Antioxidant Capacities and Total Phenolic Contents Enhancement with Acute Gamma Irradiation in Curcuma alismatifolia (Zingiberaceae) Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolics (TP) and Flavonoids (TF) Content
| Dose | Phenolic Content 1 | Flavonoid Content 2 |
|---|---|---|
| Control | 2.08 ± 0.12 a | 1.61 ± 0.48 a |
| 10 (Gy) | 2.11 ± 1.25 a | 1.88 ± 1.82 a |
| 15 (Gy) | 2.76 ± 0.32 b | 2.09 ± 0.57 b |
| 20 (Gy) | 3.15 ± 1.73 c | 2.87 ± 0.31 c |
2.2. Profiling of Phenolic and Flavonoid Compounds Using HPLC
| Phenolic Contents (µg/g Dry Sample) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dose | Gallic Acid | Salicylic Acid | Caffeic Acid | Catechin | Epicatechin | Cinnamic Acid | Ellagic Acid | Resorcinol |
| Control | ND | 406.2 ± 37.72 d | 125.2 ± 7.663 d | 212.9 ± 15.61 d | 856.4 ± 57.05 d | 1015.4 ± 76.15 d | 182.6 ± 12.12 d | 195.9 ± 16.71 d |
| 10 (Gy) | ND | 595.5 ± 51.43 c | 181.6 ± 9.12 c | 231.2 ± 17.04 c | 795.2 ± 45.32 c | 1033.1 ± 67.18 c | 204.7 ± 16.76 c | 227.5 ± 18.33 c |
| 15 (Gy) | ND | 688.1 ± 56.11 b | 203.7 ± 15.01 b | 255.6 ± 21.12 a,b | 877.1 ± 36.72 b | 1052.7 ± 56.26 b | 235.1 ± 18.27 b | 253.5 ± 21.22 b |
| 20 (Gy) | ND | 785.3 ± 34.25 a | 227.2 ± 13.02 a | 269.1 ± 23.51 a | 928.3 ± 78.94 a | 1081.5 ± 89.35 a | 269.3 ± 21.03 a | 282.1 ± 23.67 a |
| Flavonoid Contents (µg/g Dry Sample) | |||||
|---|---|---|---|---|---|
| Dose | Rutin | Naringin | Apigenin | Quercetin | Myricetin |
| Control | 1032.7 ± 67.05 d | 271.5 ± 17.01 d | ND | 964.1 ± 76.05 d | 166.1 ± 9.51 d |
| 10 (Gy) | 1286.5 ± 89.03 c | 355.1 ± 21.01 c | ND | 1025.8 ± 86.07 c | 225.6 ± 14.42 c |
| 15 (Gy) | 1545.5 ± 111.09 b | 482.9 ± 34.03 b | ND | 1131.3 ± 75.12 b | 282.5 ± 16.02 b |
| 20 (Gy) | 1704.7 ± 123.05 a | 564.1 ± 43.05 a | ND | 1292.4 ± 91.05 a | 351.1 ± 21.02 a |

2.3. Fatty Acid Composition of Irradiated Leaves
| Radiation Doses (Gy) | ||||
|---|---|---|---|---|
| Fatty Acids | Control (0) | 10 (Gy) | 15 (Gy) | 20 (Gy) |
| C12:0 | 1.17 ± 0.06 | 2.02 ± 0.11 | 1.04 ± 0.04 | 1.15 ± 0.05 |
| C14:0 | 18.61 ± 1.02 ab | 21.48 ± 1.18 a | 16.49 ± 0.91 b | 15.84 ± 0.87 b |
| C14:1 | 0.67 ± 0.04 | 0.47 ± 0.03 | 0.41 ± 0.02 | 0.46 ± 0.03 |
| C15:0 | 3.46 ± 0.19 | 3.00 ± 0.16 | 2.79 ± 0.15 | 2.28 ± 0.13 |
| C15:1 | 7.72 ± 0.42 | 7.29 ± 0.40 | 6.25 ± 0.34 | 4.66 ± 0.26 |
| C16:0 | 23.90 ± 1.31 | 23.34 ± 1.28 | 24.76 ± 1.36 | 24.46 ± 1.34 |
| C16:1 | 1.76 ± 0.10 | 2.30 ± 0.13 | 2.09 ± 0.11 | 2.03 ± 0.11 |
| C17:0 | 0.66 ± 0.04 | 0.68 ± 0.04 | 0.70 ± 0.04 | 0.64 ± 0.04 |
| C17:1 | 0.55 ± 0.03 | 0.47 ± 0.03 | 0.50 ± 0.03 | 0.66 ± 0.04 |
| C18:0 | 6.36 ± 0.35 | 5.16 ± 0.28 | 4.46 ± 0.25 | 4.90 ± 0.27 |
| C18:1n-9 | 10.65 ± 0.59 | 10.77 ± 0.59 | 12.20 ± 0.67 | 10.60 ± 0.58 |
| C18:2n-6 | 16.73 ± 0.92 | 14.06 ± 0.77 | 18.06 ± 0.99 | 17.54 ± 0.96 |
| C18:3n-3 | 18.10 ± 0.99 b | 19.29 ± 1.06 b | 20.58 ± 1.13 ab | 25.10 ± 1.38 a |
| 1 Total saturated fatty acid | 54.16 ± 2.98 a | 55.69 ± 3.06 a | 50.24 ± 2.76 b | 49.26 ± 2.71 b |
| 2 Total monounsaturated fatty acid | 19.59 ± 1.08 | 19.00 ± 1.04 | 19.36 ± 1.06 | 16.39 ± 0.90 |
| 3 Total polyunsaturated fatty acid | 34.82 ± 1.91 b | 33.36 ± 1.83 b | 38.64 ± 2.12 ab | 42.65 ± 2.34 a |
| 4 Total n-3 PUFA | 18.10 ± 0.99 b | 19.29 ± 1.06 b | 20.58 ± 1.13 ab | 25.10 ± 1.38 a |
| 5 Total n-6 PUFA | 16.73 ± 0.92 | 14.06 ± 0.77 | 18.06 ± 0.99 | 17.54 ± 0.96 |
2.4. Antioxidant Activity (DPPH, FRAP and ABTS Scavenging)
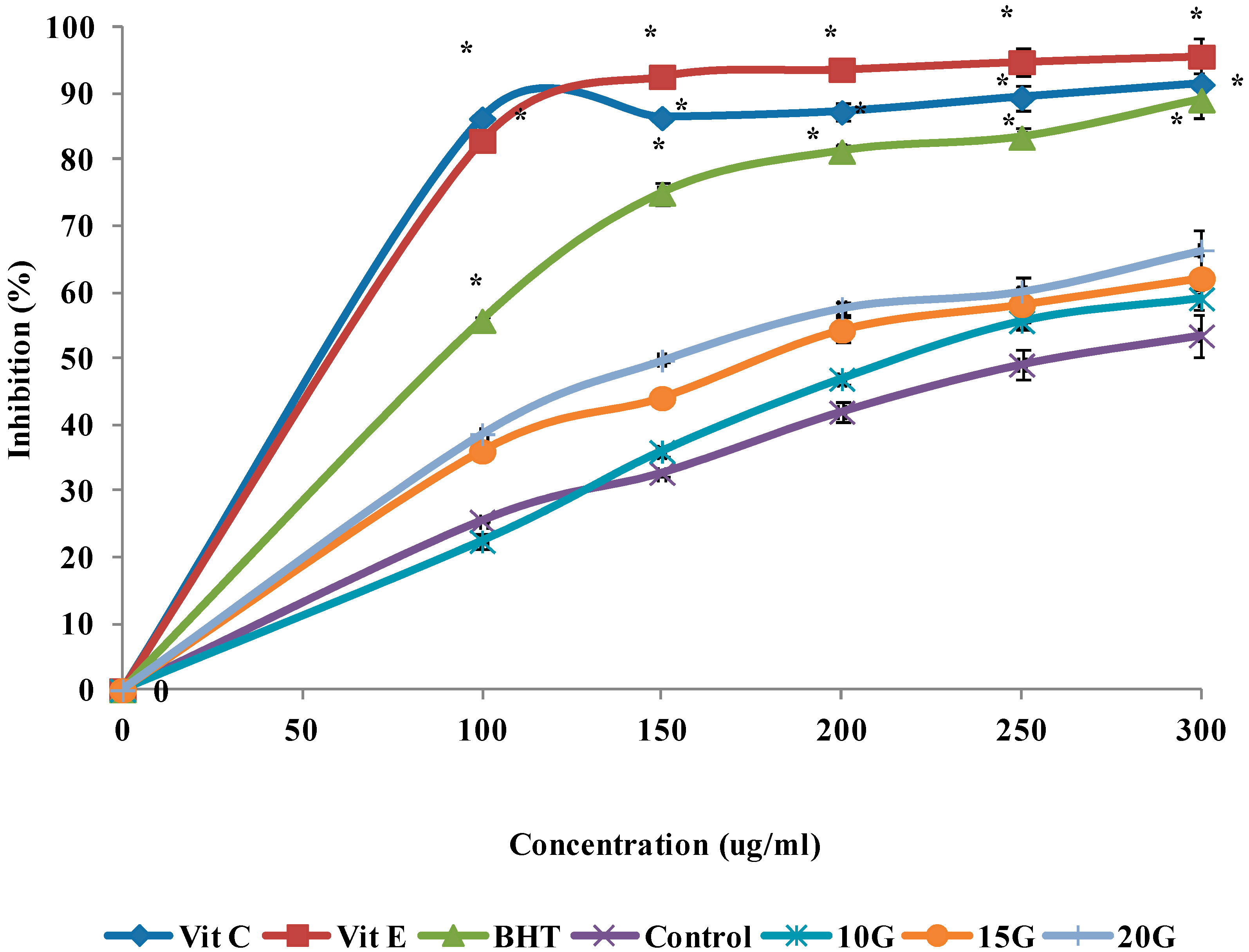
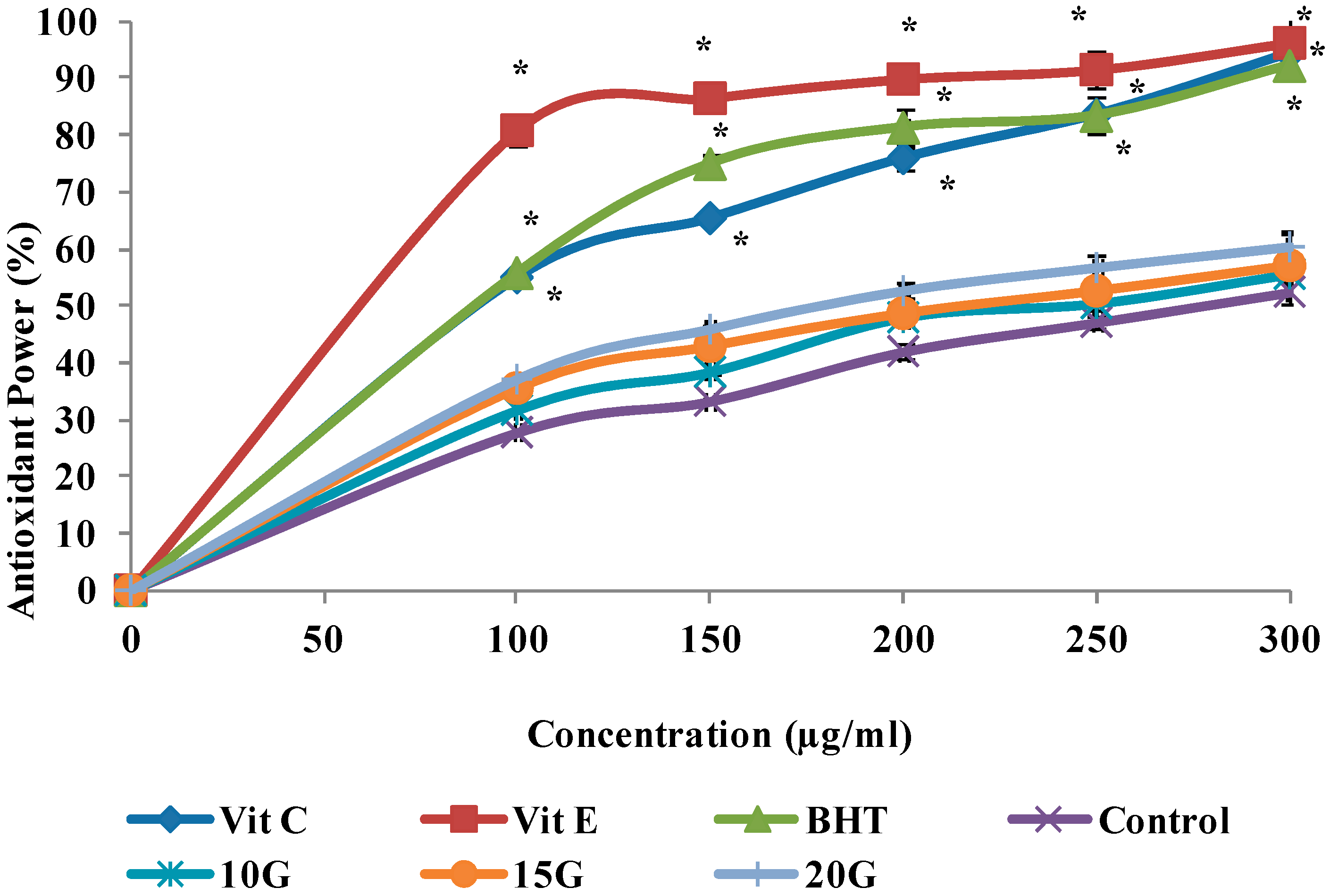
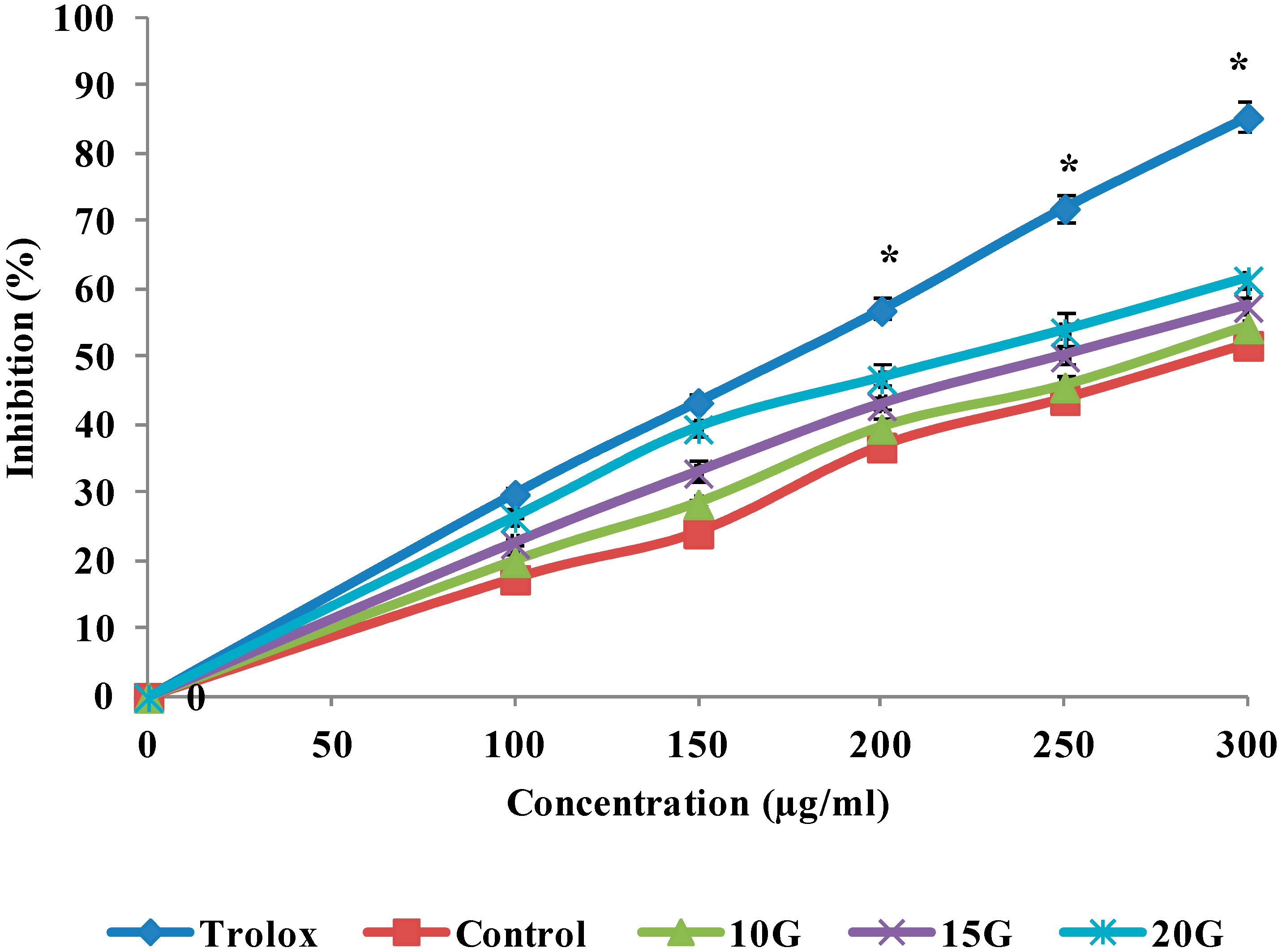
| IC50 (µg/mL) | |||
|---|---|---|---|
| Samples | Free Radical Scavenging Activity | Total Antioxidant Activity | ABTS Scavenging Activity |
| Control | 260.7 ± 1.29 a | 278.1 ± 1.42 a | 288.1 ± 1.35 a |
| 10 (Gy)3 | 218.5 ± 0.72 b | 244.8 ± 1.56 b | 275.8 ± 0.96 b |
| 15 (Gy) | 179.3 ± 1.03 c | 218.4 ± 2.05 c | 248.9 ± 1.12 c |
| 20 (Gy) | 152.6 ± 0.85 d | 180.7 ± 1.66 d | 225.2 ± 1.25 d |
| Vitamin C | 58.1 ± 1.47 f | 90.9 ± 2.11 e | ND |
| Vitamin E | 60.3 ± 3.04 f | 61.88 ± 1.86 f | ND |
| BHT | 89.7 ± 2.43 e | 89.7 ± 1.37 e | ND |
| Trolox | ND | ND | 174.47 ± 012 e |
3. Experimental
3.1. Plant Material
3.2. Planting Media and Preparation of Rhizomes for Acute Gamma Irradiation
3.3. Irradiation Treatment
3.4. Extract Preparation
3.5. Total Phenols and Flavonoids Determination
3.6. Evaluation of Phenolic and Flavonoid Compounds
3.7. Fatty Acid Profiles
3.8. Antioxidant Activity
3.8.1. DPPH Free Radical Scavenging Activity
3.8.2. Ferric Reducing Antioxidant Power (FRAP)
3.8.3. ABTS Radical Cation-Scavenging
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Finley, J.W. Bioactive compounds and designer plant foods: The need for clear guidelines to evaluate potential benefits to human health. Chronica. Hotric. 2005, 45, 6–11. [Google Scholar]
- Karimi, E.; Jaafar, H.Z.E.; Ahmad, S. Antifungal, anti-inflammatory and cytotoxicity activities of three varieties of Labisiapumilabenth: From microwave obtained extracts. BMC Complement. Altern. Med. 2013, 13, 1–10. [Google Scholar]
- Oskoueian, E.; Abdullah, N.; Hendra, R.; Karimi, E. Bioactive compounds, antioxidant, xanthine oxidase inhibitory, tyrosinase inhibitory and anti-inflammatory activities of selected agro-industrial by-products. Int. J. Mol. Sci. 2011, 12, 8610–8625. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Namuli, A.; Abdullah, N.; Sieo, C.; Zuhainis, S.; Oskoueian, E. Phytochemical compounds and antibacterial activity of Jatrophacurcas Linn extracts. J. Med. Plants Res. 2011, 5, 3982–3990. [Google Scholar]
- Hendra, R.; Ahmad, S.; Sukari, A.; Shukor, M.Y.; Oskoueian, E. Flavonoid analyses and antimicrobial activity of various parts of Phaleria macrocarpa (Scheff.) boerl Fruit. Int. J. Mol. Sci. 2011, 12, 3422–3431. [Google Scholar] [CrossRef]
- Argolo, A.C.C.; Sant-Ana, A.E.G.; Pletsch, M.; Coelho, L.C.B.B. Antioxidant activity of leaf extracts from Bauhinia monandra. Bioresour. Technol. 2004, 95, 229–233. [Google Scholar] [CrossRef]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. In vitro antioxidant activities of the methanol extracts of four Helichrysum species from Turkey. Food Chem. 2005, 90, 685–689. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Pusglove, J.W. Tropical Crops: Dicotyledons 1, Dicotyledons 2; Longmans: London, UK, 1968. [Google Scholar]
- Apavatjrut, P.; Anuntalabhochai, S.; Sirirugsa, P.; Alisi, C. Molecular markers in the identification of some early flowering Curcuma L. (Zingiberaceae) species. Ann. Bot. Lond. 1999, 84, 529–534. [Google Scholar] [CrossRef]
- Lewis, M.E. Should we be concerned about herbal remedies. J. Ethnopharmacol. 2001, 75, 141–164. [Google Scholar] [CrossRef]
- Charbaji, T.; Nabulsi, I. Effect of low doses of gamma irradiation on in vitro growth of grapevine. Plant Cell Tissue Organ Cult. 1999, 57, 129–132. [Google Scholar]
- Chakravarty, B.; Sen, S. Enhancement of regeneration potential and variability by γ-irradiationin cultured cells of Scillaindica. Biol. Plant 2001, 44, 189–193. [Google Scholar] [CrossRef]
- Cantos, E.; Garcia-Viguera, C.; Pascal-Teresa, S.D.; Tomas-Barberan, F. Effect of postharvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napolean table grapes. J. Agric. Food Chem. 2000, 48, 4606–4612. [Google Scholar] [CrossRef]
- Variyar, P.S.; Bandyopadhyay, C.; Thomas, P. Effect of c-irradiation on the phenolic acid of some Indian spices. Int. J. Food Sci. Technol. 1998, 33, 533–537. [Google Scholar] [CrossRef]
- Harrison, K.; Were, L.M. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of Almond skin extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- Guttman, A.; Khandurina, J.; Budworth, P.; Xu, W.; Hou, Y.M.; Wang, X. Analysis of combinatorial natural products by HPLC and CE. LC-GC N. Am. 2004, 22, 58–67. [Google Scholar]
- Lewis, N.F.; Madhavesh, D.A.; Qumta, U.S. Role of carotenoid pigments on radio-resistant Micococci. Can. J. Microbiol. 1974, 20, 455–459. [Google Scholar] [CrossRef]
- Work, E. Amino acids of walls of Micrococcus radiodurans. Nature 1964, 201, 1107–1109. [Google Scholar] [CrossRef]
- Lewis, N.F. Studies on the Lipids and Fatty Acid Composition of Micrococcus Radiodurans. M.S. Thesis, Oregon State University, Corvallis, OR, USA, 1968. [Google Scholar]
- Byun, M.W.; Kang, I.J.; Kwon, J.H.; Hayashi, Y.; Mori, T. Physicochemical properties of soybean oil extracted from γ-irradiated soybeans. Radiat. Phys. Chem. 1996, 47, 301–304. [Google Scholar]
- Štajner, D.; Milošević, M.; Popović, B.M. Irradiation effects on phenolic content, lipid and protein oxidation and scavenger ability of soybean seeds. Int. J. Mol. Sci. 2007, 8, 618–627. [Google Scholar] [CrossRef]
- Battino, M.; Ferri, E.; Gattavecchia, E.; Breccia, A.; Genova, M.L.; Littarru, G.P.; Lenaz, G. Mitochondrial respiratory chain features after gamma-irradiation. Free Radic. Res. 1997, 26, 431–438. [Google Scholar] [CrossRef]
- Alikamanoglu, S.; Yaycli, O.; Atak, C.; Rzakoulieva, A. Effect of magnetic field and gamma radiation on Paulowiniatomentosa tissue culture. Biotechnol. Biotechnol. Equip. 2007, 21, 129–134. [Google Scholar] [CrossRef]
- Akter, R.; Hasan, S.R.; Siddiqua, S.A.; Majumder, M.M.; Hossain, M.M.; Alam, M.A.; Ghani, A. Evaluation of analgesic and antioxidant potential of the leaves of Curcuma alismatifolia Gagnep. Stamford J. Pharm. Sci. 2008, 1, 3–9. [Google Scholar]
- Akter, R.; Hasan, S.M.; Hossain, M.M.; Jamila, M.; Chowdhury, S.S.; Mazumder, M.E.H.; Rahman, S. Antidiarrhoeal and antioxidant properties of Curcuma alismatifolia leaves. Aust. J. Basic Appl. Sci. 2010, 4, 450. [Google Scholar]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K.; Lim, K.K.; Lim, T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009, 113, 166–172. [Google Scholar] [CrossRef]
- Casarett, A.P. Radiation Chemistry; Prentice Hall: Englewood Cliffs, NJ, USA, 1968. [Google Scholar]
- Lee, J.W.; Kim, J.K.; Srinivasan, P.; Choi, J.; Kim, J.H.; Han, S.B.; Kim, D.J.; Byun, M.W. Effect of gamma irradiation on microbial analysis, antioxidant activity, sugar content and color of ready-to-use tamarind juice during storage. LWT-Food Sci. Technol. 2009, 42, 101–105. [Google Scholar] [CrossRef]
- Taheri, S.; Abdullah, T.L.; Ahmad, Z.; Abdullah, N.A.P. Effect of acute gamma irradiation on Curcuma alismatifolia varieties and detection of DNA polymorphism through SSR Marker. Biol. Med. Res. Int. 2014, 2014, 1–18. [Google Scholar]
- Quiles, J.L.; Mesa, M.D.; Ramírez-Tortosa, C.L.; Aguilera, C.M.; Battino, M.; Gil, A.; Ramírez-Tortosa, M.C. Curcuma longa extract supplementation reduces oxidative stress and attenuates aortic fatty streak development in rabbits. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1225–1231. [Google Scholar] [CrossRef]
- Bunya-atichart, K.; Saichol, K.; van Doorn, W.G. Postharvest physiology of Curcuma alismatifolia flowers. Postharvest Biol. Technol. 2004, 34, 219–226. [Google Scholar] [CrossRef]
- Crozier, A.; Jensen, E.; Lean, M.E.J.; McDonald, M.S. Quantitative analysis of flavonoids by reversed-phase high-performance liquid chromatography. J. Chromatogr. 1997, 761, 315–321. [Google Scholar] [CrossRef]
- Ismail, H.I.; Chan, K.W.; Mariod, A.A.; Ismail, M. Phenolic content and antioxidant activity of cantaloupe (cucumismelo) methanolic extracts. Food Chem. 2010, 119, 643–647. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1975, 1, 497–509. [Google Scholar]
- Ebrahimi, M.; Rajion, M.A.; Goh, Y.M.; Sazili, A.Q.; Schonewille, J.T. Effect of Linseed Oil Dietary Supplementation on Fatty Acid Composition and Gene Expression in Adipose Tissue of Growing Goats. Biomed. Res. Int. 2013, 2013, 1–11. [Google Scholar]
- Gulcin, I.; Gungor Sat, I.; Beydemir, S.; Elmastas, M.; IrfanKufrevioglu, O. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandulastoechas L.). Food Chem. 2004, 87, 393–400. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Giao, M.S.; Gonzalez-Sanjose, M.L.; Rivero-Perez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenolic content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Taheri, S.; Abdullah, T.L.; Karimi, E.; Oskoueian, E.; Ebrahimi, M. Antioxidant Capacities and Total Phenolic Contents Enhancement with Acute Gamma Irradiation in Curcuma alismatifolia (Zingiberaceae) Leaves. Int. J. Mol. Sci. 2014, 15, 13077-13090. https://doi.org/10.3390/ijms150713077
Taheri S, Abdullah TL, Karimi E, Oskoueian E, Ebrahimi M. Antioxidant Capacities and Total Phenolic Contents Enhancement with Acute Gamma Irradiation in Curcuma alismatifolia (Zingiberaceae) Leaves. International Journal of Molecular Sciences. 2014; 15(7):13077-13090. https://doi.org/10.3390/ijms150713077
Chicago/Turabian StyleTaheri, Sima, Thohirah Lee Abdullah, Ehsan Karimi, Ehsan Oskoueian, and Mahdi Ebrahimi. 2014. "Antioxidant Capacities and Total Phenolic Contents Enhancement with Acute Gamma Irradiation in Curcuma alismatifolia (Zingiberaceae) Leaves" International Journal of Molecular Sciences 15, no. 7: 13077-13090. https://doi.org/10.3390/ijms150713077
APA StyleTaheri, S., Abdullah, T. L., Karimi, E., Oskoueian, E., & Ebrahimi, M. (2014). Antioxidant Capacities and Total Phenolic Contents Enhancement with Acute Gamma Irradiation in Curcuma alismatifolia (Zingiberaceae) Leaves. International Journal of Molecular Sciences, 15(7), 13077-13090. https://doi.org/10.3390/ijms150713077




