Structural and Catalytic Differences between Two FADH2-Dependent Monooxygenases: 2,4,5-TCP 4-Monooxygenase (TftD) from Burkholderia cepacia AC1100 and 2,4,6-TCP 4-Monooxygenase (TcpA) from Cupriavidus necator JMP134
Abstract
:1. Introduction
2. Results and Discussion
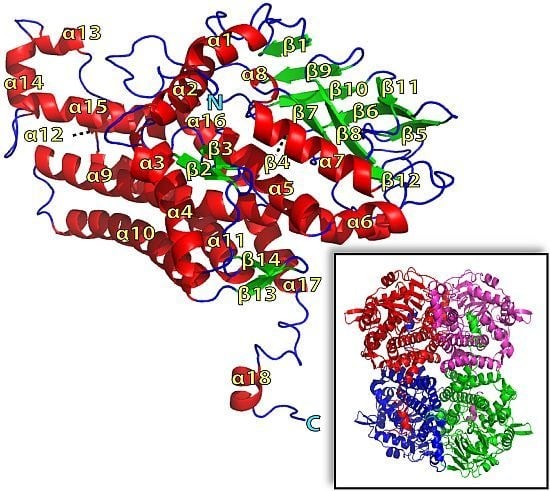
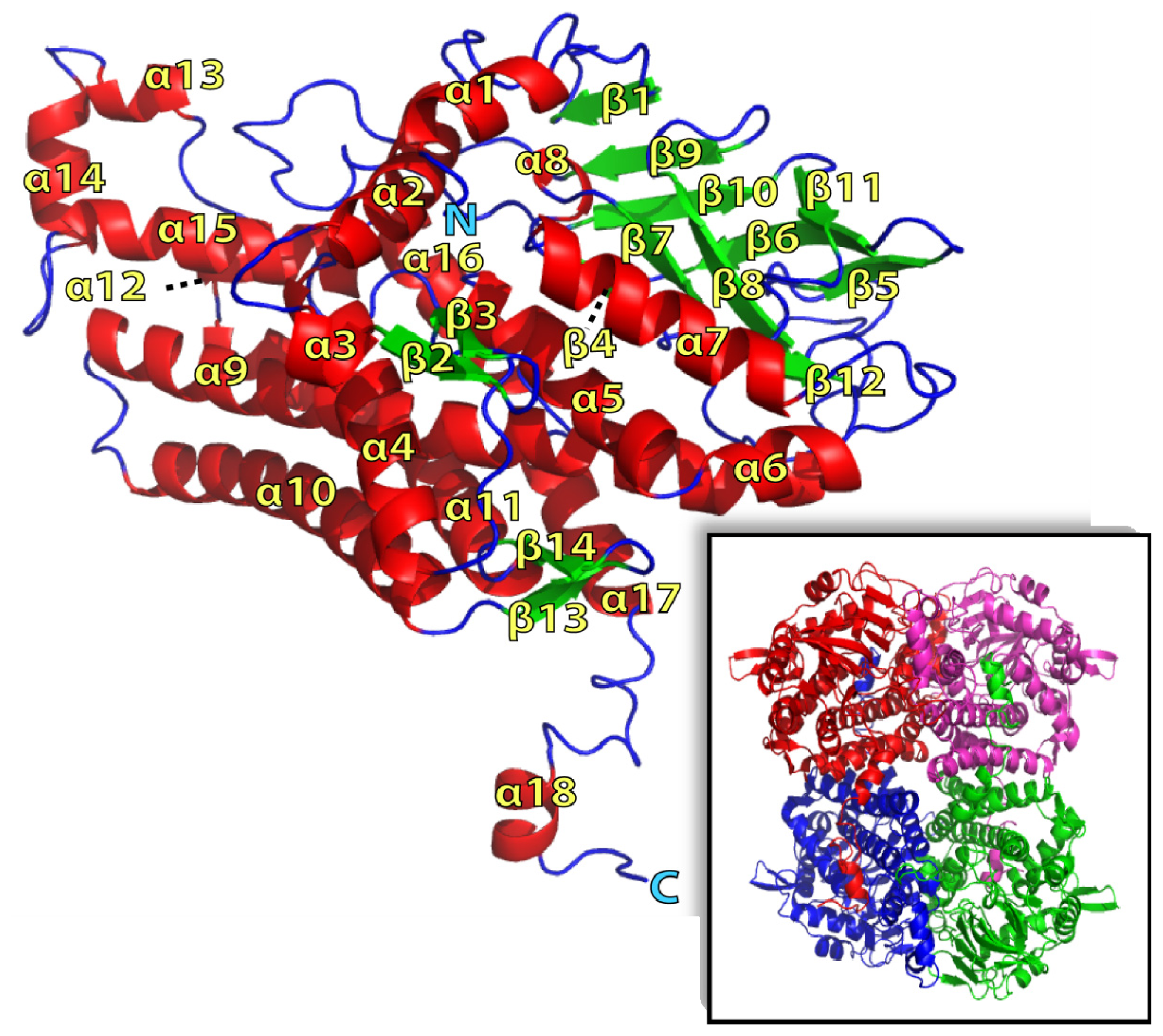
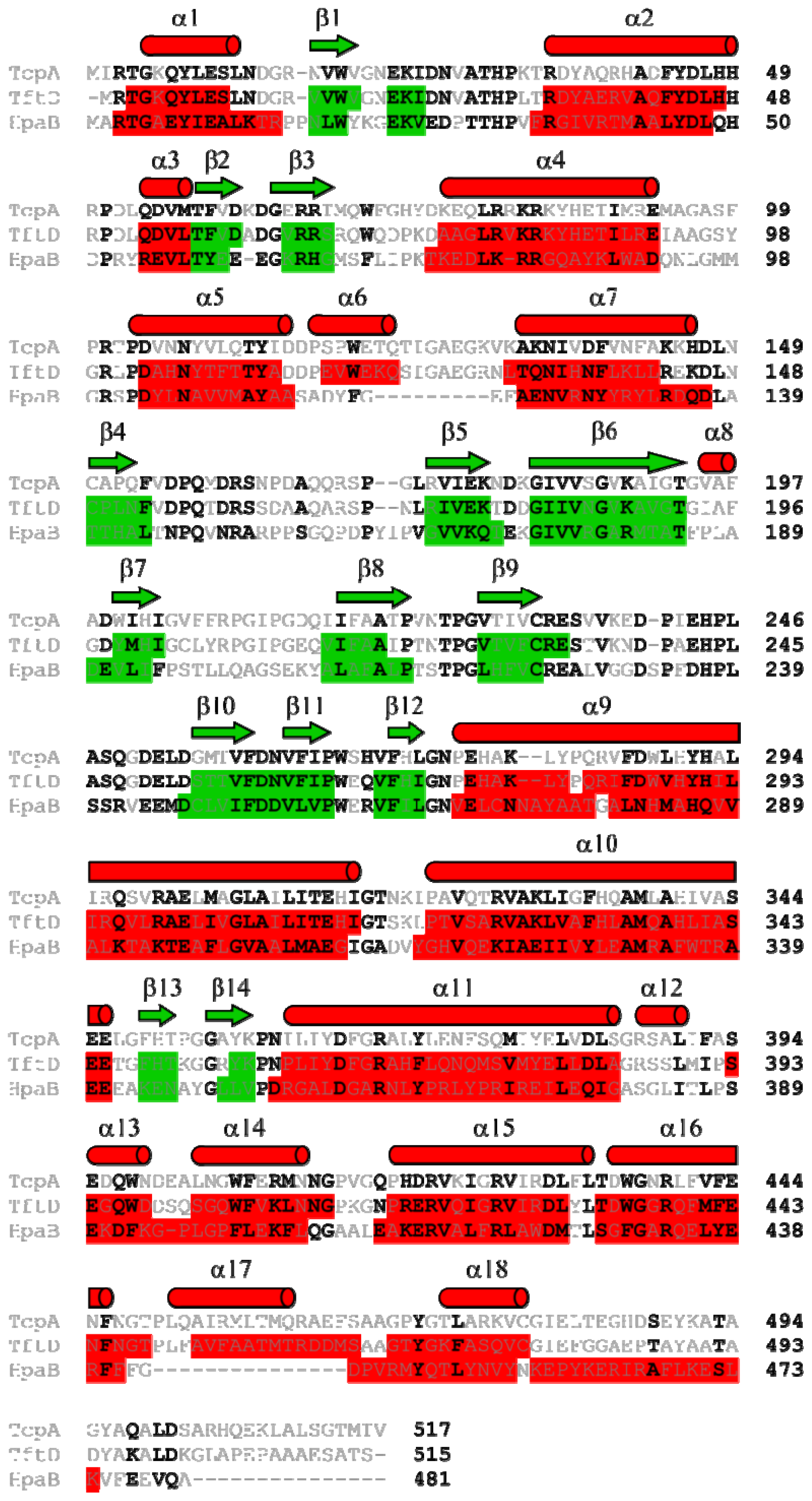
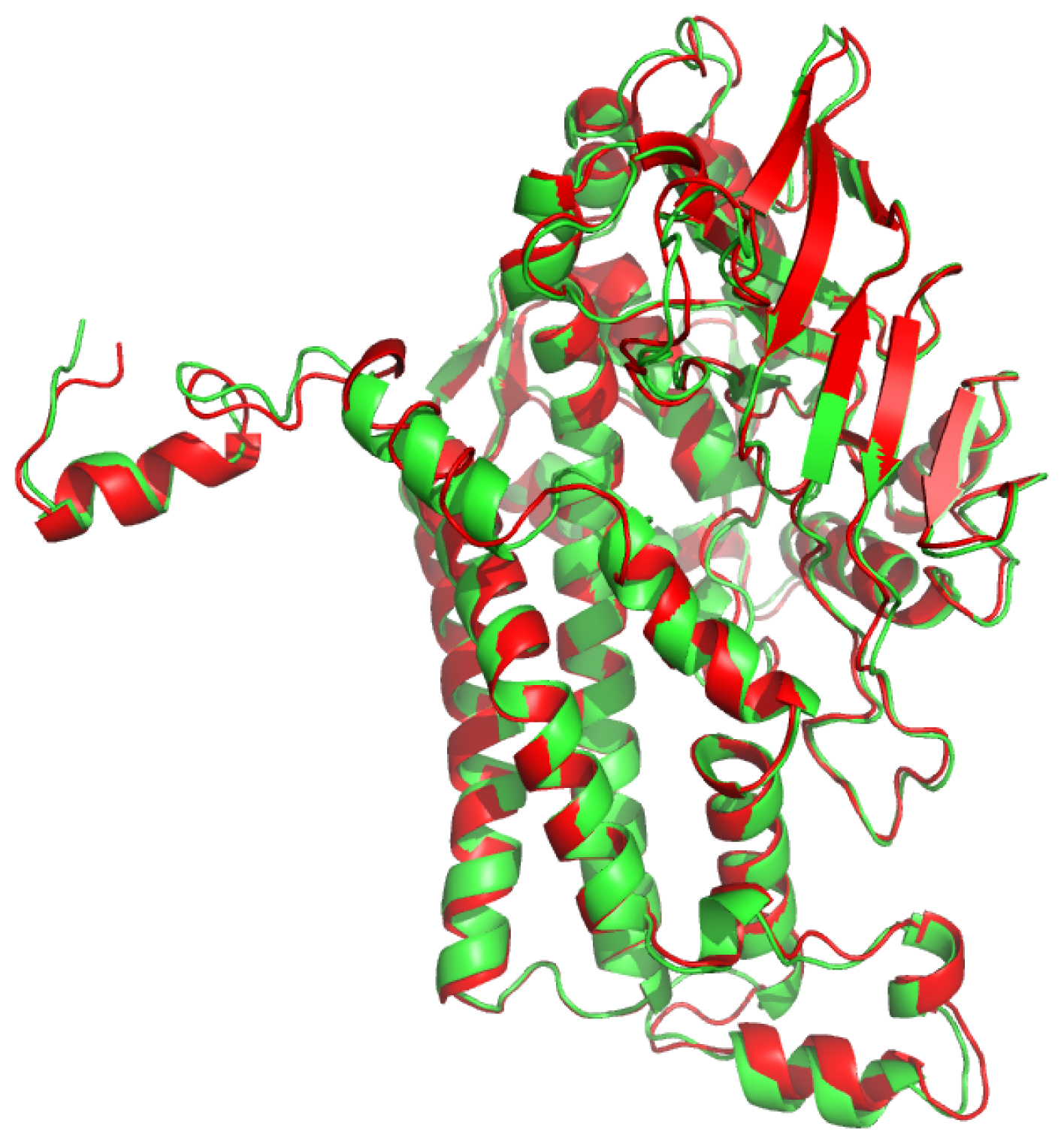
2.1. Overall Structure of TcpA
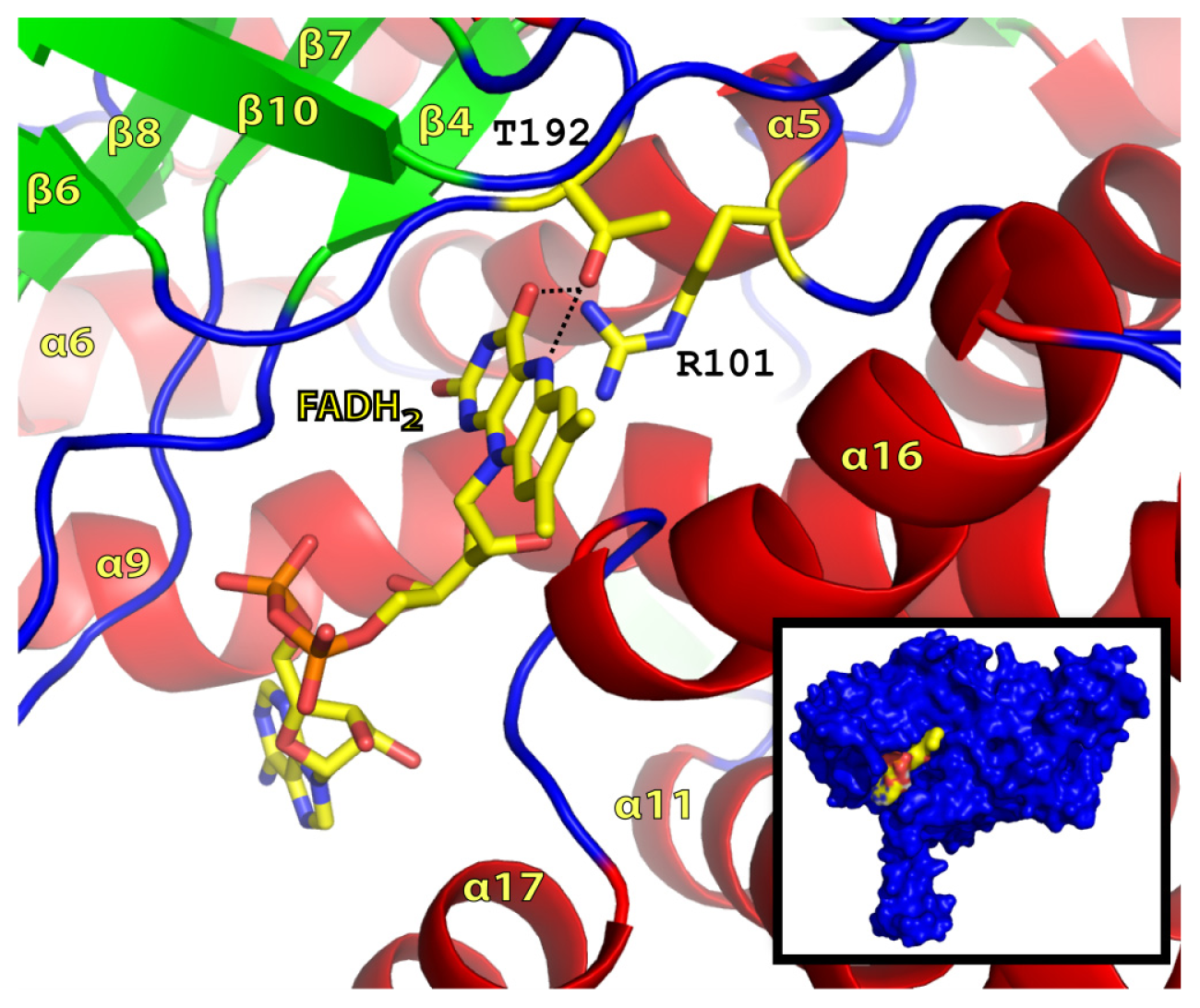
2.2. FADH2 Binding Site
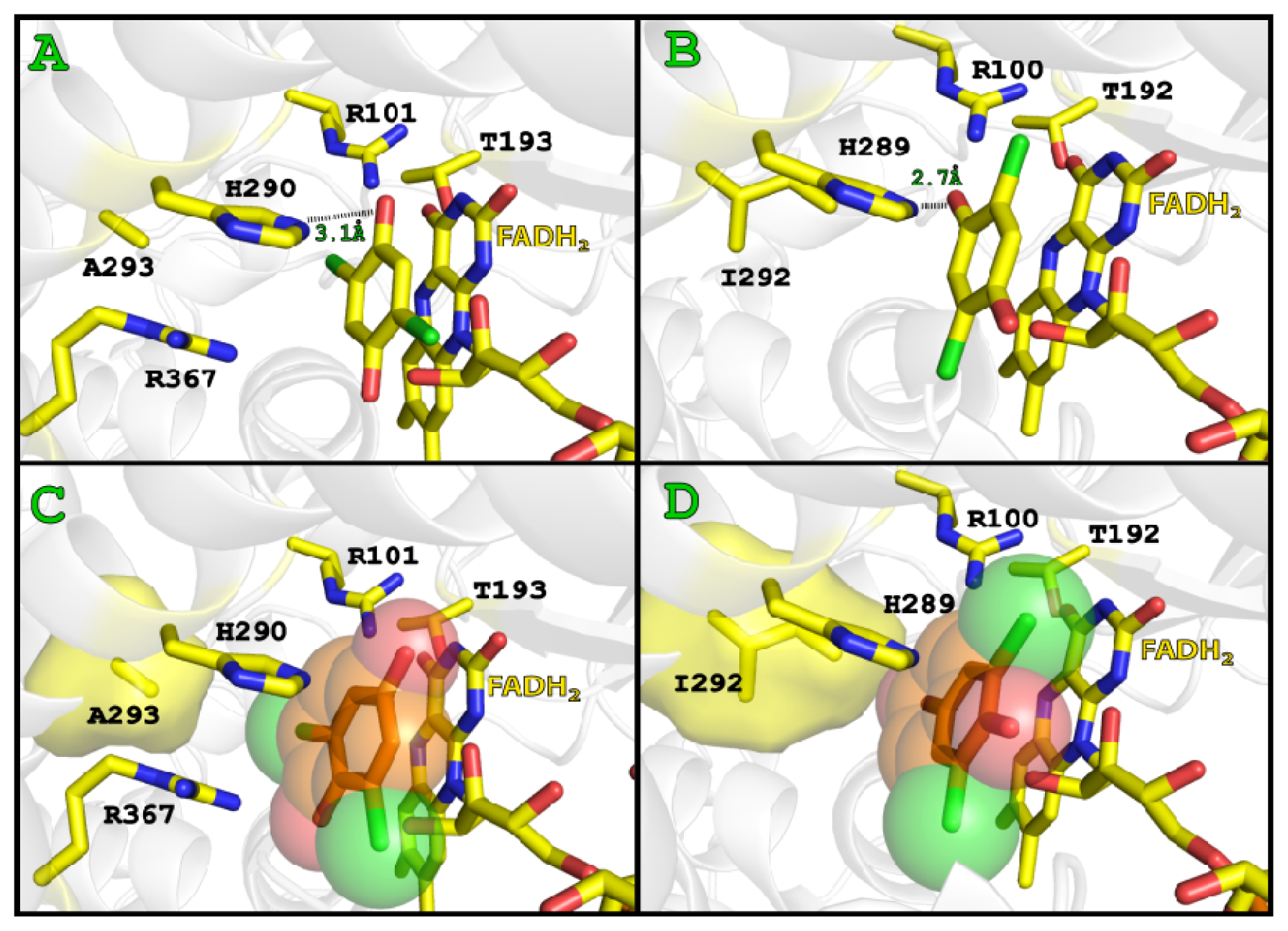
2.3. Substrate Binding
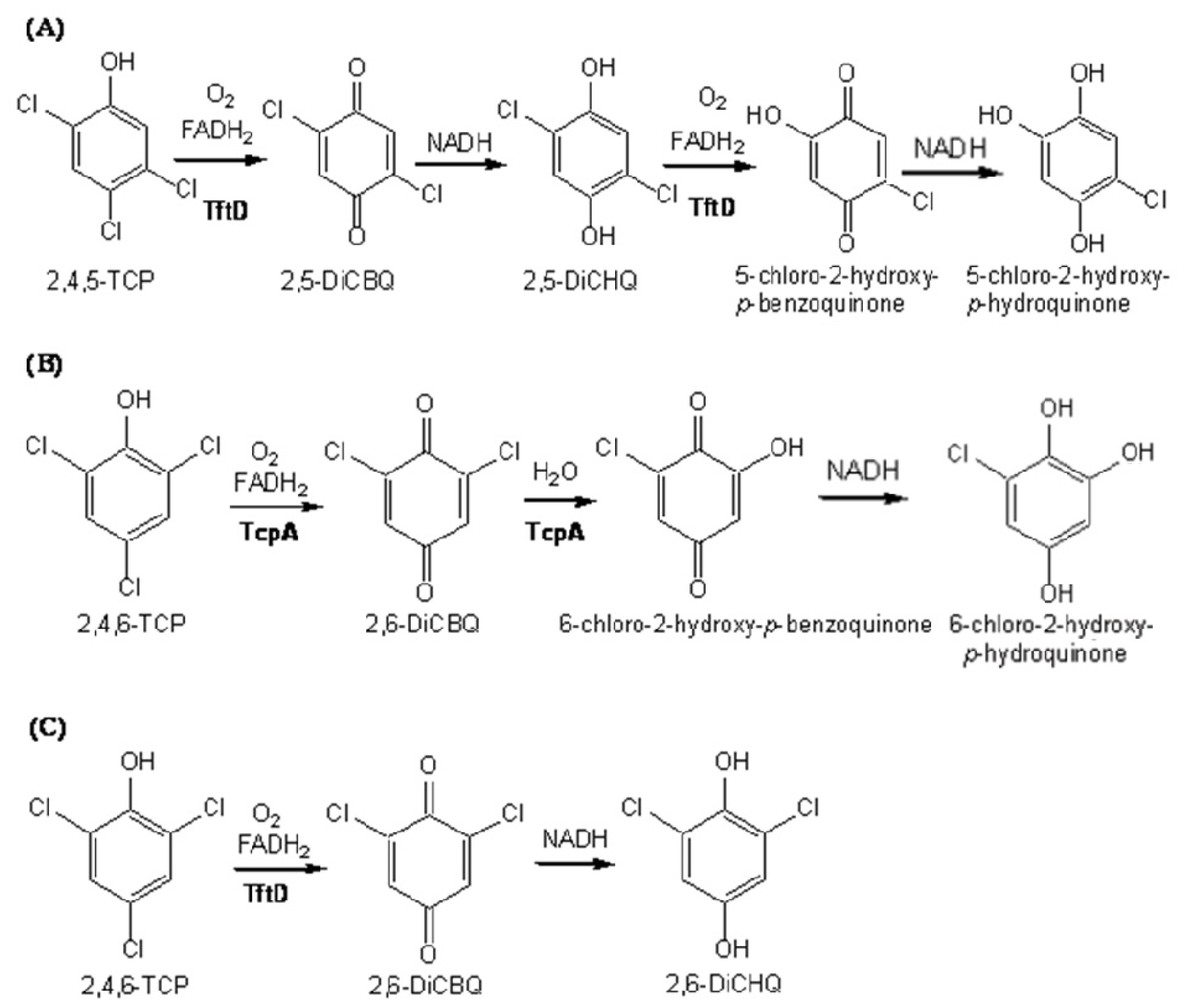
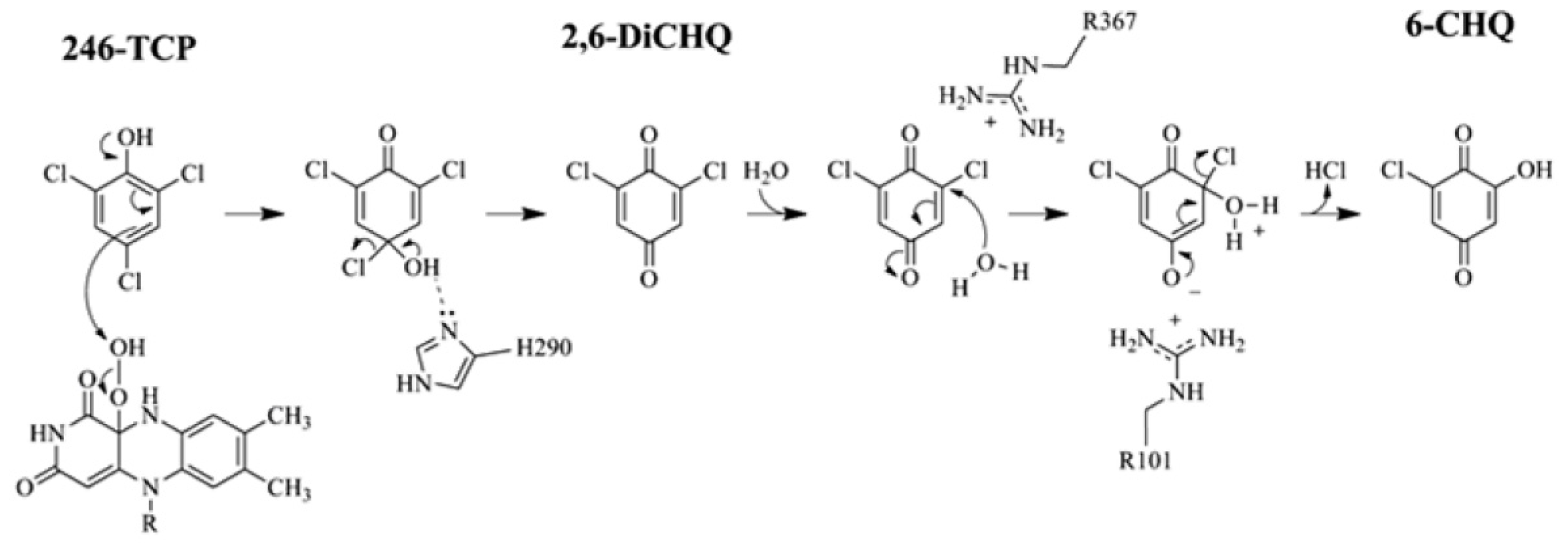
2.4. Substrate Binding Site and Reaction Mechanism: Differences between TftD and TcpA
3. Experimental Section
3.1. Expression and Purification
3.2. Crystallization and Data Collection
3.3. Structure Determination and Refinement
3.4. Molecular Docking of FADH2 and Substrates
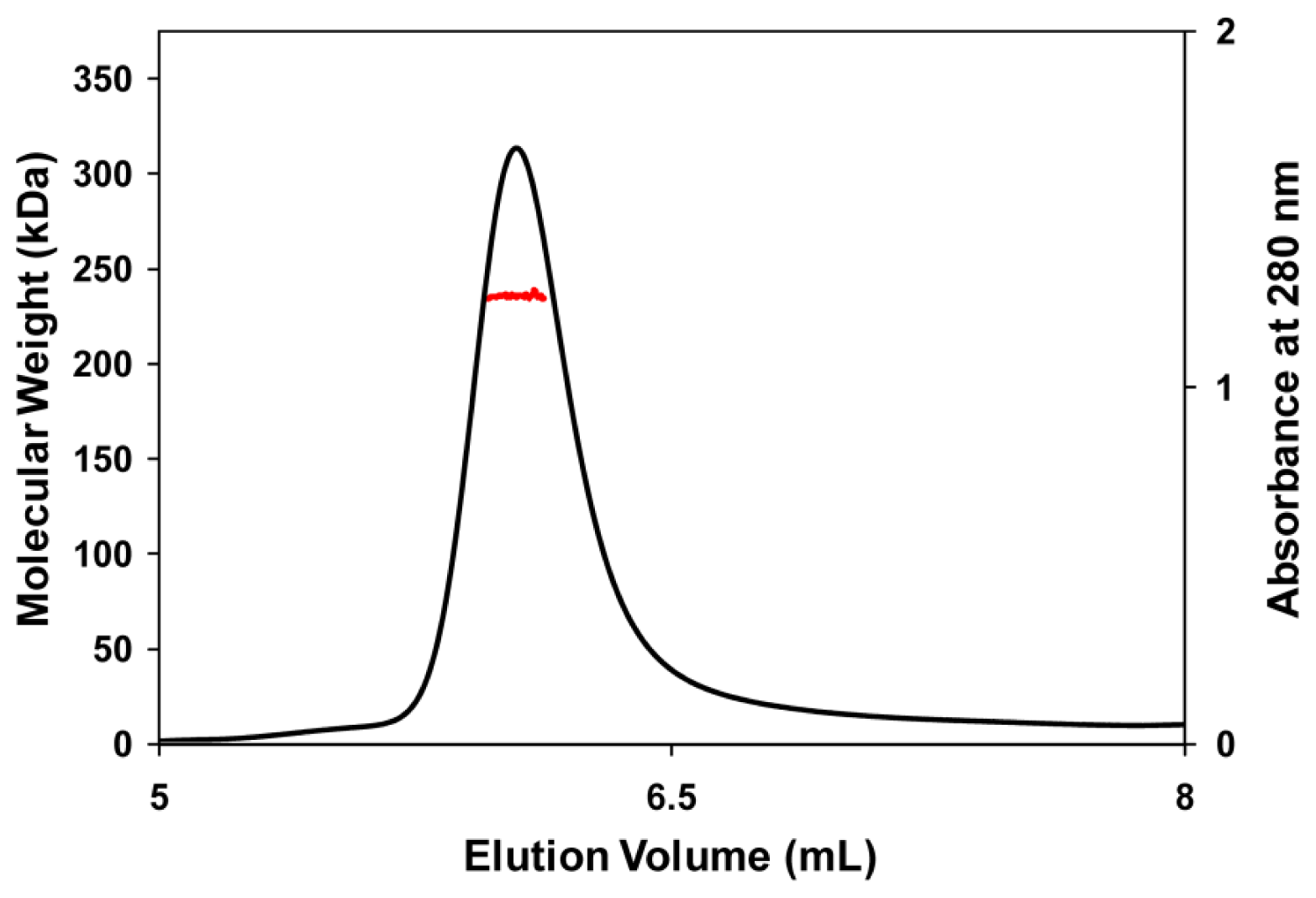
3.5. Molecular Mass Determination
4. Conclusions
Acknowledgments
References
- Czaplicka, M. Sources and transformations of chlorophenols in the natural environment. Sci. Total Environ 2004, 322, 21–39. [Google Scholar]
- U.S. EPA, Integrated Risk Information System (IRIS) on 2,4,6-Trichlorophenol. In National Center for Environmental Assessment, Office of Research and Development; U.S. Environmental Protection Agency: Washington, DC, USA; p. 1999.
- Xun, L.; Wagon, K. Purification and properties of 2,4,5-trichlorophenoxyacetate oxygenase from Pseudomonas cepacia AC1100. Appl. Environ. Microbiol 1995, 61, 3499–3502. [Google Scholar]
- Bock, C.; Kroppenstedt, R.M.; Schmidt, U.; Diekmann, H. Degradation of prochloraz and 2,4,6-trichlorophenol by environmental bacterial strains. Appl. Microbiol. Biotech 1996, 45, 257–262. [Google Scholar]
- Crosby, D.G. Environmental chemistry of pentachlorophenol. Pure Appl. Chem 1981, 53, 1052–1080. [Google Scholar]
- Firestone, D. The 2,3,7,8-trtrachlorodibenzo-para-dioxin problem: A review. Stockh. Ecol. Bull 1978, 27, 39–52. [Google Scholar]
- Hoekstra, E.; de Weerd, H.; de Leer, E.; Brinkman, U. Natural formation of chlorinated phenols, dibenzo-p-dioxins, and dibenzofurans in soil of a Douglas fir forest. Environ. Sci. Technol 1999, 33, 2543–2549. [Google Scholar]
- Kintz, P.; Tracqui, A.; Mangin, P. Accidental death caused by the absorption of 2,4-dichlorophenol through the skin. Arch. Toxicol 1992, 66, 298–299. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Chlorophenols; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 1999.
- Kilbane, J.; Chatterjee, D.; Chakrabarty, A. Biodegradation of 2,4,5-trichlorophenoxyacetic acid by a pure culture of Pseudomonas cepacia. Appl. Environ. Microbiol 1982, 44, 72–78. [Google Scholar]
- Kellogg, S.; Chatterjee, D.; Chakrabarty, A. Plasmid-assisted molecular breeding: New technique for enhanced biodegradation of persistent toxic chemicals. Science 1981, 214, 1133–1135. [Google Scholar]
- Louie, T.; Webster, C.; Xun, L. Genetic and biochemical characterization of a 2,4,6-trichlorophenol degradation pathway in Ralstonia eutropha JMP134. J. Bacteriol 2002, 184, 3492–3500. [Google Scholar]
- Xun, L.; Webster, C.M. A monooxygenase catalyzes sequential dechlorinations of 2,4,6-trichlorophenol by oxidative and hydrolytic reactions. J. Biol. Chem 2004, 279, 6696–6700. [Google Scholar]
- Xun, L. Purification and characterization of chlorophenol 4-monooxygenase from Burkholderia cepacia AC1100. J. Bacteriol 1996, 178, 2645–2649. [Google Scholar]
- Reineke, W.; Knackmuss, J.-J. Microbial degradation of haloaromatics. Ann. Rev. Microbiol 1988, 42, 263–287. [Google Scholar]
- Husain, M.; Entsch, B.; Ballou, D.P.; Massey, V.; Chapman, P.J. Fluoride elimination from substrates in hydroxylation reaction catalyzed by p-hydroxybenzoate hydroxylase. J. Biol. Chem 1980, 255, 4189–4197. [Google Scholar]
- Dai, M.; Rogers, J.B.; Warner, J.R.; Copley, S.D. A previously unrecognized step in pentachlorophenol degradation in Sphingobium chlorophenolicum is catalyzed by tetrachlorobenzoquinone reductase (PcpD). J. Bacteriol 2003, 185, 302–310. [Google Scholar]
- Haigler, B.E.; Suen, W.C.; Spain, J.C. Purification and sequence analysis of 4-methyl-5-nitrocatechol oxygenase from Burkholderia sp. strain DNT. J. Bacteriol 1996, 178, 6019–6024. [Google Scholar]
- Liu, Y.; Louie, T.; Xun, L. Purification and characterizatoin of iminodiacetate-dehydrogenase from the EDTA-degrading strain BNC1. Appl. Environ. Microbiol 2001, 67, 696–701. [Google Scholar]
- Perry, L.; Zylstra, G. Cloning of a gene cluster involved in the catabolism of p-nitrophenol by Arthrobacter sp. strain JS443 and characterization of the p-nitrophenol monooxygenase. J. Bacteriol 2007, 189, 7563–7572. [Google Scholar]
- Otto, K.; Hofstetter, K.; Röthlisberger, M.; Witholt, B.; Schmid, A. Biochemical characterization of StyAB from Pseudomonas sp. strain VLB120 as a two-component flavin-diffusible monooxygenase. J. Bacteriol 2004, 186, 5292–5302. [Google Scholar]
- Galán, B.; Díaz, E.; Prieto, M.; García, J. Functional analysis of the small component of the 4-hydroxyphenylacetate 3-monooxygenase of Escherichia coli W: A prototype of a new Flavin:NAD(P)H reductase subfamily. J. Bacteriol 2000, 182, 627–636. [Google Scholar]
- Kirchner, U.; Westphal, A.; Müller, R.; van Berkel, W. Phenol hydroxylase from Bacillus thermoglucosidasius A7, a two-protein component monooxygenase with a dual role for FAD. J. Biol. Chem 2003, 278, 47545–47553. [Google Scholar]
- Malito, E.; Alfieri, A.; Fraaije, M.; Mattevi, A. Crystal structure of a Baeyer-Villiger monooxygenase. Proc. Natl. Acad. Sci. USA 2004, 101, 13157–13162. [Google Scholar]
- Li, L.; Liu, X.; Yang, W.; Xu, F.; Wang, W.; Feng, L.; Bartlam, M.; Wang, L.; Rao, Z. Crystal structure of long-chain alkane monooxygenase (LadA) in complex with coenzyme FMN: Unveiling the long-chain alkane hydroxylase. J. Mol. Biol 2008, 376, 453–465. [Google Scholar]
- Valton, J.; Fontecave, M.; Douki, T.; Kendrew, S.; Nivière, V. An aromatic hydroxylation reaction catalyzed by a two-component FMN-dependent Monooxygenase. The ActVA-ActVB system from Streptomyces coelicolor. J. Biol. Chem 2006, 281, 27–35. [Google Scholar]
- Gisi, M.; Xun, L. Characterization of chlorophenol 4-monooxygenase (TftD) and NADH: Flavin adenine dinucleotide oxidoreductase (TftC) of Burkholderia cepacia AC1100. J. Bacteriol 2003, 185, 2786–2792. [Google Scholar]
- Massey, V. Activation of molecular oxygen by flavins and flavoproteins. J. Biol. Chem 1994, 269, 22459–22462. [Google Scholar]
- Filisetti, L.; Fontecave, M.; Nivière, V. Mechanism and substrate specificity of the flavin reductase ActVB from Streptomyces coelicolor. J. Biol. Chem 2003, 278, 296–303. [Google Scholar]
- Gao, B.; Ellis, H. Altered mechanism of the alkanesulfonate FMN reductase with the monooxygenase enzyme. Biochim. Biophys. Res. Commun 2005, 331, 1137–1145. [Google Scholar]
- Nissen, M.; Youn, B.; Knowles, B.; Ballinger, J.; Jun, S.; Belchik, S.; Xun, L.; Kang, C. Crystal structures of NADH:FMN oxidoreductase (EmoB) at different stages of catalysis. J. Biol. Chem 2008, 283, 28710–28720. [Google Scholar]
- Kim, S.; Hisano, T.; Takeda, K.; Iwasaki, W.; Ebihara, A.; Miki, K. Crystal structure of the oxygenase component (HpaB) of the 4-hydroxyphenylacetate 3-monooxygenase from Thermus thermophilus HB8. J. Biol. Chem 2007, 282, 33107–33117. [Google Scholar]
- Webb, B.N.; Ballinger, J.W.; Kim, E.; Belchik, S.M.; Lam, K.-S.; Youn, B.; Nissen, M.S.; Xun, L.; Kang, C. Characterization of Chlorophenol 4-Monooxygenase (TftD) and NADH:FAD Oxidoreductase (TftC) of Burkholderia cepacia AC1100. J. Biol. Chem 2010, 285, 2014–2027. [Google Scholar]
- Navaza, J. Implementation of molecular replacement in AMoRe. Acta Crystallogr. Sect 2001, 57, 1367–1372. [Google Scholar]
- Emsley, P.; Cowtan, K. Coot: Model-Building tools for molecular graphics. Acta Crystallogr. Sect 2004, 60, 2126–2132. [Google Scholar]
- Brunger, A.T.; Adams, P.D.; Clore, G.M.; DeLano, W.L.; Gros, P.; Grosse-Kunstleve, R.W.; Jiang, J.-S.; Kuszewski, J.; Nilges, M.; Pannu, N.S.; et al. Crystallography & NMR System: A new software suite for macromolecular structure determination. Acta Crystallogr. Sect 1998, 54, 905–921. [Google Scholar]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model 1999, 17, 57–61. [Google Scholar]
- Youn, B.; Moinuddin, S.; Davin, L.; Lewis, N.; Kang, C. Crystal structures of apo-form and binary/ternary complexes of Podophyllum secoisolariciresinol dehydrogenase, an enzyme involved in formation of health-protecting and plant defense lignans. J. Biol. Chem 2005, 280, 12917–12926. [Google Scholar]
| Data | Apo |
|---|---|
| Wavelength (Å) | 1.00 |
| Resolution (Å) | 49.5–2.0 |
| Space group | C2 |
| Cell dimensions (Å) | a = 107.07 b = 180.05 c = 110.20 α = 90.00 β = 97.87 γ = 90.00 |
| Asymmetric unit | 4 |
| Total observations | 437,758 |
| Unique reflections | 126,447 |
| Completeness (%) | 97.4 (75.5) |
| Rsym a,b | 0.010 (0.898) |
| Refinement | |
| Resolution (Å) | 49.5–2.5 |
| Number of reflections | 67,560 (95%, >1σI) |
| Rcryst c | 17.4 |
| Rfree d | 22.4 |
| r.m.s.d. bonds (Å) | 0.008 |
| r.m.s.d. angles (º) | 1.081 |
| Ramachandran Plot (%) | |
| Preferred | 95.18 |
| Allowed | 3.91 |
| Outliers | 0.91 |
| Number of atoms | |
| Protein | 15,103 |
| Water | 315 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hayes, R.P.; Webb, B.N.; Subramanian, A.K.; Nissen, M.; Popchock, A.; Xun, L.; Kang, C. Structural and Catalytic Differences between Two FADH2-Dependent Monooxygenases: 2,4,5-TCP 4-Monooxygenase (TftD) from Burkholderia cepacia AC1100 and 2,4,6-TCP 4-Monooxygenase (TcpA) from Cupriavidus necator JMP134. Int. J. Mol. Sci. 2012, 13, 9769-9784. https://doi.org/10.3390/ijms13089769
Hayes RP, Webb BN, Subramanian AK, Nissen M, Popchock A, Xun L, Kang C. Structural and Catalytic Differences between Two FADH2-Dependent Monooxygenases: 2,4,5-TCP 4-Monooxygenase (TftD) from Burkholderia cepacia AC1100 and 2,4,6-TCP 4-Monooxygenase (TcpA) from Cupriavidus necator JMP134. International Journal of Molecular Sciences. 2012; 13(8):9769-9784. https://doi.org/10.3390/ijms13089769
Chicago/Turabian StyleHayes, Robert P., Brian N. Webb, Arun Kumar Subramanian, Mark Nissen, Andrew Popchock, Luying Xun, and ChulHee Kang. 2012. "Structural and Catalytic Differences between Two FADH2-Dependent Monooxygenases: 2,4,5-TCP 4-Monooxygenase (TftD) from Burkholderia cepacia AC1100 and 2,4,6-TCP 4-Monooxygenase (TcpA) from Cupriavidus necator JMP134" International Journal of Molecular Sciences 13, no. 8: 9769-9784. https://doi.org/10.3390/ijms13089769
APA StyleHayes, R. P., Webb, B. N., Subramanian, A. K., Nissen, M., Popchock, A., Xun, L., & Kang, C. (2012). Structural and Catalytic Differences between Two FADH2-Dependent Monooxygenases: 2,4,5-TCP 4-Monooxygenase (TftD) from Burkholderia cepacia AC1100 and 2,4,6-TCP 4-Monooxygenase (TcpA) from Cupriavidus necator JMP134. International Journal of Molecular Sciences, 13(8), 9769-9784. https://doi.org/10.3390/ijms13089769