Mechanistic Investigation of ROS-Induced DNA Damage by Oestrogenic Compounds in Lymphocytes and Sperm Using the Comet Assay
Abstract
:1. Introduction
2. Results and Discussion
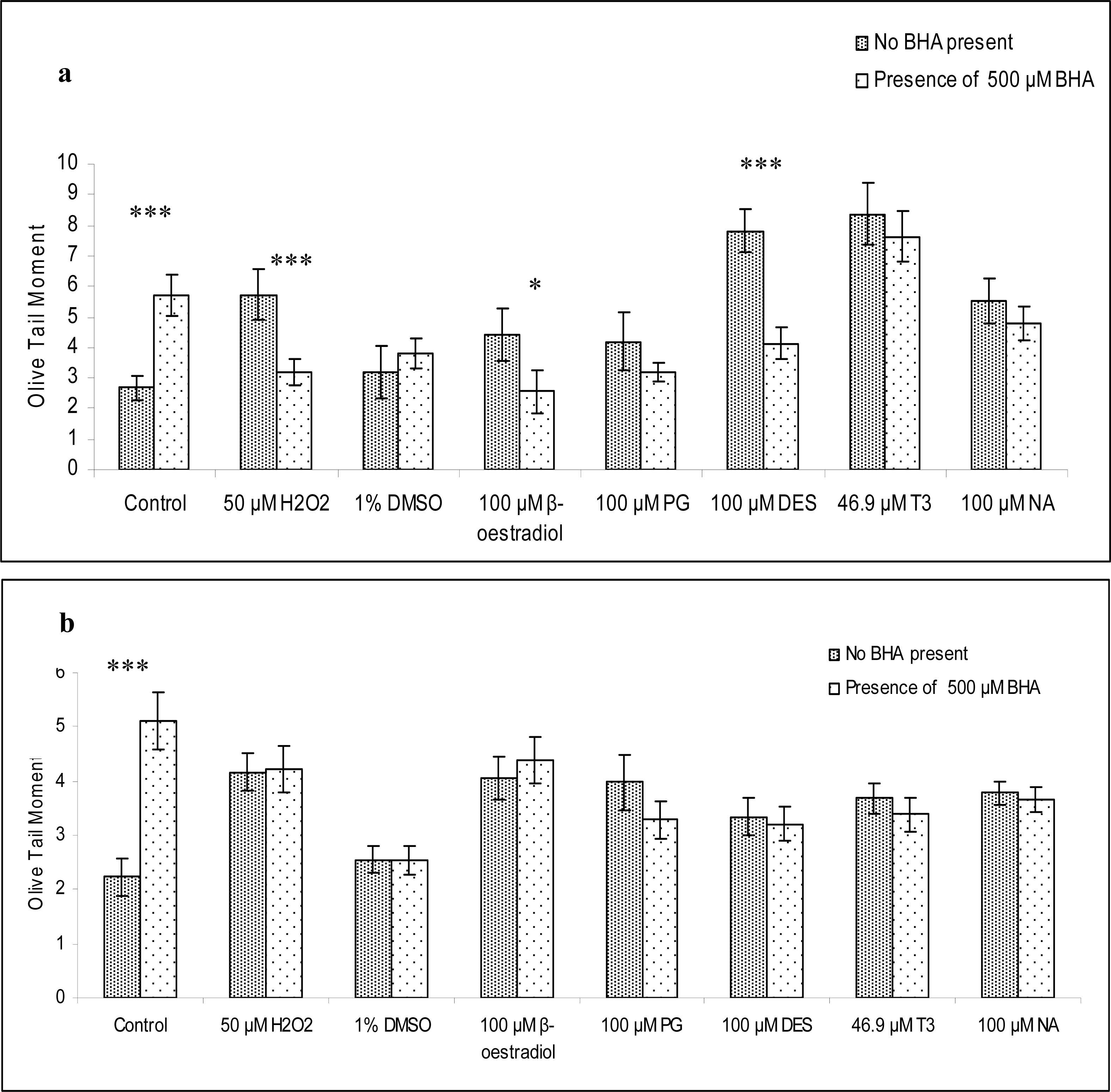
2.1. Mediation of Lipid Peroxidation in the Generation of DNA Damage
2.2. Detection of Oxidized Bases Induced by Oestrogenic Compounds in Lymphocytes and Sperm
2.3. DNA Repair after Oestrogenic Treatment
3. Materials and Methods
3.1. Human PBL Isolation
3.2. Human Semen Evaluation
3.3. Comet Assay in Human PBL
3.4. Comet Assay with Human Sperm
3.5. Evaluation of the Data
4. Conclusion
Acknowledgments
References
- Tapiero, H; Ba, GN; Tew, KD. Estrogens and environmental estrogens. Biomed. Pharmacother 2002, 56, 36–44. [Google Scholar]
- Phillips, DH. Understanding the genotoxicity of tamoxifen? Carcinogenesis 2001, 22, 839–849. [Google Scholar]
- Hankinson, SE; Eliassen, AH. Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. J. Steroid. Biochem. Mol. Biol 2007, 106, 24–30. [Google Scholar]
- Tworoger, SS; Missmer, SA; Barbieri, RL; Willett, WC; Colditz, GA; Hankinson, SE. Plasma sex hormone concentrations and subsequent risk of breast cancer among women using postmenopausal hormones. J. Natl. Cancer Inst 2005, 97, 595–602. [Google Scholar]
- Modugno, F; Ness, RB; Chen, C; Weiss, NS. Inflammation and endometrial cancer: A hypothesis. Cancer Epidemiol. Biomark. Prev 2005, 14, 2840–2847. [Google Scholar]
- Ito, K. Hormone replacement therapy and cancers: The biological roles of estrogen and progestin in tumorigenesis are different between the endometrium and breast. Tohoku J. Exp. Med 2007, 212, 1–12. [Google Scholar]
- Genazzani, AR; Gadducci, A; Gambacciani, M. Controversial issues in climacteric medicine II. Hormone replacement therapy and cancer. Climacteric 2001, 4, 181–193. [Google Scholar]
- Zheng, H; Kavanagh, JJ; Hu, W; Liao, Q; Fu, S. Hormonal therapy in ovarian cancer. Int. J. Gynecol. Cancer 2007, 17, 325–338. [Google Scholar]
- Gambacciani, M; Monteleone, P; Sacco, A; Genazzani, AR. Hormone replacement therapy and endometrial, ovarian and colorectal cancer. Best Pract. Res. Clin. Endocrinol. Metab 2003, 17, 139–147. [Google Scholar]
- Ellem, SJ; Risbridger, GP. Treating prostate cancer: A rationale for targeting local oestrogens. Nat. Rev. Cancer 2007, 7, 621–627. [Google Scholar]
- Ricke, WA; Wang, Y; Cunha, GR. Steroid hormones and carcinogenesis of the prostate: The role of estrogens. Differentiation 2007, 75, 871–882. [Google Scholar]
- West, MC; Anderson, L; McClure, N; Lewis, SE. Dietary oestrogens and male fertility potential. Hum Fertil. (Camb) 2005, 8, 197–207. [Google Scholar]
- WHO, WHO Laboratory Manual for the Examination of Human Semen and Semen–Cervical Mucus Interaction; Cambridge University Press: Cambridge, UK, 1999.
- Mueck, AO; Seeger, H. Breast cancer: Are oestrogen metabolites carcinogenic? Maturitas 2007, 57, 42–46. [Google Scholar]
- Subramanian, A; Salhab, M; Mokbel, K. Oestrogen producing enzymes and mammary carcinogenesis: A review. Breast Cancer Res. Treat 2007, 111, 191–202. [Google Scholar]
- Anderson, D; Schmid, TE; Baumgartner, A; Cemeli-Carratala, E; Brinkworth, MH; Wood, JM. Oestrogenic compounds and oxidative stress (in human sperm and lymphocytes in the Comet assay). Mutat. Res 2003, 544, 173–178. [Google Scholar]
- Cemeli, E; Schmid, TE; Anderson, D. Modulation by flavonoids of DNA damage induced by estrogen-like compounds. Environ. Mol. Mutagen 2004, 44, 420–426. [Google Scholar]
- Bhat, HK; Calaf, G; Hei, TK; Loya, T; Vadgama, JV. Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 3913–3918. [Google Scholar]
- Chen, ZH; Na, HK; Hurh, YJ; Surh, YJ. 4-Hydroxyestradiol induces oxidative stress and apoptosis in human mammary epithelial cells: Possible protection by NF-kappaB and ERK/MAPK. Toxicol. Appl. Pharmacol 2005, 208, 46–56. [Google Scholar]
- Khan, WA; Alam, K. Moinuddin. Catechol-estrogen modified DNA: A better antigen for cancer autoantibody. Arch. Biochem. Biophys 2007, 465, 293–300. [Google Scholar]
- Roy, D; Cai, Q; Felty, Q; Narayan, S. Estrogen-induced generation of reactive oxygen and nitrogen species, gene damage, and estrogen-dependent cancers. J. Toxicol. Environ. Health B Crit. Rev 2007, 10, 235–257. [Google Scholar]
- Dobrzynska, MM; Baumgartner, A; Anderson, D. Antioxidants modulate thyroid hormone- and noradrenaline-induced DNA damage in human sperm. Mutagenesis 2004, 19, 325–330. [Google Scholar]
- Buzzard, JJ; Morrison, JR; O’Bryan, MK; Song, Q; Wreford, NG. Developmental expression of thyroid hormone receptors in the rat testis. Biol. Reprod 2000, 62, 664–669. [Google Scholar]
- Karbownik, M; Lewinski, A. The role of oxidative stress in physiological and pathological processes in the thyroid gland; possible involvement in pineal-thyroid interactions. Neuro. Endocrinol. Lett 2003, 24, 293–303. [Google Scholar]
- Graham, DG. Catecholamine toxicity: A proposal for the molecular pathogenesis of manganese neurotoxicity and Parkinson’s disease. Neurotoxicology 1984, 5, 83–95. [Google Scholar]
- Djelic, N; Anderson, D. The effect of the antioxidant catalase on oestrogens, triiodothyronine, and noradrenaline in the Comet assay. Teratog. Carcinog. Mutagen 2003, 2, 69–81. [Google Scholar]
- Webster, RA. Reproductive function and pregnancy. In Henry’s Clinical Diagnosis and Management by Laboratory Methods, 21st ed; McPherson, RA, Pincus, MR, Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2006; Chapter 25. [Google Scholar]
- Rebar, RW; Erikson, GF. Menstrual cycle and fertility. In Cecil Medicine, 23rd ed; Goldman, L, Ausiello, D, Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2007; Chapter 256. [Google Scholar]
- Simons, SH; van Dijk, M; van Lingen, RA; Roofthooft, D; Boomsma, F; van den Anker, JN; Tibboel, D. Randomised controlled trial evaluating effects of morphine on plasma adrenaline/noradrenaline concentrations in newborns. Arch. Dis. Child Fetal Neonatal 2005, 90, F36–40. [Google Scholar]
- Wortsman, J. Apparent isolated elevation of serum triiodothyronine level in a patient with a thyroid nodule. Arch. Intern. Med 1988, 148, 1866–1868. [Google Scholar]
- Anderson, D; Dobrzynska, MM; Basaran, N; Basaran, A; Yu, TW. Flavonoids modulate comet assay responses to food mutagens in human lymphocytes and sperm. Mutat. Res 1998, 402, 269–277. [Google Scholar]
- Marnett, LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology 2002, 181–182, 219–222. [Google Scholar]
- Stark, G. Functional consequences of oxidative membrane damage. J. Membr. Biol 2005, 205, 1–16. [Google Scholar]
- Goetz, ME; Luch, A. Reactive species: A cell damaging rout assisting to chemical carcinogens. Cancer Lett 2008, 266, 73–83. [Google Scholar]
- Aitken, J; Fisher, H. Reactive oxygen species generation and human spermatozoa: The balance of benefit and risk. Bioessays 1994, 16, 259–267. [Google Scholar]
- Madhavi, DL; Salunkhe, DK. Antioxidants. In Food Additive Toxicology; Maga, JA, Tu, AT, Eds.; Marcel Dekker Inc: New York, NY, USA, 1995; pp. 89–110. [Google Scholar]
- Cemeli, E; Wagner, ED; Anderson, D; Richardson, SD; Plewa, MJ. Modulation of the cytotoxicity and genotoxicity of the drinking water disinfection product iodo acetic acid by suppressors of oxidative stress. Environ. Sci. Technol 2006, 40, 1878–1883. [Google Scholar]
- Slamenová, D; Horváthová, E; Robichová, S; Hrusovská, L; Gábelová, A; Kleibl, K; Jakubíková, J; Sedlák, J. Molecular and cellular influences of butylated hydroxyanisole on Chinese hamster V79 cells treated with N-methyl-N’-nitro-N-nitrosoguanidine: Antimutagenicity of butylated hydroxyanisole. Environ. Mol. Mutagen 2003, 41, 28–36. [Google Scholar]
- Sasaki, YF; Kawaguchi, S; Kamaya, A; Ohshita, M; Kabasawa, K; Iwama, K; Taniguchi, K; Tsuda, S. The comet assay with 8 mouse organs: Results with 39 currently used food additives. Mutat. Res 2002, 519, 103–119. [Google Scholar]
- Bennetts, LE; de Iuliis, GN; Nixon, B; Kime, M; Zelski, K; McVicar, CM; Lewis, SE; Aitken, RJ. Impact of estrogenic compounds on DNA integrity in human spermatozoa: Evidence for cross-linking and redox cycling activities. Mutat. Res 2008, 641, 1–11. [Google Scholar]
- Gedik, CM; Collins, A. Establishing the background level of base oxidation in human lymphocyte DNA: Results of an interlaboratory validation study. FASEB J 2005, 19, 82–84. [Google Scholar]
- Anderson, MA; Hellman, BE. Different roles of FPG and Endo III on catechol-induced DNA damage in extended-term cultures of human lymphocytes and L5178Y mouse lymphoma cells. Toxicol. in Vitro 2005, 19, 779–786. [Google Scholar]
- Rajapakse, N; Butterworth, M; Kortenkamp, A. Detection of DNA strand breaks and oxidized DNA bases at the single-cell level resulting from exposure to estradiol and hydroxylated metabolites. Environ. Mol. Mutagen 2005, 45, 397–404. [Google Scholar]
- Collins, AR. Investigating oxidative DNA damage and its repair using the comet assay. Mutat. Res 2009, 681, 24–32. [Google Scholar]
- Humphreys, V; Martin, RM; Ratcliffe, B; Duthie, S; Wood, S; Gunnell, D; Collins, AR. Age-related increases in DNA repair and antioxidant protection: A comparison of the Boyd Orr Cohort of elderly subjects with a younger population sample. Age Ageing 2007, 36, 521–526. [Google Scholar]
- Kruszewski, M; Wojewodzka, M; Iwanenko, T; Collins, AR; Szumiel, I. Application of the comet assay for monitoring DNA damage in workers exposed to chronic low-dose irradiation. II. Base damage. Mutat. Res 1998, 416, 37–57. [Google Scholar]
- Palus, J; Rydzynski, K; Dziubaltowska, E; Wyszynska, K; Natarajan, AT; Nilsson, R. Genotoxic effects of occupational exposure to lead and cadmium. Mutat. Res 2003, 540, 19–28. [Google Scholar]
- Olsen, AK; Duale, N; Bjoras, M; Larsen, CT; Wiger, R; Holme, JA; Seeberg, EC; Brunborg, G. Limited repair of 8-hydroxy-7,8-dihydroguanine residues in human testicular cells. Nucl. Acids Res 2003, 31, 1351–1363. [Google Scholar]
- Gonzalez, C; Najera, O; Cortes, E; Toledo, G; Lopez, L; Betancourt, M; Ortiz, R. Hydrogen peroxide-induced DNA damage and DNA repair in lymphocytes from malnourished children. Environ. Mol. Mutagen 2002, 39, 33–42. [Google Scholar]
- Cavallo, D; Ursini, CL; Setini, A; Chianese, C; Piegari, P; Perniconi, B; Iavicoli, S. Evaluation of oxidative damage and inhibition of DNA repair in an in vitro study of nickel exposure. Toxicol. in Vitro 2003, 17, 603–607. [Google Scholar]
- Starcevic, SL; Diotte, NM; Zukowski, KL; Cameron, MJ; Novak, RF. Oxidative DNA damage and repair in a cell lineage model of human proliferative breast disease (PBD). Toxicol. Sci 2003, 75, 74–81. [Google Scholar]
- Alvarez, JG; Touchstone, JC; Blasco, L; Storey, BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J. Androl 1987, 8, 338–348. [Google Scholar]
- De Jonge, C; Barratt, CL. Gamete donation: A question of anonymity. Fertil. Steril 2006, 85, 500–501. [Google Scholar]
- Morris, ID; Ilott, S; Dixon, L; Brison, DR. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum. Reprod 2002, 17, 990–998. [Google Scholar]
- Henderson, L; Wolfreys, A; Fedyk, J; Bourner, C; Windebank, S. The ability of the Comet assay to discriminate between genotoxins and cytotoxins. Mutagenesis 1998, 13, 89–94. [Google Scholar]
- Tice, RR; Agurell, E; Anderson, D; Burlinson, B; Hartmann, A; Kobayashi, H; Miyamae, Y; Rojas, E; Ryu, JC; Sasaki, YF. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen 2000, 35, 206–221. [Google Scholar]
- Collins, AR; Dusinska, M; Gedik, CM; Stetina, R. Oxidative damage to DNA: Do we have a reliable biomarker? Environ. Health Perspect 1996, 104, 465–469. [Google Scholar]
- Collins, AR; Duthie, SJ; Dobson, VL. Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogenesis 1993, 14, 1733–1735. [Google Scholar]
- Kumaravel, TS; Jha, AN. Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat. Res 2006, 605, 7–16. [Google Scholar]

| Compound | Treatment | Lymphocytes | Sperm | |
|---|---|---|---|---|
| 120 min treatment | 120 min followed by 24 h repair (Olive tail moment) | 120 min (Olive tail moment) | ||
| Control | Buffer | 3.65 ± 0.28 | 1.89 ± 0.32 | 2.94 ± 0.24 |
| FPG | 6.29 ± 0.85 (**) | 2.34 ± 0.72 (n.s.) | 3.96 ± 0.34 (**) | |
| EndoIII | 6.36 ± 0.55 (**) | 2.08 ± 1.65 (n.s.) | 4.12 ± 0.36 (**) | |
| H2O2 | 50 μM | 17.13 ± 1.22 | 5.44 ± 1.36 | 4.98 ± 0.40 |
| FPG | 16.72 ± 1.21 (n.s.) | 6.51 ± 1.71 (n.s.) | 4.91 ± 0.52 (n.s.) | |
| EndoIII | 18.45 ± 1.16 (n.s.) | 5.94 ± 1.80 (n.s.) | 5.03 ± 0.47 (n.s.) | |
| 17 β-oestradiol | 100 μM | 8.01 ± 1.72 | 2.51 ± 0.70 | 5.52 ± 0.67 |
| FPG | 8.89 ± 1.43 (n.s.) | 2.97 ± 0.73 (n.s.) | 6.24 ± 1.07 (n.s.) | |
| EndoIII | 9.04 ±1.57 (n.s.) | 3.01 ±0.65 (n.s.) | 3.65 ± 0.50 (**) | |
| Progesterone | 100 μM | 7.81 ± 1.34 | 2.76 ± 0.64 | 6.35 ± 0.57 |
| FPG | 7.82 ± 1.23 (n.s.) | 2.89 ± 0.63 (n.s.) | 5.44 ± 0.65 (n.s.) | |
| EndoIII | 7.04 ±1.09 (n.s.) | 2.94 ±0.53 (n.s.) | 5.82 ± 0.92 (n.s.) | |
| DES | 100 μM | 22.62 ± 3.73 | 3.03 ± 0.86 | 6.55 ± 0.81 |
| FPG | 24.90 ± 4.22 (n.s.) | 4.80 ± 1.30 (n.s.) | 5.48 ± 0.56 (n.s.) | |
| EndoIII | 24.01 ± 3.24 (n.s.) | 3.28 ± 1.56 (n.s.) | 4.92 ± 0.75 (n.s.) | |
| T3 | 46.9 μM | 6.63 ± 1.03 | 2.02 ± 0.29 | 4.11 ± 1.12 |
| FPG | 6.99 ± 1.31 (n.s.) | 2.53 ± 0.33 (n.s.) | 4.12 ± 0.43 (n.s.) | |
| EndoIII | 6.09 ± 0.94 (n.s.) | 2.28 ± 0.26 (n.s.) | 4.45 ± 0.89 (n.s.) | |
| Noradrenaline | 100 μM | 13.08 ± 2.91 | 2.72 ± 0.75 | 3.39 ± 0.70 |
| FPG | 13.04 ± 1.30 (n.s.) | 3.93 ± 1.23 (n.s.) | 3.25 ± 0.77 (n.s.) | |
| EndoIII | 13.50 ± 1.14 (n.s.) | 2.78 ± 0.57 (n.s.) | 2.79 ± 0.34 (n.s.) | |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cemeli, E.; Anderson, D. Mechanistic Investigation of ROS-Induced DNA Damage by Oestrogenic Compounds in Lymphocytes and Sperm Using the Comet Assay. Int. J. Mol. Sci. 2011, 12, 2783-2796. https://doi.org/10.3390/ijms12052783
Cemeli E, Anderson D. Mechanistic Investigation of ROS-Induced DNA Damage by Oestrogenic Compounds in Lymphocytes and Sperm Using the Comet Assay. International Journal of Molecular Sciences. 2011; 12(5):2783-2796. https://doi.org/10.3390/ijms12052783
Chicago/Turabian StyleCemeli, Eduardo, and Diana Anderson. 2011. "Mechanistic Investigation of ROS-Induced DNA Damage by Oestrogenic Compounds in Lymphocytes and Sperm Using the Comet Assay" International Journal of Molecular Sciences 12, no. 5: 2783-2796. https://doi.org/10.3390/ijms12052783
APA StyleCemeli, E., & Anderson, D. (2011). Mechanistic Investigation of ROS-Induced DNA Damage by Oestrogenic Compounds in Lymphocytes and Sperm Using the Comet Assay. International Journal of Molecular Sciences, 12(5), 2783-2796. https://doi.org/10.3390/ijms12052783




