the kidney. Sci. China Life Sci. 2015, 58, 649–657. [Google Scholar] [CrossRef]
- May, C.J.; Saleem, M.; Welsh, G.I. Podocyte dedifferentiation: A specialized process for a specialized cell. Front. Endocrinol. 2014, 5, 148. [Google Scholar] [CrossRef]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef]
- Hills, C.E.; Squires, P.E. The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 2011, 22, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Neilson, J.R.; Bayle, J.H.; Gestwicki, J.E.; Kuo, A.; Stankunas, K.; Graef, I.A.; Crabtree, G.R. A field of myocardial-endocardial nfat signaling underlies heart valve morphogenesis. Cell 2004, 118, 649–663. [Google Scholar]
- Zhu, L.; Qi, X.Y.; Aoudjit, L.; Mouawad, F.; Baldwin, C.; Nattel, S.; Takano, T. Nuclear factor of activated T cells mediates RhoA-induced fibronectin upregulation in glomerular podocytes. Am. J. Physiol. Ren. Physiol. 2013, 304, F849–F862. [Google Scholar]
- Chen, Y.Q.; Wang, X.X.; Yao, X.M.; Zhang, D.L.; Yang, X.F.; Tian, S.F.; Wang, N.S. Microrna-195 promotes apoptosis in mouse podocytes via enhanced caspase activity driven by BCL2 insufficiency. Am. J. Nephrol. 2011, 34, 549–559. [Google Scholar] [CrossRef]
- Long, J.; Wang, Y.; Wang, W.; Chang, B.H.; Danesh, F.R. Identification of microrna-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J. Biol. Chem. 2010, 285, 23457–23465. [Google Scholar] [CrossRef]
- Du, R.; Sun, W.; Xia, L.; Zhao, A.; Yu, Y.; Zhao, L.; Wang, H.; Huang, C.; Sun, S. Hypoxia-induced down-regulation of microrna-34a promotes EMT by targeting the notch signaling pathway in tubular epithelial cells. PLoS ONE 2012, 7, e30771. [Google Scholar] [CrossRef] [PubMed]
- Tang, O.; Chen, X.M.; Shen, S.; Hahn, M.; Pollock, C.A. Mirna-200b represses transforming growth factor-β1-induced EMT and fibronectin expression in kidney proximal tubular cells. Am. J. Physiol. Ren. Physiol. 2013, 304, F1266–F1273. [Google Scholar] [CrossRef]
- Harvey, S.J.; Jarad, G.; Cunningham, J.; Goldberg, S.; Schermer, B.; Harfe, B.D.; McManus, M.T.; Benzing, T.; Miner, J.H. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. JASN 2008, 19, 2150–2158. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, C.; Chen, H.; Li, L.; Tu, Y.; Liu, C.; Shi, S.; Zen, K.; Liu, Z. Evaluation of micrornas mir-196a, mir-30a-5P, and mir-490 as biomarkers of disease activity among patients with FSGS. CJASN 2014, 9, 1545–1552. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, M.; Lan, H.; Yu, X. MiR-30a negatively regulates TGF-β1-induced epithelial-mesenchymal transition and peritoneal fibrosis by targeting Snai1. Am. J. Pathol. 2013, 183, 808–819. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Mudduluru, G.; Ceppi, P.; Muppala, S.; Kozlowski, M.; Niklinski, J.; Papotti, M.; Allgayer, H. MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. Int. J. Cancer 2012, 130, 2044–2053. [Google Scholar] [CrossRef]
- Liu, K.; Guo, L.; Guo, Y.; Zhou, B.; Li, T.; Yang, H.; Yin, R.; Xi, T. AEG-1 3’-untranslated region functions as a ceRNA in inducing epithelial-mesenchymal transition of human non-small cell lung cancer by regulating miR-30a activity. Eur. J. Cell Biol. 2015, 94, 22–31. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Zhang, X.; Xu, X.; Xing, H.; Zhang, Y.; Li, W.; Yu, H.; Zeng, J.; Jia, J. RUNX3 regulates vimentin expression via miR-30a during epithelial-mesenchymal transition in gastric cancer cells. J. Cell. Mol. Med. 2014, 18, 610–623. [Google Scholar] [CrossRef]
- Liu, Z.; Tu, K.; Liu, Q. Effects of microRNA-30a on migration, invasion and prognosis of hepatocellular carcinoma. FEBS Lett. 2014, 588, 3089–3097. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.P.; Tay, Y.C.; Harris, D.C. Progressive Adriamycin nephropathy in mice: Sequence of histologic and immunohistochemical events. Kidney Int. 2000, 58, 1797–1804. [Google Scholar] [CrossRef]
- Fogo, A.B. Animal models of FSGS: Lessons for pathogenesis and treatment. Semin. Nephrol. 2003, 23, 161–171. [Google Scholar] [CrossRef]
- Wang, Y.; Jarad, G.; Tripathi, P.; Pan, M.; Cunningham, J.; Martin, D.R.; Liapis, H.; Miner, J.H.; Chen, F. Activation of NFAT signaling in podocytes causes glomerulosclerosis. JASN 2010, 21, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M.R. Inherited podocytopathies: FSGS and nephrotic syndrome from a genetic viewpoint. JASN 2002, 13, 3016–3023. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Luo, C.; Hogan, P.G. Transcription factors of the NFAT family: Regulation and function. Annu. Rev. Immunol. 1997, 15, 707–747. [Google Scholar] [CrossRef]
- Welsh, G.I.; Saleem, M.A. The podocyte cytoskeleton—Key to a functioning glomerulus in health and disease. Nat. Rev. Nephrol. 2012, 8, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K. The role of podocyte injury in chronic kidney disease. Jpn. J. Clin. Immunol. 2015, 38, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Kriz, W.; Gretz, N.; Lemley, K.V. Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int. 1998, 54, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Floege, J.; Kriz, W.; Schulze, M.; Susani, M.; Kerjaschki, D.; Mooney, A.; Couser, W.G.; Koch, K.M. Basic fibroblast growth factor augments podocyte injury and induces glomerulosclerosis in rats with experimental membranous nephropathy. J. Clin. Investig. 1995, 96, 2809–2819. [Google Scholar] [CrossRef] [PubMed]
- Stieger, N.; Worthmann, K.; Schiffer, M. The role of metabolic and haemodynamic factors in podocyte injury in diabetes. Diabetes Metab. Res. Rev. 2011, 27, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zheng, C.; Fan, Y.; Zeng, C.; Chen, Z.; Qin, W.; Zhang, C.; Zhang, W.; Wang, X.; Zhu, X.; et al. Downregulation of microRNA-30 facilitates podocyte injury and is prevented by glucocorticoids. JASN 2014, 25, 92–104. [Google Scholar] [CrossRef]
- Liu, Y.H. New insights into epithelial-mesenchymal transition in kidney fibrosis. JASN 2010, 21, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Schlondorff, J.; del Camino, D.; Carrasquillo, R.; Lacey, V.; Pollak, M.R. TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am. J. Physiol. Cell Physiol. 2009, 296, C558–C569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, R.; Shi, W.; Liang, X.; Liu, S.; Ye, Z.; Yu, C.; Chen, Y.; Zhang, B.; Wang, W.; et al. NFAT2 inhibitor ameliorates diabetic nephropathy and podocyte injury in db/db mice. Br. J. Pharmacol. 2013, 170, 426–439. [Google Scholar] [CrossRef]
- Faul, C.; Donnelly, M.; Merscher-Gomez, S.; Chang, Y.H.; Franz, S.; Delfgaauw, J.; Chang, J.M.; Choi, H.Y.; Campbell, K.N.; Kim, K.; et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 2008, 14, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, L.; Shi, W.; Zhang, B.; Liang, X.; Liu, S.; Wang, W. NFAT2 mediates high glucose-induced glomerular podocyte apoptosis through increased Bax expression. Exp. Cell Res. 2013, 319, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Saleem, M.; Mathieson, P.W. Podocyte culture: Tricks of the trade. Nephrology 2012, 17, 525–531. [Google Scholar] [CrossRef]
- Mundel, P.; Reiser, J.; Zuniga Mejia Borja, A.; Pavenstadt, H.; Davidson, G.R.; Kriz, W.; Zeller, R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp. Cell Res. 1997, 236, 248–258. [Google Scholar] [CrossRef]
- Kolfschoten, I.G.; van Leeuwen, B.; Berns, K.; Mullenders, J.; Beijersbergen, R.L.; Bernards, R.; Voorhoeve, P.M.; Agami, R. A genetic screen identifies PITX1 as a suppressor of RAS activity and tumorigenicity. Cell 2005, 121, 849–858. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, R.; Zhou, L.; Zhou, Y.; Zhao, Y.; Li, Q.; Ni, D.; Hu, Y.; Long, Y.; Liu, J.; Lyu, Z.; et al. MiR-30a Inhibits the Epithelial—Mesenchymal Transition of Podocytes through Downregulation of NFATc3. Int. J. Mol. Sci. 2015, 16, 24032-24047. https://doi.org/10.3390/ijms161024032
Peng R, Zhou L, Zhou Y, Zhao Y, Li Q, Ni D, Hu Y, Long Y, Liu J, Lyu Z, et al. MiR-30a Inhibits the Epithelial—Mesenchymal Transition of Podocytes through Downregulation of NFATc3. International Journal of Molecular Sciences. 2015; 16(10):24032-24047. https://doi.org/10.3390/ijms161024032
Chicago/Turabian StylePeng, Rui, Li Zhou, Yuru Zhou, Ya Zhao, Qianyin Li, Dongsheng Ni, Yanxia Hu, Yaoshui Long, Jianing Liu, Zhongshi Lyu, and et al. 2015. "MiR-30a Inhibits the Epithelial—Mesenchymal Transition of Podocytes through Downregulation of NFATc3" International Journal of Molecular Sciences 16, no. 10: 24032-24047. https://doi.org/10.3390/ijms161024032
APA StylePeng, R., Zhou, L., Zhou, Y., Zhao, Y., Li, Q., Ni, D., Hu, Y., Long, Y., Liu, J., Lyu, Z., Mao, Z., Yuan, Y., Huang, L., Zhao, H., Li, G., & Zhou, Q. (2015). MiR-30a Inhibits the Epithelial—Mesenchymal Transition of Podocytes through Downregulation of NFATc3. International Journal of Molecular Sciences, 16(10), 24032-24047. https://doi.org/10.3390/ijms161024032