C57BL/KsJ-db/db-ApcMin/+ Mice Exhibit an Increased Incidence of Intestinal Neoplasms
Abstract
:1. Introduction
2. Materilas and Methods
2.1. Animals
2.2. Experimental Procedure
2.3. Pathological and Immunohistochemical Analyses
2.4. Blood Chemistry
2.5. RNA Extraction and Quantitative Real-Time RT-PCR Analysis
2.6. Statistical Analysis
3. Results
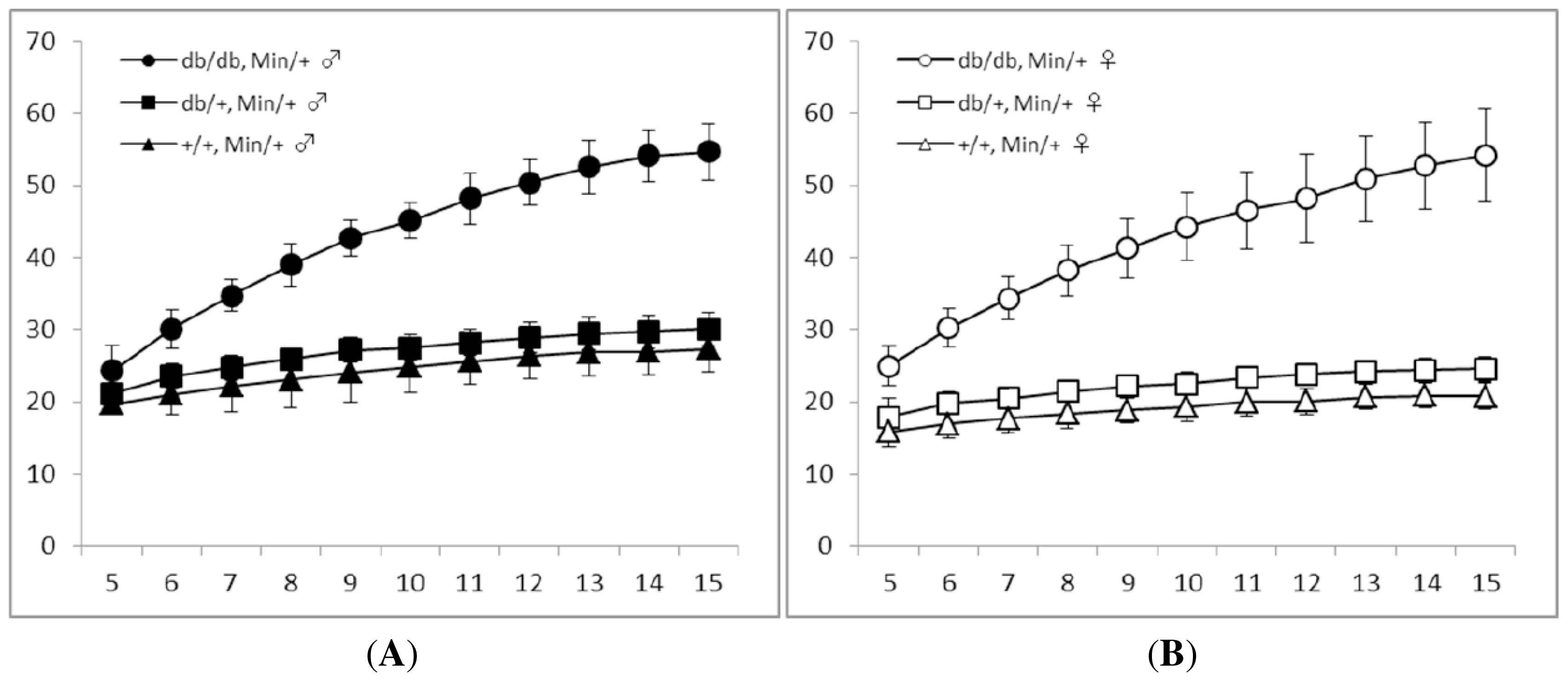
3.1. General Observations
3.2. Serum Levels of Glucose, Total Cholesterol, Triglyceride and Insulin in Experimental Mice
3.3. Tumors in the Intestinal Tract
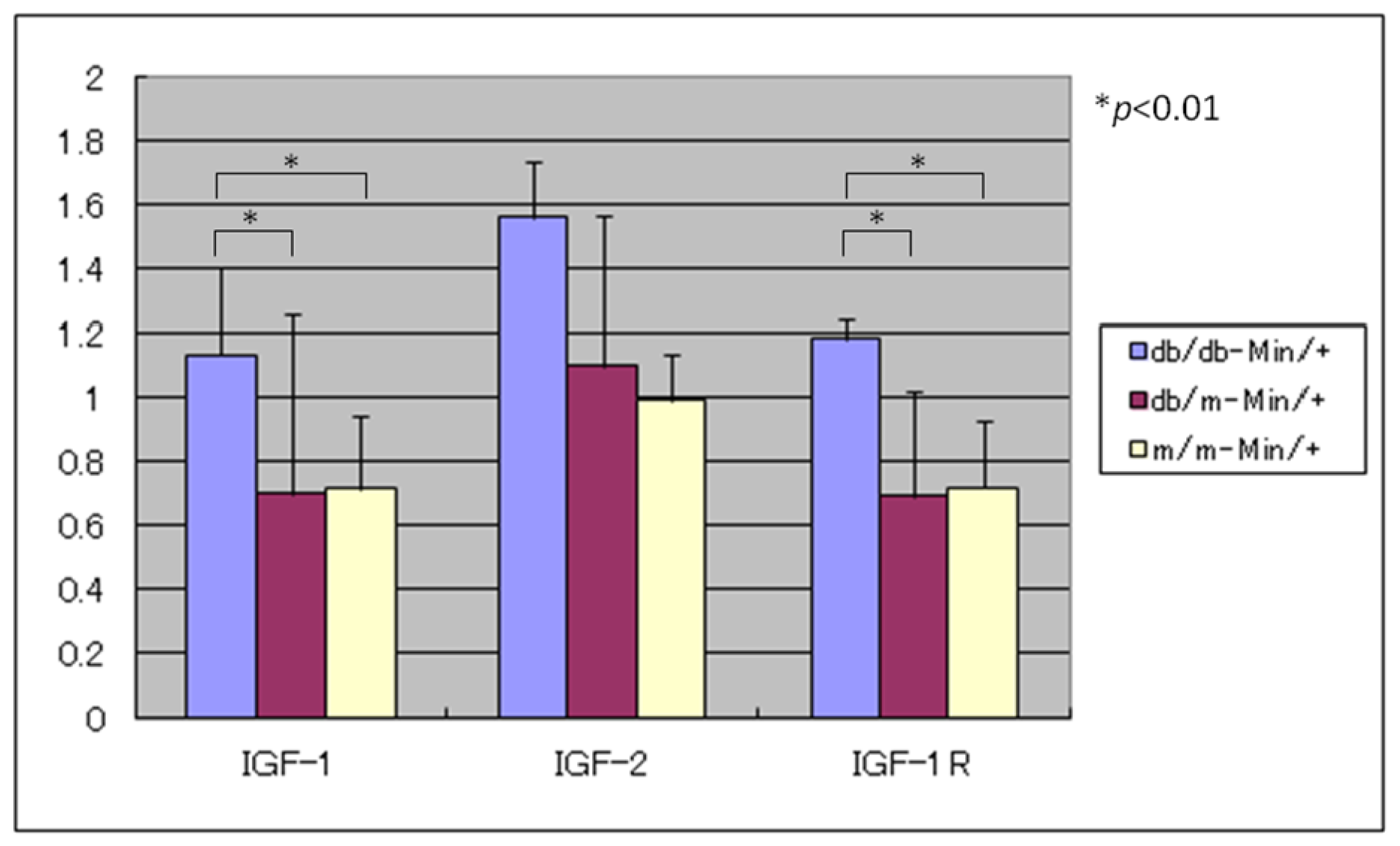
3.4. Expression Levels of IGF-1, IGF-2, and IGF-1R mRNAs
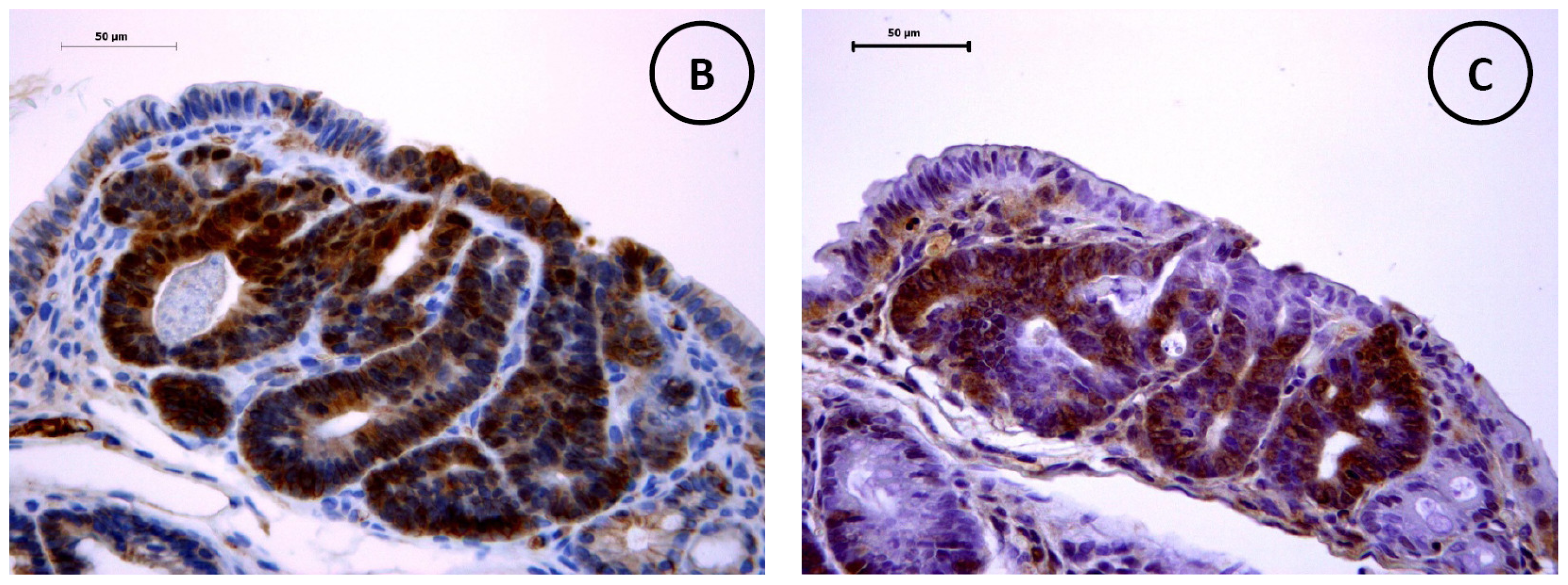
3.5. Immunohistochemistry of β-Catenin and IGF-IR in the Colonic Tumors
4. Discussion
Supplementary Material
ijms-12-08133-s001.pdfAcknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Bergstrom, A.; Pisani, P.; Tenet, V.; Wolk, A.; Adami, H.O. Overweight as an avoidable cause of cancer in Europe. Int. J. Cancer 2001, 91, 421–430. [Google Scholar]
- Murphy, T.K.; Calle, E.E.; Rodriguez, C.; Kahn, H.S.; Thun, M.J. Body mass index and colon cancer mortality in a large prospective study. Am. J. Epidemiol 2000, 152, 847–854. [Google Scholar]
- LeRoith, D.; Novosyadlyy, R.; Gallagher, E.J.; Lann, D.; Vijayakumar, A.; Yakar, S. Obesity and type 2 diabetes are associated with an increased risk of developing cancer and a worse prognosis; epidemiological and mechanistic evidence. Exp. Clin. Endocrinol. Diabetes 2008, 116, S4–S6. [Google Scholar]
- Chang, C.K.; Ulrich, C.M. Hyperinsulinaemia and hyperglycaemia: Possible risk factors of colorectal cancer among diabetic patients. Diabetologia 2003, 46, 595–607. [Google Scholar]
- Becker, S.; Dossus, L.; Kaaks, R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch. Physiol. Biochem 2009, 115, 86–96. [Google Scholar]
- Jalving, M.; Gietema, J.A.; Lefrandt, J.D.; de Jong, S.; Reyners, A.K.; Gans, R.O.; de Vries, E.G. Metformin: Taking away the candy for cancer? Eur. J. Cancer 2010, 46, 2369–2380. [Google Scholar]
- Hosono, K.; Endo, H.; Takahashi, H.; Sugiyama, M.; Sakai, E.; Uchiyama, T.; Suzuki, K.; Iida, H.; Sakamoto, Y.; Yoneda, K.; Koide, T.; Tokoro, C.; Abe, Y.; Inamori, M.; Nakagama, H.; Nakajima, A. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev. Res. (Phila) 2010, 3, 1077–1083. [Google Scholar]
- Gallagher, E.J.; LeRoith, D. Insulin, insulin resistance, obesity, and cancer. Curr. Diabet. Rep 2010, 10, 93–100. [Google Scholar]
- Renehan, A.G.; Frystyk, J.; Flyvbjerg, A. Obesity and cancer risk: The role of the insulin-IGF axis. Trends Endocrinol. Metab 2006, 17, 328–336. [Google Scholar]
- Shimizu, M.; Sakai, H.; Shirakami, Y.; Yasuda, Y.; Kubota, M.; Terakura, D.; Baba, A.; Ohno, T.; Hara, Y.; Tanaka, T.; Moriwaki, H. Preventive effects of (−)-epigallocatechin gallate on diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db Mice. Cancer Prev. Res. (Phila) 2011, 4, 396–403. [Google Scholar]
- Hirose, Y.; Hata, K.; Kuno, T.; Yoshida, K.; Sakata, K.; Yamada, Y.; Tanaka, T.; Reddy, B.S.; Mori, H. Enhancement of development of azoxymethane-induced colonic premalignant lesions in C57BL/KsJ-db/db mice. Carcinogenesis 2004, 25, 821–825. [Google Scholar]
- Hirose, Y.; Kuno, T.; Yamada, Y.; Sakata, K.; Katayama, M.; Yoshida, K.; Qiao, Z.; Hata, K.; Yoshimi, N.; Mori, H. Azoxymethane-induced beta-catenin-accumulated crypts in colonic mucosa of rodents as an intermediate biomarker for colon carcinogenesis. Carcinogenesis 2003, 24, 107–111. [Google Scholar]
- Hayashi, K.; Suzuki, R.; Miyamoto, S.; Shin-Ichiroh, Y.; Kohno, H.; Sugie, S.; Takashima, S.; Tanaka, T. Citrus auraptene suppresses azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db mice. Nutr. Cancer 2007, 58, 75–84. [Google Scholar]
- Suzuki, R.; Kohno, H.; Yasui, Y.; Hata, K.; Sugie, S.; Miyamoto, S.; Sugawara, K.; Sumida, T.; Hirose, Y.; Tanaka, T. Diet supplemented with citrus unshiu segment membrane suppresses chemically induced colonic preneoplastic lesions and fatty liver in male db/db mice. Int. J. Cancer 2007, 120, 252–258. [Google Scholar]
- Moser, A.R.; Pitot, H.C.; Dove, W.F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 1990, 247, 322–324. [Google Scholar]
- Hata, K.; Tanaka, T.; Kohno, H.; Suzuki, R.; Qiang, S.H.; Yamada, Y.; Oyama, T.; Kuno, T.; Hirose, Y.; Hara, A.; Mori, H. Beta-catenin-accumulated crypts in the colonic mucosa of juvenile ApcMin/+ mice. Cancer Lett 2006, 239, 123–128. [Google Scholar]
- Yamada, Y.; Hata, K.; Hirose, Y.; Hara, A.; Sugie, S.; Kuno, T.; Yoshimi, N.; Tanaka, T.; Mori, H. Microadenomatous lesions involving loss of Apc heterozygosity in the colon of adult Apc(Min/+) mice. Cancer Res 2002, 62, 6367–6370. [Google Scholar]
- Corpet, D.E.; Pierre, F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur. J. Cancer 2005, 41, 1911–1922. [Google Scholar] [Green Version]
- Dove, W.F.; Gould, K.A.; Luongo, C.; Moser, A.R.; Shoemaker, A.R. Emergent issues in the genetics of intestinal neoplasia. Cancer Surv 1995, 25, 335–355. [Google Scholar]
- DuBois, R.N.; Giardiello, F.M.; Smalley, W.E. Nonsteroidal anti-inflammatory drugs, eicosanoids, and colorectal cancer prevention. Gastroenterol. Clin. North Am 1996, 25, 773–791. [Google Scholar]
- Gupta, R.A.; Dubois, R.N. Controversy: PPARgamma as a target for treatment of colorectal cancer. Am. J. Physiol. Gastrointest. Liver Physiol 2002, 283, G266–G269. [Google Scholar]
- Mutoh, M.; Niho, N.; Komiya, M.; Takahashi, M.; Ohtsubo, R.; Nakatogawa, K.; Ueda, K.; Sugimura, T.; Wakabayashi, K. Plasminogen activator inhibitor-1 (Pai-1) blockers suppress intestinal polyp formation in Min mice. Carcinogenesis 2008, 29, 824–829. [Google Scholar]
- Niho, N.; Takahashi, M.; Shoji, Y.; Takeuchi, Y.; Matsubara, S.; Sugimura, T.; Wakabayashi, K. Dose-dependent suppression of hyperlipidemia and intestinal polyp formation in Min mice by pioglitazone, a PPAR gamma ligand. Cancer Sci 2003, 94, 960–964. [Google Scholar]
- Tanaka, T.; Kohno, H.; Suzuki, R.; Hata, K.; Sugie, S.; Niho, N.; Sakano, K.; Takahashi, M.; Wakabayashi, K. Dextran sodium sulfate strongly promotes colorectal carcinogenesis in Apc(Min/+) mice: Inflammatory stimuli by dextran sodium sulfate results in development of multiple colonic neoplasms. Int. J. Cancer 2006, 118, 25–34. [Google Scholar]
- Singh, P.; Rubin, N. Insulinlike growth factors and binding proteins in colon cancer. Gastroenterology 1993, 105, 1218–1237. [Google Scholar]
- Belfiore, A. The role of insulin receptor isoforms and hybrid insulin/IGF-I receptors in human cancer. Curr. Pharm. Des 2007, 13, 671–686. [Google Scholar]
- Komninou, D.; Ayonote, A.; Richie, J.P., Jr; Rigas, B. Insulin resistance and its contribution to colon carcinogenesis. Exp. Biol. Med. (Maywood) 2003, 228, 396–405. [Google Scholar]
- Shimizu, M.; Shirakami, Y.; Iwasa, J.; Shiraki, M.; Yasuda, Y.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Supplementation with branched-chain amino acids inhibits azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Clin. Cancer Res 2009, 15, 3068–3075. [Google Scholar]
- Lee, W.M.; Lu, S.; Medline, A.; Archer, M.C. Susceptibility of lean and obese Zucker rats to tumorigenesis induced by N-methyl-N-nitrosourea. Cancer Lett 2001, 162, 155–160. [Google Scholar]
- Weber, R.V.; Stein, D.E.; Scholes, J.; Kral, J.G. Obesity potentiates AOM-induced colon cancer. Dig. Dis. Sci 2000, 45, 890–895. [Google Scholar]
- Berster, J.M.; Goke, B. Type 2 diabetes mellitus as risk factor for colorectal cancer. Arch. Physiol. Biochem 2008, 114, 84–98. [Google Scholar]
- Giouleme, O.; Diamantidis, M.D.; Katsaros, M.G. Is diabetes a causal agent for colorectal cancer? Pathophysiological and molecular mechanisms. World J. Gastroenterol 2011, 17, 444–448. [Google Scholar]
- Seow, A.; Yuan, J.M.; Koh, W.P.; Lee, H.P.; Yu, M.C. Diabetes mellitus and risk of colorectal cancer in the Singapore Chinese Health Study. J. Natl. Cancer Inst 2006, 98, 135–138. [Google Scholar]
- Giovannucci, E. Insulin, insulin-like growth factors and colon cancer: A review of the evidence. J. Nutr 2001, 131, 3109S–3120S. [Google Scholar]
- Tsugane, S.; Inoue, M. Insulin resistance and cancer: Epidemiological evidence. Cancer Sci 2010, 101, 1073–1079. [Google Scholar]
- Tran, T.T.; Medline, A.; Bruce, W.R. Insulin promotion of colon tumors in rats. Cancer Epidemiol. Biomarkers Prev 1996, 5, 1013–1015. [Google Scholar]
- Rosenzweig, S.A.; Atreya, H.S. Defining the pathway to insulin-like growth factor system targeting in cancer. Biochem. Pharmacol 2010, 80, 1115–1124. [Google Scholar]
- Lee, P.D.; Giudice, L.C.; Conover, C.A.; Powell, D.R. Insulin-like growth factor binding protein-1: Recent findings and new directions. Proc. Soc. Exp. Biol. Med 1997, 216, 319–357. [Google Scholar]
- Suikkari, A.M.; Koivisto, V.A.; Rutanen, E.M.; Yki-Jarvinen, H.; Karonen, S.L.; Seppala, M. Insulin regulates the serum levels of low molecular weight insulin-like growth factor-binding protein. J. Clin. Endocrinol. Metab 1988, 66, 266–272. [Google Scholar]
- Mutoh, M.; Akasu, T.; Takahashi, M.; Niho, N.; Yoshida, T.; Sugimura, T.; Wakabayashi, K. Possible involvement of hyperlipidemia in increasing risk of colorectal tumor development in human familial adenomatous polyposis. Jpn. J. Clin. Oncol 2006, 36, 166–171. [Google Scholar]
- Sturmer, T.; Buring, J.E.; Lee, I.M.; Gaziano, J.M.; Glynn, R.J. Metabolic abnormalities and risk for colorectal cancer in the physicians’ health study. Cancer Epidemiol. Biomarkers Prev 2006, 15, 2391–2397. [Google Scholar]
- Tabuchi, M.; Kitayama, J.; Nagawa, H. Hyperglycemia and hypertriglyceridemia may associate with the adenoma-carcinoma transition in colorectal epithelial cells. J. Gastroenterol. Hepatol 2008, 23, 985–987. [Google Scholar]
- Takahashi, H.; Yoneda, K.; Tomimoto, A.; Endo, H.; Fujisawa, T.; Iida, H.; Mawatari, H.; Nozaki, Y.; Ikeda, T.; Akiyama, T.; Yoneda, M.; Inamori, M.; Abe, Y.; Saito, S.; Nakajima, A.; Nakagama, H. Life style-related diseases of the digestive system: Colorectal cancer as a life style-related disease: From carcinogenesis to medical treatment. J. Pharmacol. Sci 2007, 105, 129–132. [Google Scholar]
- Niho, N.; Takahashi, M.; Kitamura, T.; Shoji, Y.; Itoh, M.; Noda, T.; Sugimura, T.; Wakabayashi, K. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Res 2003, 63, 6090–6095. [Google Scholar]
- Yasuda, Y.; Shimizu, M.; Shirakami, Y.; Sakai, H.; Kubota, M.; Hata, K.; Hirose, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Sci 2010, 101, 1701–1707. [Google Scholar]
- Niho, N.; Mutoh, M.; Komiya, M.; Ohta, T.; Sugimura, T.; Wakabayashi, K. Improvement of hyperlipidemia by indomethacin in Min mice. Int. J. Cancer 2007, 121, 1665–1669. [Google Scholar]
- Niho, N.; Mutoh, M.; Takahashi, M.; Tsutsumi, K.; Sugimura, T.; Wakabayashi, K. Concurrent suppression of hyperlipidemia and intestinal polyp formation by NO-1886, increasing lipoprotein lipase activity in Min mice. Proc. Natl. Acad. Sci. USA 2005, 102, 2970–2974. [Google Scholar]
- Endo, H.; Hosono, K.; Uchiyama, T.; Sakai, E.; Sugiyama, M.; Takahashi, H.; Nakajima, N.; Wada, K.; Takeda, K.; Nakagama, H.; Nakajima, A. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut 2011, 60, 1363–1371. [Google Scholar]

| Gender | Genotype | No. of mice examined | Body weight (g) | Body length (cm) | BMI (g/cm2) | |
|---|---|---|---|---|---|---|
| db/db type | ApcMin/+ type | |||||
| Males | db/db | Min/+ | 18 | 54.7 ± 3.9 a,b | 9.38 ± 0.30 c | 0.62 ± 0.04 b |
| db/m | Min/+ | 23 | 30.2 ± 2.3 d | 9.23 ± 0.38 | 0.35 ± 0.03 e | |
| m/m | Min/+ | 11 | 27.3 ± 3.2 f | 9.00 ± 0.30 | 0.34 ± 0.05 f | |
| Females | db/db | Min/+ | 13 | 54.2 ± 6.4 g | 9.30 ± 0.24 h | 0.63 ± 0.08 g |
| db/m | Min/+ | 19 | 24.5 ± 1.8 | 9.13 ± 0.33 | 0.30 ± 0.03 | |
| m/m | Min/+ | 10 | 20.8 ± 1.8 | 8.75 ± 0.23 | 0.27 ± 0.03 | |
| Gender | Genotype | No. of mice examined | Glucose (mg/dL) | Total cholesterol (mg/dL) | Triglyceride (mg/dL) | Insulin (ng/dl) | |
|---|---|---|---|---|---|---|---|
| db/db type | ApcMin/+ type | ||||||
| Males | db/db | Min/+ | 18 | 250.6 ± 35.6 a,b | 161.8 ± 28.7 b | 193.1 ± 14.8 c | 3.3 ± 1.4 b |
| db/m | Min/+ | 23 | 185.7 ± 27.6 d | 109.4 ± 13.1 | 162.6 ± 27.6 | 0.4 ± 0.2 | |
| m/m | Min/+ | 11 | 187.0 ± 21.8 e | 96.1 ± 13.0 | 165.2 ± 21.5 | 0.3 ± 0.1 | |
| Females | db/db | Min/+ | 13 | 226.2 ± 28.7 f | 145.1 ± 18.5 g | 215.0 ± 38.0 h,i | 3.5 ± 1.1 f |
| db/m | Min/+ | 19 | 181.6 ± 24.5 | 119.1 ± 12.5 | 182.3 ± 29.8 | 0.3 ± 0.1 | |
| m/m | Min/+ | 10 | 195.0 ± 20.4 | 115.3 ± 10.3 | 170.9 ± 18.2 | 0.3 ± 0.1 | |
| Gender | Genotype | No. of mice examined | Total number of adenomas/mouse (incidence) | Small intestine (incidence) | Colon (incidence) | |
|---|---|---|---|---|---|---|
| db/db type | ApcMin/+ type | |||||
| Males | db/db | Min/+ | 18 | 60.5 ± 14.6 a,b (18/18, 100%) | 58.8 ± 13.7 b (18/18, 100%) | 1.7 ± 2.6 (12/18, 66.7% c) |
| db/m | Min/+ | 23 | 32.1 ± 7.5 (23/23, 100%) | 31.6 ± 6.7 (23/23, 100%) | 0.6 ± 1.2 (5/23, 21.8%) | |
| m/m | Min/+ | 11 | 30.5 ± 7.7 (11/11, 100%) | 29.5 ± 6.1 (11/11, 100%) | 0.9 ± 2.1 (3/11, 27.3%) | |
| Females | db/db | Min/+ | 13 | 57.8 ± 12.7 d (13/13, 100%) | 58.6 ± 12.3 d (13/13, 100%) | 1.2 ± 1.5 (7/13, 53.8%) |
| db/m | Min/+ | 19 | 32.6 ± 5.9 (19/19, 100%) | 32.4 ± 5.9 (19/19, 100%) | 0.2 ± 0.4 (4/19, 21.1%) | |
| m/m | Min/+ | 10 | 30.9 ± 6.3 (10/10, 100%) | 30.7 ± 6.3 (10/10, 100%) | 0.2 ± 0.4 (2/10, 20.0%) | |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hata, K.; Kubota, M.; Shimizu, M.; Moriwaki, H.; Kuno, T.; Tanaka, T.; Hara, A.; Hirose, Y. C57BL/KsJ-db/db-ApcMin/+ Mice Exhibit an Increased Incidence of Intestinal Neoplasms. Int. J. Mol. Sci. 2011, 12, 8133-8145. https://doi.org/10.3390/ijms12118133
Hata K, Kubota M, Shimizu M, Moriwaki H, Kuno T, Tanaka T, Hara A, Hirose Y. C57BL/KsJ-db/db-ApcMin/+ Mice Exhibit an Increased Incidence of Intestinal Neoplasms. International Journal of Molecular Sciences. 2011; 12(11):8133-8145. https://doi.org/10.3390/ijms12118133
Chicago/Turabian StyleHata, Kazuya, Masaya Kubota, Masahito Shimizu, Hisataka Moriwaki, Toshiya Kuno, Takuji Tanaka, Akira Hara, and Yoshinobu Hirose. 2011. "C57BL/KsJ-db/db-ApcMin/+ Mice Exhibit an Increased Incidence of Intestinal Neoplasms" International Journal of Molecular Sciences 12, no. 11: 8133-8145. https://doi.org/10.3390/ijms12118133
APA StyleHata, K., Kubota, M., Shimizu, M., Moriwaki, H., Kuno, T., Tanaka, T., Hara, A., & Hirose, Y. (2011). C57BL/KsJ-db/db-ApcMin/+ Mice Exhibit an Increased Incidence of Intestinal Neoplasms. International Journal of Molecular Sciences, 12(11), 8133-8145. https://doi.org/10.3390/ijms12118133






