Processed Aloe vera Gel Ameliorates Cyclophosphamide-Induced Immunotoxicity
Abstract
:1. Introduction
2. Results
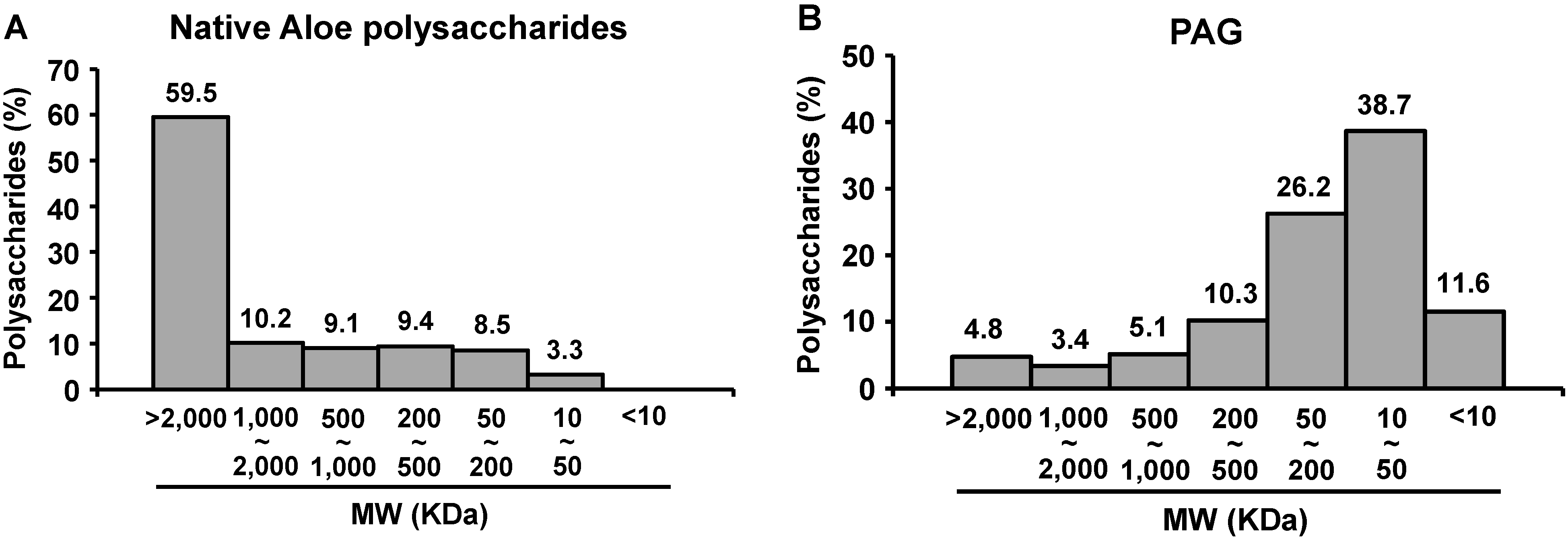
2.1. Molecular Size Distribution of PAG Polysaccharides
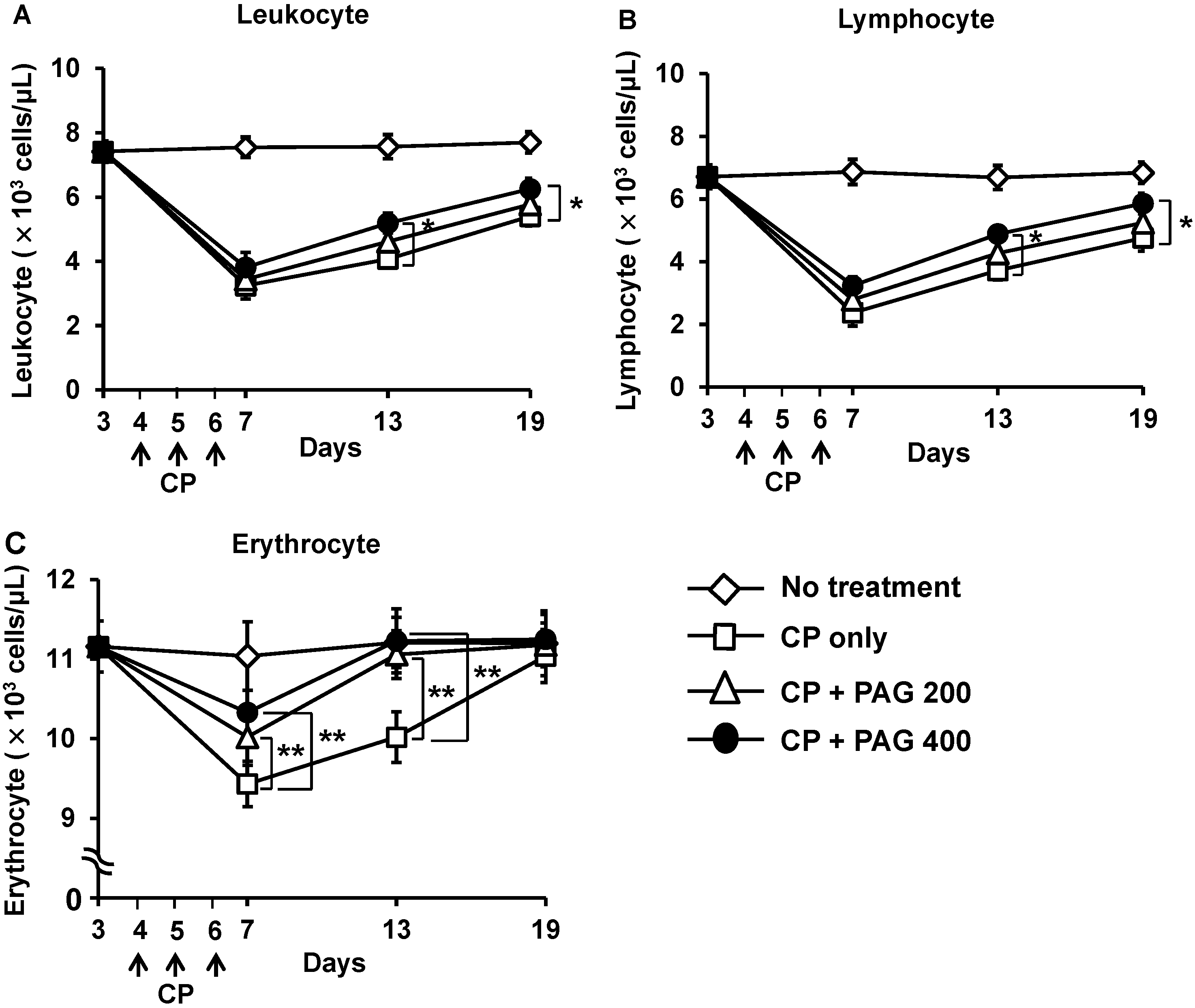
2.2. Effect of Orally Administered PAG on CP-Induced Lymphopenia and Erythropenia
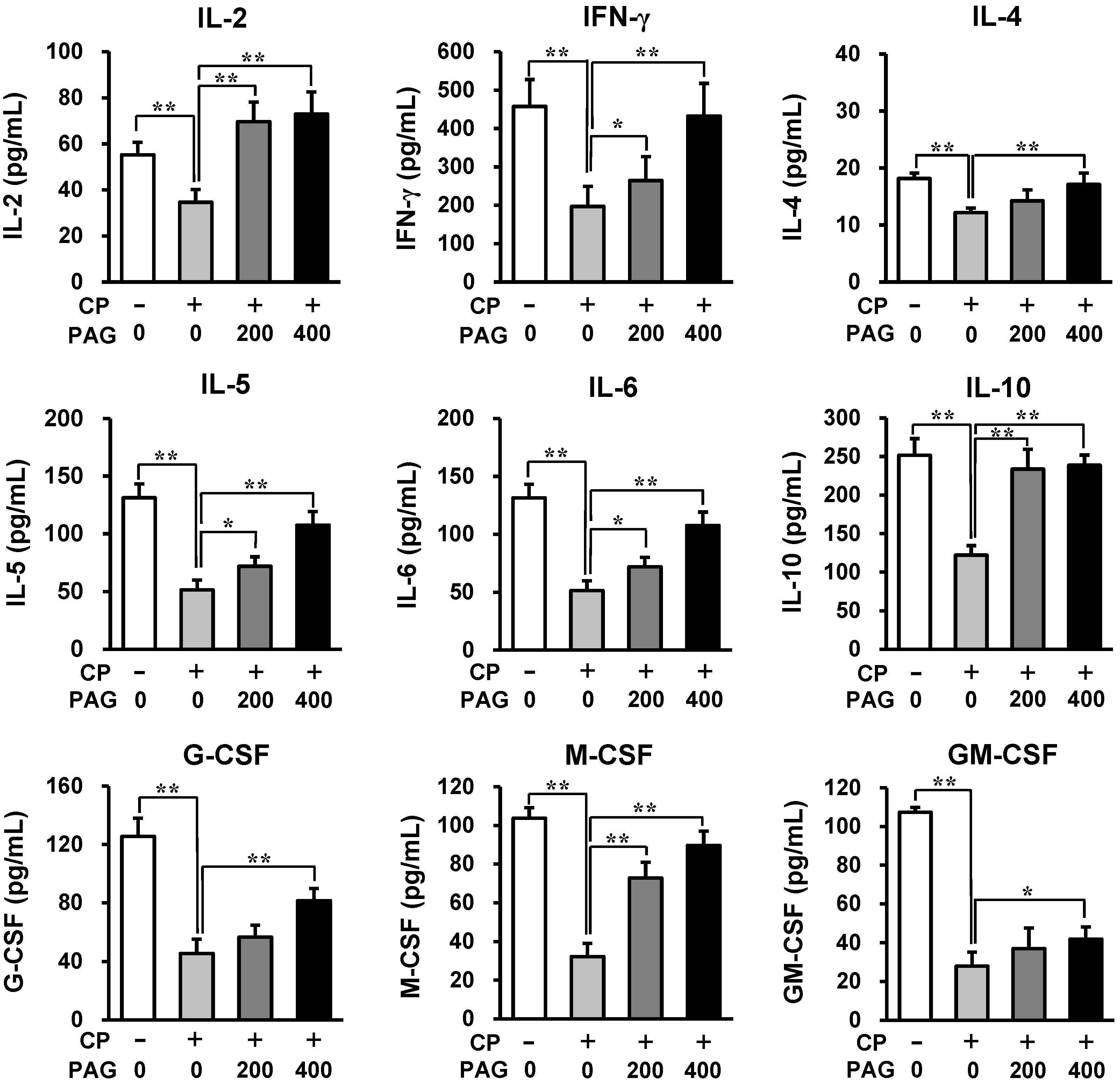
2.3. Oral Administration of PAG Preserves the Cytokine-Producing Capability of Peyer’s Patch Cells in CP-Treated Mice
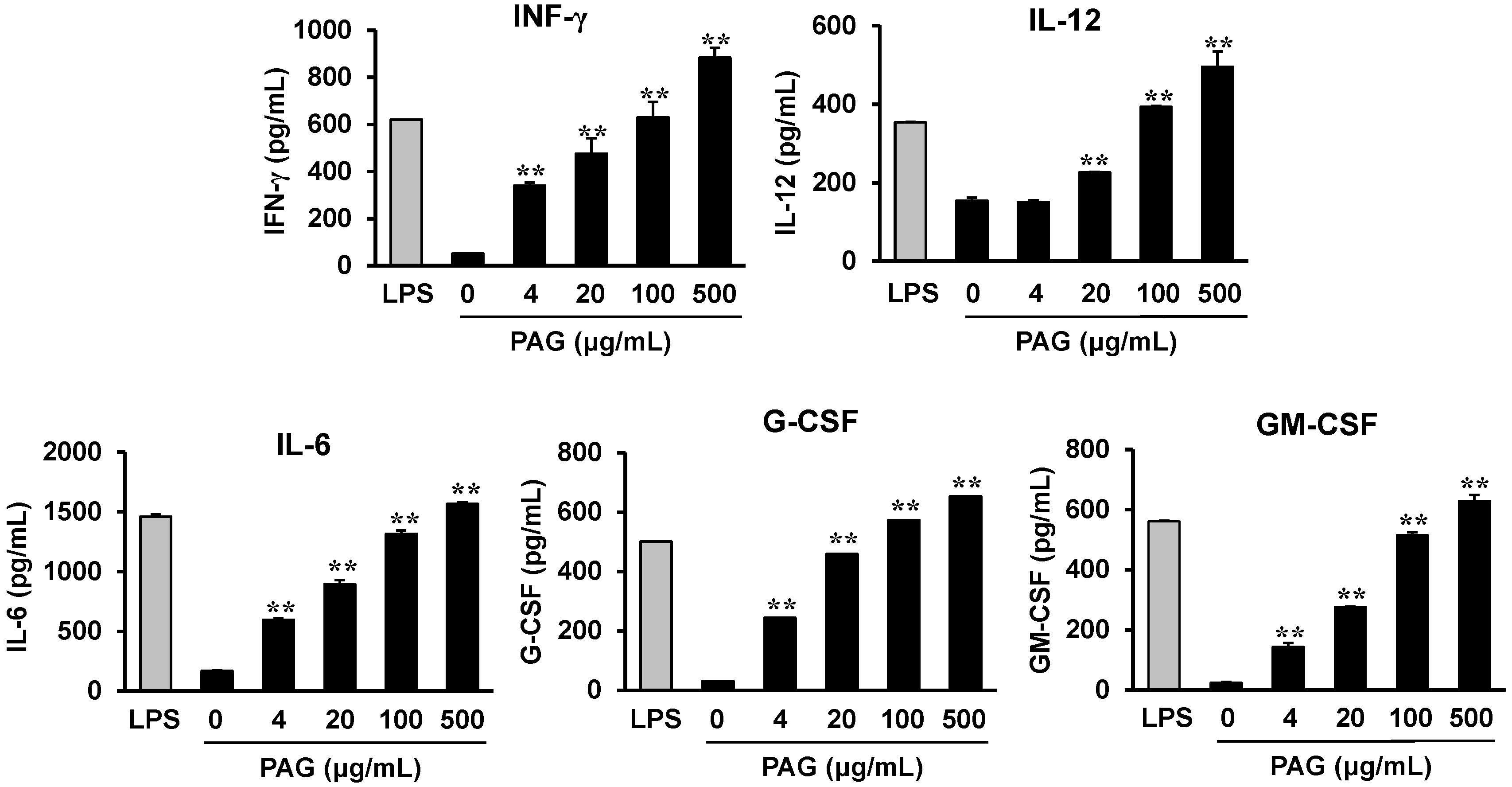
2.4. PAG Polysaccharides Activate Peyer’s Patch Cells to Produce Hematopoietic Cytokines
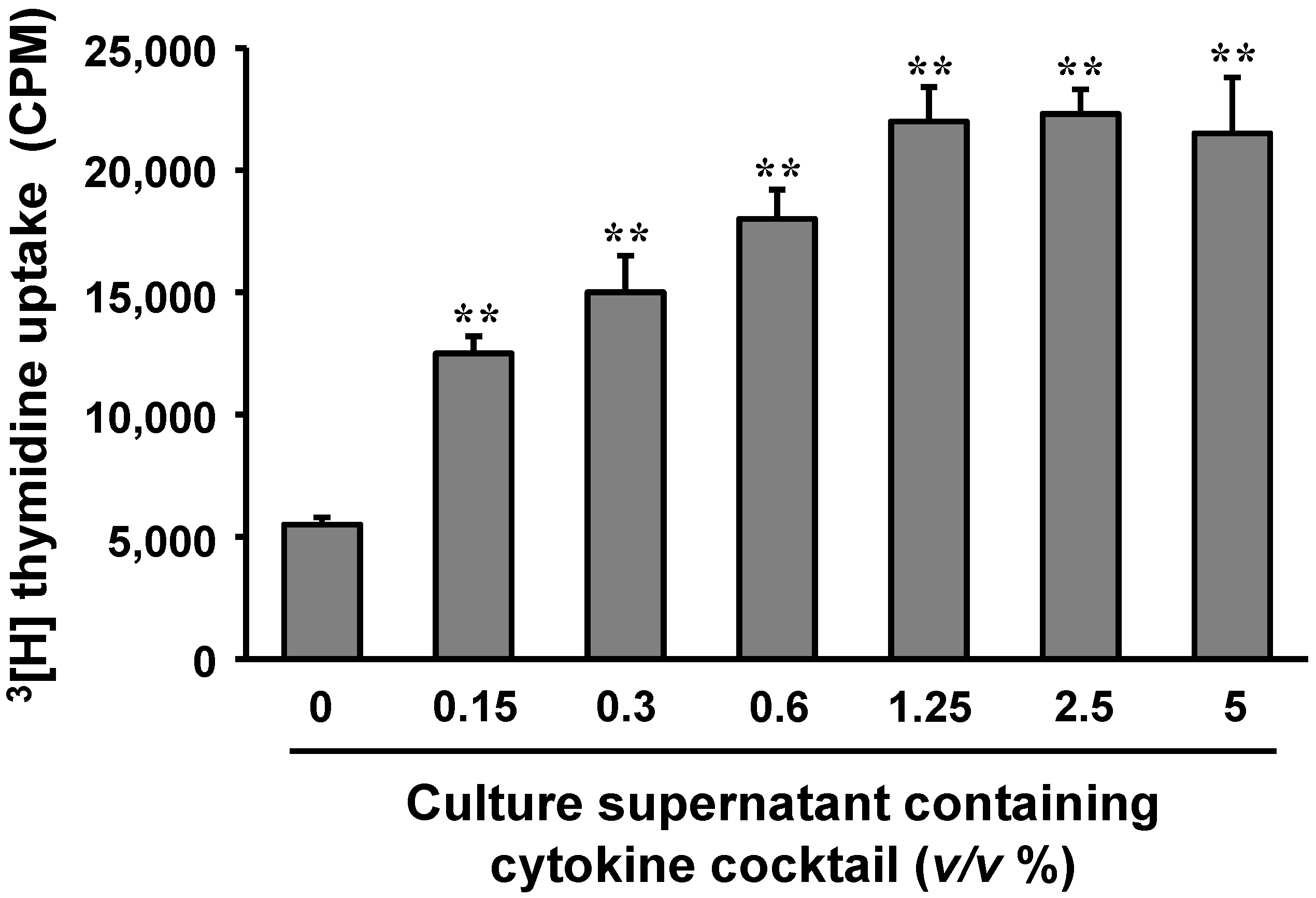
2.5. Cytokines Produced by Polysaccharide-Stimulated Peyer’s Patch Cells Promote Bone Marrow Cell Proliferation
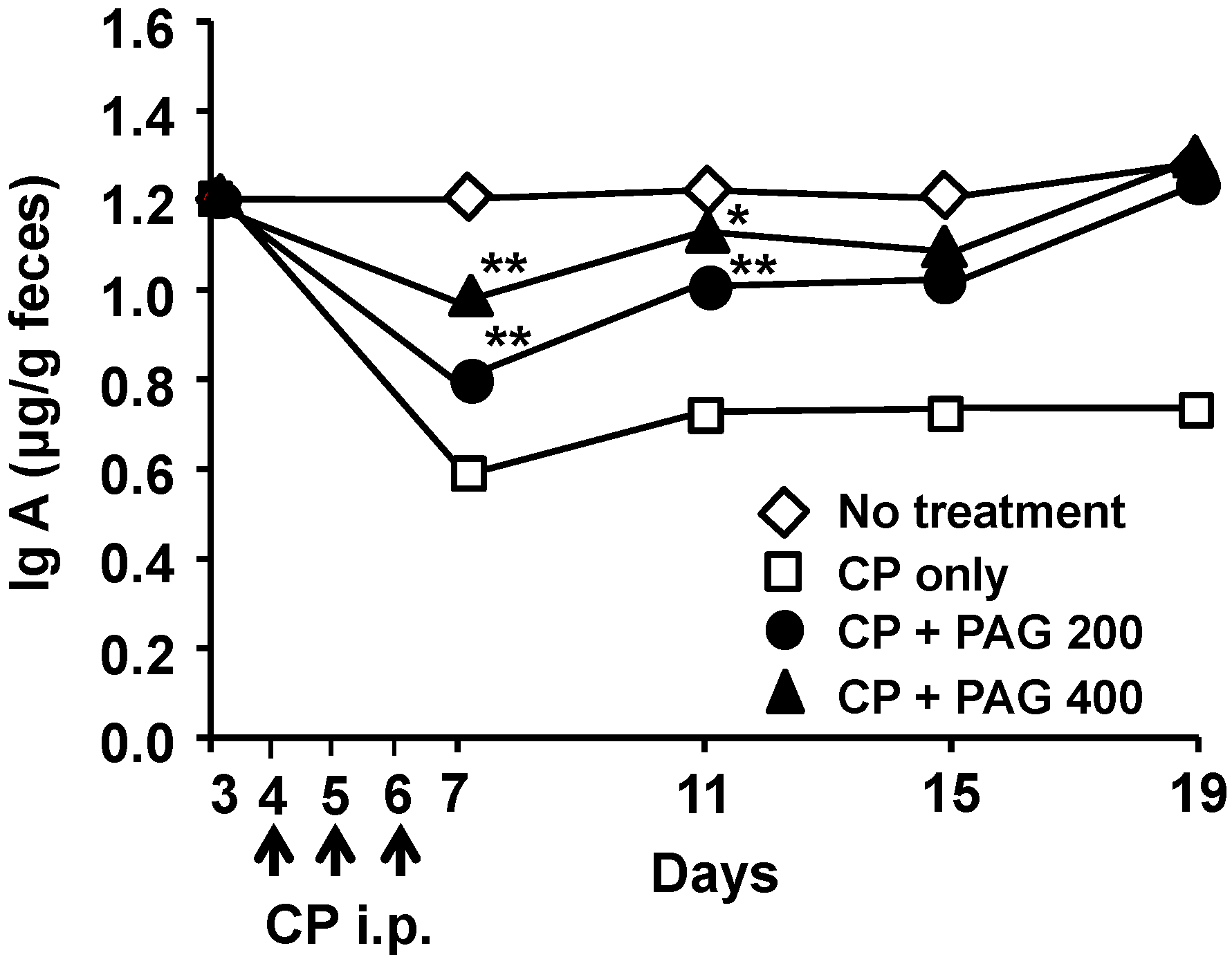
2.6. Oral Administration of PAG Restores CP-Suppressed Immunoglobulin A (IgA) Secretion in the Intestine
3. Discussion
4. Experimental Section
4.1. Preparation and Molecular Weight Determination of PAG
4.2. Animals, Immunosuppression, and Treatment Protocol
4.3. Blood Analysis
4.4. Peyer’s Patch Cell Preparation and Culture
4.5. Cytokine Production and Measurement
4.6. Measurement of IgA Content in the Feces
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Manna, S.; McAnalley, B.H. Determination of the position of the O-acetyl group in a beta-(1–4)-mannan (acemannan) from Aloe barbardensis Miller. Carbohydr. Res. 1993, 241, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Yagi, A.; Makino, K.; Nishioka, I.; Kuchino, Y. Aloe mannan, ploysaccharide, from Aloe arborescens var. natalensis. Planta Med. 1977, 31, 17–20. [Google Scholar] [PubMed]
- Gowda, D.C.; Neelisiddaiah, B.; Anjaneyalu, Y.V. Structural studies of polysaccharides from Aloe vera. Carbohydr. Res. 1979, 72, 201–205. [Google Scholar] [CrossRef]
- Peng, S.Y.; Norman, J.; Curtin, G.; Corrier, D.; McDaniel, H.R.; Busbee, D. Decreased mortality of Norman murine sarcoma in mice treated with the immunomodulator, Acemannan. Mol. Biother. 1991, 3, 79–87. [Google Scholar] [PubMed]
- Harris, C.; Pierce, K.; King, G.; Yates, K.M.; Hall, J.; Tizard, I. Efficacy of acemannan in treatment of canine and feline spontaneous neoplasms. Mol. Biother. 1991, 3, 207–213. [Google Scholar] [PubMed]
- King, G.K.; Yates, K.M.; Greenlee, P.G.; Pierce, K.R.; Ford, C.R.; McAnalley, B.H.; Tizard, I.R. The effect of acemannan immunostimulant in combination with surgery and radiation therapy on spontaneous canine and feline fibrosarcomas. J. Am. Anim. Hosp. Assoc. 1995, 31, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Oh, S.T.; Song, S.; Kim, M.R.; Kim, D.S.; Woo, S.S.; Jo, T.H.; Park, Y.I.; Lee, C.K. Identification of optimal molecular size of modified Aloe polysaccharides with maximum immunomodulatory activity. Int. Immunopharmacol. 2005, 5, 271–279. [Google Scholar] [PubMed]
- Sheets, M.A.; Unger, B.A.; Giggleman, G.F., Jr.; Tizard, I.R. Studies of the effect of acemannan on retrovirus infections: clinical stabilization of feline leukemia virus-infected cats. Mol. Biother. 1991, 3, 41–45. [Google Scholar]
- Yates, K.M.; Rosenberg, L.J.; Harris, C.K.; Bronstad, D.C.; King, G.K.; Biehle, G.A.; Walker, B.; Ford, C.R.; Hall, J.E.; Tizard, I.R. Pilot study of the effect of acemannan in cats infected with feline immunodeficiency virus. Vet. Immunol. Immunopathol. 1992, 35, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Saka, W.A.; Akhigbe, R.E.; Ishola, O.S.; Ashamu, E.A.; Olayemi, O.T.; Adeleke, G.E. Hepatotherapeutic effect of Aloe vera in alcohol-induced hepatic damage. Pak. J. Biol. Sci. 2011, 14, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tizard, I.R. Activation of a mouse macrophage cell line by acemannan: The major carbohydrate fraction from Aloe vera gel. Immunopharmacology 1996, 35, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, L.; Kemp, M.C.; Tizard, I.R. Acemannan, a beta-(1,4)-acetylated mannan, induces nitric oxide production in macrophage cell line RAW 264.7. Mol. Pharmacol. 1996, 50, 878–884. [Google Scholar] [PubMed]
- Djeraba, A.; Quere, P. In vivo macrophage activation in chickens with Acemannan, a complex carbohydrate extracted from Aloe vera. Int. J. Immunopharmacol. 2000, 22, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Stuart, R.W.; Lefkowitz, D.L.; Lincoln, J.A.; Howard, K.; Gelderman, M.P.; Lefkowitz, S.S. Upregulation of phagocytosis and candidicidal activity of macrophages exposed to the immunostimulant acemannan. Int. J. Immunopharmacol. 1997, 19, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Lee, Y.R.; Lee, Y.H.; Lee, M.K.; Park, Y.I.; Lee, S.; Kim, K.; Lee, C.K. In vivo evidence of the immunomodulatory activity of orally administered Aloe vera gel. Arch. Pharm. Res. 2010, 33, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lee, M.K.; Yun, Y.P.; Kim, Y.; Kim, J.S.; Kim, Y.S.; Kim, K.; Han, S.S.; Lee, C.K. Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int. Immunopharmacol. 2001, 1, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Young, S.D.; Whissell, M.; Noble, J.C.; Cano, P.O.; Lopez, P.G.; Germond, C.J. Phase II clinical trial results involving treatment with low-dose daily oral cyclophosphamide, weekly vinblastine, and rofecoxib in patients with advanced solid tumors. Clin. Cancer Res. 2006, 12, 3092–3098. [Google Scholar] [CrossRef] [PubMed]
- Slavin, R.E.; Millan, J.C.; Mullins, G.M. Pathology of high dose intermittent cyclophosphamide therapy. Hum. Pathol. 1975, 6, 693–709. [Google Scholar] [CrossRef]
- Fishman, P.; Bar-Yehuda, S.; Barer, F.; Madi, L.; Multani, A.S.; Pathak, S. The A3 adenosine receptor as a new target for cancer therapy and chemoprotection. Exp. Cell Res. 2001, 269, 230–236. [Google Scholar] [CrossRef]
- Lohrmann, H.P. The problem of permanent bone marrow damage after cytotoxic drug treatment. Oncology 1984, 41, 180–184. [Google Scholar] [CrossRef]
- Wang, M.; Meng, X.Y.; Yang, R.L.; Qin, T.; Wang, X.Y.; Zhang, K.Y.; Fei, C.Z.; Li, Y.; Hu, Y.; Xue, F.Q. Cordyceps militaris polysaccharides can enhance the immunity and antioxidation activity in immunosuppressed mice. Carbohydr. Polym. 2012, 89, 461–466. [Google Scholar] [CrossRef]
- Chen, X.; Nie, W.; Fan, S.; Zhang, J.; Wang, Y.; Lu, J.; Jin, L. A polysaccharide from Sargassum fusiforme protects against immunosuppression in cyclophosphamide-treated mice. Carbohydr. Polym. 2012, 90, 1114–1119. [Google Scholar] [CrossRef]
- Mei, Y.X.; Chen, H.X.; Zhang, J.; Zhang, X.D.; Liang, Y.X. Protective effect of chitooligosaccharides against cyclophosphamide-induced immunosuppression in mice. Int. J. Biol. Macromol. 2013, 62, 330–335. [Google Scholar] [CrossRef]
- Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003, 3, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Frey, A.; Kraehenbuhl, J.P. Epithelial M cells: Gateways for mucosal infection and immunization. Cell 1996, 86, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Kelsall, B. Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages. Mucosal. Immunol. 2008, 1, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Lee, Y.-H.; Kim, K.-H.; Im, S.-A.; Lee, C.-K. Induction of potent antigen-specific cytotoxic T cell response by PLGA-nanoparticles containing antigen and TLR agonist. Immune Netw. 2013, 13, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, T.T.; Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 2005, 307, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Kobie, J.J.; Lee, F.E.; Quataert, S.A. T helper cytokine patterns: Defined subsets, random expression, and external modulation. Immunol. Res. 2009, 45, 173–184. [Google Scholar] [CrossRef]
- Jansen, J.H.; Kluin-Nelemans, J.C.; Van, D.J.; Wientjens, G.J.; Willemze, R.; Fibbe, W.E. Interleukin 6 is a permissive factor for monocytic colony formation by human hematopoietic progenitor cells. J. Exp. Med. 1992, 175, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Kim, J.K.; Kim, Y.; Lee, M.K.; Kim, K.; Kang, J.K.; Hofmeister, R.; Durum, S.K.; Han, S.S. Generation of macrophages from early T progenitors in vitro in vitro. J. Immunol. 2001, 166, 5964–5969. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.; Zhang, H.H.; Matthews, V.; Weinstock, J.; Nice, E.C.; Ernst, M.; Rose-John, S.; Burgess, A.W. IL6/sIL6R complex contributes to emergency granulopoietic responses in G-CSF- and GM-CSF-deficient mice. Blood 2008, 111, 3978–3985. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Wei, J.J.; Yuan, Y.; Sun, R.; Li, D.; Luo, J.; Liao, S.J.; Zhou, Y.H.; Shu, Y.; Wang, Q.; et al. IL-6 cooperates with G-CSF to induce protumor function of neutrophils in bone marrow by enhancing STAT3 activation. J. Immunol. 2013, 190, 5882–5893. [Google Scholar] [CrossRef] [PubMed]
- Gee, K.; Guzzo, C.; Che Mat, N.F.; Ma, W.; Kumar, A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm. Allergy Drug Targets 2009, 8, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Santaolalla, R.; Abreu, M.T. Innate immunity in the small intestine. Curr. Opin. Gastroenterol. 2012, 28, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, H.; Kwon, J.; Lee, S.; Kong, H.; Im, S.A.; Lee, Y.H.; Lee, Y.R.; Oh, S.T.; Jo, T.H.; et al. Hypoglycemic and hypolipidemic effects of processed Aloe vera gel in a mouse model of non-insulin-dependent diabetes mellitus. Phytomedicine 2009, 16, 856–863. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, S.-A.; Kim, K.-H.; Kim, H.-S.; Lee, K.-H.; Shin, E.; Do, S.-G.; Jo, T.H.; Park, Y.I.; Lee, C.-K. Processed Aloe vera Gel Ameliorates Cyclophosphamide-Induced Immunotoxicity. Int. J. Mol. Sci. 2014, 15, 19342-19354. https://doi.org/10.3390/ijms151119342
Im S-A, Kim K-H, Kim H-S, Lee K-H, Shin E, Do S-G, Jo TH, Park YI, Lee C-K. Processed Aloe vera Gel Ameliorates Cyclophosphamide-Induced Immunotoxicity. International Journal of Molecular Sciences. 2014; 15(11):19342-19354. https://doi.org/10.3390/ijms151119342
Chicago/Turabian StyleIm, Sun-A, Ki-Hyang Kim, Hee-Suk Kim, Ki-Hwa Lee, Eunju Shin, Seon-Gil Do, Tae Hyung Jo, Young In Park, and Chong-Kil Lee. 2014. "Processed Aloe vera Gel Ameliorates Cyclophosphamide-Induced Immunotoxicity" International Journal of Molecular Sciences 15, no. 11: 19342-19354. https://doi.org/10.3390/ijms151119342
APA StyleIm, S.-A., Kim, K.-H., Kim, H.-S., Lee, K.-H., Shin, E., Do, S.-G., Jo, T. H., Park, Y. I., & Lee, C.-K. (2014). Processed Aloe vera Gel Ameliorates Cyclophosphamide-Induced Immunotoxicity. International Journal of Molecular Sciences, 15(11), 19342-19354. https://doi.org/10.3390/ijms151119342



