G Protein-Coupled Receptors: What a Difference a ‘Partner’ Makes
Abstract
:1. Introduction
2. Interacting Proteins that Modulate Cell-Surface Localization of GPCRs
2.1. ninaA and RanBP2: Modulators of Opsin Receptors
2.2. RTPs and REEPs: Traffic of the Odorant Receptors
2.3. Protein S100-A10 and Its Role in Depression
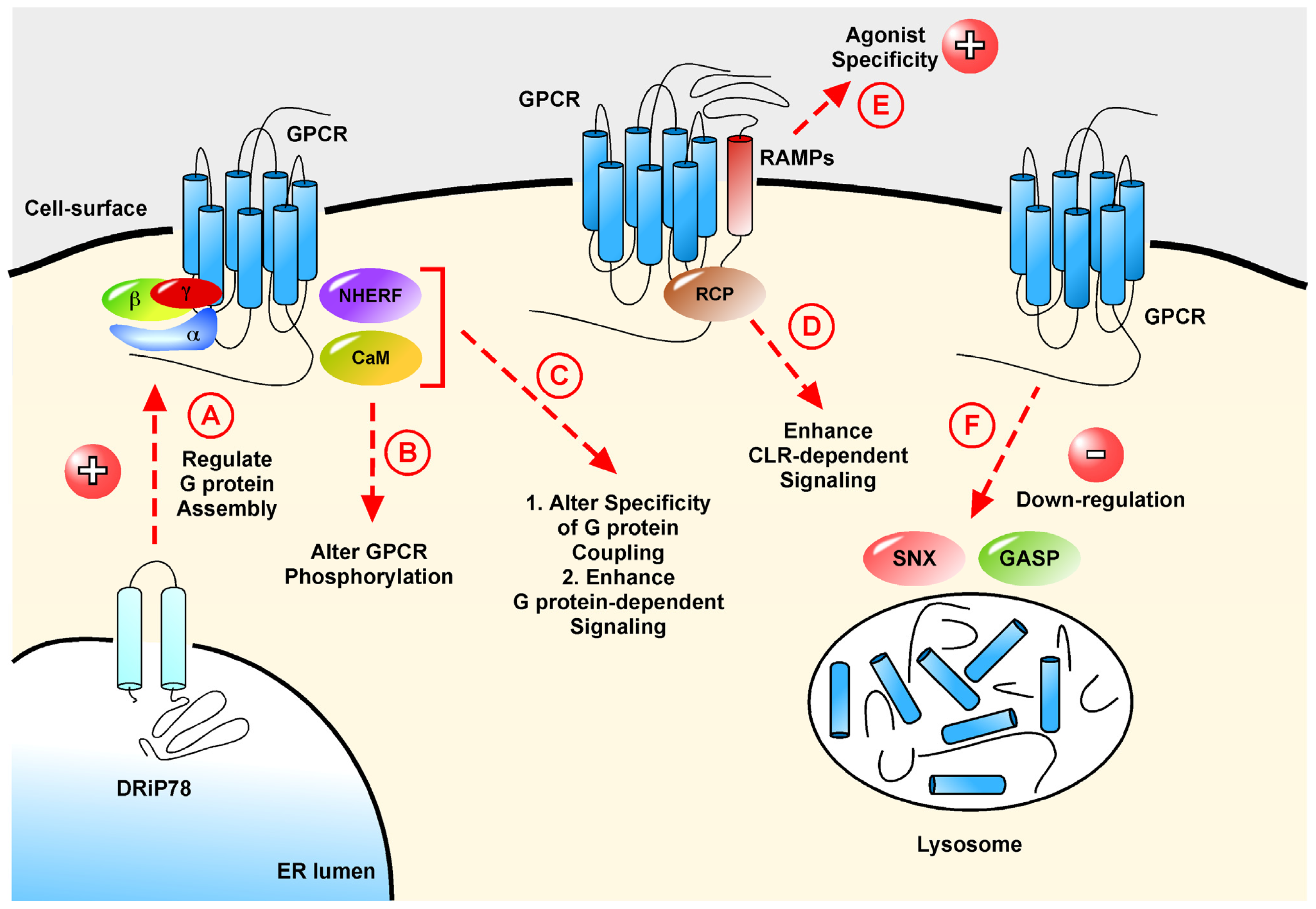
2.4. GASPs and SNXs: Interacting Proteins in GPCR Down-Regulation
3. Interacting Proteins that Modulate Cell-Surface Localization and Signaling of GPCRs
3.1. RAMPs, Co-Receptors of the Calcitonin Receptor Family
3.2. RAMPs, Beyond the Calcitonin Receptor Family
3.3. MRAPs and Their Vital Role in Stress
3.4. DRiP78, a Regulator of the G Protein•GPCR Complex
3.5. The PDZ-Domain Containing Family of Proteins
4. Interacting Proteins That Modulate GPCR Signaling
4.1. RCP and CLR: Proof Three Is not a Crowd
4.2. Calmodulin
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine |
| 5-HTR | 5-HT receptor |
| δ, κ | μ receptors |
| δ, κ | μ opioid receptors |
| ACTH | adrenal corticotrophic hormone |
| ADM | adrenomedullin |
| AM receptor | adrenomedullin receptor |
| AT1 receptor | angiotensin II receptor type 1 |
| CaM | calmodulin |
| cAMP | cyclic AMP |
| CGRP | calcitonin gene-related peptide |
| CLR | calcitonin receptor-like receptor |
| CRF1 receptor | corticotropin releasing factor receptor 1 |
| CRF | corticotropin releasing factor |
| CT | calcitonin |
| CTR | calcitonin receptor |
| DLG1 | disks large homolog 1 |
| D receptor | dopamine receptor |
| DRiP78 | dopamine-receptor-interacting protein of 78 kDa |
| ER | endoplasmic reticulum |
| ErbB1 | epidermal growth factor receptor |
| FGD | familial glucocorticoid deficiency |
| GASP | GPCR-associated sorting protein |
| GPCR | G protein-coupled receptor |
| GPK | GPCR kinase |
| HSP | heat shock protein |
| IAPP | islet amyloid polypeptide |
| MCR | melanocortin receptor |
| MRAP | melanocortin 2 receptor accessory protein |
| MSH | melanocyte-stimulating hormone |
| mGlu receptor | metabotropic glutamate receptor |
| NHERF | Na+/H+ exchanger regulatory factor |
| ninaA | neither inactivated nor after potential A |
| ODR-4 | odorant protein 4 |
| OR | odorant receptor |
| PAR | proteinase-activated receptor |
| PDZ | postsynaptic density protein 95, drosophila disc and zonula occludens protein 1 |
| PKC | protein kinase C |
| PLC | phospholipase C |
| PTH | parathyroid hormone |
| RAMP | receptor activity-modifying protein |
| RanBP2 | Ran binding protein 2 |
| RCP | receptor component protein |
| REEP | receptor expression enhancing protein |
| Rh1 | rhodopsin 1 |
| RTP | receptor transporting protein |
| SNX | sorting nexin |
| VIP | vasoactive intestinal peptide |
| V2 receptor | vasopressin 2 receptor |
| VPAC receptor | VIP and pituitary adenylate cyclase-activating polypeptide receptor |
| Yip | Ypt-interacting protein |
References
- Fredriksson, R.; Lagerstrom, M.C.; Lundin, L.G.; Schioth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol 2003, 63, 1256–1272. [Google Scholar]
- Kristiansen, K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: Molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol. Ther 2004, 103, 21–80. [Google Scholar]
- Ellgaard, L.; Molinari, M.; Helenius, A. Setting the standards: Quality control in the secretory pathway. Science 1999, 286, 1882–1888. [Google Scholar]
- Petaja-Repo, U.E.; Hogue, M.; Laperriere, A.; Bhalla, S.; Walker, P.; Bouvier, M. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J. Biol. Chem 2001, 276, 4416–4423. [Google Scholar]
- Cook, L.B.; Zhu, C.C.; Hinkle, P.M. Thyrotropin-releasing hormone receptor processing: Role of ubiquitination and proteasomal degradation. Mol. Endocrinol 2003, 17, 1777–1791. [Google Scholar]
- Ellis, J. Proteins as molecular chaperones. Nature 1987, 328, 378–379. [Google Scholar]
- Bukau, B.; Horwich, A.L. The Hsp70 and Hsp60 chaperone machines. Cell 1998, 92, 351–366. [Google Scholar]
- Craig, E.A. The heat shock response. CRC Crit. Rev. Biochem 1985, 18, 239–280. [Google Scholar]
- Vos, M.J.; Hageman, J.; Carra, S.; Kampinga, H.H. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry 2008, 47, 7001–7011. [Google Scholar]
- Kelley, W.L. The J-domain family and the recruitment of chaperone power. Trends Biochem. Sci 1998, 23, 222–227. [Google Scholar]
- Morello, J.P.; Salahpour, A.; Laperriere, A.; Bernier, V.; Arthus, M.F.; Lonergan, M.; Petäjä-Repo, U.; Angers, S.; Morin, D.; Bichet, D.G.; et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J. Clin. Invest 2000, 105, 887–895. [Google Scholar]
- Noorwez, S.M.; Kuksa, V.; Imanishi, Y.; Zhu, L.; Filipek, S.; Palczewski, K.; Kaushal, S. Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. J. Biol. Chem 2003, 278, 14442–14450. [Google Scholar]
- Cabrera-Vera, T.M.; Vanhauwe, J.; Thomas, T.O.; Medkova, M.; Preininger, A.; Mazzoni, M.R.; Hamm, H.E. Insights into G protein structure, function, and regulation. Endocr. Rev 2003, 24, 765–781. [Google Scholar]
- Kjeldgaard, M.; Nyborg, J.; Clark, B.F. The GTP binding motif: Variations on a theme. FASEB. J 1996, 10, 1347–1368. [Google Scholar]
- Hamm, H.E. The many faces of G protein signaling. J. Biol. Chem 1998, 273, 669–672. [Google Scholar]
- Claing, A.; Laporte, S.A.; Caron, M.G.; Lefkowitz, R.J. Endocytosis of G protein-coupled receptors: Roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog. Neurobiol 2002, 66, 61–79. [Google Scholar]
- Ferguson, S.S. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol. Rev 2001, 53, 1–24. [Google Scholar]
- Benovic, J.L.; Strasser, R.H.; Caron, M.G.; Lefkowitz, R.J. Beta-adrenergic receptor kinase: Identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc. Natl. Acad. Sci. USA 1986, 83, 2797–2801. [Google Scholar]
- Strasser, R.H.; Benovic, J.L.; Caron, M.G.; Lefkowitz, R.J. Beta-agonist- and prostaglandin E1-induced translocation of the beta-adrenergic receptor kinase: Evidence that the kinase may act on multiple adenylate cyclase-coupled receptors. Proc. Natl. Acad. Sci. USA 1986, 83, 6362–6366. [Google Scholar]
- Premont, R.T.; Gainetdinov, R.R. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu. Rev. Physiol 2007, 69, 511–534. [Google Scholar]
- Attramadal, H.; Arriza, J.L.; Aoki, C.; Dawson, T.M.; Codina, J.; Kwatra, M.M.; Snyder, S.H.; Caron, M.G.; Lefkowitz, R.J. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J. Biol. Chem 1992, 267, 17882–17890. [Google Scholar]
- Lohse, M.J.; Benovic, J.L.; Codina, J.; Caron, M.G.; Lefkowitz, R.J. Beta-Arrestin: A protein that regulates beta-adrenergic receptor function. Science 1990, 248, 1547–1550. [Google Scholar]
- Evron, T.; Daigle, T.L.; Caron, M.G. GRK2: Multiple roles beyond G protein-coupled receptor desensitization. Trends Pharmacol. Sci 2012, 33, 154–164. [Google Scholar]
- Murphy, J.E.; Padilla, B.E.; Hasdemir, B.; Cottrell, G.S.; Bunnett, N.W. Endosomes: A legitimate platform for the signaling train. Proc. Natl. Acad. Sci. USA 2009, 106, 17615–17622. [Google Scholar]
- Shenoy, S.K.; Lefkowitz, R.J. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol. Sci 2011, 32, 521–533. [Google Scholar]
- Pálfy, M.; Reményi, A.; Korcsmáros, T. Endosomal crosstalk: Meeting points for signaling pathways. Trends Cell Biol 2012, 22, 447–456. [Google Scholar]
- Walther, C.; Ferguson, S.S.G. Arrestins: Role in the desensitization, sequestration, and vesicular trafficking of G protein-coupled receptors. Prog. Mol. Biol. Transl. Sci 2013, 118, 93–113. [Google Scholar]
- Marchese, A.A.; Trejo, J.J. Ubiquitin-dependent regulation of G protein-coupled receptor trafficking and signaling. Cell Signal 2013, 25, 707–716. [Google Scholar]
- Stamnes, M.A.; Shieh, B.H.; Chuman, L.; Harris, G.L.; Zuker, C.S. The cyclophilin homolog ninaA is a tissue-specific integral membrane protein required for the proper synthesis of a subset of Drosophila rhodopsins. Cell 1991, 65, 219–227. [Google Scholar]
- Shieh, B.H.; Stamnes, M.A.; Seavello, S.; Harris, G.L.; Zuker, C.S. The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature 1989, 338, 67–70. [Google Scholar]
- Colley, N.J.; Baker, E.K.; Stamnes, M.A.; Zuker, C.S. The cyclophilin homolog ninaA is required in the secretory pathway. Cell 1991, 67, 255–263. [Google Scholar]
- Ferreira, P.A.; Nakayama, T.A.; Pak, W.L.; Travis, G.H. Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature 1996, 383, 637–640. [Google Scholar]
- Saito, H.; Kubota, M.; Roberts, R.W.; Chi, Q.; Matsunami, H. RTP family members induce functional expression of mammalian odorant receptors. Cell 2004, 119, 679–691. [Google Scholar]
- Behrens, M.; Bartelt, J.; Reichling, C.; Winnig, M.; Kuhn, C.; Meyerhof, W. Members of RTP and REEP gene families influence functional bitter taste receptor expression. J. Biol. Chem 2006, 281, 20650–20659. [Google Scholar]
- Décaillot, F.M.; Rozenfeld, R.; Gupta, A.; Devi, L.A. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc. Natl. Acad. Sci. USA 2008, 105, 16045–16050. [Google Scholar]
- Svenningsson, P.; Chergui, K.; Rachleff, I.; Flajolet, M.; Zhang, X.; Yacoubi El, M.; Vaugeois, J.-M.; Nomikos, G.G.; Greengard, P. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science 2006, 311, 77–80. [Google Scholar]
- Warner-Schmidt, J.L.; Flajolet, M.; Maller, A.; Chen, E.Y.; Qi, H.; Svenningsson, P.; Greengard, P. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J. Neurosci 2009, 29, 1937–1946. [Google Scholar]
- Whistler, J.L.; Enquist, J.; Marley, A.; Fong, J.; Gladher, F.; Tsuruda, P.; Murray, S.R.; von Zastrow, M. Modulation of postendocytic sorting of G protein-coupled receptors. Science 2002, 297, 615–620. [Google Scholar]
- Bartlett, S.E.; Enquist, J.; Hopf, F.W.; Lee, J.H.; Gladher, F.; Kharazia, V.; Waldhoer, M.; Mailliard, W.S.; Armstrong, R.; Bonci, A.; et al. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 11521–11526. [Google Scholar]
- Simonin, F.; Karcher, P.; Boeuf, J.J.; Matifas, A.; Kieffer, B.L. Identification of a novel family of G protein-coupled receptor associated sorting proteins. J. Neurochem 2004, 89, 766–775. [Google Scholar]
- Wang, Y.; Zhou, Y.; Szabo, K.; Haft, C.R.; Trejo, J. Down-regulation of protease-activated receptor-1 is regulated by sorting nexin 1. Mol. Biol. Cell 2002, 13, 1965–1976. [Google Scholar]
- Gullapalli, A.; Wolfe, B.L.; Griffin, C.T.; Magnuson, T.; Trejo, J. An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: Evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins. Mol. Biol. Cell 2006, 17, 1228–1238. [Google Scholar]
- Heydorn, A.; Sondergaard, B.P.; Ersboll, B.; Holst, B.; Nielsen, F.C.; Haft, C.R.; Whistler, J.; Schwartz, T.W. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP). J. Biol. Chem 2004, 279, 54291–54303. [Google Scholar]
- Brady, A.E.; Limbird, L.E. G protein-coupled receptor interacting proteins: Emerging roles in localization and signal transduction. Cell Signal 2002, 14, 297–309. [Google Scholar]
- Rivero-Müller, A.; Jonas, K.C.; Hanyaloglu, A.C.; Huhtaniemi, I. Di/oligomerization of GPCRs-mechanisms and functional significance. Prog. Mol. Biol. Transl. Sci 2013, 117, 163–185. [Google Scholar]
- Wang, P.; Heitman, J. The cyclophilins. Genome Biol 2005, 6, 226. [Google Scholar]
- Baker, E.K.; Colley, N.J.; Zuker, C.S. The cyclophilin homolog NinaA functions as a chaperone, forming a stable complex in vivo with its protein target rhodopsin. EMBO J 1994, 13, 4886–4895. [Google Scholar]
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar]
- McClintock, T.S.; Landers, T.M.; Gimelbrant, A.A.; Fuller, L.Z.; Jackson, B.A.; Jayawickreme, C.K.; Lerner, M.R. Functional expression of olfactory-adrenergic receptor chimeras and intracellular retention of heterologously expressed olfactory receptors. Brain Res. Mol. Brain Res 1997, 48, 270–278. [Google Scholar]
- Lu, M.; Echeverri, F.; Moyer, B.D. Endoplasmic reticulum retention, degradation, and aggregation of olfactory G-protein coupled receptors. Traffic 2003, 4, 416–433. [Google Scholar]
- Dwyer, N.D.; Troemel, E.R.; Sengupta, P.; Bargmann, C.I. Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell 1998, 93, 455–466. [Google Scholar]
- Murrell, J.R.; Hunter, D.D. An olfactory sensory neuron line, odora, properly targets olfactory proteins and responds to odorants. J. Neurosci 1999, 19, 8260–8270. [Google Scholar]
- Gimelbrant, A.A.; Haley, S.L.; McClintock, T.S. Olfactory receptor trafficking involves conserved regulatory steps. J. Biol. Chem 2001, 276, 7285–7290. [Google Scholar]
- McClintock, T.S.; Sammeta, N. Trafficking prerogatives of olfactory receptors. Neuroreport 2003, 14, 1547–1552. [Google Scholar]
- Parmentier, M.; Libert, F.; Schurmans, S.; Schiffmann, S.; Lefort, A.; Eggerickx, D.; Ledent, C.; Mollereau, C.; Gérard, C.; Perret, J. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature 1992, 355, 453–455. [Google Scholar]
- Spehr, M.; Gisselmann, G.; Poplawski, A.; Riffell, J.A.; Wetzel, C.H.; Zimmer, R.K.; Hatt, H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 2003, 299, 2054–2058. [Google Scholar]
- Levitt, E.S.; Clark, M.J.; Jenkins, P.M.; Martens, J.R.; Traynor, J.R. Differential effect of membrane cholesterol removal on mu- and delta-opioid receptors: A parallel comparison of acute and chronic signaling to adenylyl cyclase. J. Biol. Chem 2009, 284, 22108–22122. [Google Scholar]
- Park, S.H.; Zhu, P.-P.; Parker, R.L.; Blackstone, C. Hereditary spastic paraplegia proteins REEP1, spastin, and atlastin-1 coordinate microtubule interactions with the tubular ER network. J. Clin. Invest 2010, 120, 1097–1110. [Google Scholar]
- Pfeffer, S.; Aivazian, D. Targeting Rab GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell. Biol 2004, 5, 886–896. [Google Scholar]
- Voeltz, G.K.; Prinz, W.A.; Shibata, Y.; Rist, J.M.; Rapoport, T.A. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 2006, 124, 573–586. [Google Scholar]
- Björk, S.; Hurt, C.M.; Ho, V.K.; Angelotti, T. REEPs are membrane shaping adapter proteins that modulate specific G protein-coupled receptor trafficking by affecting ER cargo capacity. PLoS One 2013, 8, e76366. [Google Scholar]
- Gerke, V.; Weber, K. The regulatory chain in the p36-kd substrate complex of viral tyrosine-specific protein kinases is related in sequence to the S-100 protein of glial cells. EMBO J 1985, 4, 2917–2920. [Google Scholar]
- Okuse, K.; Malik-Hall, M.; Baker, M.D.; Poon, W.-Y.L.; Kong, H.; Chao, M.V.; Wood, J.N. Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature 2002, 417, 653–656. [Google Scholar]
- Girard, C.; Tinel, N.; Terrenoire, C.; Romey, G.; Lazdunski, M.; Borsotto, M. p11, an annexin II subunit, an auxiliary protein associated with the background K+ channel, TASK-1. EMBO J 2002, 21, 4439–4448. [Google Scholar]
- Mai, J.; Finley, R.L.; Waisman, D.M.; Sloane, B.F. Human procathepsin B interacts with the annexin II tetramer on the surface of tumor cells. J. Biol. Chem 2000, 275, 12806–12812. [Google Scholar]
- Zobiack, N.; Rescher, U.; Ludwig, C.; Zeuschner, D.; Gerke, V. The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol. Biol. Cell 2003, 14, 4896–4908. [Google Scholar]
- Marenholz, I.; Heizmann, C.W.; Fritz, G. S100 proteins in mouse and man: From evolution to function and pathology (including an update of the nomenclature). Biochem. Biophys. Res. Commun 2004, 322, 1111–1122. [Google Scholar]
- Warner-Schmidt, J.L.; Schmidt, E.F.; Marshall, J.J.; Rubin, A.J.; Arango-Lievano, M.; Kaplitt, M.G.; Ibañez-Tallon, I.; Heintz, N.; Greengard, P. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc. Natl. Acad. Sci. USA 2012, 109, 11360–11365. [Google Scholar]
- Schmidt, E.F.; Warner-Schmidt, J.L.; Otopalik, B.G.; Pickett, S.B.; Greengard, P.; Heintz, N. Identification of the cortical neurons that mediate antidepressant responses. Cell 2012, 149, 1152–1163. [Google Scholar]
- Oh, Y.-S.; Gao, P.; Lee, K.-W.; Ceglia, I.; Seo, J.-S.; Zhang, X.; Ahn, J.-H.; Chait, B.T.; Patel, D.J.; Kim, Y.; et al. SMARCA3, a chromatin-remodeling factor, is required for p11-dependent antidepressant action. Cell 2013, 152, 831–843. [Google Scholar]
- Svenningsson, P.; Kim, Y.; Warner-Schmidt, J.; Oh, Y.-S.; Greengard, P. p11 and its role in depression and therapeutic responses to antidepressants. Nat. Rev. Neurosci 2013, 14, 673–680. [Google Scholar]
- Cowen, P.J. Serotonin and depression: Pathophysiological mechanism or marketing myth? Trends Pharmacol. Sci 2008, 29, 433–436. [Google Scholar]
- O’Neill, M.F.; Conway, M.W. Role of 5-HT(1A) and 5-HT(1B) receptors in the mediation of behavior in the forced swim test in mice. Neuropsychopharmacology 2001, 24, 391–398. [Google Scholar]
- Lucas, G.; Rymar, V.V.; Du, J.; Mnie-Filali, O.; Bisgaard, C.; Manta, S.; Lambas-Senas, L.; Wiborg, O.; Haddjeri, N.; Piñeyro, G.; et al. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron 2007, 55, 712–725. [Google Scholar]
- Kang, D.S.; Tian, X.; Benovic, J.L. β-Arrestins and G protein-coupled receptor trafficking. Meth. Enzymol 2013, 521, 91–108. [Google Scholar]
- Abu-Helo, A.; Simonin, F. Identification and biological significance of G protein-coupled receptor associated sorting proteins (GASPs). Pharmacol. Ther 2010, 126, 244–250. [Google Scholar]
- Bornert, O.; Møller, T.C.; Boeuf, J.; Candusso, M.-P.; Wagner, R.; Martinez, K.L.; Simonin, F. Identification of a novel protein-protein interaction motif mediating interaction of GPCR-associated sorting proteins with G protein-coupled receptors. PLoS One 2013, 8, e56336. [Google Scholar]
- Ponting, C.P. Novel domains in NADPH oxidase subunits, sorting nexins, and PtdIns 3-kinases: Binding partners of SH3 domains? Protein Sci 1996, 5, 2353–2357. [Google Scholar]
- Kurten, R.C.; Cadena, D.L.; Gill, G.N. Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science 1996, 272, 1008–1010. [Google Scholar]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998, 393, 333–339. [Google Scholar]
- Muff, R.; Buhlmann, N.; Fischer, J.A.; Born, W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology 1999, 140, 2924–2927. [Google Scholar]
- Christopoulos, G.; Perry, K.J.; Morfis, M.; Tilakaratne, N.; Gao, Y.; Fraser, N.J.; Main, M.J.; Foord, S.M.; Sexton, P.M. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol 1999, 56, 235–242. [Google Scholar]
- Christopoulos, A.; Christopoulos, G.; Morfis, M.; Udawela, M.; Laburthe, M.; Couvineau, A.; Kuwasako, K.; Tilakaratne, N.; Sexton, P.M. Novel receptor partners and function of receptor activity-modifying proteins. J. Biol. Chem 2003, 278, 3293–3297. [Google Scholar]
- Wootten, D.; Lindmark, H.; Kadmiel, M.; Willcockson, H.; Caron, K.M.; Barwell, J.; Drmota, T.; Poyner, D.R. Receptor activity modifying proteins (RAMPs) interact with the VPAC2 receptor and CRF1 receptors and modulate their function. Br. J. Pharmacol 2013, 168, 822–834. [Google Scholar]
- Bouschet, T.; Martin, S.; Henley, J.M. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J. Cell. Sci 2005, 118, 4709–4720. [Google Scholar]
- Metherell, L.A.; Chapple, J.P.; Cooray, S.; David, A.; Becker, C.; Rüschendorf, F.; Naville, D.; Begeot, M.; Khoo, B.; Nürnberg, P.; et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat. Genet 2005, 37, 166–170. [Google Scholar]
- Sebag, J.A.; Hinkle, P.M. Regions of melanocortin 2 (MC2) receptor accessory protein necessary for dual topology and MC2 receptor trafficking and signaling. J. Biol. Chem 2009, 284, 610–618. [Google Scholar]
- Chan, L.F.; Webb, T.R.; Chung, T.-T.; Meimaridou, E.; Cooray, S.N.; Guasti, L.; Chapple, J.P.; Egertová, M.; Elphick, M.R.; Cheetham, M.E.; et al. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc. Natl. Acad. Sci. USA 2009, 106, 6146–6151. [Google Scholar]
- Bermak, J.C.; Li, M.; Bullock, C.; Zhou, Q.Y. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat. Cell. Biol 2001, 3, 492–498. [Google Scholar]
- Málaga-Diéguez, L.; Yang, Q.; Bauer, J.; Pankevych, H.; Freissmuth, M.; Nanoff, C. Pharmacochaperoning of the A1 adenosine receptor is contingent on the endoplasmic reticulum. Mol. Pharmacol 2010, 77, 940–952. [Google Scholar]
- Leclerc, P.C.; Auger-Messier, M.; Lanctot, P.M.; Escher, E.; Leduc, R.; Guillemette, G. A polyaromatic caveolin-binding-like motif in the cytoplasmic tail of the type 1 receptor for angiotensin II plays an important role in receptor trafficking and signaling. Endocrinology 2002, 143, 4702–4710. [Google Scholar]
- Dupré, D.J.; Robitaille, M.; Richer, M.; Ethier, N.; Mamarbachi, A.M.; Hébert, T.E. Dopamine receptor-interacting protein 78 acts as a molecular chaperone for Ggamma subunits before assembly with Gbeta. J. Biol. Chem 2007, 282, 13703–13715. [Google Scholar]
- Kuang, Y.-Q.; Charette, N.; Frazer, J.; Holland, P.J.; Attwood, K.M.; Dellaire, G.; Dupré, D.J. Dopamine receptor-interacting protein 78 acts as a molecular chaperone for CCR5 chemokine receptor signaling complex organization. PLoS One 2012, 7, e40522. [Google Scholar]
- Mahon, M.J.; Donowitz, M.; Yun, C.C.; Segre, G.V. Na(+)/H(+) exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature 2002, 417, 858–861. [Google Scholar]
- Mahon, M.J.; Segre, G.V. Stimulation by parathyroid hormone of a NHERF-1-assembled complex consisting of the parathyroid hormone I receptor, phospholipase Cbeta, and actin increases intracellular calcium in opossum kidney cells. J. Biol. Chem 2004, 279, 23550–23558. [Google Scholar]
- Wang, B.; Bisello, A.; Yang, Y.; Romero, G.G.; Friedman, P.A. NHERF1 regulates parathyroid hormone receptor membrane retention without affecting recycling. J. Biol. Chem 2007, 282, 36214–36222. [Google Scholar]
- Wang, B.; Yang, Y.; Abou-Samra, A.B.; Friedman, P.A. NHERF1 regulates parathyroid hormone receptor desensitization: Interference with beta-arrestin binding. Mol. Pharmacol 2009, 75, 1189–1197. [Google Scholar]
- Ardura, J.A.; Wang, B.; Watkins, S.C.; Vilardaga, J.-P.; Friedman, P.A. Dynamic Na+-H+ exchanger regulatory factor-1 association and dissociation regulate parathyroid hormone receptor trafficking at membrane microdomains. J. Biol. Chem 2011, 286, 35020–35029. [Google Scholar]
- Oh, Y.-S.; Jo, N.W.; Choi, J.W.; Kim, H.S.; Seo, S.-W.; Kang, K.-O.; Hwang, J.-I.; Heo, K.; Kim, S.-H.; Kim, Y.-H.; et al. NHERF2 specifically interacts with LPA2 receptor and defines the specificity and efficiency of receptor-mediated phospholipase C-beta3 activation. Mol. Cell. Biol 2004, 24, 5069–5079. [Google Scholar]
- Fam, S.R.; Paquet, M.; Castleberry, A.M.; Oller, H.; Lee, C.J.; Traynelis, S.F.; Smith, Y.; Yun, C.C.; Hall, R.A. P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc. Natl. Acad. Sci. USA 2005, 102, 8042–8047. [Google Scholar]
- Paquet, M.; Asay, M.J.; Fam, S.R.; Inuzuka, H.; Castleberry, A.M.; Oller, H.; Smith, Y.; Yun, C.C.; Traynelis, S.F.; Hall, R.A. The PDZ scaffold NHERF-2 interacts with mGluR5 and regulates receptor activity. J. Biol. Chem 2006, 281, 29949–29961. [Google Scholar]
- Dunn, H.A.; Walther, C.; Godin, C.M.; Hall, R.A.; Ferguson, S.S.G. Role of SAP97 protein in the regulation of corticotropin-releasing factor receptor 1 endocytosis and extracellular signal-regulated kinase 1/2 signaling. J. Biol. Chem 2013, 288, 15023–15034. [Google Scholar]
- Jones, M.C. Therapies for diabetes: Pramlintide and exenatide. Am. Fam. Phys 2007, 75, 1831–1835. [Google Scholar]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol 1990, 28, 183–187. [Google Scholar]
- Overgaard, K.; Hansen, M.A.; Jensen, S.B.; Christiansen, C. Effect of salcatonin given intranasally on bone mass and fracture rates in established osteoporosis: A dose-response study. BMJ 1992, 305, 556–561. [Google Scholar]
- Lin, H.Y.; Harris, T.L.; Flannery, M.S.; Aruffo, A.; Kaji, E.H.; Gorn, A.; Kolakowski, L.F.J.; Yamin, M.; Lodish, H.F.; Goldring, S.R. Expression cloning and characterization of a porcine renal calcitonin receptor. Trans. Assoc. Am. Phys 1991, 104, 265–272. [Google Scholar]
- Njuki, F.; Nicholl, C.G.; Howard, A.; Mak, J.C.; Barnes, P.J.; Girgis, S.I.; Legon, S. A new calcitonin-receptor-like sequence in rat pulmonary blood vessels. Clin. Sci 1993, 85, 385–388. [Google Scholar]
- Fluhmann, B.; Muff, R.; Hunziker, W.; Fischer, J.A.; Born, W. A human orphan calcitonin receptor-like structure. Biochem. Biophys. Res. Commun 1995, 206, 341–347. [Google Scholar]
- Aiyar, N.; Rand, K.; Elshourbagy, N.A.; Zeng, Z.; Adamou, J.E.; Bergsma, D.J.; Li, Y. A cDNA encoding the calcitonin gene-related peptide type 1 receptor. J. Biol. Chem 1996, 271, 11325–11329. [Google Scholar]
- Hilairet, S.; Belanger, C.; Bertrand, J.; Laperriere, A.; Foord, S.M.; Bouvier, M. Agonist-promoted internalization of a ternary complex between calcitonin receptor-like receptor, receptor activity-modifying protein 1 (RAMP1), and beta-arrestin. J. Biol. Chem 2001, 276, 42182–42190. [Google Scholar]
- Fraser, N.J.; Wise, A.; Brown, J.; McLatchie, L.M.; Main, M.J.; Foord, S.M. The amino terminus of receptor activity modifying proteins is a critical determinant of glycosylation state and ligand binding of calcitonin receptor-like receptor. Mol. Pharmacol 1999, 55, 1054–1059. [Google Scholar]
- Hilairet, S.; Foord, S.M.; Marshall, F.H.; Bouvier, M. Protein-protein interaction and not glycosylation determines the binding selectivity of heterodimers between the calcitonin receptor-like receptor and the receptor activity-modifying proteins. J. Biol. Chem 2001, 276, 29575–29581. [Google Scholar]
- Hay, D.L.; Howitt, S.G.; Conner, A.C.; Schindler, M.; Smith, D.M.; Poyner, D.R. CL/RAMP2 and CL/RAMP3 produce pharmacologically distinct adrenomedullin receptors: A comparison of effects of adrenomedullin22–52, CGRP8–37 and BIBN4096BS. Br. J. Pharmacol 2003, 140, 477–486. [Google Scholar]
- Bomberger, J.M.; Parameswaran, N.; Hall, C.S.; Aiyar, N.; Spielman, W.S. Novel function for receptor activity-modifying proteins (RAMPs) in post-endocytic receptor trafficking. J. Biol. Chem 2005, 280, 9297–9307. [Google Scholar]
- Cottrell, G.S.; Padilla, B.; Pikios, S.; Roosterman, D.; Steinhoff, M.; Grady, E.F.; Bunnett, N.W. Post-endocytic sorting of calcitonin receptor-like receptor and receptor activity-modifying protein 1. J. Biol. Chem 2007, 282, 12260–12271. [Google Scholar]
- Roh, J.; Chang, C.L.; Bhalla, A.; Klein, C.; Hsu, S.Y. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J. Biol. Chem 2004, 279, 7264–7274. [Google Scholar]
- Oliver, K.R.; Kane, S.A.; Salvatore, C.A.; Mallee, J.J.; Kinsey, A.M.; Koblan, K.S.; Keyvan-Fouladi, N.; Heavens, R.P.; Wainwright, A.; Jacobson, M.; et al. Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur. J. Neurosci 2001, 14, 618–628. [Google Scholar]
- Nikitenko, L.L.; Brown, N.S.; Smith, D.M.; MacKenzie, I.Z.; Bicknell, R.; Rees, M.C. Differential and cell-specific expression of calcitonin receptor-like receptor and receptor activity modifying proteins in the human uterus. Mol. Hum. Reprod 2001, 7, 655–664. [Google Scholar]
- Sarkar, A.; Dickerson, I.M. Cloning, characterization, and expression of a calcitonin receptor from guinea pig brain. J. Neurochem 1997, 69, 455–464. [Google Scholar]
- Caron, K.M.; Smithies, O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc. Natl. Acad. Sci. USA 2001, 98, 615–619. [Google Scholar]
- Dackor, R.T.; Fritz-Six, K.; Dunworth, W.P.; Gibbons, C.L.; Smithies, O.; Caron, K.M. Hydrops fetalis, cardiovascular defects, and embryonic lethality in mice lacking the calcitonin receptor-like receptor gene. Mol. Cell. Biol 2006, 26, 2511–2518. [Google Scholar]
- Dackor, R.; Fritz-Six, K.; Smithies, O.; Caron, K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J. Biol. Chem 2007, 282, 18094–18099. [Google Scholar]
- Kadmiel, M.; Fritz-Six, K.; Pacharne, S.; Richards, G.O.; Li, M.; Skerry, T.M.; Caron, K.M. Research resource: Haploinsufficiency of receptor activity-modifying protein-2 (RAMP2) causes reduced fertility, hyperprolactinemia, skeletal abnormalities, and endocrine dysfunction in mice. Mol. Endocrinol 2011, 25, 1244–1253. [Google Scholar]
- Tsujikawa, K.; Yayama, K.; Hayashi, T.; Matsushita, H.; Yamaguchi, T.; Shigeno, T.; Ogitani, Y.; Hirayama, M.; Kato, T.; Fukada, S.-I.; et al. Hypertension and dysregulated proinflammatory cytokine production in receptor activity-modifying protein 1-deficient mice. Proc. Natl. Acad. Sci. USA 2007, 104, 16702–16707. [Google Scholar]
- Laburthe, M.; Couvineau, A.; Marie, J.C. VPAC receptors for VIP and PACAP. Recept. Channels 2002, 8, 137–153. [Google Scholar]
- Montecucchi, P.C.; Anastasi, A.; de Castiglione, R.; Erspamer, V. Isolation and amino acid composition of sauvagine. An active polypeptide from methanol extracts of the skin of the South American frog Phyllomedusa sauvagei. Int. J. Pept. Protein Res 1980, 16, 191–199. [Google Scholar]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev 2000, 21, 55–89. [Google Scholar]
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci 2013, 34, 518–530. [Google Scholar]
- Pittman, Q.J.; Veale, W.L.; Lederis, K. Central neurohypophyseal peptide pathways: Interactions with endocrine and other autonomic functions. Peptides 1982, 3, 515–520. [Google Scholar]
- Lutz-Bucher, B.; Koch, B.; Mialhe, C.; Briaud, B. Involvement of vasopressin in corticotropin-releasing effect of hypothalamic median eminence extract. Neuroendocrinology 1980, 30, 178–182. [Google Scholar]
- Bradbury, A.F.; Smyth, D.G.; Snell, C.R. Prohormones of beta-melanotropin (beta-melanocyte-stimulating hormone, beta-MSH) and corticotropin (adrenocorticotropic hormone, ACTH): Structure and activation. Ciba Found. Symp 1976, 41, 61–75. [Google Scholar]
- Dores, R.M.; Baron, A.J. Evolution of POMC: Origin, phylogeny, posttranslational processing, and the melanocortins. Ann. N.Y. Acad. Sci 2011, 1220, 34–48. [Google Scholar]
- Cone, R.D.; Mountjoy, K.G.; Robbins, L.S.; Nadeau, J.H.; Johnson, K.R.; Roselli-Rehfuss, L.; Mortrud, M.T. Cloning and functional characterization of a family of receptors for the melanotropic peptides. Ann. N.Y. Acad. Sci 1993, 680, 342–363. [Google Scholar]
- Mountjoy, K.G.; Robbins, L.S.; Mortrud, M.T.; Cone, R.D. The cloning of a family of genes that encode the melanocortin receptors. Science 1992, 257, 1248–1251. [Google Scholar]
- Yang, Y. Structure, function and regulation of the melanocortin receptors. Eur. J. Pharmacol 2011, 660, 125–130. [Google Scholar]
- Clark, A.J.; McLoughlin, L.; Grossman, A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet 1993, 341, 461–462. [Google Scholar]
- Chung, T.T.; Webb, T.R.; Chan, L.F.; Cooray, S.N.; Metherell, L.A.; King, P.J.; Chapple, J.P.; Clark, A.J.L. The majority of adrenocorticotropin receptor (melanocortin 2 receptor) mutations found in familial glucocorticoid deficiency type 1 lead to defective trafficking of the receptor to the cell surface. J. Clin. Endocrinol. Metable 2008, 93, 4948–4954. [Google Scholar]
- Clark, A.J.; Weber, A. Adrenocorticotropin insensitivity syndromes. Endocr. Rev 1998, 19, 828–843. [Google Scholar]
- Clark, A.J.L.; Metherell, L.A.; Cheetham, M.E.; Huebner, A. Inherited ACTH insensitivity illuminates the mechanisms of ACTH action. Trends Endocrinol. Metable 2005, 16, 451–457. [Google Scholar]
- Noon, L.A.; Franklin, J.M.; King, P.J.; Goulding, N.J.; Hunyady, L.; Clark, A.J.L. Failed export of the adrenocorticotrophin receptor from the endoplasmic reticulum in non-adrenal cells: Evidence in support of a requirement for a specific adrenal accessory factor. J. Endocrinol 2002, 174, 17–25. [Google Scholar]
- Sebag, J.A.; Hinkle, P.M. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc. Natl. Acad. Sci. USA 2007, 104, 20244–20249. [Google Scholar]
- Webb, T.R.; Chan, L.; Cooray, S.N.; Cheetham, M.E.; Chapple, J.P.; Clark, A.J.L. Distinct melanocortin 2 receptor accessory protein domains are required for melanocortin 2 receptor interaction and promotion of receptor trafficking. Endocrinology 2009, 150, 720–726. [Google Scholar]
- Mancias, J.D.; Goldberg, J. Exiting the endoplasmic reticulum. Traffic 2005, 6, 278–285. [Google Scholar]
- Nishimura, N.; Balch, W.E. A di-acidic signal required for selective export from the endoplasmic reticulum. Science 1997, 277, 556–558. [Google Scholar]
- Kappeler, F.; Klopfenstein, D.R.; Foguet, M.; Paccaud, J.P.; Hauri, H.P. The recycling of ERGIC-53 in the early secretory pathway. ERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J. Biol. Chem 1997, 272, 31801–31808. [Google Scholar]
- Fiedler, K.; Veit, M.; Stamnes, M.A.; Rothman, J.E. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science 1996, 273, 1396–1399. [Google Scholar]
- Chen, J.; Huang, Y.; Wu, H.; Ni, X.; Cheng, H.; Fan, J.; Gu, S.; Gu, X.; Cao, G.; Ying, K.; et al. Molecular cloning and characterization of a novel human J-domain protein gene (HDJ3) from the fetal brain. J. Hum. Genet 2003, 48, 217–221. [Google Scholar]
- Dupré, D.J.; Robitaille, M.; Ethier, N.; Villeneuve, L.R.; Mamarbachi, A.M.; Hébert, T.E. Seven transmembrane receptor core signaling complexes are assembled prior to plasma membrane trafficking. J. Biol. Chem 2006, 281, 34561–34573. [Google Scholar]
- Lukov, G.L.; Hu, T.; McLaughlin, J.N.; Hamm, H.E.; Willardson, B.M. Phosducin-like protein acts as a molecular chaperone for G protein betagamma dimer assembly. EMBO J 2005, 24, 1965–1975. [Google Scholar]
- Humrich, J.; Bermel, C.; Bünemann, M.; Härmark, L.; Frost, R.; Quitterer, U.; Lohse, M.J. Phosducin-like protein regulates G-protein betagamma folding by interaction with tailless complex polypeptide-1alpha: Dephosphorylation or splicing of PhLP turns the switch toward regulation of Gbetagamma folding. J. Biol. Chem 2005, 280, 20042–20050. [Google Scholar]
- Kennedy, M.B. Origin of PDZ (DHR, GLGF) domains. Trends Biochem. Sci 1995, 20, 350. [Google Scholar]
- Ritter, S.L.; Hall, R.A. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell. Biol 2009, 10, 819–830. [Google Scholar]
- Romero, G.; von Zastrow, M.; Friedman, P.A. Role of PDZ proteins in regulating trafficking, signaling, and function of GPCRs: Means, motif, and opportunity. Adv. Pharmacol 2011, 62, 279–314. [Google Scholar]
- Bretscher, A.; Edwards, K.; Fehon, R.G. ERM proteins and merlin: Integrators at the cell cortex. Nat. Rev. Mol. Cell. Biol 2002, 3, 586–599. [Google Scholar]
- Rochdi, M.D.; Watier, V.; la Madeleine, C.; Nakata, H.; Kozasa, T.; Parent, J.-L. Regulation of GTP-binding protein alpha q (Galpha q) signaling by the ezrin-radixin-moesin-binding phosphoprotein-50 (EBP50). J. Biol. Chem 2002, 277, 40751–40759. [Google Scholar]
- Lee-Kwon, W.; Kim, J.H.; Choi, J.W.; Kawano, K.; Cha, B.; Dartt, D.A.; Zoukhri, D.; Donowitz, M. Ca2+-dependent inhibition of NHE3 requires PKC alpha which binds to E3KARP to decrease surface NHE3 containing plasma membrane complexes. Am. J. Physiol. Cell. Physiol 2003, 285, C1527–36. [Google Scholar]
- Cao, T.T.; Deacon, H.W.; Reczek, D.; Bretscher, A.; von Zastrow, M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature 1999, 401, 286–290. [Google Scholar]
- Li, J.-G.; Chen, C.; Liu-Chen, L.-Y. Ezrin-radixin-moesin-binding phosphoprotein-50/Na+/H+ exchanger regulatory factor (EBP50/NHERF) blocks U50,488H-induced down-regulation of the human kappa opioid receptor by enhancing its recycling rate. J. Biol. Chem 2002, 277, 27545–27552. [Google Scholar]
- Gage, R.M.; Kim, K.A.; Cao, T.T.; von Zastrow, M. A transplantable sorting signal that is sufficient to mediate rapid recycling of G protein-coupled receptors. J. Biol. Chem 2001, 276, 44712–44720. [Google Scholar]
- Nisar, S.P.; Cunningham, M.; Saxena, K.; Pope, R.J.; Kelly, E.; Mundell, S.J. Arrestin scaffolds NHERF1 to the P2Y12 receptor to regulate receptor internalization. J. Biol. Chem 2012, 287, 24505–24515. [Google Scholar]
- Cai, C.; Li, H.; Kangasniemi, A.; Pihlajamaa, T.; Ossowski Von, L.; Kerkelä, K.; Schulz, S.; Rivera, C.; Keinänen, K. Somatostatin receptor subtype 1 is a PDZ ligand for synapse-associated protein 97 and a potential regulator of growth cone dynamics. Neuroscience 2008, 157, 833–843. [Google Scholar]
- Bécamel, C.; Gavarini, S.; Chanrion, B.; Alonso, G.; Galéotti, N.; Dumuis, A.; Bockaert, J.; Marin, P. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J. Biol. Chem 2004, 279, 20257–20266. [Google Scholar]
- Gardner, L.A.; Naren, A.P.; Bahouth, S.W. Assembly of an SAP97-AKAP79-cAMP-dependent protein kinase scaffold at the type 1 PSD-95/DLG/ZO1 motif of the human beta(1)-adrenergic receptor generates a receptosome involved in receptor recycling and networking. J. Biol. Chem 2007, 282, 5085–5099. [Google Scholar]
- Beuming, T.; Skrabanek, L.; Niv, M.Y.; Mukherjee, P.; Weinstein, H. PDZBase: A protein-protein interaction database for PDZ-domains. Bioinformatics 2005, 21, 827–828. [Google Scholar]
- Chabre, O.; Conklin, B.R.; Lin, H.Y.; Lodish, H.F.; Wilson, E.; Ives, H.E.; Catanzariti, L.; Hemmings, B.A.; Bourne, H.R. A recombinant calcitonin receptor independently stimulates 3′,5′-cyclic adenosine monophosphate and Ca2+/inositol phosphate signaling pathways. Mol. Endocrinol 1992, 6, 551–556. [Google Scholar]
- Force, T.; Bonventre, J.V.; Flannery, M.R.; Gorn, A.H.; Yamin, M.; Goldring, S.R. A cloned porcine renal calcitonin receptor couples to adenylyl cyclase and phospholipase C. Am. J. Physiol 1992, 262, F1110–F1115. [Google Scholar]
- Chakraborty, M.; Chatterjee, D.; Kellokumpu, S.; Rasmussen, H.; Baron, R. Cell cycle-dependent coupling of the calcitonin receptor to different G proteins. Science 1991, 251, 1078–1082. [Google Scholar]
- Luebke, A.E.; Dahl, G.P.; Roos, B.A.; Dickerson, I.M. Identification of a protein that confers calcitonin gene-related peptide responsiveness to oocytes by using a cystic fibrosis transmembrane conductance regulator assay. Proc. Natl. Acad. Sci. USA 1996, 93, 3455–3460. [Google Scholar]
- Wang, D.; Sadée, W.; Quillan, J.M. Calmodulin binding to G protein-coupling domain of opioid receptors. J. Biol. Chem 1999, 274, 22081–22088. [Google Scholar]
- Bofill-Cardona, E.; Kudlacek, O.; Yang, Q.; Ahorn, H.; Freissmuth, M.; Nanoff, C. Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J. Biol. Chem 2000, 275, 32672–32680. [Google Scholar]
- Turner, J.H.; Raymond, J.R. Interaction of calmodulin with the serotonin 5-hydroxytryptamine2A receptor. A putative regulator of G protein coupling and receptor phosphorylation by protein kinase C. J. Biol. Chem 2005, 280, 30741–30750. [Google Scholar]
- Mahon, M.J.; Shimada, M. Calmodulin interacts with the cytoplasmic tails of the parathyroid hormone 1 receptor and a sub-set of class b G-protein coupled receptors. FEBS Lett 2005, 579, 803–807. [Google Scholar]
- Nickols, H.H.; Shah, V.N.; Chazin, W.J.; Limbird, L.E. Calmodulin interacts with the V2 vasopressin receptor: Elimination of binding to the C terminus also eliminates arginine vasopressin-stimulated elevation of intracellular calcium. J. Biol. Chem 2004, 279, 46969–46980. [Google Scholar]
- Nakajima, Y.; Yamamoto, T.; Nakayama, T.; Nakanishi, S. A relationship between protein kinase C phosphorylation and calmodulin binding to the metabotropic glutamate receptor subtype 7. J. Biol. Chem 1999, 274, 27573–27577. [Google Scholar]
- Sorensen, S.D.; Macek, T.A.; Cai, Z.; Saugstad, J.A.; Conn, P.J. Dissociation of protein kinase-mediated regulation of metabotropic glutamate receptor 7 (mGluR7) interactions with calmodulin and regulation of mGluR7 function. Mol. Pharmacol 2002, 61, 1303–1312. [Google Scholar]
- Prado, M.A.; Evans-Bain, B.; Oliver, K.R.; Dickerson, I.M. The role of the CGRP-receptor component protein (RCP) in adrenomedullin receptor signal transduction. Peptides 2001, 22, 1773–1781. [Google Scholar]
- Naghashpour, M.; Rosenblatt, M.I.; Dickerson, I.M.; Dahl, G.P. Inhibitory effect of calcitonin gene-related peptide on myometrial contractility is diminished at parturition. Endocrinology 1997, 138, 4207–4214. [Google Scholar]
- Rosenblatt, M.I.; Dahl, G.P.; Dickerson, I.M. Characterization and localization of the rabbit ocular calcitonin gene-related peptide (CGRP)-receptor component protein (RCP). Invest. Ophthalmol. Vis. Sci 2000, 41, 1159–1167. [Google Scholar]
- Han, Z.Q.; Coppock, H.A.; Smith, D.M.; Van Noorden, S.; Makgoba, M.W.; Nicholl, C.G.; Legon, S. The interaction of CGRP and adrenomedullin with a receptor expressed in the rat pulmonary vascular endothelium. J. Mol. Endocrinol 1997, 18, 267–272. [Google Scholar]
- Buhlmann, N.; Leuthauser, K.; Muff, R.; Fischer, J.A.; Born, W. A receptor activity modifying protein (RAMP)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human RAMP1. Endocrinology 1999, 140, 2883–2890. [Google Scholar]
- Evans, B.N.; Rosenblatt, M.I.; Mnayer, L.O.; Oliver, K.R.; Dickerson, I.M. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J. Biol. Chem 2000, 275, 31438–31443. [Google Scholar]
- Ma, W.; Chabot, J.G.; Powell, K.J.; Jhamandas, K.; Dickerson, I.M.; Quirion, R. Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience 2003, 120, 677–694. [Google Scholar]
- Egea, S.C.; Dickerson, I.M. Direct interactions between calcitonin-like receptor (CLR) and CGRP-receptor component protein (RCP) regulate CGRP receptor signaling. Endocrinology 2012, 153, 1850–1860. [Google Scholar]
- Miret, J.J.; Rakhilina, L.; Silverman, L.; Oehlen, B. Functional expression of heteromeric calcitonin gene-related peptide and adrenomedullin receptors in yeast. J. Biol. Chem 2002, 277, 6881–6887. [Google Scholar]
- Cheung, W.Y. Cyclic 3′,5′-nucleotide phosphodiesterase. Demonstration of an activator. Biochem. Biophys. Res. Commun 1970, 38, 533–538. [Google Scholar]
- Kakiuchi, S.; Yamazaki, R. Calcium dependent phosphodiesterase activity and its activating factor (PAF) from brain studies on cyclic 3′,5′-nucleotide phosphodiesterase (3). Biochem. Biophys. Res. Commun 1970, 41, 1104–1110. [Google Scholar]
- Babu, Y.S.; Sack, J.S.; Greenhough, T.J.; Bugg, C.E.; Means, A.R.; Cook, W.J. Three-dimensional structure of calmodulin. Nature 1985, 315, 37–40. [Google Scholar]
- Yamniuk, A.P.; Vogel, H.J. Calmodulin’s flexibility allows for promiscuity in its interactions with target proteins and peptides. Mol. Biotechnol 2004, 27, 33–57. [Google Scholar]
- Tan, C.M.; Brady, A.E.; Nickols, H.H.; Wang, Q.; Limbird, L.E. Membrane trafficking of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol 2004, 44, 559–609. [Google Scholar]
 represents glycosylation.
represents glycosylation.
 represents glycosylation.
represents glycosylation.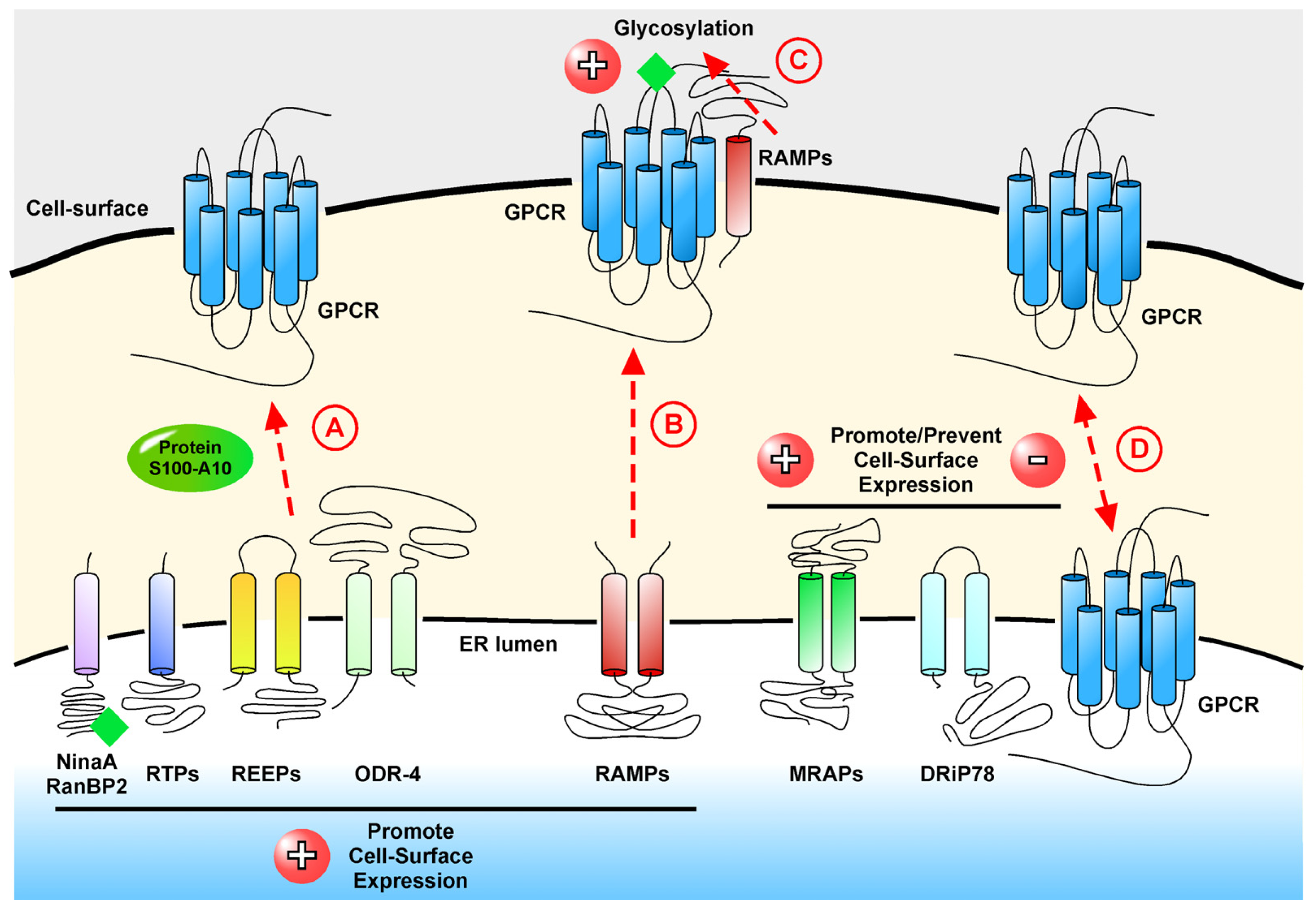
| GPCR(s) | Interacting protein(s) | Function(s) | References |
|---|---|---|---|
| Rh1 receptor | ninaA | Promote correct folding and cell-surface expression | [29–31] |
| Red/green opsins | RanBP2 | Potentially promote correct folding and cell-surface expression | [32] |
| ORs | RTP1-2 and REEP1 | Promote cell-surface expression. Co-localize at the cell-surface with ORs | [33] |
| TAS2Rs | RTP3-4 | Promote cell-surface expression | [34] |
| δ-μ receptor heterodimer | RTP4 | Promote cell-surface expression | [35] |
| 5-HT1BR and 5-HT4R | S100-A10 | Promote cell-surface expression | [36,37] |
| δ receptor | GASPs | Promote down-regulation of the receptor | [38] |
| β2-adrenoceptor | GASPs | Promote down-regulation of the receptor | [38] |
| D2 receptor | GASPs | Promote down-regulation of the receptor | [39] |
| μ receptor | GASPs | Promote down-regulation of the receptor | [40] |
| κ receptor | GASPs | Promote down-regulation of the receptor | [40] |
| β1-adrenoceptor | GASPs | Promote down-regulation of the receptor | [40] |
| CTR | GASPs | Promote down-regulation of the receptor | [40] |
| PAR1 | SNX-1 | Promote internalization of the receptor | [41,42] |
| oxytocin receptor | SNX-1 | Promote internalization of the receptor | [43] |
| δ receptor | SNX-1 | Promote internalization of the receptor | [43] |
| Neurokinin 1 receptor | SNX-1 | Promote internalization of the receptor | [43] |
| GPCR | Interacting protein(s) | Function(s) | References |
|---|---|---|---|
| CLR | RAMPs | Promote cell-surface expression, receptor glycosylation and induce ligand binding specificity | [80] |
| CTR | RAMPs | Induce ligand binding specificity to IAPP | [81,82] |
| VPAC1 receptor | RAMPs | Increase the coupling of Gαq-subunit with the receptor | [83] |
| PTH1 receptor | RAMP2 | ? | [83] |
| Glucagon receptor | RAMP2 | ? | [83] |
| PTH2 receptor | RAMP3 | ? | [83] |
| VPAC2 receptor | RAMPs | Increase the coupling of Gαi/o-subunit to the receptor, but RAMP3 | [84] |
| CRF1 receptor | RAMP2 | Promote cell-surface expression and increase Ca2+ mobilization | [84] |
| Calcium Sensing receptor | RAMP1 and 3 | Promote cell-surface expression and receptor glycosylation | [85] |
| MCRs | MRAPs | Regulate differently the cell-surface expression depending on the MCR and also can modulate MCR-mediated signaling | [86–88] |
| D1 receptor | DRiP78 | Retain receptor in ER | [89] |
| Muscarinic acetylcholine 2 receptor | DRiP78 | Retain receptor in ER | [89] |
| Adenosine 1 receptor | DRiP78 | Retain receptor in ER | [90] |
| AT1 receptor | DRiP78 | Promote cell-surface expression | [91] |
| β2-adrenoceptor | DRiP78 | Promote the coupling of the βγ-subunit | [92] |
| Chemokine receptor 5 | DRiP78 | Retain receptor in ER and promote the coupling of the βγ-subunit | [93] |
| PTH1 receptor | NHERF1-2 | Switch the coupling of Gαs to Gαq of the receptor. | [94,95] |
| Inhibit internalization and desensitization of the receptor | [96–98] | ||
| LPA2 receptor | NHERF2 | Potentiate PLC signaling | [99] |
| P2Y1 receptor | NHERF2 | Potentiate PLC signaling | [100] |
| mGlu5 receptor | NHERF2 | Potentiate PLC signaling | [101] |
| CRF1 receptor | DLG1 | Inhibit receptor agonist-induced internalization and promote | [102] |
| ERK1/2 signaling | |||
| GPCR | Interacting protein | Function(s) | References |
|---|---|---|---|
| CLR | RCP | Enhance CGRP- and ADM-mediated signaling | [168] |
| μ receptor | CaM | Impair G protein coupling | [169] |
| D2 receptor | CaM | Impair G protein coupling | [170] |
| 5-HT2AR | CaM | Impair G protein coupling | [171] |
| PTH1 receptor | CaM | Inhibit Gα-mediated PLC activation | [172] |
| V2 receptor | CaM | Enhance Ca2+ mobilization | [173] |
| mGlu7 receptor | CaM | Regulate GPCR phosphorylation | [174,175] |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Roux, B.T.; Cottrell, G.S. G Protein-Coupled Receptors: What a Difference a ‘Partner’ Makes. Int. J. Mol. Sci. 2014, 15, 1112-1142. https://doi.org/10.3390/ijms15011112
Roux BT, Cottrell GS. G Protein-Coupled Receptors: What a Difference a ‘Partner’ Makes. International Journal of Molecular Sciences. 2014; 15(1):1112-1142. https://doi.org/10.3390/ijms15011112
Chicago/Turabian StyleRoux, Benoît T., and Graeme S. Cottrell. 2014. "G Protein-Coupled Receptors: What a Difference a ‘Partner’ Makes" International Journal of Molecular Sciences 15, no. 1: 1112-1142. https://doi.org/10.3390/ijms15011112
APA StyleRoux, B. T., & Cottrell, G. S. (2014). G Protein-Coupled Receptors: What a Difference a ‘Partner’ Makes. International Journal of Molecular Sciences, 15(1), 1112-1142. https://doi.org/10.3390/ijms15011112




