Antioxidant Properties of Aminoethylcysteine Ketimine Decarboxylated Dimer: A Review
Abstract
:1. Introduction
2. Sulfur-Containing Antioxidants
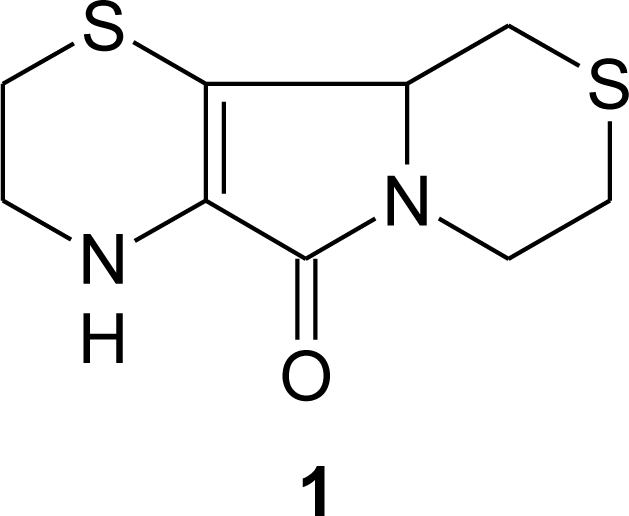
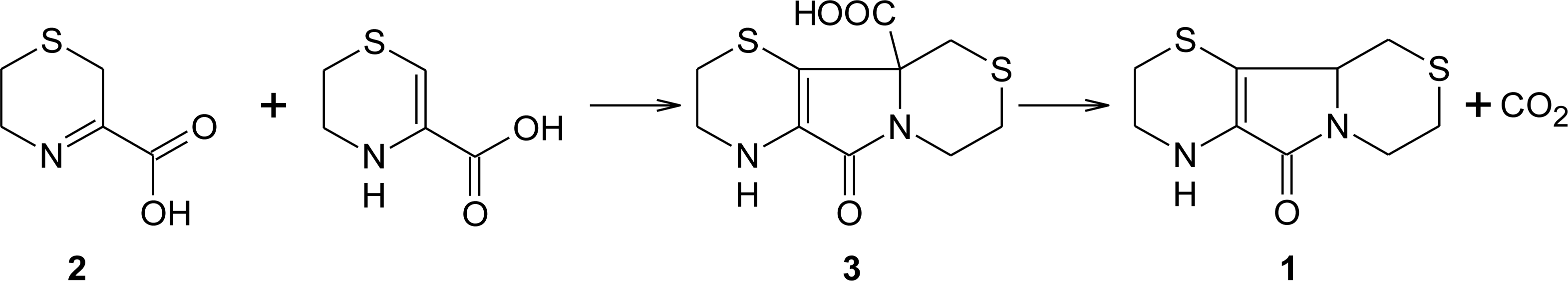
3. Aminoethylcysteine Ketimine Decarboxylated Dimer
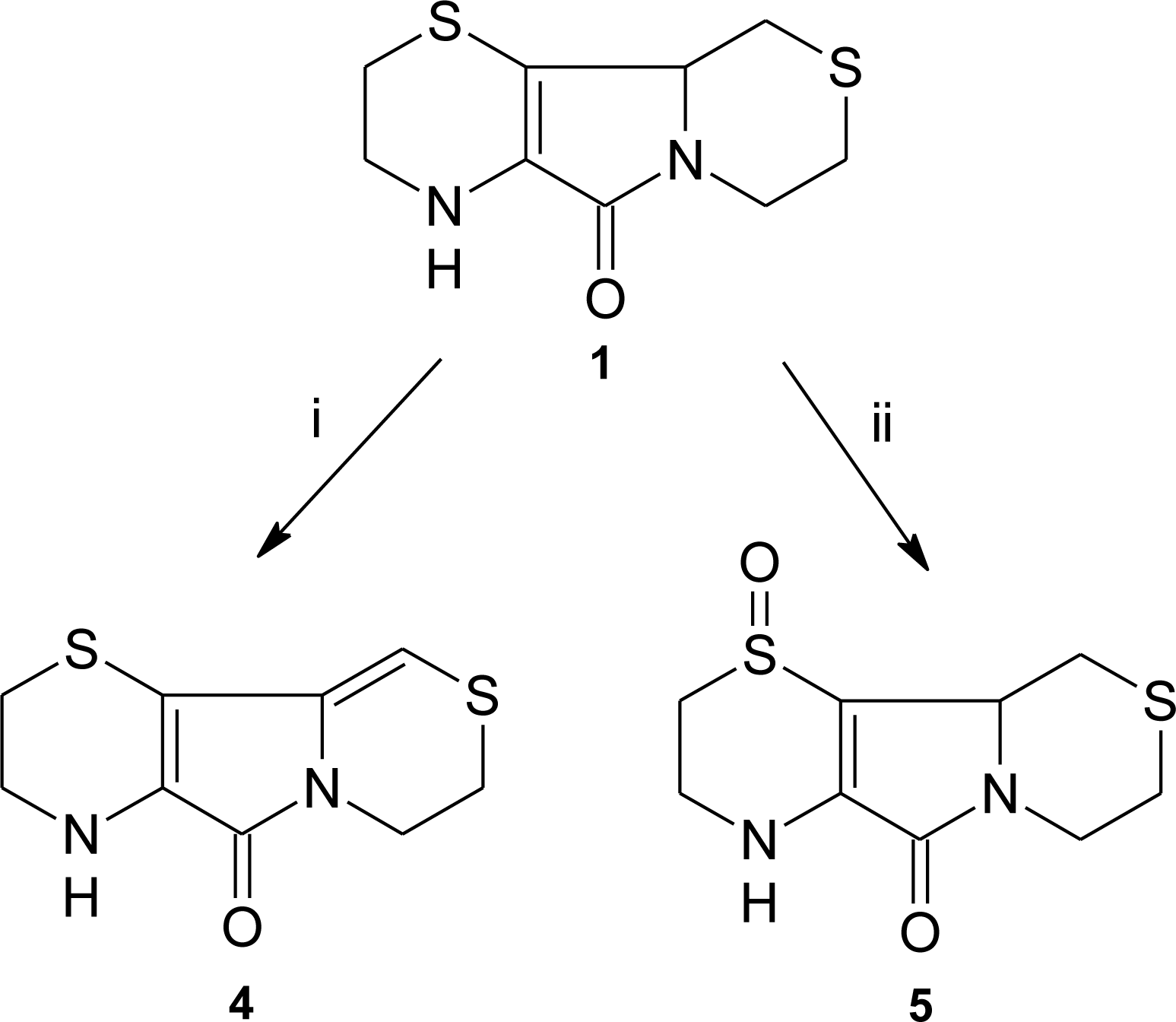
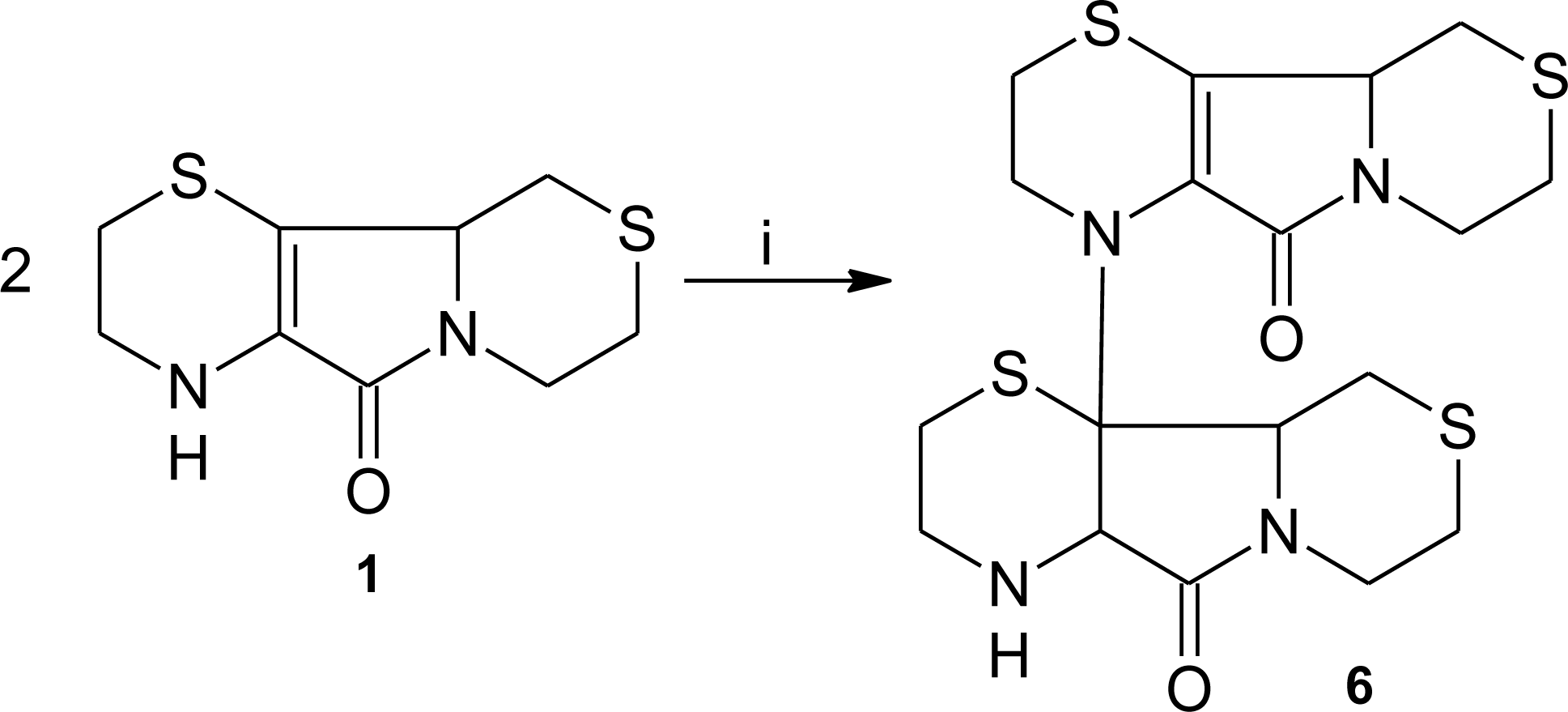
3.1. Aminoethylcysteine Ketimine Decarboxylated Dimer as Scavenger of Reactive Oxygen Species
3.2. Aminoethylcysteine Ketimine Decarboxylated Dimer as Scavenger of Reactive Nitrogen Species
4. Conclusions
Acknowledgments
References
- Davies, K. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp 1995, 61, 1–31. [Google Scholar]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol 1997, 82, 291–295. [Google Scholar]
- Bjelakovic, G; Nikolova, D; Gluud, LL; Simonetti, RG; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar]
- Matill, HA. Antioxidants. Ann. Rev. Biochem 1947, 16, 177–192. [Google Scholar]
- Vertuani, S; Angusti, A; Manfredini, S. The antioxidants and pro-antioxidants network: An overview. Curr. Pharm. Des 2004, 10, 1677–1694. [Google Scholar]
- Miller, RA; Britigan, BE. Role of oxidant in microbial pathophysiology. Clin. Microbiol. Rev 1997, 10, 1–18. [Google Scholar]
- Valko, M; Leibfritz, D; Moncol, J; Cronin, MTD; Mazur, M; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol 2007, 39, 44–84. [Google Scholar]
- Gosslau, A; Chen, KY. Nutraceuticals, apoptosis, and disease prevention. Nutrition 2004, 20, 95–102. [Google Scholar]
- Law, MR; Morris, JK. By how much does fruit and vegetable consumption reduce the risk of ischaemic heart disease? Eur. J. Clin. Nutr 1998, 52, 549–556. [Google Scholar]
- Gundgaard, J; Nielsen, JN; Olsen, J; Sørensen, J. Increased intake of fruit and vegetables: Estimation of impact in terms of life expectancy and healthcare costs. Public Health Nutr 2003, 6, 25–30. [Google Scholar]
- Brigelius-Flohé, R; Traber, MG. Vitamin E: Function and metabolism. FASEB J 1999, 13, 1145–1155. [Google Scholar]
- Migliore, L; Coppedè, F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat. Res 2009, 674, 73–84. [Google Scholar]
- Mitscher, LA; Telikepalli, H; McGhee, E; Shankel, DM. Natural antimutagenic agents. Mutat. Res 1996, 350, 142–143. [Google Scholar]
- Owen, RW; Giacosa, A; Hull, WE; Haubner, R; Spiegelhalder, B; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Canc 2000, 36, 1235–1247. [Google Scholar]
- Sala, A; Recio, MD; Giner, RM; Manez, S; Tournier, H; Schinella, G; Rios, JL. Anti-inflammatory and antioxidant properties of Helichrysum italicum. J. Pharm. Pharmacol 2002, 54, 365–371. [Google Scholar]
- Cavallini, D; Ricci, G; Duprè, S; Pecci, L; Costa, M; Matarese, RM; Pensa, B; Antonucci, A; Solinas, SP; Fontana, M. Sulfur-containing cyclic ketimines and imino acids. A novel family of endogenous products in the search for a role. Eur. J. Biochem 1991, 202, 217–23. [Google Scholar]
- Nardini, M; Matarese, RM; Pecci, L; Antonucci, A; Ricci, G; Cavallini, D. Detection of 2H-1,4-thiazine-5,6-dihydro-3-carboxylic acid(aminoethylcysteine ketimine) in the bovine brain. Biochem. Biophys. Res. Commun 1990, 166, 1251–1256. [Google Scholar]
- Ricci, G; Vesci, L; Nardini, M; Arduini, A; Storto, S; Rosato, N; Cavallini, D. Detection of 2H-1,4-thiazine-5,6-dihydro-3,5-dicarboxylic acid (lanthionine ketimine) in the bovine brain by a fluorimetric assay. Biochim. Biophys. Acta 1989, 990, 211–215. [Google Scholar]
- Cavallini, D; Pecci, L; Matarese, RM; Ricci, G; Achilli, M. Gas-chromatographic mass-spectrometric detection of 1,4-hexahydrothiazepi±ne-3,5-dicarboxylic acid (cyclothionine) in bovine brain. J. Biol. Chem 1985, 29, 15577–15579. [Google Scholar]
- Matarese, RM; Pecci, L; Ricci, G; Nardini, M; Antonucci, A; Cavallini, D. Hexahydro-1,4-thiazepine-3,5-dicarboxylic acid and thiomorpholine-3,5-dicarboxylic acid are present in normal human urine. Proc. Natl. Acad. Sci. USA 1987, 84, 5111–5114. [Google Scholar]
- Yu, S; Sugahara, K; Zhang, J; Ageta, T; Kodama, H; Fontana, M; Duprè, S. Simultaneous determination of urinary cystathionine, lanthionine, (S)-(2-aminoethyl)-l-cysteine and their cyclic compounds using liquid chromatography-mass spectrometry with atmospheric pressure chemical ionization. J. Chrom. B 1997, 698, 301–307. [Google Scholar]
- Matarese, RM; Macone, A; Antonini, R; Maggio, A; Antonucci, A. Identification of aminoethylcysteine ketimine decarboxylated dimer in human plasma. J. Chromatogr. B 1999, 732, 137–144. [Google Scholar]
- Matarese, RM; Macone, A; Maggio, A; Cavallini, D. Aminoethylcysteine ketimine decarboxylated dimer detected in normal human urine by gas-liquid chromatography, selected ion monitoring, and mass spectrometry. J. Chromatogr. B 1996, 683, 269–272. [Google Scholar]
- Matarese, RM; Macone, A; Crescentini, G; Duprè, S; Cavallini, D. Detection of a decarboxylated dimer of aminoethylcysteine ketimine in bovine cerebellum. Neurochem. Int 1998, 32, 365–368. [Google Scholar]
- Macone, A; Nardini, M; Antonucci, A; Maggio, A; Matarese, RM. Identification of aminoethylcysteine ketimine decarboxylated dimer, a natural antioxidant, in dietary vegetables. J. Agric. Food Chem 2002, 50, 2169–2172. [Google Scholar]
- Jacob, C; Giles, GI; Giles, NM; Sies, H. Sulfur and selenium: The role of oxidation state in protein structure and function. Angew. Chem. Int. Ed 2003, 42, 4742–4258. [Google Scholar]
- Giles, NM; Watts, AB; Giles, GI; Fry, FH; Littlechild, JA; Jacob, C. metal and redox modulation of cysteine protein function. Chem. Biol 2003, 10, 677–693. [Google Scholar]
- Jacob, C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep 2006, 23, 851–863. [Google Scholar]
- Atmaca, G. Antioxidant effects of sulfur-containing amino acids. Yonsei Med. J 2004, 45, 776–788. [Google Scholar]
- Hermann, P. Zur reaktion von halogenbrenztrabensaure mit thiolaminen. Chem. Ber 1961, 94, 442–445. [Google Scholar]
- Pinto, JT; Khomenko, T; Szabo, S; McLaren, GD; Denton, TT; Krasnikov, BF; Jeitner, TM; Cooper, AJL. Measurement of sulfur-containing compounds involved in the metabolism and transport of cysteamine and cystamine. Regional differences in cerebral metabolism. J. Chromatogr. B 2009, 877, 3434–3441. [Google Scholar]
- Greenberg, DM. Metabolic Pathways; Greenberg, DM, Ed.; Academic press: New York, NY, USA, 1975; pp. 505–520. [Google Scholar]
- Pecci, L; Montefoschi, G; Antonucci, A; Cavallini, D. Antioxidant properties of the decarboxylated dimer of aminoethylcysteine ketimine. Physiol. Chem. Phys. Med. NMR 1995, 27, 223–229. [Google Scholar]
- Pecci, L; Fontana, M; Montefoschi, G; Cavallini, D. Aminoethylcysteine ketimine decarboxylated dimer protects submitochondrial particles from lipid peroxidation at a concentration not inhibitory of electron transport. Biochem. Biophys. Res. Commun 1994, 205, 264–268. [Google Scholar]
- Antonucci, A; Pecci, L; Coccia, R; Fontana, M; Cavallini, D. The oxidation of aminoethylcysteine ketimine dimer by oxygen reactive species. Amino Acids 1994, 7, 83–88. [Google Scholar]
- Fontana, M; Pecci, L; Macone, A; Cavallini, D. Antioxidant properties of the decarboxylated dimer of aminoethylcysteine ketimine: Assessment of its ability to scavenge peroxynitrite. Free Radic. Res 1998, 29, 435–440. [Google Scholar]
- Macone, A; Caiazzo, A; Antonucci, A; Fochi, I; Nardini, M; Duprè, S; Matarese, RM. Synthesis and characterization of a dehydrogenation product arising from the oxidation of aminoethylcysteine ketimine decarboxylated dimer. J. Nat. Prod 2007, 70, 1046–1048. [Google Scholar]
- Pecci, L; Antonucci, A; Pinnen, F; Cavallini, D. Identification of an oxidation product of aminoethylcysteine ketimine dimer. Amino Acids 2000, 18, 61–67. [Google Scholar]
- Mannina, L; Viel, S; Duprè, S; Pecci, L; Fontana, M; Pinnen, F; Antonucci, A; Segre, L. Structural elucidation of the oxidation product of aminoethylcysteine ketimine decarboxylated dimer by peroxynitrite. Tetrahedron 2004, 60, 4151–4157. [Google Scholar]
- Steinberg, D; Parthasarathy, S; Carew, TE; Khoo, JC; Witztum, JL. Beyond cholesterol. modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med 1989, 320, 915–924. [Google Scholar]
- Witztum, JL; Steinberg, D. Role of oxidized low-density lipoprotein in atherogenesis. J. Clin. Invest 1991, 88, 1785–1792. [Google Scholar]
- Steinbrecher, UP; Zhang, HF; Lougheed, M. Role of oxidatively modified LDL in atherosclerosis. Free Radic. Biol. Med 1990, 9, 155–168. [Google Scholar]
- Esterbauer, H; Gebicki, J; Puhl, H; Jürgens, G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic. Biol. Med 1992, 13, 341–390. [Google Scholar]
- Matarese, RM; Macone, A; Fontana, M; Duprè, S; Cavallini, D. Antioxidant activity of aminoethylcysteine ketimine decarboxylated dimer on copper induced LDL oxidation. Biochem. Mol. Biol. Int 1998, 46, 829–837. [Google Scholar]
- Giessauf, A; Steiner, E; Esterbauer, H. Early destruction of tryptophan residues of apolipoprotein B is a vitamin E-independent process during copper-mediated oxidation of LDL. Biochim. Biophys. Acta 1995, 1256, 221–232. [Google Scholar]
- Noguchi, N; Gotoh, N; Niki, E. Dynamics of the oxidation of low density lipoprotein induced by free radicals. Biochim. Biophys. Acta 1993, 1168, 348–357. [Google Scholar]
- Halliwell, B; Gutteridge, JM. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol 1990, 186, 1–85. [Google Scholar]
- Smith, C; Mitchinson, MJ; Aruoma, OI; Halliwell, B. Stimulation of lipid peroxidation and hydroxyl-radical generation by the contents of human atherosclerotic lesions. Biochem. J 1992, 286, 901–905. [Google Scholar]
- Macone, A; Matarese, RM; Gentili, V; Antonucci, A; Duprè, S; Nardini, M. Effect of aminoethylcysteine ketimine decarboxylated dimer, a natural sulfur compound present in human plasma, on tert-butyl hydroperoxide-induced oxidative stress in human monocytic U937 cells. Free Radic. Res 2004, 38, 705–714. [Google Scholar]
- Devaraj, S; Li, D; Jialal, I. The effects of alpha tocopherol supplementation on monocyte function. decreased lipid oxidation, interleukin 1 beta secretion, and monocyte adhesion to endothelium. J. Clin. Invest 1996, 98, 756–763. [Google Scholar]
- Weber, C; Erl, W. Modulation of vascular cell activation, function, and apoptosis: Role of antioxidants and nuclear factor-kappa B. Curr. Top. Cell. Regul 2000, 36, 217–235. [Google Scholar]
- Jarvis, WD; Kolesnick, RN; Fornari, FA; Traylor, RS; Gewirtz, DA; Grant, S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc. Natl. Acad. Sci. USA 1994, 91, 73–77. [Google Scholar]
- Quillet-Mary, A; Jaffrézou, JP; Mansat, V; Bordier, C; Naval, J; Laurent, G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J. Biol. Chem 1997, 272, 21388–21395. [Google Scholar]
- Nardini, M; Macone, A; Matarese, RM. Determination of aminoethylcysteine ketimine decarboxylated dimer in human plasma and cultured cells by high performance liquid chromatography with electrochemical detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 2003, 795, 319–327. [Google Scholar]
- Masaki, N; Kyle, ME; Farber, JL. Tert-butyl hydroperoxide kills cultured hepatocytes by peroxidizing membrane lipids. Arch. Biochem. Biophys 1989, 269, 390–399. [Google Scholar]
- Radi, R. Nitric oxide, oxidants and protein nitration. Proc. Natl. Acad. Sci. USA 2004, 101, 4003–4008. [Google Scholar]
- Koppenol, WH; Moreno, JJ; Prior, WA; Ischiropoulos, H; Beckman, JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol 1992, 5, 834–842. [Google Scholar]
- Huie, RE; Padmaja, S. The reaction of NO with superoxide. Free Radic. Res. Commun 1993, 18, 195–199. [Google Scholar]
- Pryor, WA; Squadrito, GL. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am J Physiol 1995, 268, L699–L722. [Google Scholar]
- Szabo, C; Ohshima, H. DNA damage induced by peroxynitrite: Subsequent biological effects. Nitric Oxide 1997, 1, 373–385. [Google Scholar]
- Trujillo, M; Radi, R. Peroxynitrite reaction with the reduced and the oxidized forms of lipoic acid: New insight into the reaction of peroxynitrite with thiols. Arch. Biochem. Biophys 2002, 397, 91–98. [Google Scholar]
- Alvarez, B; Radi, R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids 2003, 25, 295–311. [Google Scholar]
- Kurz, CR; Kissner, R; Nauser, T; Perrin, D; Koppenol, WH. Rapid scavenging of peroxynitrous acid by monohydroascorbate. Free Radic. Biol. Med 2003, 35, 1529–1537. [Google Scholar]
- Pacher, P; Beckman, JS; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev 2007, 87, 315–424. [Google Scholar]
- Szabo, C; Ischiropoulos, H; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov 2007, 6, 662–680. [Google Scholar]
- Goldstein, S; Czapski, G. Direct and indirect oxidations by peroxynitrite. Inorg. Chem 1995, 34, 4041–4048. [Google Scholar]
- Richeson, CE; Mulder, P; Bowry, VW; Ingold, KU. The complex chemistry of peroxynitrite decomposition: New insights. J. Am. Chem. Soc 1998, 12, 7211–7219. [Google Scholar]
- Merenyi, G; Lind, J. Free radical formation in the peroxynitrous (ONOOH)/peroxynitrite (ONOO−) system. Chem. Res. Toxicol. 1998, 11, 243–246. [Google Scholar]
- Lymar, SV; Hurst, JK. Rapid reaction between peroxynitrite ion and carbon dioxide: Implications for biological activity. J. Am. Chem. Soc 1995, 117, 8867–8868. [Google Scholar]
- Denicola, A; Freeman, BA; Trujillo, M; Radi, R. Peroxynitrite reaction with carbon dioxide/bicarbonate: Kinetics and influence on peroxynitrite-mediated oxidations. Arch. Biochem. Biophys 1996, 333, 49–58. [Google Scholar]
- Goldstein, S; Czapski, G. The effect of bicarbonate on oxidation by peroxynitrite: Implication for its biological activity. Inorg. Chem 1997, 36, 5113–5117. [Google Scholar]
- Augusto, O; Bonini, MG; Amanso, AM; Linares, E; Santos, CCX; De Menezes, SL. Nitrogen dioxide and carbonate radical anion: Two emerging radicals in biology. Free Radic. Biol. Med 2002, 32, 841–859. [Google Scholar]
- Radi, R; Beckman, JS; Bush, KM; Freeman, BA. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys 1991, 288, 481–487. [Google Scholar]
- Radi, R; Beckman, JS; Bush, KM; Freeman, BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem 1991, 266, 4244–4250. [Google Scholar]
- Ischiropoulos, H. Biological tyrosine nitration: A pathophysiological function of nitric oxide and reactive oxygen species. Arch. Biochem. Biophys 1998, 356, 1–11. [Google Scholar]
- Greenacre, SA; Ischiropoulos, H. Tyrosine nitration: Localization, quantification, consequences for protein function and signal transduction. Free Radic. Res 2001, 34, 541–581. [Google Scholar]
- Pryor, WA; Jin, X; Squadrito, GL. One- and two-electron oxidation of methionine by peroxynitrite. Proc. Natl. Acad. Sci. USA 1994, 91, 11173–11177. [Google Scholar]
- Moreno, JJ; Pryor, WA. Inactivation of α1-antiproteinase inhibitor by peroxynitrite. Chem. Res. Toxicol 1992, 5, 425–431. [Google Scholar]
- Graham, A; Hogg, N; Kalyanaraman, B; O′Leary, V; Darley-Usmar, V; Moncada, S. Peroxynitrite modification of low-density lipoprotein leads to recognition by the macrophage scavenger receptor. FEBS Lett 1993, 330, 181–185. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Macone, A.; Fontana, M.; Barba, M.; Botta, B.; Nardini, M.; Ghirga, F.; Calcaterra, A.; Pecci, L.; Matarese, R.M. Antioxidant Properties of Aminoethylcysteine Ketimine Decarboxylated Dimer: A Review. Int. J. Mol. Sci. 2011, 12, 3072-3084. https://doi.org/10.3390/ijms12053072
Macone A, Fontana M, Barba M, Botta B, Nardini M, Ghirga F, Calcaterra A, Pecci L, Matarese RM. Antioxidant Properties of Aminoethylcysteine Ketimine Decarboxylated Dimer: A Review. International Journal of Molecular Sciences. 2011; 12(5):3072-3084. https://doi.org/10.3390/ijms12053072
Chicago/Turabian StyleMacone, Alberto, Mario Fontana, Marco Barba, Bruno Botta, Mirella Nardini, Francesca Ghirga, Andrea Calcaterra, Laura Pecci, and Rosa Marina Matarese. 2011. "Antioxidant Properties of Aminoethylcysteine Ketimine Decarboxylated Dimer: A Review" International Journal of Molecular Sciences 12, no. 5: 3072-3084. https://doi.org/10.3390/ijms12053072
APA StyleMacone, A., Fontana, M., Barba, M., Botta, B., Nardini, M., Ghirga, F., Calcaterra, A., Pecci, L., & Matarese, R. M. (2011). Antioxidant Properties of Aminoethylcysteine Ketimine Decarboxylated Dimer: A Review. International Journal of Molecular Sciences, 12(5), 3072-3084. https://doi.org/10.3390/ijms12053072








