Chitosan Interaction with Iron from Yoghurt Using an In Vitro Digestive Model: Comparative Study with Plant Dietary Fibers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Fibers
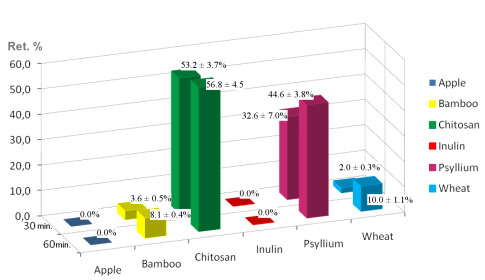
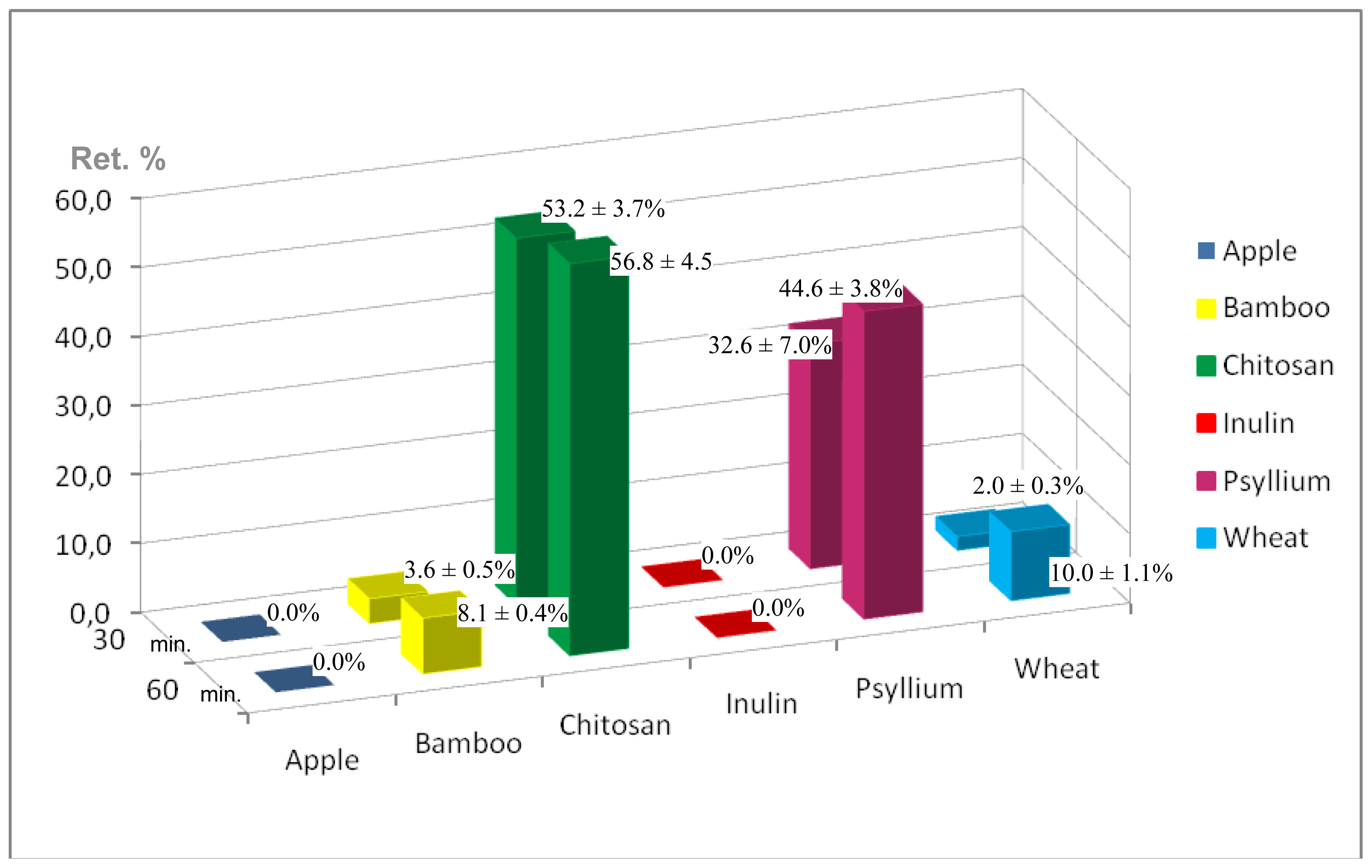
2.2. Digestive Chemical Model and Iron Retention Percentages
3. Experimental Section
3.1. Chitosan
3.2. Plant Fibers
3.3. Analysis for Dietary Fiber
3.4. Yoghurt Preparation
3.5. Digestive Chemical Experimental Model
3.6. Iron Content Determination
3.7. Iron Retention Percentage Calculations
3.8. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Arai, K; Minumari, K; Fujita, T. On the toxicity of chitosan. Bull. Tokai Reg. Fish. Res. Lab 1968, 56, 889–892. [Google Scholar]
- Kumar, MNVR; Muzzarelli, RAA; Muzzarelli, C; Sashiwa, H; Domb, AJ. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev 2004, 104, 6017–6084. [Google Scholar]
- Harish Prashanth, KV; Lakshman, K; Shamala, TR; Tharanathan, RN. Biodegradation of chitosan-graft-polymethylmethacrylate films. Int. Biodeterior. Biodegrad 2005, 56, 115–120. [Google Scholar]
- Cummings, TJ; Mann, J; Nishida, C; Vorster, H. Dietary fibre: An agreed definition. Lancet 2009, 373, 365–366. [Google Scholar]
- Harris, SS; Pijls, L. Dietary fibre: Refining a definition. Lancet 2009, 374, 28. [Google Scholar]
- Phillips, GO; Cui, SW. An introduction: Evolution and finalisation of the regulatory definition of dietary. Food. Hydrocolloid 2010, 25, 139–143. [Google Scholar]
- Harish Prashanth, KV; Tharanathan, RN. Chitin/chitosan: Modifications and their unlimited application potential. Trends Food Sci. Technol 2007, 18, 117–131. [Google Scholar]
- Elleuch, M; Bedigian, D; Roiseux, O; Besbes, S; Blecker, C; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterization, technological functionality and commercial applications: A review. Food Chem 2011, 124, 411–421. [Google Scholar]
- Beard, J; Stoltzfus, RJ. Iron-deficiency anemia: Reexamining the nature and magnitude of the public health problem. J. Nutr 2001, 131, 563S–703S. [Google Scholar]
- Yasuda, K; Roneker, KR; Miller, DD; Welch, RM; Lei, XG. Supplemental dietary insulin affects the bioavailability of iron in corn and soybean meal to young pigs. J. Nutr 2006, 136, 3033–3038. [Google Scholar]
- Thompson, SA; Weber, CW. Influence of pH on the binding of copper, zinc and iron in six fiber sources. J. Food. Sci 1979, 44, 752–754. [Google Scholar]
- Laszlo, JA. Mineral binding properties of soy hull. Modelling mineral interactions with an insoluble dietary fiber source. J. Agric. Food. Chem 1987, 35, 593–600. [Google Scholar]
- Torre, M; Rodriguez, AR; Saura-Calixto, F. Interactions of Fe(II), Ca(I1) and Fe(II1) with high dietary fibre materials: A physicochemical approach. Food Chem 1995, 54, 23–31. [Google Scholar]
- Leigh, MJ; Miller, DD. Effects of pH and chelating agents on iron binding by dietary fiber. Implications for iron availability. Am. J. Clin. Nutr 1983, 38, 202–213. [Google Scholar]
- Shen, H; Luten, J; Robberecht, H; Bindels, J; Deelstra, H. Modification of an in vitro method for estimating the bioavailability of zinc and calcium from foods. Z. Lebensm. Unters. Forsch 1994, 199, 442–445. [Google Scholar]
- Argyri, K; Birba, A; Miller, DD; Komaitis, M; Kapsokefalou, M. Predicting relative concentrations of bioavailable iron in foods using in vitro digestion: New developments. Food Chem 2009, 113, 602–607. [Google Scholar]
- Vitali, D; Vedrina-Dragojević, I; Šebečić, B; Vujić, L. Impact of modifying tea-biscuit composition on phytate levels and iron content and availability. Food Chem 2007, 102, 82–89. [Google Scholar]
- Kumar, V; Sinha, AK; Makkar, HPS; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem 2010, 120(4), 945–959. [Google Scholar]
- Hur, SJ; Lim, BO; Decker, EA; McClements, DJ. In vitro human digestion models for food applications. Food Chem 2011, 125, 1–12. [Google Scholar]
- Laparra, JM; Tako, E; Glahn, RP; Miller, DD. Supplemental inulin does not enhance iron bioavailability to Caco-2 cells from milk- or soy-based, probiotic-containing, yoghurts but incubation at 37 °C does. Food Chem 2008, 109, 122–128. [Google Scholar]
- Yeung, CK; Glahn, RP; Welch, RM; Miller, DD. Prebiotics and iron bioavailability—Is there a connection? J. Food Sci 2005, 70, 88–92. [Google Scholar]
- Drago, SR; Valencia, ME. Effect of fermentation on iron, zinc, and calcium availability from iron-fortified dairy products. J. Food Sci 2002, 67, 3130–3134. [Google Scholar]
- Dello Staffolo, M; Bertola, N; Martino, M; Bevilacqua, A. Influence of dietary fibre addition on sensory and rheological properties of yoghurt. Int. Dairy J 2004, 14, 263–268. [Google Scholar]
- Rodríguez, M; Albertengo, L. Interaction between chitosan and oil under stomach and duodenal digestive chemical conditions. Biosci. Biotechnol. Biochem 2005, 69, 2057–2062. [Google Scholar]
- Rodríguez, MS; Montero, M; Dello Staffolo, M; Martino, M; Bevilacqua, A; Albertengo, L. Chitosan influence on glucose and calcium availability from yoghurt: In vitro comparative study with plants fibre. Carbohyd. Polym 2008, 74, 797–801. [Google Scholar]
- Tungland, B; Meyer, D. Nondigestible oligo- and polysaccharides (dietary fiber): Their physiology and role in human health and food. Compr. Rev. Food Sci. Food Saf 2002, 3, 73–91. [Google Scholar]
- Total, soluble, and insoluble dietary fibre in foods. AOAC Official Methods 991.43. In Official Methods of Analysis, 16th ed; Cunniff, P (Ed.) AOAC International: Arlington, VA, USA, 1995; Volume Chapter 32, pp. 7–9.
- Van Craeyveld, V; Delcour, JA; Courtin, CM. Extractability and chemical and enzymic degradation of psyllium (Plantago ovata Forsk) seed husk arabinoxylans. Food Chem 2009, 112, 812–819. [Google Scholar]
- Sudha, ML; Baskaran, V; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem 2007, 104, 686–692. [Google Scholar]
- Dierenfeld, ES; Hintz, HF; Robertson, JB; van Soest, PJ; Oftedal, OT. Utilization of bamboo by the giant panda. J. Nutr 1982, 112, 636–641. [Google Scholar]
- Robertson, JB; Van Soest, PJ. The detergent system of analysis and its applications to human foods. In The Analysis of Dietary Fiber and Food; James, WPT, Theander, O, Eds.; Marcel Dekker: New York, NY, USA, 1981; pp. 139–153. [Google Scholar]
- Escarnot, E; Agneessens, R; Wathelet, B; Paquot, M. Quantitative and qualitative study of spelt and wheat fibres in varying milling fractions. Food Chem 2010, 122, 857–863. [Google Scholar]
- Miret, S; Simpson, RJ; McKie, AT. Physiology and molecular biology of dietary iron absorption. Annu. Rev. Nutr 2003, 23, 283–301. [Google Scholar]
- Bouhallab, S; Bouglé, D. Biopeptides of milk: Caseinophosphopeptides and mineral bioavailability. Reprod. Nutr. Dev 2004, 44, 493–498. [Google Scholar]
- Sun-Waterhouse, D; Farr, J; Wibisono, R; Saleh, Z. Fruit-based functional foods I: Production of food-grade apple fibre ingredients. Int. J. Food Sci. Technol 2008, 43, 2113–2122. [Google Scholar]
- Chan, JKC; Wypyszyk, VA. Forgotten natural dietary fiber: Psyllium mucilloid. Cereal Food. World 1988, 33, 919–922. [Google Scholar]
- Fischer, MH; Yu, N; Gray, GR; Ralph, J; Andersond, L; Marletta, JA. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk). Carbohyd. Res 2004, 339, 2009–2017. [Google Scholar]
- Anal, AK; Tobiassen, A; Flanagan, J; Singh, H. Preparation and characterization of nanoparticles formed by chitosan–caseinate interactions. Colloid. Surface. B 2008, 64, 104–110. [Google Scholar]
- Ausar, SF; Bianco, ID; Badini, RG; Castagna, LF; Modesti, NM; Landa, CA; Beltramo, DM. Characterization of casein micelle precipitation by chitosans. J. Dairy Sci 2001, 84, 361–369. [Google Scholar]
- Van den Heuvel, EG; Schaafsma, G; Muys, T; van Dokkum, W. Nondigestible oligosaccharides do not interfere with calcium and nonheme-iron absorption in young, healthy men. Am. J. Clin. Nutr 1998, 67, 445–451. [Google Scholar]
- Bosscher, D; van Caillie-Bertrand, M; van Cauwenbergh, R; Deelstra, H. Availabilities of calcium iron and zinc from dairy infant formulas is affected by soluble dietary fibres and modified starch fractions. Nutrition 2003, 19, 641–645. [Google Scholar]
- Azorín-Ortuño, M; Urbán, C; Cerón, JJ; Tecles, F; Allende, A; Tomás-Barberán, FA; Espín, JC. Effect of low inulin doses with different polymerisation degree on lipid metabolism, mineral absorption, and intestinal microbiota in rats with fat-supplemented diet. Food Chem 2009, 113, 1058–1065. [Google Scholar]
- Fairweather-Tait, S; Lynch, S; Hotz, C; Hurrell, R; Abrahamse, L; Beebe, S; Bering, S; Bukhave, K; Glahn, R; Hambidge, M; et al. The usefulness of in vitro models to predict the bioavailability of iron and zinc: A consensus statement from the Harvest Plus expert consultation. Int. J. Vitam. Nutr. Res 2005, 7, 371–374. [Google Scholar]
- Moreira, M; Abraham, A; De Antoni, G. Technological properties of milks fermented with thermophilic lactic acid bacteria at suboptimal temperature. J. Dairy Sci 2000, 83, 395–400. [Google Scholar]
- Tamine, A; Robinson, R. Yoghurt Science and Technology, 3rd ed; CRC Press: Boca Raton, FL, USA, 2007; Volume Chapter 2. [Google Scholar]
- Fernández-García, E; McGregor, JU. Fortification of sweetened plain yoghurt with insoluble dietary fibre. Z. Lebensm. Unters. Forsch 1997, 204, 433–437. [Google Scholar]


| Fiber | Insoluble fiber (g/100g) | % Insoluble fiber | Soluble fiber (g/100g) | % Soluble fiber | Total fiber |
|---|---|---|---|---|---|
| Apple | 44.8 ± 0.4 | 77.1 | 13.3 ± 0.7 | 22.9 | 58.1 ± 1.0 |
| Bamboo | 91.4 ± 0.5 | 95.9 | 3.2 ± 0.8 | 3.4 | 95.3 ± 0.9 |
| Chitosan | 98.0 ± 1.0 | 100 | nd | nd | 98.0 ± 1.0 |
| Inulin | nd | nd | ≥85.5 | 100 | ≥85.5 |
| Psyllium | 37.5 ± 0.6 | 82.9 | 7.1 ± 0.5 | 15.7 | 45.2 ± 0.8 |
| Wheat | 92.1 ± 0.6 | 97.6 | 2.3 ± 0.6 | 2.4 | 94.4 ± 1.1 |
| Fiber | ADF | NDF | Lignin | Cellulose | Hemicellulose |
|---|---|---|---|---|---|
| Apple | 38.6 ± 0.9 | 44.3 ± 0.7 | 8.4 ± 0.8 | 30.2 ± 1.7 | 5.7 ± 1.6 |
| Bamboo | 50.2 ± 0.7 | 90.4 ± 0.6 | 5.0 ± 0.3 | 45.2 ± 1.0 | 40.2 ± 1.7 |
| Psyllium | 7.3 ± 0.4 | 36.8 ± 0.9 | 0.8 ± 0.1 | 6.5 ± 0.4 | 29.5 ± 1.3 |
| Wheat | 74.8 ± 0.3 | 89.7 ± 0.6 | 2.6 ± 0.4 | 72.2 ± 0.7 | 14.9 ± 0.9 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dello Staffolo, M.; Martino, M.; Bevilacqua, A.; Montero, M.; Rodríguez, M.S.; Albertengo, L. Chitosan Interaction with Iron from Yoghurt Using an In Vitro Digestive Model: Comparative Study with Plant Dietary Fibers. Int. J. Mol. Sci. 2011, 12, 4647-4660. https://doi.org/10.3390/ijms12074647
Dello Staffolo M, Martino M, Bevilacqua A, Montero M, Rodríguez MS, Albertengo L. Chitosan Interaction with Iron from Yoghurt Using an In Vitro Digestive Model: Comparative Study with Plant Dietary Fibers. International Journal of Molecular Sciences. 2011; 12(7):4647-4660. https://doi.org/10.3390/ijms12074647
Chicago/Turabian StyleDello Staffolo, Marina, Miriam Martino, Alicia Bevilacqua, Mirta Montero, María Susana Rodríguez, and Liliana Albertengo. 2011. "Chitosan Interaction with Iron from Yoghurt Using an In Vitro Digestive Model: Comparative Study with Plant Dietary Fibers" International Journal of Molecular Sciences 12, no. 7: 4647-4660. https://doi.org/10.3390/ijms12074647
APA StyleDello Staffolo, M., Martino, M., Bevilacqua, A., Montero, M., Rodríguez, M. S., & Albertengo, L. (2011). Chitosan Interaction with Iron from Yoghurt Using an In Vitro Digestive Model: Comparative Study with Plant Dietary Fibers. International Journal of Molecular Sciences, 12(7), 4647-4660. https://doi.org/10.3390/ijms12074647