RETRACTED: Dextran Sulfate Sodium Inhibits Alanine Synthesis in Caco-2 Cells
Abstract
:1. Introduction
2. Results and Discussion
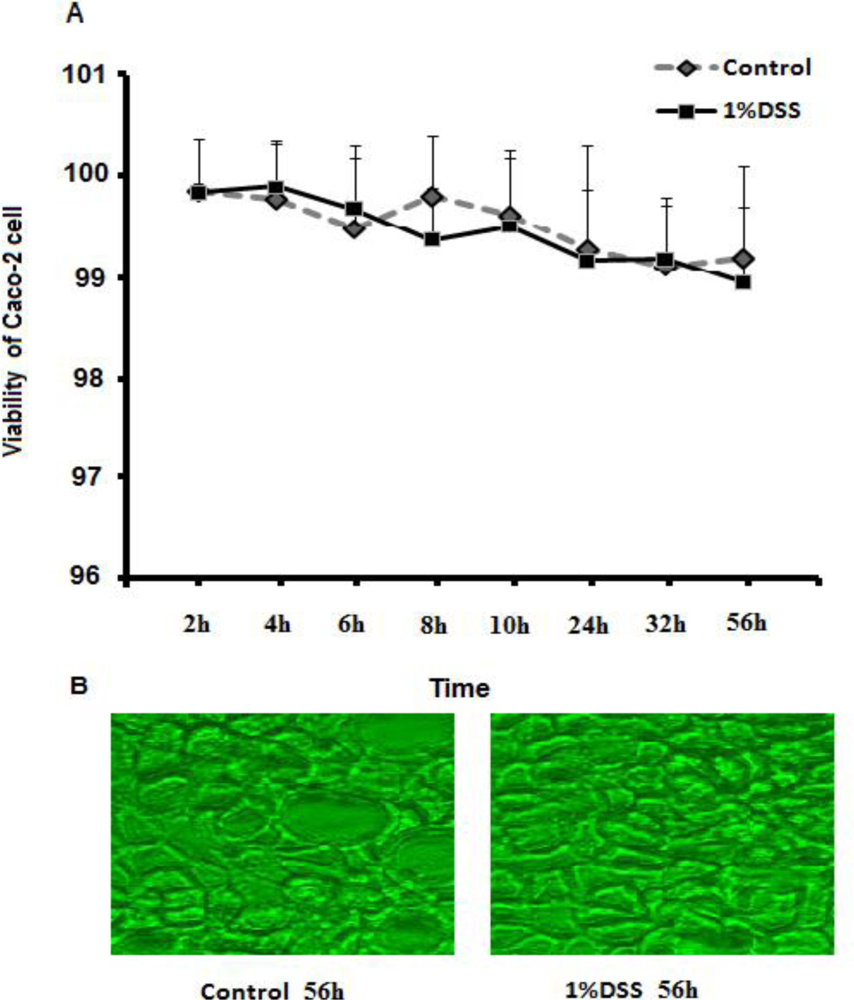
2.1. Cell Viability of Caco-2 Cells Treated with DSS Is Similar to Control
2.2. Interleukin-6 Level Increases with DSS Incubation
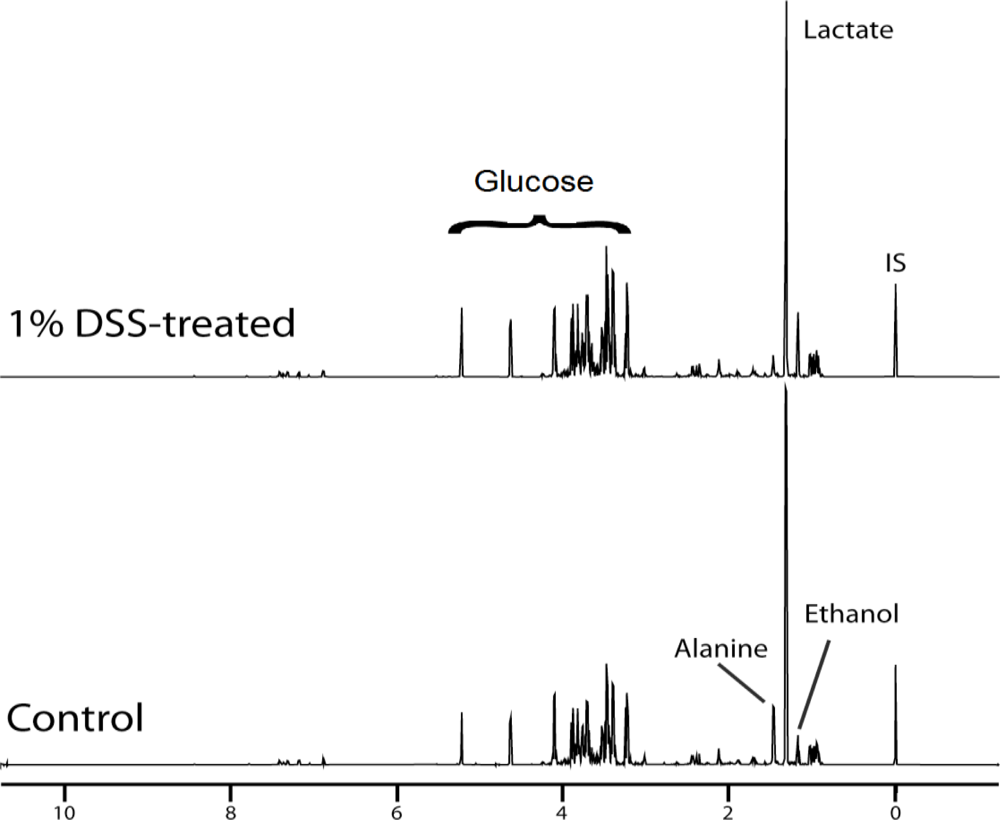
2.3. DSS Induces Changes in 1H NMR Spectra of Supernatant Derived from Culture of Caco-2 Cells
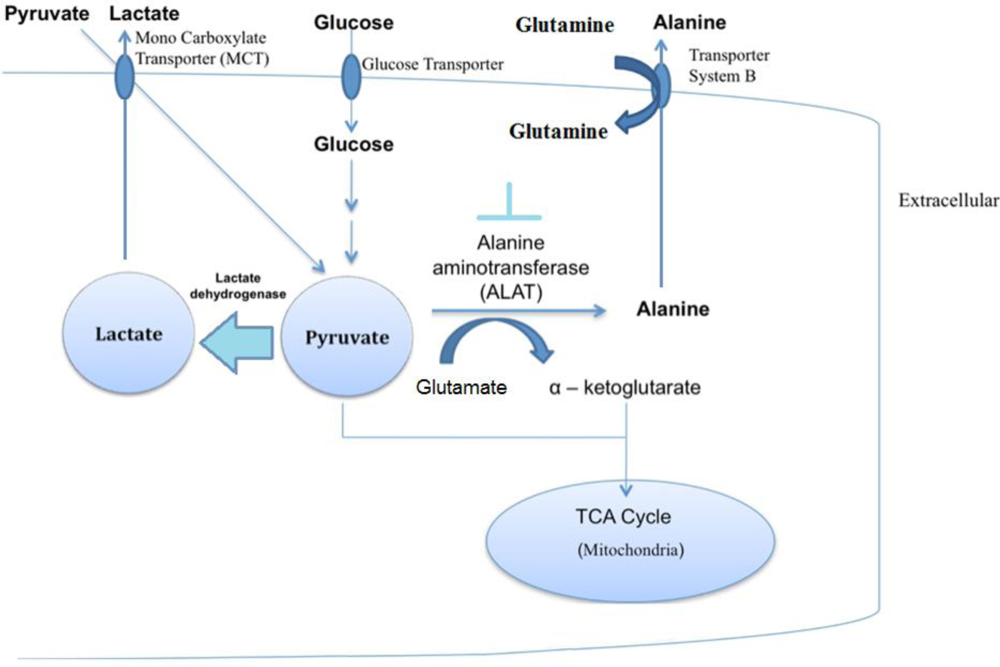
2.4. Discussion
3. Experimental Section
3.1. Caco-2 Preparation and Reagents
3.2. Caco-2 Cells Viability Test
3.3. IL-6 Assay
3.4. NMR Sample Preparation, Spectroscopy and Analysis
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Okayasu, I; Hatakeyama, S; Yamada, M; Ohkusa, T; Inagaki, Y; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [Green Version]
- Yamada, M; Ohkusa, T; Okayasu, I. Occurrence of dysplasia and adenocarcinoma after experimental chronic ulcerative colitis in hamsters induced by dextran sulphate sodium. Gut 1992, 33, 1521–1527. [Google Scholar] [Green Version]
- Abrams, DI; Kuno, S; Wong, R; Jeffords, K; Nash, M; Molaghan, JB; Gorter, R; Ueno, R. Oral dextran sulfate (UA001) in the treatment of the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. Ann. Intern. Med 1989, 110, 183–188. [Google Scholar] [Green Version]
- Dieleman, LA; Ridwan, BU; Tennyson, GS; Beagley, KW; Bucy, RP; Elson, CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 1994, 107, 1643–1652. [Google Scholar] [Green Version]
- Ye, Z; Liu, Z; Henderson, A; Lee, K; Hostetter, J; Wannemuehler, M; Hendrich, S. Increased CYP4B1 mRNA is associated with the inhibition of dextran sulfate sodium-induced colitis by caffeic acid in mice. Exp. Biol. Med 2009, 234, 605–616. [Google Scholar] [Green Version]
- Breider, MA; Eppinger, M; Gough, A. Intercellular adhesion molecule-1 expression in dextran sodium sulfate-induced colitis in rats. Vet. Pathol 1997, 34, 598–604. [Google Scholar] [Green Version]
- Ni, J; Chen, SF; Hollander, D. Effects of dextran sulphate sodium on intestinal epithelial cells and intestinal lymphocytes. Gut 1996, 39, 234–241. [Google Scholar] [Green Version]
- Melgar, S; Karlsson, A; Michaëlsson, E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: Correlation between symptoms and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol 2005, 288, 1328–1338. [Google Scholar] [Green Version]
- Araki, Y; Mukaisyo, K; Sugihara, H; Fujiyama, Y; Hattori, T. Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium-induced colitis in mice. Oncol. Rep 2010, 24, 869–874. [Google Scholar] [Green Version]
- Araki, Y; Sugihara, H; Hattori, T. In vitro effects of dextran sulfate sodium on a Caco-2 cell line and plausible mechanisms for dextran sulfate sodium-induced colitis. Oncol. Rep 2006, 16, 1357–1362. [Google Scholar] [Green Version]
- Souba, WW; Pan, M; Stevens, BR. Kinetics of the sodium-dependent glutamine transporter in human intestinal cell confluent monolayers. Biochem. Biophys. Res. Commun 1992, 188, 746–753. [Google Scholar] [Green Version]
- Bröer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev 2008, 88, 249–286. [Google Scholar] [Green Version]
- Curran, PF; Schultz, SG; Chez, RA; Fuisz, RE. Kinetic relations of the Na-amino acid interaction at the mucosal border of intestine. J. Gen. Physiol 1967, 50, 1261–1286. [Google Scholar] [Green Version]
- Vadstrup, S. Subnormal alanine aminotransferase values in blood of patients with Crohn disease. Scand. J. Gastroenterol 2004, 39, 554–556. [Google Scholar] [Green Version]
- Mendes, FD; Levy, C; Enders, FB; Loftus, EV; Angulo, P; Lindor, KD. Abnormal hepatic biochemistries in patients with inflammatory bowel disease. Am. J. Gastroenterol 2007, 102, 344–350. [Google Scholar] [Green Version]
- Schatorjé, E; Hoekstra, H. Transient hypertransaminasemia in paediatric patients with Crohn disease undergoing initial treatment with enteral nutrition. J. Pediatr. Gastroenterol. Nutr 2010, 51, 336–340. [Google Scholar] [Green Version]
- Mazzon, E; Puzzolo, D; Caputi, AP; Cuzzocrea, S. Role of IL-10 in hepatocyte tight junction alteration in mouse model of experimental colitis. Mol. Med 2002, 8, 353–366. [Google Scholar] [Green Version]
- Chen, C; Shah, YM; Morimura, K; Krausz, KW; Miyazaki, M; Richardson, TA; Morgan, ET; Ntambi, JM; Idle, JR; Gonzalez, FJ. Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab 2008, 7, 135–147. [Google Scholar] [Green Version]
- Balasubramanian, K; Kumar, S; Singh, RR; Sharma, U; Ahuja, V; Makharia, GK; Jagannathan, NR. Metabolism of the colonic mucosa in patients with inflammatory bowel diseases: An in vitro proton magnetic resonance spectroscopy study. Magn. Reson. Imaging 2009, 27, 79–86. [Google Scholar] [Green Version]
- Marchesi, JR; Holmes, E; Khan, F; Kochhar, S; Scanlan, P; Shanahan, F; Wilson, ID; Wang, Y. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J. Proteome Res 2007, 6, 546–551. [Google Scholar] [Green Version]
- Bregenzer, N; Hartmann, A; Strauch, U; Schölmerich, J; Andus, T; Bollheimer, LC. Increased insulin resistance and beta cell activity in patients with Crohn’s disease. Inflamm. Bowel Dis 2006, 12, 53–56. [Google Scholar] [Green Version]
- Oberlinner, C; Zober, A; Nawroth, PP; Humpert, PM; Morcos, M. Alanine-aminotransferase levels predict impaired glucose tolerance in a worksite population. Acta Diabetol 2010, 47, 161–165. [Google Scholar] [Green Version]
- Anemaet, IG; González, JD; Salgado, MC; Giralt, M; Fernández, F; Baanante, IV; Metón, I. Transactivation of cytosolic alanine aminotransferase gene promoter by p300 and c-Myb. J. Mol. Endocrinol 2010, 45, 119–132. [Google Scholar] [Green Version]
- Hu, J; Reddy, MB; Hendrich, S; Murphy, PA. Soyasaponin I and sapongenol B have limited absorption by Caco-2 intestinal cells and limited bioavailability in women. J. Nutr 2004, 134, 1867–1873. [Google Scholar] [Green Version]
- Weljie, AM; Newton, J; Mercier, P; Carlson, E; Slupsky, CM. Targeted profiling: Quantitative analysis of 1H NMR metabolomics data. Anal. Chem 2006, 78, 4430–4442. [Google Scholar] [Green Version]
 ) Caco-2 cells. Supernatants were collected for each of control and DSS-treated cells at 2, 6, 8, 10, 24, 32, and 56 hours, and IL-6 levels were measured, and averaged. Results therefore represent the mean of 28 determinations ± SD, p = 0.00003.
) Caco-2 cells. Supernatants were collected for each of control and DSS-treated cells at 2, 6, 8, 10, 24, 32, and 56 hours, and IL-6 levels were measured, and averaged. Results therefore represent the mean of 28 determinations ± SD, p = 0.00003.
 ) Caco-2 cells. Supernatants were collected for each of control and DSS-treated cells at 2, 6, 8, 10, 24, 32, and 56 hours, and IL-6 levels were measured, and averaged. Results therefore represent the mean of 28 determinations ± SD, p = 0.00003.
) Caco-2 cells. Supernatants were collected for each of control and DSS-treated cells at 2, 6, 8, 10, 24, 32, and 56 hours, and IL-6 levels were measured, and averaged. Results therefore represent the mean of 28 determinations ± SD, p = 0.00003.
 ) Caco-2 cell culture supernatants. All metabolites in cell culture supernatants were collected for each of control and DSS-treated cells at 10, 24, 32, and 56 hours, and metabolites were measured, and averaged. Results therefore represent the mean of 16 determinations ± SD, and * p < 0.00001.
) Caco-2 cell culture supernatants. All metabolites in cell culture supernatants were collected for each of control and DSS-treated cells at 10, 24, 32, and 56 hours, and metabolites were measured, and averaged. Results therefore represent the mean of 16 determinations ± SD, and * p < 0.00001.
 ) Caco-2 cell culture supernatants. All metabolites in cell culture supernatants were collected for each of control and DSS-treated cells at 10, 24, 32, and 56 hours, and metabolites were measured, and averaged. Results therefore represent the mean of 16 determinations ± SD, and * p < 0.00001.
) Caco-2 cell culture supernatants. All metabolites in cell culture supernatants were collected for each of control and DSS-treated cells at 10, 24, 32, and 56 hours, and metabolites were measured, and averaged. Results therefore represent the mean of 16 determinations ± SD, and * p < 0.00001.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ye, Z.; Mishchuk, D.O.; Stephens, N.S.; Slupsky, C.M. RETRACTED: Dextran Sulfate Sodium Inhibits Alanine Synthesis in Caco-2 Cells. Int. J. Mol. Sci. 2011, 12, 2325-2335. https://doi.org/10.3390/ijms12042325
Ye Z, Mishchuk DO, Stephens NS, Slupsky CM. RETRACTED: Dextran Sulfate Sodium Inhibits Alanine Synthesis in Caco-2 Cells. International Journal of Molecular Sciences. 2011; 12(4):2325-2335. https://doi.org/10.3390/ijms12042325
Chicago/Turabian StyleYe, Zhong, Darya O. Mishchuk, Natasha S. Stephens, and Carolyn M. Slupsky. 2011. "RETRACTED: Dextran Sulfate Sodium Inhibits Alanine Synthesis in Caco-2 Cells" International Journal of Molecular Sciences 12, no. 4: 2325-2335. https://doi.org/10.3390/ijms12042325
APA StyleYe, Z., Mishchuk, D. O., Stephens, N. S., & Slupsky, C. M. (2011). RETRACTED: Dextran Sulfate Sodium Inhibits Alanine Synthesis in Caco-2 Cells. International Journal of Molecular Sciences, 12(4), 2325-2335. https://doi.org/10.3390/ijms12042325



