Mechanistic Understanding of Toxicity from Nanocatalysts
Abstract
:1. Introduction
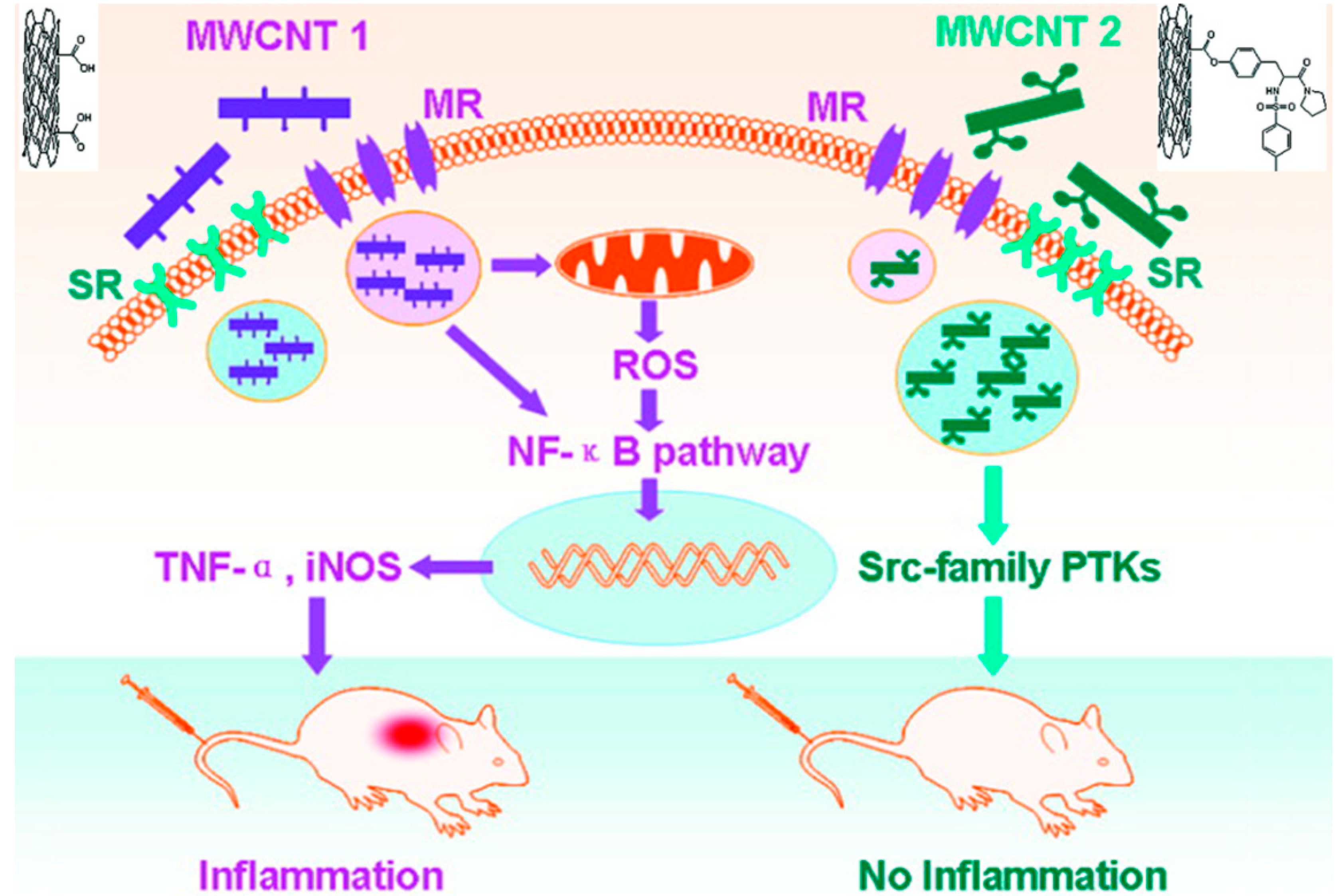
2. Carbon-Based Nanomaterials
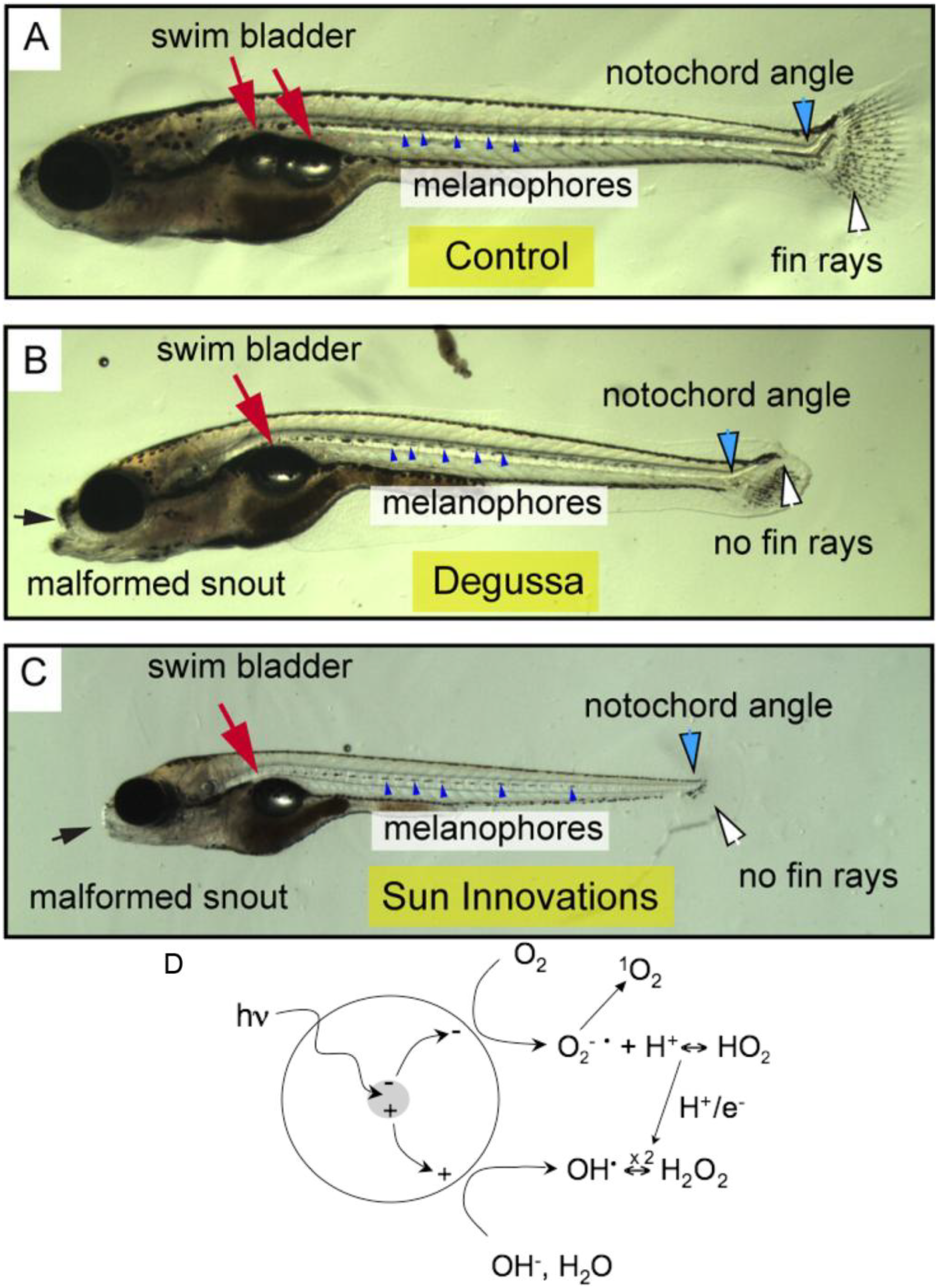
3. Titanium Dioxide Nanoparticles
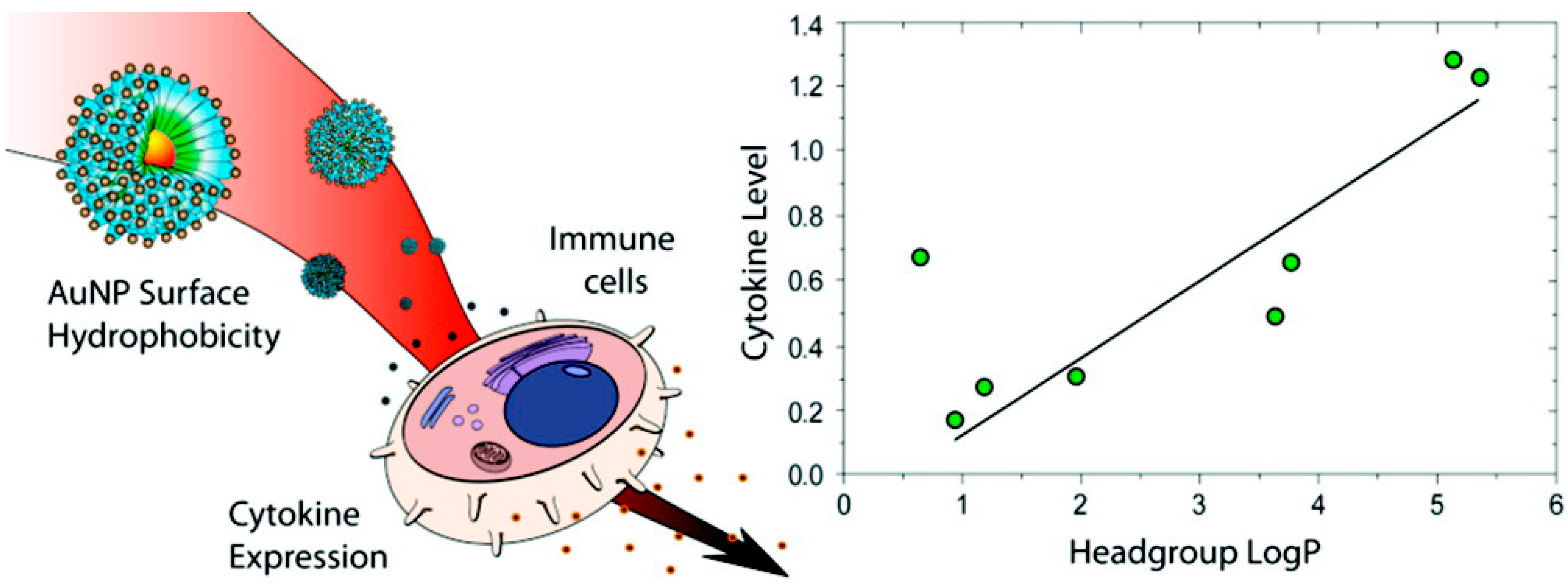
4. Gold Nanoparticles
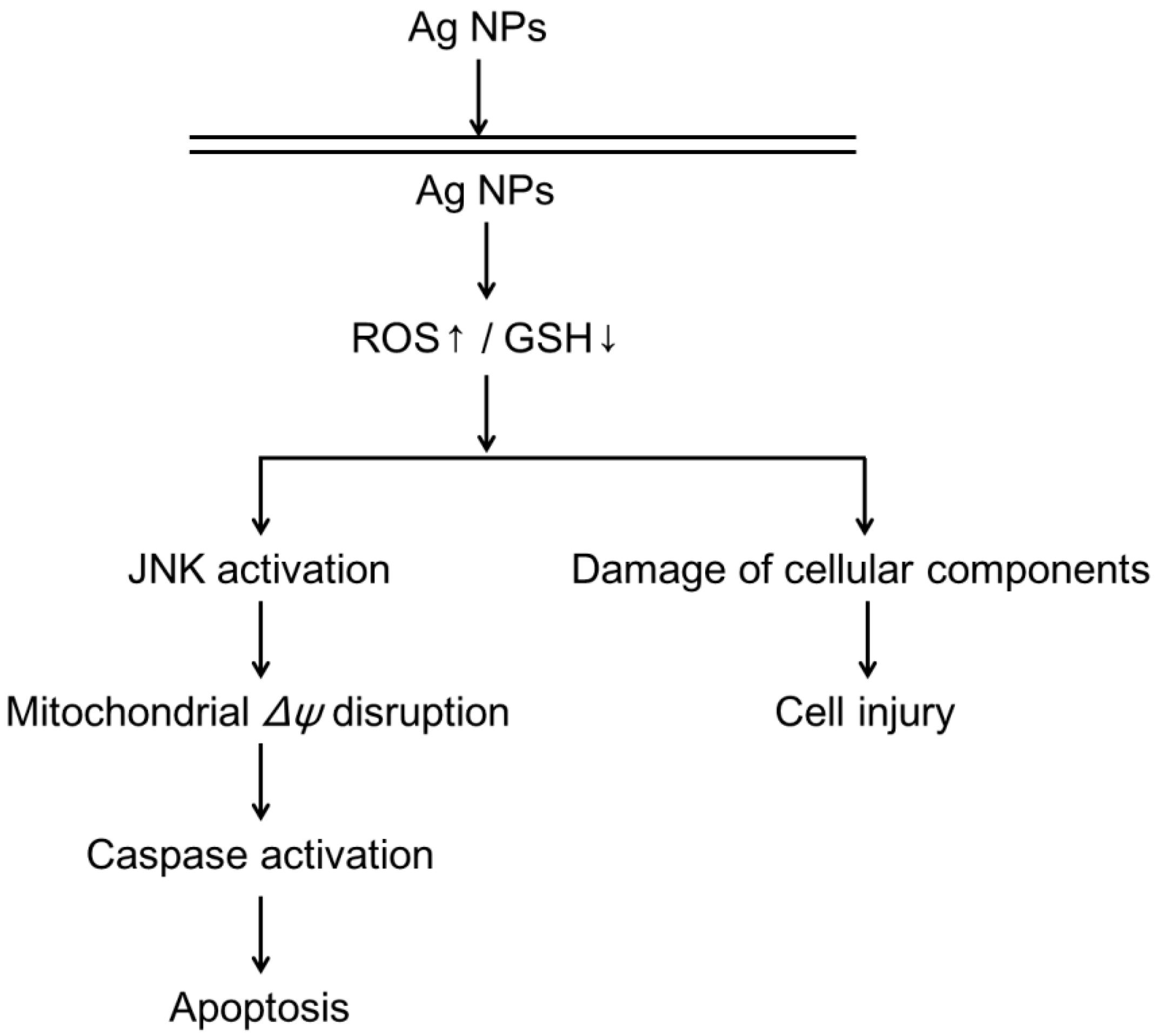
5. Silver Nanoparticles
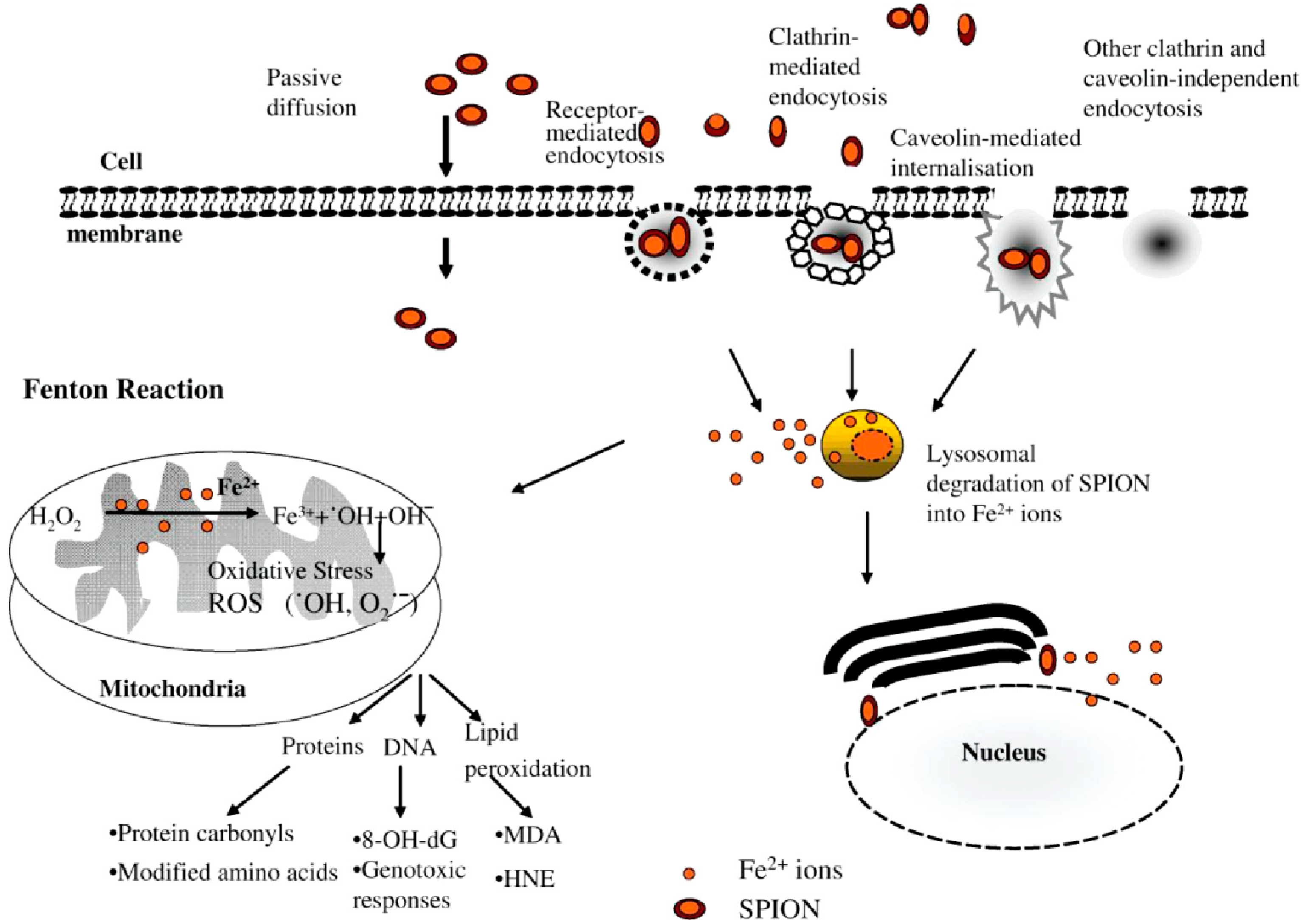
6. Iron Nanoparticles
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Polshettiwar, V.; Varma, R.S. Green chemistry by nano-catalysis. Green Chem. 2010, 12, 743–754. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Chaudret, B.; Serp, P.; Philippot, K. Nanomaterials in Catalysis, 1st ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Li, Y.; Zhou, Z.; Yu, G.; Chen, W.; Chen, Z. CO catalytic oxidation on iron-embedded graphene: Computational quest for low-cost nanocatalysts. J. Phys. Chem. C 2010, 114, 6250–6254. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, P.; Yin, Y.; Zhang, H.; Cai, C. Effects of structure, composition, and carbon support properties on the electrocatalytic activity of Pt-Ni-graphene nanocatalysts for the methanol oxidation. Appl.Catal. B Environ. 2012, 111, 208–217. [Google Scholar]
- Yin, S.-F.; Xu, B.-Q.; Ng, C.-F.; Au, C.-T. Nano Ru/CNTs: A highly active and stable catalyst for the generation of COx-free hydrogen in ammonia decomposition. Appl. Catal. B Environ. 2004, 48, 237–241. [Google Scholar] [CrossRef]
- Cheng, X.; Wu, B.; Yang, Y.; Xiang, H.; Li, Y. Fischer-Tropsch synthesis in polyethylene glycol with amorphous iron nanocatalysts prepared by chemical reduction in various solvents. J. Mol. Catal. A Chem. 2010, 329, 103–109. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Z.; Ke, X.; Jaatinen, E.; Xie, T.; Wang, D.; Guo, C.; Zhao, J.; Zhu, H. Supported silver nanoparticles as photocatalysts under ultraviolet and visible light irradiation. Green Chem. 2010, 12, 414–419. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, B.; Wang, S. ZnO/Au hybrid nanoarchitectures: Wet-chemical synthesis and structurally enhanced photocatalytic performance. Environ. Sci. Technol. 2009, 43, 8968–8973. [Google Scholar] [CrossRef]
- Arshadi, M.; Ghiaci, M.; Ensafi, A.; Karimi-Maleh, H.; Suib, S.L. Oxidation of ethylbenzene using some recyclable cobalt nanocatalysts: The role of linker and electrochemical study. J. Mol. Catal. A Chem. 2011, 338, 71–83. [Google Scholar]
- Gao, F.; Zhang, L.; Huang, S. Zinc oxide catalyzed growth of single-walled carbon nanotubes. Appl. Surf. Sci. 2010, 256, 2323–2326. [Google Scholar]
- Tseng, Y.-H.; Kuo, C.-S.; Huang, C.-H.; Li, Y.-Y.; Chou, P.-W.; Cheng, C.-L.; Wong, M.-S. Visible-light-responsive nano-TiO2 with mixed crystal lattice and its photocatalytic activity. Nanotechnology 2006, 17. [Google Scholar] [CrossRef]
- Liu, B.; Chen, J.; Zhong, X.; Cui, K.; Zhou, H.; Kuang, Y. Preparation and electrocatalytic properties of Pt-SiO2 nanocatalysts for ethanol electrooxidation. J. Colloid Interface Sci. 2007, 307, 139–144. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Photocatalytic activity of CdS and Ag2S quantum dots deposited on poly(amidoamine) functionalized carbon nanotubes. Appl. Catal. B Environ. 2011, 110, 99–107. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, F.; Zeng, H.-Y.; Guo, F. Production of glucose by hydrolysis of cellulose at 423K in the presence of activated hydrotalcite nanoparticles. Bioresour. Technol. 2011, 102, 8017–8021. [Google Scholar] [CrossRef]
- Zarkesh, J.; Hashemi, R.; Ghaedian, M.; Khakdaman, H.R.; Ahmadpanah, S.J.; Khadzhiev, S.; Kadiev, H. HRH: Nano Catalytic Process to Upgrade Extra Heavy Crude/residual Oils. In Presented at 19th World Petroleum Congress, Madrid, Spain, 29 June–3 July 2008.
- Zisimopoulos, E.G.; Tsogas, G.Z.; Giokas, D.L.; Kapakoglou, N.I.; Vlessidis, A.G. Indirect chemiluminescence-based detection of mefenamic acid in pharmaceutical formulations by flow injection analysis and effect of gold nanocatalysts. Talanta 2009, 79, 893–899. [Google Scholar] [CrossRef]
- Li, J.; Yan, R.; Xiao, B.; Liang, D.T.; Du, L. Development of nano-NiO/Al2O3 catalyst to be used for tar removal in biomass gasification. Environ. Sci. Technol. 2008, 42, 6224–6229. [Google Scholar] [CrossRef]
- Chorianopoulos, N.G.; Tsoukleris, D.S.; Panagou, E.Z.; Falaras, P.; Nychas, G.J.E. Use of titanium dioxide (TiO2) photocatalysts as alternative means for Listeria monocytogenes biofilm disinfection in food processing. Food Microbiol. 2011, 28, 164–170. [Google Scholar] [CrossRef]
- Nanocatalysts—A Global Strategic Business Report; Global Industry Analysts, Inc.: San Jose, CA, USA, 2009; p. 291.
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Da Ros, T.; Prato, M. Medicinal chemistry with fullerenes and fullerene derivatives. Chem. Commun. 1999, 663–669. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Zhou, O.Z. Applications of carbon nanotubes. In Carbon Nanotubes; Springer: Berlin, Germany, 2001; pp. 391–425. [Google Scholar]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Guo, S.; Pan, X.; Gao, H.; Yang, Z.; Zhao, J.; Bao, X. Probing the electronic effect of carbon nanotubes in catalysis: NH3 synthesis with Ru nanoparticles. Chem. Eur. J. 2010, 16, 5379–5384. [Google Scholar] [CrossRef]
- Ma, H.; Wang, L.; Chen, L.; Dong, C.; Yu, W.; Huang, T.; Qian, Y. Pt nanoparticles deposited over carbon nanotubes for selective hydrogenation of cinnamaldehyde. Catal. Commun. 2007, 8, 452–456. [Google Scholar] [CrossRef]
- Selvaraj, V.; Alagar, M. Pt and Pt-Ru nanoparticles decorated polypyrrole/multiwalled carbon nanotubes and their catalytic activity towards methanol oxidation. Electrochem. Commun. 2007, 9, 1145–1153. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Wang, J.-G.; Song, J.; Li, Q.; Dong, M.; Liu, C.-J. CO oxidation over graphene supported palladium catalyst. Appl. Catal. B Environ. 2012, 125, 189–196. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Fiorito, S.; Serafino, A.; Andreola, F.; Bernier, P. Effects of fullerenes and single-wall carbon nanotubes on murine and human macrophages. Carbon 2006, 44, 1100–1105. [Google Scholar] [CrossRef]
- Porter, A.E.; Muller, K.; Skepper, J.; Midgley, P.; Welland, M. Uptake of C60 by human monocyte macrophages, its localization and implications for toxicity: Studied by high resolution electron microscopy and electron tomography. Acta Biomater. 2006, 2, 409–419. [Google Scholar] [CrossRef]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B. The differential cytotoxicity of water-soluble fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Sayes, C.M.; Gobin, A.M.; Ausman, K.D.; Mendez, J.; West, J.L.; Colvin, V.L. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials 2005, 26, 7587–7595. [Google Scholar] [CrossRef]
- Saathoff, J.G.; Inman, A.O.; Xia, X.R.; Riviere, J.E.; Monteiro-Riviere, N.A. In vitro toxicity assessment of three hydroxylated fullerenes in human skin cells. Toxicol. In Vitro 2011, 25, 2105–2112. [Google Scholar] [CrossRef]
- Isakovic, A.; Markovic, Z.; Todorovic-Markovic, B.; Nikolic, N.; Vranjes-Djuric, S.; Mirkovic, M.; Dramicanin, M.; Harhaji, L.; Raicevic, N.; Nikolic, Z. Distinct cytotoxic mechanisms of pristine versus hydroxylated fullerene. Toxicol. Sci. 2006, 91, 173–183. [Google Scholar] [CrossRef]
- Vileno, B.; Marcoux, P.R.; Lekka, M.; Sienkiewicz, A.; Fehér, T.; Forró, L. Spectroscopic and photophysical properties of a highly derivatized C60 fullerol. Adv. Funct. Mater. 2006, 16, 120–128. [Google Scholar] [CrossRef]
- Wielgus, A.R.; Zhao, B.; Chignell, C.F.; Hu, D.-N.; Roberts, J.E. Phototoxicity and cytotoxicity of fullerol in human retinal pigment epithelial cells. Toxicol. Appl. Pharmacol. 2010, 242, 79–90. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Inman, A.O. Challenges for assessing carbon nanomaterial toxicity to the skin. Carbon 2006, 44, 1070–1078. [Google Scholar] [CrossRef]
- Cui, D.; Tian, F.; Ozkan, C.S.; Wang, M.; Gao, H. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol. Lett. 2005, 155, 73–85. [Google Scholar] [CrossRef]
- Pulskamp, K.; Diabaté, S.; Krug, H.F. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef]
- Ding, L.; Stilwell, J.; Zhang, T.; Elboudwarej, O.; Jiang, H.; Selegue, J.P.; Cooke, P.A.; Gray, J.W.; Chen, F.F. Molecular characterization of the cytotoxic mechanism of multiwall carbon nanotubes and nano-onions on human skin fibroblast. Nano Lett. 2005, 5, 2448–2464. [Google Scholar] [CrossRef]
- Tian, F.; Cui, D.; Schwarz, H.; Estrada, G.G.; Kobayashi, H. Cytotoxicity of single-wall carbon nanotubes on human fibroblasts. Toxicol. In Vitro 2006, 20, 1202–1212. [Google Scholar] [CrossRef]
- Sayes, C.M.; Liang, F.; Hudson, J.L.; Mendez, J.; Guo, W.; Beach, J.M.; Moore, V.C.; Doyle, C.D.; West, J.L.; Billups, W.E. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol. Lett. 2006, 161, 135–142. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Lalwani, G.; Zhang, K.; Yang, J.Y.; Neville, K.; Sitharaman, B. Cell specific cytotoxicity and uptake of graphene nanoribbons. Biomaterials 2013, 34, 283–293. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, Y.; Liang, L.; Wei, M.; Hu, W.; Li, X.; Huang, Q. Effect of graphene oxide on undifferentiated and retinoic acid-differentiated SH-SY5Y cells line. Nanoscale 2012, 4, 3861–3866. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Mittal, S.; Shukla, R.K.; Pandey, A.K.; Dhakate, S.R.; Pasricha, R.; Dhawan, A. Toxicity of graphene in normal human lung cells (BEAS-2B). J. Biomed. Nanotechnol. 2011, 7, 106–107. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Akhavan, A. Size-dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials 2012, 33, 8017–8025. [Google Scholar] [CrossRef]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Ogami, A.; Yamamoto, K.; Morimoto, Y.; Fujita, K.; Hirohashi, M.; Oyabu, T.; Myojo, T.; Nishi, K.; Kadoya, C.; Todoroki, M. Pathological features of rat lung following inhalation and intratracheal instillation of C60 fullerene. Inhal. Toxicol. 2011, 23, 407–416. [Google Scholar] [CrossRef]
- Sayes, C.M.; Marchione, A.A.; Reed, K.L.; Warheit, D.B. Comparative pulmonary toxicity assessments of C60 water suspensions in rats: Few differences in fullerene toxicity in vivo in contrast to in vitro profiles. Nano Lett. 2007, 7, 2399–2406. [Google Scholar] [CrossRef]
- Morimoto, Y.; Kobayashi, N.; Shinohara, N.; Myojo, T.; Tanaka, I.; Nakanishi, J. Hazard assessments of manufactured nanomaterials. J. Occup. Health 2010, 52, 325–334. [Google Scholar] [CrossRef]
- Fujita, K.; Morimoto, Y.; Ogami, A.; Myojyo, T.; Tanaka, I.; Shimada, M.; Wang, W.-N.; Endoh, S.; Uchida, K.; Nakazato, T. Gene expression profiles in rat lung after inhalation exposure to C60 fullerene particles. Toxicology 2009, 258, 47–55. [Google Scholar] [CrossRef]
- Trpkovic, A.; Todorovic-Markovic, B.; Trajkovic, V. Toxicity of pristine versus functionalized fullerenes: Mechanisms of cell damage and the role of oxidative stress. Arch. Toxicol. 2012, 86, 1809–1827. [Google Scholar] [CrossRef]
- Yamashita, K.; Yoshioka, Y.; Pan, H.; Taira, M.; Ogura, T.; Nagano, T.; Aoyama, M.; Nagano, K.; Abe, Y.; Kamada, H.; et al. Biochemical and hematologic effects of polyvinylpyrrolidone-wrapped fullerene C-60 after oral administration. Pharmazie 2013, 68, 54–57. [Google Scholar]
- Ema, M.; Matsuda, A.; Kobayashi, N.; Naya, M.; Nakanishi, J. Dermal and ocular irritation and skin sensitization studies of fullerene C-60 nanoparticles. Cutan. Ocul. Toxicol. 2013, 32, 128–134. [Google Scholar] [CrossRef]
- Takahashi, M.; Kato, H.; Doi, Y.; Hagiwara, A.; Hirata-Koizumi, M.; Ono, A.; Kubota, R.; Nishimura, T.; Hirose, A. Sub-acute oral toxicity study with fullerene C60 in rats. J. Toxicol. Sci. 2012, 37, 353–361. [Google Scholar] [CrossRef]
- Chou, C.-C.; Hsiao, H.-Y.; Hong, Q.-S.; Chen, C.-H.; Peng, Y.-W.; Chen, H.-W.; Yang, P.-C. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008, 8, 437–445. [Google Scholar] [CrossRef]
- Lam, C.W.; James, J.T.; McCluskey, R.; Hunter, R.L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004, 77, 126–134. [Google Scholar]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kisin, E.R.; Mercer, R.; Murray, A.R.; Johnson, V.J.; Potapovich, A.I.; Tyurina, Y.Y.; Gorelik, O.; Arepalli, S.; Schwegler-Berry, D.; et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L698–L708. [Google Scholar]
- Warheit, D.B.; Laurence, B.R.; Reed, K.L.; Roach, D.H.; Reynolds, G.A.M.; Webb, T.R. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. 2004, 77, 117–125. [Google Scholar]
- Ma-Hock, L.; Treumann, S.; Strauss, V.; Brill, S.; Luizi, F.; Mertler, M.; Wiench, K.; Gamer, A.O.; van Ravenzwaay, B.; Landsiedel, R. Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 months. Toxicol. Sci. 2009, 112, 468–481. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Gao, J.; Wal, R.V.; Gigliotti, A.; Burchiel, S.W.; McDonald, J.D. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol. Sci. 2007, 100, 203–214. [Google Scholar] [CrossRef]
- Schipper, M.L.; Nakayama-Ratchford, N.; Davis, C.R.; Kam, N.W.S.; Chu, P.; Liu, Z.; Sun, X.; Dai, H.; Gambhir, S.S. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 2008, 3, 216–221. [Google Scholar] [CrossRef]
- Meng, J.; Yang, M.; Jia, F.; Xu, Z.; Kong, H.; Xu, H. Immune responses of BALB/c mice to subcutaneously injected multi-walled carbon nanotubes. Nanotoxicology 2011, 5, 583–591. [Google Scholar] [CrossRef]
- Mitchell, L.; Lauer, F.; Burchiel, S.; McDonald, J. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat. Nanotechnol. 2009, 4, 451–456. [Google Scholar] [CrossRef]
- Minnikanti, S.; Pereira, M.G.; Jaraiedi, S.; Jackson, K.; Costa-Neto, C.M.; Li, Q.; Peixoto, N. In vivo electrochemical characterization and inflammatory response of multiwalled carbon nanotube-based electrodes in rat hippocampus. J. Neural Eng. 2010, 7. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, Q.; Mu, Q.; Bai, Y.; Li, L.; Zhou, H.; Butch, E.R.; Powell, T.B.; Snyder, S.E.; Jiang, G. Steering carbon nanotubes to scavenger receptor recognition by nanotube surface chemistry modification partially alleviates NF-κB activation and reduces its immunotoxicity. ACS Nano 2011, 5, 4581–4591. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Y.; Zhang, J.; Mu, Q.; Zhang, W.; Butch, E.R.; Snyder, S.E.; Yan, B. Repeated administrations of carbon nanotubes in male mice cause reversible testis damage without affecting fertility. Nat. Nanotechnol. 2010, 5, 683–689. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of graphene oxide. Nanoscale Res. Lett. 2011, 6, 8:1–8:8. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, K.; Feng, L.; Liu, Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon 2011, 49, 4040–4049. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Pacurari, M.; Schwegler-Berry, D.; Friend, S.; Leonard, S.S.; Mercer, R.R.; Vallyathan, V.; Castranova, V. Raw single-walled carbon nanotube-induced cytotoxic effects in human bronchial epithelial cells: Comparison to asbestos. Toxicol. Environ. Chem. 2011, 93, 1045–1072. [Google Scholar] [CrossRef]
- Thurnherr, T.; Brandenberger, C.; Fischer, K.; Diener, L.; Manser, P.; Maeder-Althaus, X.; Kaiser, J.-P.; Krug, H.F.; Rothen-Rutishauser, B.; Wick, P. A comparison of acute and long-term effects of industrial multiwalled carbon nanotubes on human lung and immune cells in vitro. Toxicol. Lett. 2011, 200, 176–186. [Google Scholar] [CrossRef]
- Hirano, S.; Fujitani, Y.; Furuyama, A.; Kanno, S. Uptake and cytotoxic effects of multi-walled carbon nanotubes in human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2010, 249, 8–15. [Google Scholar] [CrossRef]
- Zhou, H.; Mu, Q.; Gao, N.; Liu, A.; Xing, Y.; Gao, S.; Zhang, Q.; Qu, G.; Chen, Y.; Liu, G. A nano-combinatorial library strategy for the discovery of nanotubes with reduced protein-binding, cytotoxicity, and immune response. Nano Lett. 2008, 8, 859–865. [Google Scholar] [CrossRef]
- Ingale, S.V.; Wagh, P.B.; Tripathi, A.K.; Dudwadkar, A.S.; Gamre, S.S.; Rao, P.T.; Singh, I.K.; Gupta, S.C. Photo catalytic oxidation of TNT using TiO2-SiO2 nano-composite aerogel catalyst prepared using sol-gel process. J. Sol. Gel Sci. Technol. 2011, 58, 682–688. [Google Scholar] [CrossRef]
- Tominaga, Y.; Kubo, T.; Hosoya, K. Surface modification of TiO2 for selective photodegradation of toxic compounds. Catal. Commun. 2011, 12, 785–789. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.; Brown, R.; Hashib, M.A. Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: A Review. Water Air Soil. Pollut. 2011, 215, 3–29. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; el Khakani, M.A. Photoelectrocatalytic oxidation of chlortetracycline using Ti/TiO2 photo-anode with simultaneous H2O2 production. Electrochim. Acta 2013, 87, 18–31. [Google Scholar] [CrossRef]
- Kwon, S.; Fan, M.; Cooper, A.T.; Yang, H. Photocatalytic applications of micro-and nano-TiO2 in environmental engineering. Crit. Rev. Environ. Sci. Technol. 2008, 38, 197–226. [Google Scholar] [CrossRef]
- Khataee, A.; Kasiri, M.; Alidokht, L. Application of response surface methodology in the optimization of photocatalytic removal of environmental pollutants using nanocatalysts. Environ. Technol. 2011, 32, 1669–1684. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Hyndman, K.; Denslow, N.D.; Barber, D.S. Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicol. Sci. 2009, 107, 404–415. [Google Scholar]
- Andersson-Willman, B.; Gehrmann, U.; Cansu, Z.; Buerki-Thurnherr, T.; Krug, H.F.; Gabrielsson, S.; Scheynius, A. Effects of subtoxic concentrations of TiO2 and ZnO nanoparticles on human lymphocytes, dendritic cells and exosome production. Toxicol. Appl. Pharmacol. 2012, 264, 94–103. [Google Scholar] [CrossRef]
- Mo, Y.; Zhu, X.; Hu, X.; Tollerud, D.J.; Zhang, Q. Cytokine and NO release from peripheral blood neutrophils after exposure to metal nanoparticles: In vitro and ex vivo studies. Nanotoxicology 2008, 2, 79–87. [Google Scholar] [CrossRef]
- Bregoli, L.; Chiarini, F.; Gambarelli, A.; Sighinolfi, G.; Gatti, A.M.; Santi, P.; Martelli, A.M.; Cocco, L. Toxicity of antimony trioxide nanoparticles on human hematopoietic progenitor cells and comparison to cell lines. Toxicology 2009, 262, 121–129. [Google Scholar] [CrossRef]
- Dechsakulthorn, F.; Hayes, A.; Bakand, S.; Joeng, L.; Winder, C. In vitro cytotoxicity assessment of selected nanoparticles using human skin fibroblasts. AATEX 2007, 14, 397–400. [Google Scholar]
- Jin, C.-Y.; Zhu, B.-S.; Wang, X.-F.; Lu, Q.-H. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem. Res. Toxicol. 2008, 21, 1871–1877. [Google Scholar] [CrossRef]
- Li, S.-Q.; Zhu, R.-R.; Zhu, H.; Xue, M.; Sun, X.-Y.; Yao, S.-D.; Wang, S.-L. Nanotoxicity of TiO2 nanoparticles to erythrocyte in vitro. Food Chem. Toxicol. 2008, 46, 3626–3631. [Google Scholar] [CrossRef]
- Wang, J.J.; Sanderson, B.J.; Wang, H. Cyto-and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 628, 99–106. [Google Scholar]
- Barillet, S.; Simon-Deckers, A.; Herlin-Boime, N.; Mayne-L’Hermite, M.; Reynaud, C.; Cassio, D.; Gouget, B.; Carriere, M. Toxicological consequences of TiO2, SiC nanoparticles and multi-walled carbon nanotubes exposure in several mammalian cell types: An in vitro study. J. Nanopart. Res. 2010, 12, 61–73. [Google Scholar] [CrossRef]
- Wilhelmi, V.; Fischer, U.; van Berlo, D.; Schulze-Osthoff, K.; Schins, R.P.F.; Albrecht, C. Evaluation of apoptosis induced by nanoparticles and fine particles in RAW 264.7 macrophages: Facts and artefacts. Toxicol. In Vitro 2012, 26, 323–334. [Google Scholar] [CrossRef]
- Vevers, W.F.; Jha, A.N. Genotoxic and cytotoxic potential of titanium dioxide (TiO2) nanoparticles on fish cells in vitro. Ecotoxicology 2008, 17, 410–420. [Google Scholar] [CrossRef]
- Aruoja, V.; Dubourguier, H.-C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef]
- Menard, A.; Drobne, D.; Jemec, A. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ. Pollut. 2011, 159, 677–684. [Google Scholar] [CrossRef]
- Kamat, P.V. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J. Phys. Chem. B 2002, 106, 7729–7744. [Google Scholar]
- Fabian, E.; Landsiedel, R.; Ma-Hock, L.; Wiench, K.; Wohlleben, W.; van Ravenzwaay, B. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch. Toxicol. 2008, 82, 151–157. [Google Scholar] [CrossRef]
- Liu, H.; Ma, L.; Zhao, J.; Liu, J.; Yan, J.; Ruan, J.; Hong, F. Biochemical toxicity of nano-anatase TiO2 particles in mice. Biol. Trace Element. 2009, 129, 170–180. [Google Scholar] [CrossRef]
- Li, N.; Ma, L.; Wang, J.; Zheng, L.; Liu, J.; Duan, Y.; Liu, H.; Zhao, X.; Wang, S.; Wang, H.; et al. Interaction between nano-anatase TiO2 and liver DNA from mice In Vivo. Nanoscale Res. Lett. 2010, 5, 108–115. [Google Scholar] [CrossRef]
- Liu, R.; Yin, L.; Pu, Y.; Liang, G.; Zhang, J.; Su, Y.; Xiao, Z.; Ye, B. Pulmonary toxicity induced by three forms of titanium dioxide nanoparticles via intra-tracheal instillation in rats. Prog. Nat. Sci. 2009, 19, 573–579. [Google Scholar] [CrossRef]
- Jonasson, S.; Gustafsson, A.; Koch, B.; Bucht, A. Inhalation exposure of nano-scaled titanium dioxide (TiO2) particles alters the inflammatory responses in asthmatic mice. Inhal. Toxicol. 2013, 25, 179–191. [Google Scholar] [CrossRef]
- Wamer, W.G.; Yin, J.-J.; Wei, R.R. Oxidative damage to nucleic acids photosensitized by titanium dioxide. Free Radic. Biol. Med. 1997, 23, 851–858. [Google Scholar] [CrossRef]
- Gurr, J.-R.; Wang, A.S.S.; Chen, C.-H.; Jan, K.-Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef]
- Ramires, P.A.; Romito, A.; Cosentino, F.; Milella, E. The influence of titania/hydroxyapatite composite coatings on in vitro osteoblasts behaviour. Biomaterials 2001, 22, 1467–1474. [Google Scholar] [CrossRef]
- Reeves, J.F.; Davies, S.J.; Dodd, N.J.F.; Jha, A.N. Hydroxyl radicals (OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2008, 640, 113–122. [Google Scholar] [CrossRef]
- Brunet, L.; Lyon, D.Y.; Hotze, E.M.; Alvarez, P.J.; Wiesner, M.R. Comparative photoactivity and antibacterial properties of C60 fullerenes and titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 4355–4360. [Google Scholar] [CrossRef]
- Bar-Ilan, O.; Chuang, C.C.; Schwahn, D.J.; Yang, S.; Joshi, S.; Pedersen, J.A.; Hamers, R.J.; Peterson, R.E.; Heideman, W. TiO2 nanoparticle exposure and illumination during zebrafish development: Mortality at parts per billion concentrations. Environ. Sci. Technol. 2013, 47, 4726–4733. [Google Scholar] [CrossRef]
- Bar-Ilan, O.; Louis, K.M.; Yang, S.P.; Pedersen, J.A.; Hamers, R.J.; Peterson, R.E.; Heideman, W. Titanium dioxide nanoparticles produce phototoxicity in the developing zebrafish. Nanotoxicology 2012, 6, 670–679. [Google Scholar] [CrossRef]
- Yang, S.P.; Bar-Ilan, O.; Peterson, R.E.; Heideman, W.; Hamers, R.J.; Pedersen, J.A. Influence of humic acid on titanium dioxide nanoparticle toxicity to developing zebrafish. Environ. Sci. Technol. 2013, 47, 4718–4725. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef]
- Corma, A.; Domine, M.E. Gold supported on a mesoporous CeO2 matrix as an efficient catalyst in the selective aerobic oxidation of aldehydes in the liquid phase. Chem. Commun. 2005, 4042–4044. [Google Scholar] [CrossRef]
- Biella, S.; Castiglioni, G.; Fumagalli, C.; Prati, L.; Rossi, M. Application of gold catalysts to selective liquid phase oxidation. Catal. Today 2002, 72, 43–49. [Google Scholar] [CrossRef]
- Zheng, N.; Stucky, G.D. Promoting gold nanocatalysts in solvent-free selective aerobic oxidation of alcohols. Chem. Commun. 2007, 3862–3864. [Google Scholar] [CrossRef]
- Chandrasekharan, N.; Kamat, P.V. Improving the photoelectrochemical performance of nanostructured TiO2 films by adsorption of gold nanoparticles. J. Phys. Chem. B 2000, 104, 10851–10857. [Google Scholar]
- Chi, Y.-S.; Lin, H.-P.; Mou, C.-Y. CO oxidation over gold nanocatalyst confined in mesoporous silica. Appl. Catal. A Gen. 2005, 284, 199–206. [Google Scholar] [CrossRef]
- Comotti, M.; Li, W.-C.; Spliethoff, B.; Schüth, F. Support effect in high activity gold catalysts for CO oxidation. J. Am. Chem. Soc. 2006, 128, 917–924. [Google Scholar] [CrossRef]
- Liu, J.-H.; Wang, A.-Q.; Chi, Y.-S.; Lin, H.-P.; Mou, C.-Y. Synergistic effect in an Au-Ag alloy nanocatalyst: CO oxidation. J. Phys. Chem. B 2005, 109, 40–43. [Google Scholar]
- Kim, S.T.; Saha, K.; Kim, C.; Rotello, V.M. The role of surface functionality in determining nanoparticle cytotoxicity. Acc. Chem. Res. 2013, 46, 681–691. [Google Scholar] [CrossRef]
- Thomas, M.; Klibanov, A.M. Conjugation to gold nanoparticles enhances polyethylenimine’s transfer of plasmid DNA into mammalian cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9138–9143. [Google Scholar] [CrossRef]
- Su, G.; Zhou, H.; Mu, Q.; Zhang, Y.; Li, L.; Jiao, P.; Jiang, G.; Yan, B. Effective surface charge density determines the electrostatic attraction between nanoparticles and cells. J. Phys. Chem. C 2012, 116, 4993–4998. [Google Scholar]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Miranda, O.R.; Thompson, M.A.; Pabelick, C.M.; Bhattacharya, R.; Robertson, J.D.; Rotello, V.M.; Prakash, Y.; Mukherjee, P. Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett. 2010, 10, 2543–2548. [Google Scholar] [CrossRef]
- Tkachenko, A.G.; Xie, H.; Coleman, D.; Glomm, W.; Ryan, J.; Anderson, M.F.; Franzen, S.; Feldheim, D.L. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J. Am. Chem. Soc. 2003, 125, 4700–4701. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Patra, H.K.; Banerjee, S.; Chaudhuri, U.; Lahiri, P.; Dasgupta, A.K. Cell selective response to gold nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 111–119. [Google Scholar] [CrossRef]
- Moyano, D.F.; Goldsmith, M.; Solfiell, D.J.; Landesman-Milo, D.; Miranda, O.R.; Peer, D.; Rotello, V.M. Nanoparticle hydrophobicity dictates immune response. J. Am. Chem. Soc. 2012, 134, 3965–3967. [Google Scholar] [CrossRef]
- Cho, W.-S.; Cho, M.; Jeong, J.; Choi, M.; Cho, H.-Y.; Han, B.S.; Kim, S.H.; Kim, H.O.; Lim, Y.T.; Chung, B.H. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2009, 236, 16–24. [Google Scholar] [CrossRef]
- Cho, W.-S.; Kim, S.; Han, B.S.; Son, W.C.; Jeong, J. Comparison of gene expression profiles in mice liver following intravenous injection of 4 and 100nm-sized PEG-coated gold nanoparticles. Toxicol. Lett. 2009, 191, 96–102. [Google Scholar] [CrossRef]
- Lasagna-Reeves, C.; Gonzalez-Romero, D.; Barria, M.; Olmedo, I.; Clos, A.; Ramanujam, V.S.; Urayama, A.; Vergara, L.; Kogan, M.J.; Soto, C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2010, 393, 649–655. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Hung, Y.-C.; Liau, I.; Huang, G.S. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res. Lett. 2009, 4, 858–864. [Google Scholar] [CrossRef]
- Bar-Ilan, O.; Albrecht, R.M.; Fako, V.E.; Furgeson, D.Y. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small 2009, 5, 1897–1910. [Google Scholar] [CrossRef]
- Browning, L.M.; Lee, K.J.; Huang, T.; Nallathamby, P.D.; Lowman, J.E.; Xu, X.-H.N. Random walk of single gold nanoparticles in zebrafish embryos leading to stochastic toxic effects on embryonic developments. Nanoscale 2009, 1, 138–152. [Google Scholar] [CrossRef]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Li, J.J.; Hartono, D.; Ong, C.-N.; Bay, B.-H.; Yung, L.-Y.L. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [CrossRef]
- Jia, H.Y.; Liu, Y.; Zhang, X.J.; Han, L.; Du, L.B.; Tian, Q.; Xu, Y.C. Potential oxidative stress of gold nanoparticles by induced-NO releasing in serum. J. Am. Chem. Soc. 2008, 131, 40–41. [Google Scholar]
- Bhattacharya, R.; Mukherjee, P.; Xiong, Z.; Atala, A.; Soker, S.; Mukhopadhyay, D. Gold nanoparticles inhibit VEGF165-induced proliferation of HUVEC cells. Nano Lett. 2004, 4, 2479–2481. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Sheikpranbabu, S.; BarathManiKanth, S.; Haribalaganesh, R.; Ramkumarpandian, S.; Gurunathan, S. Gold nanoparticles inhibit vascular endothelial growth factor-induced angiogenesis and vascular permeability via Src dependent pathway in retinal endothelial cells. Angiogenesis 2011, 14, 29–45. [Google Scholar] [CrossRef]
- Staroverov, S.A.; Aksinenko, N.M.; Gabalov, K.P.; Vasilenko, O.A.; Vidyasheva, I.V.; Shchyogolev, S.Y.; Dykman, L.A. Effect of gold nanoparticles on the respiratory activity of peritoneal macrophages. Gold Bull. 2009, 42, 153–156. [Google Scholar] [CrossRef]
- Ernest, V.; Shiny, P.; Mukherjee, A.; Chandrasekaran, N. Silver nanoparticles: A potential nanocatalyst for the rapid degradation of starch hydrolysis by α-amylase. Carbohydr. Res. 2012, 352, 60–64. [Google Scholar] [CrossRef]
- Li, Z.; Divakara, S.G.; Richards, R.M.; Geckeler, K.; Nishide, H. Oxidation catalysis by nanoscale gold, silver, and copper. Adv. Nanomater. 2010, 333–364. [Google Scholar]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Wei, J.; Whangbo, M.H. Ag@AgCl: A highly efficient and stable photocatalyst active under visible light. Angew. Chem. Int. Ed. 2008, 47, 7931–7933. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J. Photocatalytic activity of Ag/ZnO heterostructure nanocatalyst: Correlation between structure and property. J. Phys. Chem. C 2008, 112, 10773–10777. [Google Scholar] [CrossRef]
- Lam, P.; Chan, E.; Ho, W.; Liew, C. In vitro cytotoxicity testing of a nanocrystalline silver dressing (Acticoat) on cultured keratinocytes. Br. J. Biomed. Sci. 2004, 61, 125–127. [Google Scholar]
- Paddle-Ledinek, J.E.; Nasa, Z.; Cleland, H.J. Effect of different wound dressings on cell viability and proliferation. Plast. Reconstr. Surg. 2006, 117, 110S–118S. [Google Scholar] [CrossRef]
- Poon, V.K.; Burd, A. In vitro cytotoxity of silver: Implication for clinical wound care. Burns 2004, 30, 140–147. [Google Scholar] [CrossRef]
- Shin, S.-H.; Ye, M.-K.; Kim, H.-S.; Kang, H.-S. The effects of nano-silver on the proliferation and cytokine expression by peripheral blood mononuclear cells. Int. Immunopharmacol. 2007, 7, 1813–1818. [Google Scholar] [CrossRef]
- Braydich-Stolle, L.; Hussain, S.; Schlager, J.J.; Hofmann, M.-C. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol. Sci. 2005, 88, 412–419. [Google Scholar] [CrossRef]
- Hussain, S.; Hess, K.; Gearhart, J.; Geiss, K.; Schlager, J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro 2005, 19, 975–984. [Google Scholar] [CrossRef]
- Ahamed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef]
- Samberg, M.E.; Oldenburg, S.J.; Monteiro-Riviere, N.A. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ. Health Perspect. 2010, 118, 407–413. [Google Scholar]
- Kim, T.H.; Kim, M.; Park, H.S.; Shin, U.S.; Gong, M.S.; Kim, H.W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. Part A 2012, 100, 1033–1043. [Google Scholar]
- Takenaka, S.; Karg, E.; Roth, C.; Schulz, H.; Ziesenis, A.; Heinzmann, U.; Schramel, P.; Heyder, J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ. Health Perspect. 2001, 109 (Suppl. 4), 547–551. [Google Scholar] [CrossRef]
- Sung, J.H.; Ji, J.H.; Yoon, J.U.; Kim, D.S.; Song, M.Y.; Jeong, J.; Han, B.S.; Han, J.H.; Chung, Y.H.; Kim, J. Lung function changes in Sprague-Dawley rats after prolonged inhalation exposure to silver nanoparticles. Inhal. Toxicol. 2008, 20, 567–574. [Google Scholar] [CrossRef]
- Loeschner, K.; Hadrup, N.; Qvortrup, K.; Larsen, A.; Gao, X.; Vogel, U.; Mortensen, A.; Lam, H.R.; Larsen, E.H. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part. Fibre Toxicol. 2011, 8, 18:1–18:14. [Google Scholar]
- Tang, J.; Xiong, L.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Yuan, F.; Xi, T. Distribution, translocation and accumulation of silver nanoparticles in rats. J. Nanosci. Nanotechnol. 2009, 9, 4924–4932. [Google Scholar] [CrossRef]
- Ji, J.H.; Jung, J.H.; Kim, S.S.; Yoon, J.-U.; Park, J.D.; Choi, B.S.; Chung, Y.H.; Kwon, I.H.; Jeong, J.; Han, B.S. Twenty-eight-day inhalation toxicity study of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2007, 19, 857–871. [Google Scholar] [CrossRef]
- Hyun, J.-S.; Lee, B.S.; Ryu, H.Y.; Sung, J.H.; Chung, K.H.; Yu, I.J. Effects of repeated silver nanoparticles exposure on the histological structure and mucins of nasal respiratory mucosa in rats. Toxicol. Lett. 2008, 182, 24–28. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Choi, Y.-J.; Jung, E.-J.; Yin, H.-Q.; Kwon, J.-T.; Kim, J.-E.; Im, H.-T.; Cho, M.-H.; Kim, J.-H.; Kim, H.-Y. Genomics-based screening of differentially expressed genes in the brains of mice exposed to silver nanoparticles via inhalation. J. Nanopart. Res. 2010, 12, 1567–1578. [Google Scholar] [CrossRef]
- Rahman, M.; Wang, J.; Patterson, T.; Saini, U.; Robinson, B.; Newport, G.; Murdock, R.; Schlager, J.; Hussain, S.; Ali, S. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009, 187, 15–21. [Google Scholar] [CrossRef]
- Cha, K.; Hong, H.-W.; Choi, Y.-G.; Lee, M.J.; Park, J.H.; Chae, H.-K.; Ryu, G.; Myung, H. Comparison of acute responses of mice livers to short-term exposure to nano-sized or micro-sized silver particles. Biotechnol. Lett. 2008, 30, 1893–1899. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef]
- Kim, Y.S.; Song, M.Y.; Park, J.D.; Song, K.S.; Ryu, H.R.; Chung, Y.H.; Chang, H.K.; Lee, J.H.; Oh, K.H.; Kelman, B.J. Subchronic oral toxicity of silver nanoparticles. Part. Fibre Toxicol. 2010, 7, 20:1–20:11. [Google Scholar]
- Asharani, P.; Wu, Y.L.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Lee, K.J.; Nallathamby, P.D.; Browning, L.M.; Osgood, C.J.; Xu, X.-H.N. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano 2007, 1, 133–143. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar]
- Hsin, Y.-H.; Chen, C.-F.; Huang, S.; Shih, T.-S.; Lai, P.-S.; Chueh, P.J. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol. Lett. 2008, 179, 130–139. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef]
- AshaRani, P.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2008, 3, 279–290. [Google Scholar]
- Park, E.-J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. In Vitro 2010, 24, 872–878. [Google Scholar] [CrossRef]
- Lubick, N. Nanosilver toxicity: Ions, nanoparticles—or both? Environ. Sci. Technol. 2008, 42, 8617–8617. [Google Scholar] [CrossRef]
- Kawata, K.; Osawa, M.; Okabe, S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ. Sci. Technol. 2009, 43, 6046–6051. [Google Scholar] [CrossRef]
- Wang, Y.; Davis, B.H. Fischer-Tropsch synthesis. Conversion of alcohols over iron oxide and iron carbide catalysts. Appl. Catal. A Gen. 1999, 180, 277–285. [Google Scholar] [CrossRef]
- Shi, F.; Tse, M.K.; Pohl, M.M.; Brückner, A.; Zhang, S.; Beller, M. Tuning catalytic activity between homogeneous and heterogeneous catalysis: Improved activity and selectivity of free nano-Fe2O3 in selective oxidations. Angew. Chem. Int. Ed. 2007, 46, 8866–8868. [Google Scholar] [CrossRef]
- Bandara, J.; Klehm, U.; Kiwi, J. Raschig rings-Fe2O3 composite photocatalyst activate in the degradation of 4-chlorophenol and Orange II under daylight irradiation. Appl. Catal. B Environ. 2007, 76, 73–81. [Google Scholar] [CrossRef]
- Brebu, M.; Uddin, M.A.; Muto, A.; Sakata, Y.; Vasile, C. Catalytic degradation of acrylonitrile-butadiene-styrene into fuel oil 1. The effect of iron oxides on the distribution of nitrogen-containing compounds. Energy Fuels 2001, 15, 559–564. [Google Scholar] [CrossRef]
- Li, X.-Q.; Elliott, D.W.; Zhang, W.-X. Zero-valent iron nanoparticles for abatement of environmental pollutants: Materials and engineering aspects. Crit. Rev. Solid State Mater. Sci. 2006, 31, 111–122. [Google Scholar]
- Soenen, S.J.; Nuytten, N.; de Meyer, S.F.; de Smedt, S.C.; de Cuyper, M. High intracellular iron oxide nanoparticle concentrations affect cellular cytoskeleton and focal adhesion kinase-mediated signaling. Small 2010, 6, 832–842. [Google Scholar] [CrossRef]
- Kim, H.S.; Choi, Y.; Song, I.C.; Moon, W.K. Magnetic resonance imaging and biological properties of pancreatic islets labeled with iron oxide nanoparticles. NMR Biomed. 2009, 22, 852–856. [Google Scholar] [CrossRef]
- Berry, C.C.; Charles, S.; Wells, S.; Dalby, M.J.; Curtis, A.S. The influence of transferrin stabilised magnetic nanoparticles on human dermal fibroblasts in culture. Int. J. Pharm. 2004, 269, 211–225. [Google Scholar]
- Gupta, A.K.; Gupta, M. Cytotoxicity suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials 2005, 26, 1565–1573. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wells, S. Surface-modified superparamagnetic nanoparticles for drug delivery: Preparation, characterization, and cytotoxicity studies. NanoBiosci. IEEE Trans. 2004, 3, 66–73. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Simchi, A.; Imani, M.; Shokrgozar, M.A.; Milani, A.S.; Häfeli, U.O.; Stroeve, P. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf. B Biointerfaces 2010, 75, 300–309. [Google Scholar] [CrossRef]
- Häfeli, U.O.; Riffle, J.S.; Harris-Shekhawat, L.; Carmichael-Baranauskas, A.; Mark, F.; Dailey, J.P.; Bardenstein, D. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol. Pharm. 2009, 6, 1417–1428. [Google Scholar] [CrossRef]
- Keenan, C.R.; Goth-Goldstein, R.; Lucas, D.; Sedlak, D.L. Oxidative stress induced by zero-valent iron nanoparticles and Fe (II) in human bronchial epithelial cells. Environ. Sci. Technol. 2009, 43, 4555–4560. [Google Scholar] [CrossRef]
- Hanini, A.; Schmitt, A.; Kacem, K.; Chau, F.; Ammar, S.; Gavard, J. Evaluation of iron oxide nanoparticle biocompatibility. Int. J. Nanomed. 2011, 6, 787–794. [Google Scholar]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem. Rev. Columb. 2012, 112, 2323–2338. [Google Scholar] [CrossRef]
- Lübbe, A.S.; Bergemann, C.; Riess, H.; Schriever, F.; Reichardt, P.; Possinger, K.; Matthias, M.; Dörken, B.; Herrmann, F.; Gürtler, R. Clinical experiences with magnetic drug targeting: A phase I study with 4'-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996, 56, 4686–4693. [Google Scholar]
- Marchal, G.; van Hecke, P.; Demaerel, P.; Decrop, E.; Kennis, C.; Baert, A.; van der Schueren, E. Detection of liver metastases with superparamagnetic iron oxide in 15 patients: Results of MR imaging at 1.5 T. Am. J. Roentgenol. 1989, 152, 771–775. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1. [Google Scholar] [CrossRef]
- Gupta, A.K.; Curtis, A.S. Surface modified superparamagnetic nanoparticles for drug delivery: Interaction studies with human fibroblasts in culture. J. Mater. Sci. Mater. Med. 2004, 15, 493–496. [Google Scholar]
- Arbab, A.S.; Wilson, L.B.; Ashari, P.; Jordan, E.K.; Lewis, B.K.; Frank, J.A. A model of lysosomal metabolism of dextran coated superparamagnetic iron oxide (SPIO) nanoparticles: Implications for cellular magnetic resonance imaging. NMR Biomed. 2005, 18, 383–389. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, C.; Jia, J.; Zhai, S. Mechanistic Understanding of Toxicity from Nanocatalysts. Int. J. Mol. Sci. 2014, 15, 13967-13992. https://doi.org/10.3390/ijms150813967
Jiang C, Jia J, Zhai S. Mechanistic Understanding of Toxicity from Nanocatalysts. International Journal of Molecular Sciences. 2014; 15(8):13967-13992. https://doi.org/10.3390/ijms150813967
Chicago/Turabian StyleJiang, Cuijuan, Jianbo Jia, and Shumei Zhai. 2014. "Mechanistic Understanding of Toxicity from Nanocatalysts" International Journal of Molecular Sciences 15, no. 8: 13967-13992. https://doi.org/10.3390/ijms150813967
APA StyleJiang, C., Jia, J., & Zhai, S. (2014). Mechanistic Understanding of Toxicity from Nanocatalysts. International Journal of Molecular Sciences, 15(8), 13967-13992. https://doi.org/10.3390/ijms150813967





