The Protective Effects of α-Lipoic Acid on Kidneys in Type 2 Diabetic Goto-Kakisaki Rats via Reducing Oxidative Stress
Abstract
:1. Introduction
2. Results
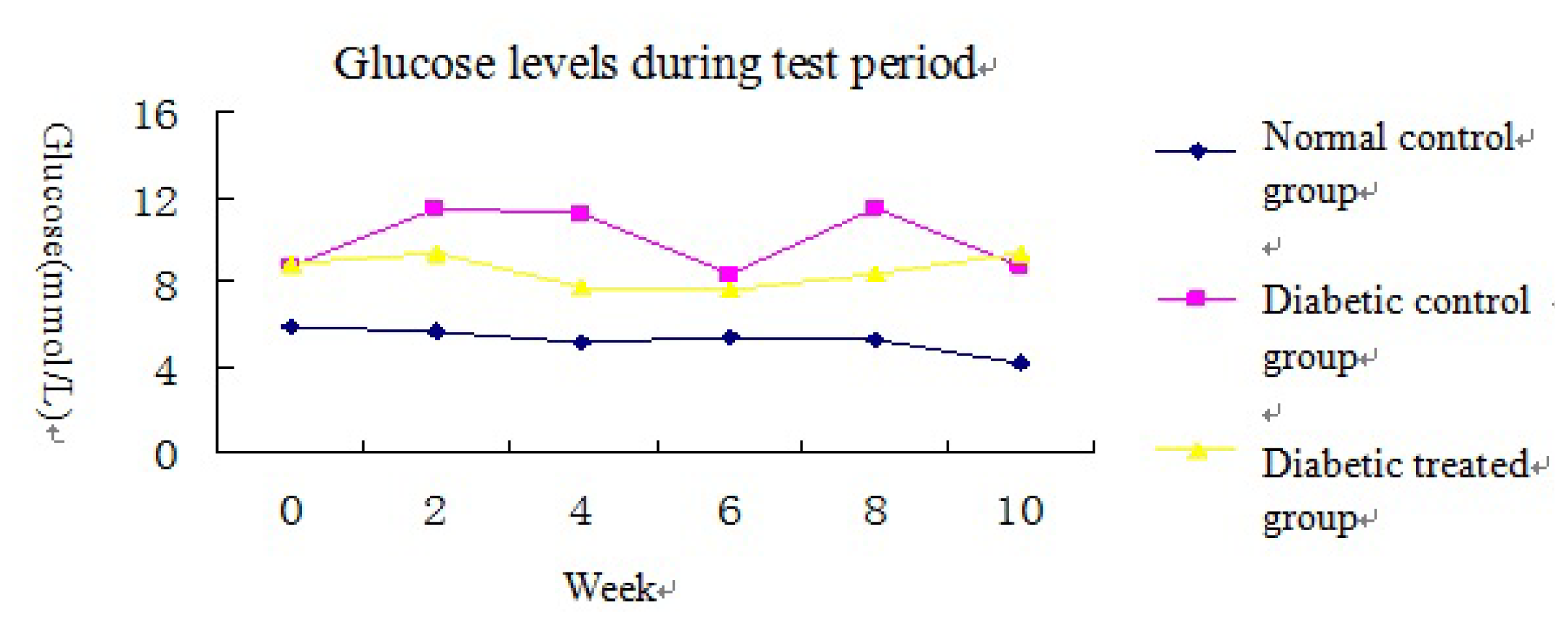
2.1. Blood Glucose and Body/Kidney Weight Determination
2.2. Urine Protein Excretion (UPE)
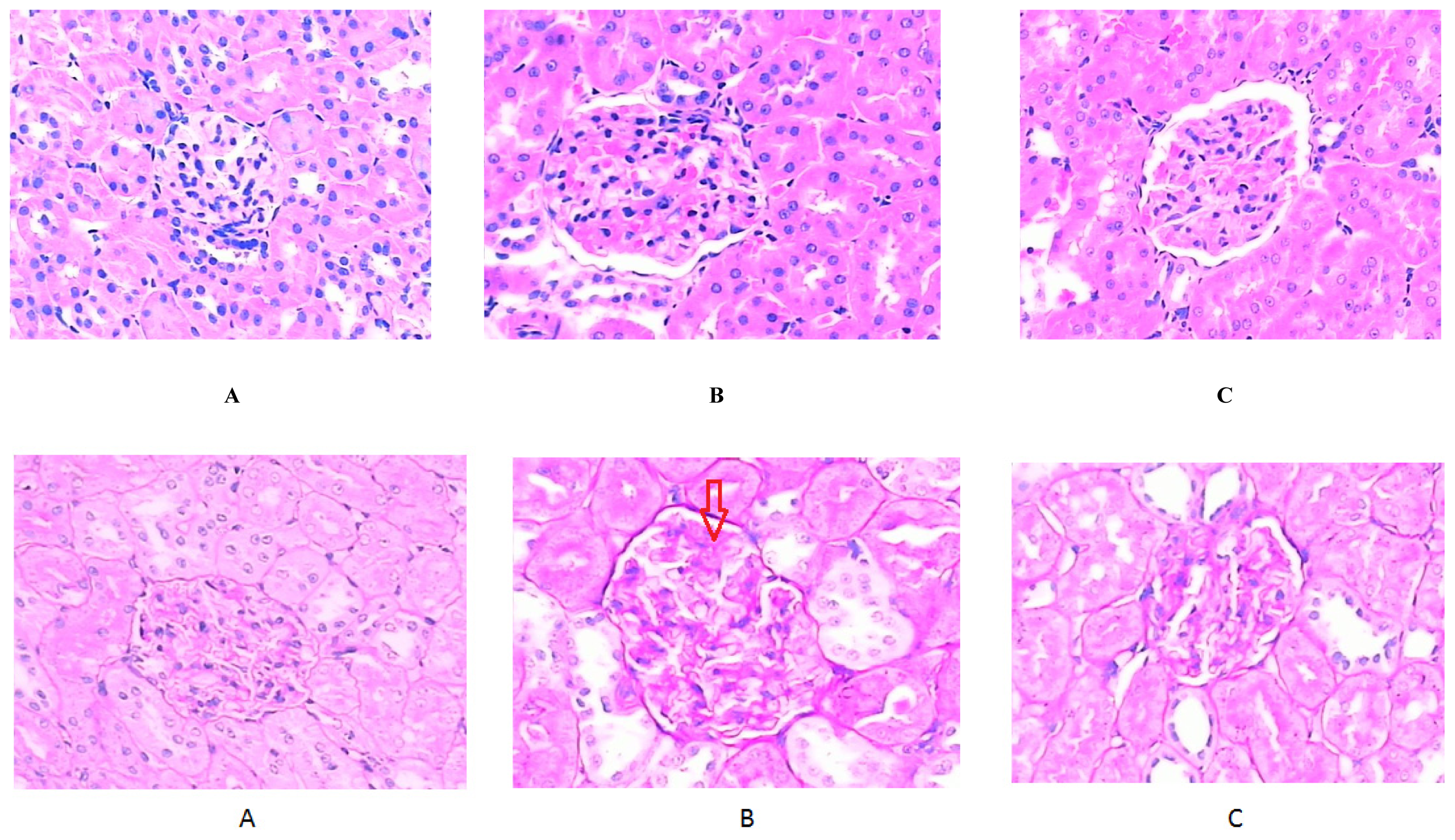
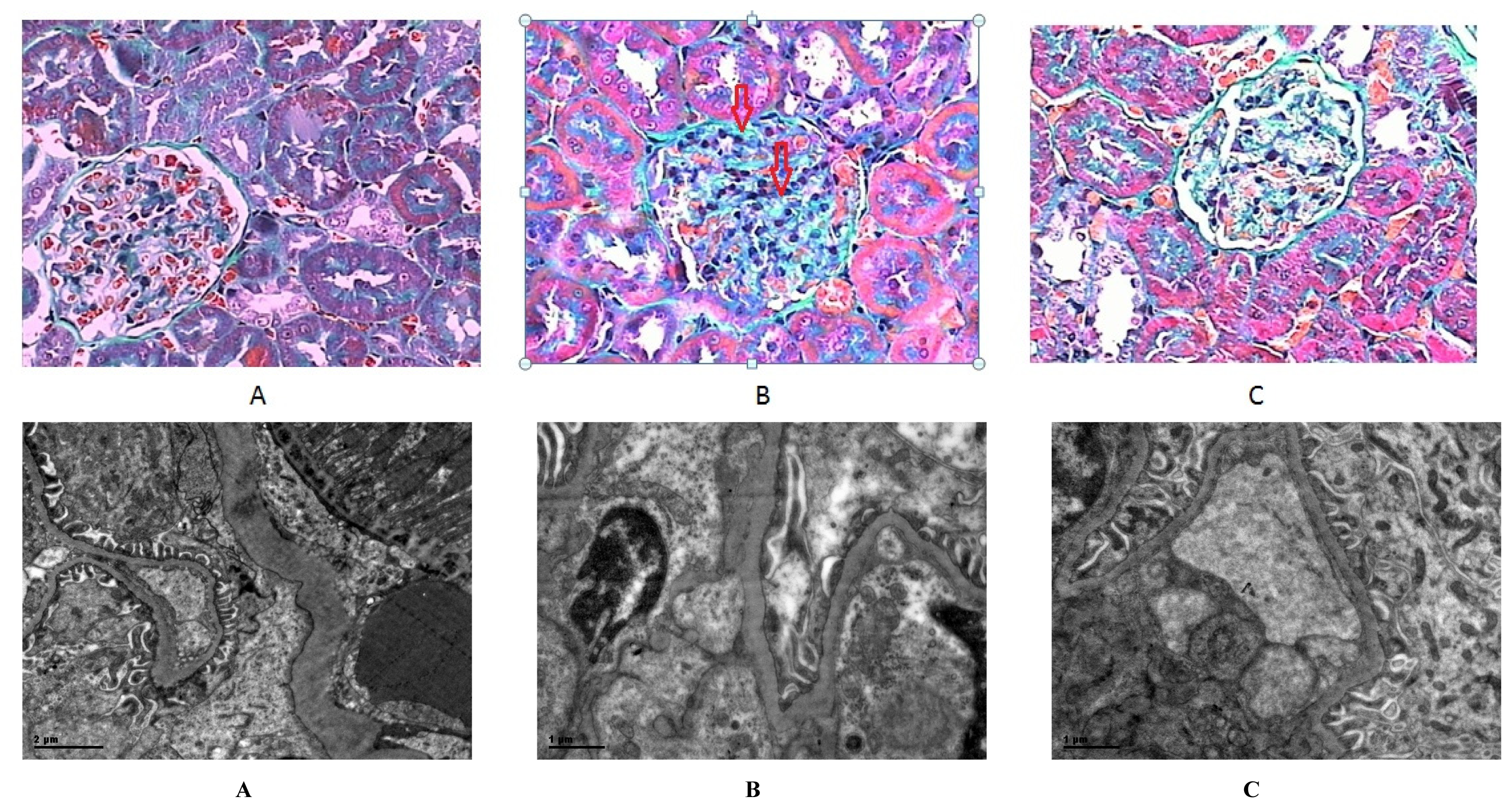
2.3. Morphology and Realtime Reverse Transcriptase (RT)-PCR
2.4. Biochemical Analysis
2.5. Correlation Analyses
3. Discussion
4. Methods
4.1. Blood Glucose Evaluation
4.2. Urine Protein Excretion (UPE)
4.3. Morphology and Realtime Reverse Transcriptase (RT)-PCR
- B-actin: 5′CCTGTACGCCAACACAGTGC3′, 5′ATACTCCTGCTTGCTGATCC3′;
- p22phox: 5′GGACGCTTCACGCAGTGGTA3′, 5′GGACAGCAGTAAGTGGAGGACA3′;
- p47phox: 5′ATGGGACTGCCCGTGAAGAT3′, 5′GGATGATGGGACCCGTGATG3′;
- NF-κB: 5′ACTGCCGGGATGGCTTCTAT3′, 5′CTGGATGCGCTGGCTAATGG3′.
4.4. Biochemical Analysis
4.5. Statistical Analysis
5. Conclusions
Acknowledgements
Disclosures
References
- Markell, M. S.; Friedman, E.A. Diabetic nephropathy: Management of the end-stage patients. Diabetes Care 1992, 15, 1226–1238. [Google Scholar]
- Susztak, K.; Raff, A. C.; Schiffer, M.; Böttinger, E.P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 2006, 55, 225–233. [Google Scholar]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar]
- Horie, K.; Miyata, T.; Maeda, K.; Miyata, S.; Sugiyama, S.; Sakai, H.; van Ypersole de Strihou, C.; Monnier, V. M.; Witztum, J. L.; Kurokawa, K. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J. Clin. Invest 1997, 100, 2995–3004. [Google Scholar]
- Dobashi, K.; Asayama, K.; Hayashibe, H.; Uchida, N.; Kobayashi, M.; Kawaoi, A.; Kato, K. Effect of diabetes mellitus induced by streptozotocin on renal superoxide dismutases in the rat. A radioimmunoassay and immunohistochemical study. Virchows Archiv. B Cell Pathol. Incl. Mol. Pathol 1991, 60, 67–72. [Google Scholar]
- Schnackenberg, C. G.; Wilcox, C.S. The SOD mimetic tempol restores vasodilation in afferent arterioles of experimental diabetes. Kidney Int 2001, 59, 1859–1864. [Google Scholar]
- Melhem, M. F.; Craven, P. A.; Liachenko, J.; DeRubertis, F.R. α-lipoic acid attenuates hyperglycemia and prevents glomerular mesangial expansion in diabetes. J. Am. Soc. Nephrol 2002, 13, 108–116. [Google Scholar]
- Melhem, M. F.; Craven, P. A.; Derubertis, F.R. Effects of dietary supplementation of alpha-lipoic acid on early glomerular injury in diabetes mellitus. J. Am. Soc. Nephrol 2001, 12, 124–133. [Google Scholar]
- Trachtman, H.; Futterweit, S.; Maesaka, J.; Ma, C.; Valderrama, E.; Fuchs, A.; Tarectecan, A. A.; Rao, P. S.; Sturman, J. A.; Boles, T.H. Taurine ameliorates chronic streptozocin-induced diabetic nephropathy in rats. Am. J. Physiol 1995, 269, F429–F438. [Google Scholar]
- Yi, X.; Nickeleit, V.; James, L. R.; Maeda, N. α-Lipoic acid protects diabetic apolipoprotein E-deficient mice from nephropathy. J. Diabetes Complicat 2011, 25, 193–201. [Google Scholar]
- Sadi, G.; Eryilmaz, N.; Tütüncüoğlu, E.; Cingir, Ş.; Güray, T. Changes in expression profiles of antioxidant enzymes in diabetic rat kidneys. Diabetes Metab. Res. Rev. 2012, 28, 228–235. [Google Scholar]
- Arambašić, J.; Mihailović, M.; Uskoković, A.; Dinić, S.; Grdović, N.; Marković, J.; Poznanović, G.; Bajec, D.; Vidaković, M. Alpha-lipoic acid upregulates antioxidant enzyme gene expression and enzymatic activity in diabetic rat kidneys through an O-GlcNAc-dependent mechanism. Eur. J. Nutr. 2012. [Google Scholar] [CrossRef]
- Maritum, A. C.; Sanders, R. A.; Watkins, J.B. Effects of alpha-lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats. J. Nutr. Biochem 2003, 14, 288–294. [Google Scholar]
- Malarkodi, K. P.; Sivaprasad, R.; Varalakshmi, P. Effect of lipoic acid on the oxidoreductive status of red blood cells in rats subject to oxidative stress by chronic administration of adriamycin. Hum. Exp. Toxicol 2004, 23, 129–135. [Google Scholar]
- Nascimento, N. R.; Costa-e-Forti, A.; Peter, A. A.; Fonteles, M.C. Free radical scavengers improve the impaired endothelium-dependent responses in aorta and kidneys of diabetic rabbits. Diabetes Res. Clin. Pract 2003, 61, 145–153. [Google Scholar]
- Satoh, M.; Fujimoto, S.; Haruna, Y.; Arakawa, S.; Horike, H.; Komai, N.; Sasaki, T.; Tsujioka, K.; Makino, H.; Kashihara, N. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am. J. Physiol 2005, 288, F1144–F1152. [Google Scholar]
- Bhatti, F.; Mankhey, R. W.; Asico, L.; Quinn, M. T.; Welch, W. J.; Maric, C. Mechanisms of antioxidant and pro-oxidant effects of a-lipoic acid in the diabetic and nondiabetic kidney. Kidney Int 2005, 67, 1371–1380. [Google Scholar]
- Ha, H.; Kim, K. Pathogenesis of diabetic nephropathy: The role of oxidative stress and protein kinase C. Diabetes Res. Clin. Pract 1999, 45, 147–151. [Google Scholar]
- Asaba, K.; Tojo, A.; Onozato, M. L.; Goto, A.; Quinn, M. T.; Fujita, T.; Wilcox, C.S. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int 2005, 7, 1890–1898. [Google Scholar]
- Qi, W.; Chen, X.; Holian, J.; Mreich, E.; Twigg, S.; Gilbert, R. E.; Pollock, C.A. Transforming growth factor-beta1 differentially mediates fibronectin and inflammatory cytokine expression in kidney tubular cells. Am. J. Physiol. Renal Physiol 2006, 291, F1070–F1077. [Google Scholar]
- Lee, K. Y.; Ito, K.; Hayashi, R.; Mreich, E.; Twigg, S.; Gilbert, R. E.; Pollock, C.A. NF-kappaB and activator protein 1 response elements and the role of histone modifications in IL-1beta-induced TGF-beta1 gene transcription. J. Immunol 2006, 176, 603–615. [Google Scholar]
- Liu, Z.; Li, S.; Chen, C. The clinical pathological characters of podocytes in diabetic nephropathy patients. J. Nephrol. Dial. Transpl 2003, 12, 144–148. [Google Scholar]
- Siu, B.; Saha, J.; Smoyer, W. E.; Sullivan, K. A.; Brosius, F.C., 3rd. Reduction in podocyte density as a pathologic feature in early diabetic nephropathy in rodents: Prevention by lipoic acid treatment. BMC Nephrol. 2006, 7, 6–17. [Google Scholar]
| Normal control | Diabetic control | ALA-treated diabetic | |
|---|---|---|---|
| Blood glucose (mmol/L) | 4.2 ± 0.4 | 8.7 ± 4.0 * | 9.4 ± 2.1 * |
| Body weight (g) | 436.3 ± 83.8 | 305.8 ± 8.4 ** | 327 ± 19.6 ** |
| Kidney weight (g) | 0.7 ± 0.0 | 1.5 ± 0.1 ** | 1.2 ± 0.1 **Δ |
| Kidney/body weight (%) | 0.16 ± 0.03 | 0.50 ± 0.03 ** | 0.41 ± 0.08 *Δ |
| Non control | Diabetic control | ALA-treated diabetic | |
|---|---|---|---|
| UPE (mg/24 h) | 8.5 ± 6.1 | 12.0 ± 3.8 * | 9.7 ± 3.8 |
| Glomerulosclerotic index | 1.25 ± 0.50 | 2.50 ± 0.58 * | 2.0 ± 0.71 |
| SM (μm2) | 65.6 ± 13.5 | 209.9 ± 77.0 ** | 99.5 ± 32.8 ΔΔ |
| SG (μm2) | 844.4 ± 190.7 | 1573.2 ± 208.7 ** | 1325.2 ± 320.9 * |
| SM/SG (%) | 7.9 ± 1.5 | 13.4 ± 4.4 * | 8.0 ± 3.6 Δ |
| GBM (μm) | 0.25 ± 0.06 | 0.39 ± 0.11 ** | 0.29 ± 0.06 ΔΔ |
| Normal control | Diabetic control | ALA-treated diabetic | |
|---|---|---|---|
| NF-κB (×10−3) | 13.8 ± 5.6 | 46.9 ± 37.4 * | 29.7 ± 6.5 |
| p22phox (×10−5) | 10.0 ± 1.8 | 32.9 ± 37.7 * | 2.7 ± 1.9 |
| p47phox (×10−4) | 6.6 ± 4.3 | 19.5 ± 17.4 * | 8.6 ± 2.4 |
| Normal control | Diabetic control | ALA-treated diabetic | |
|---|---|---|---|
| GSH (mg/mgprot) | 74.7 ± 2.0 | 63.6 ± 4.4 ** | 69.4 ± 4.8 Δ |
| SOD (U/mgprot) | 9.3 ± 5.5 | 0.5 ± 0.2 ** | 4.8 ± 1.7 Δ |
| VE (μg/mgprot) | 0.09 ± 0.02 | 0.04 ± 0.01 ** | 0.06 ± 0.01 Δ |
| VC (μg/mgprot) | 1.8 ± 0.2 | 1.4 ± 0.1 ** | 1.7 ± 1.2 Δ |
| MDA (nmol/mgprot) | 1.3 ± 0.2 | 1.7 ± 0.4 | 1.1 ± 0.3 Δ |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Feng, B.; Yan, X.-F.; Xue, J.-L.; Xu, L.; Wang, H. The Protective Effects of α-Lipoic Acid on Kidneys in Type 2 Diabetic Goto-Kakisaki Rats via Reducing Oxidative Stress. Int. J. Mol. Sci. 2013, 14, 6746-6756. https://doi.org/10.3390/ijms14046746
Feng B, Yan X-F, Xue J-L, Xu L, Wang H. The Protective Effects of α-Lipoic Acid on Kidneys in Type 2 Diabetic Goto-Kakisaki Rats via Reducing Oxidative Stress. International Journal of Molecular Sciences. 2013; 14(4):6746-6756. https://doi.org/10.3390/ijms14046746
Chicago/Turabian StyleFeng, Bo, Xin-Feng Yan, Jun-Li Xue, Lei Xu, and Hua Wang. 2013. "The Protective Effects of α-Lipoic Acid on Kidneys in Type 2 Diabetic Goto-Kakisaki Rats via Reducing Oxidative Stress" International Journal of Molecular Sciences 14, no. 4: 6746-6756. https://doi.org/10.3390/ijms14046746
APA StyleFeng, B., Yan, X.-F., Xue, J.-L., Xu, L., & Wang, H. (2013). The Protective Effects of α-Lipoic Acid on Kidneys in Type 2 Diabetic Goto-Kakisaki Rats via Reducing Oxidative Stress. International Journal of Molecular Sciences, 14(4), 6746-6756. https://doi.org/10.3390/ijms14046746




