Rapid and Green Analytical Method for the Determination of Quinoline Alkaloids from Cinchona succirubra Based on Microwave-Integrated Extraction and Leaching (MIEL) Prior to High Performance Liquid Chromatography
Abstract
:1. Introduction
2. Experimental Section
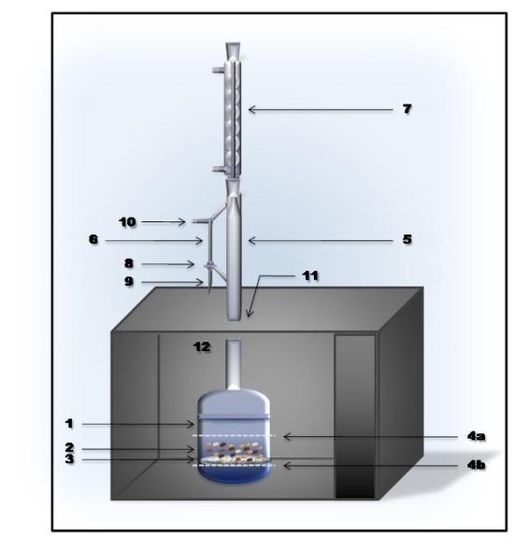
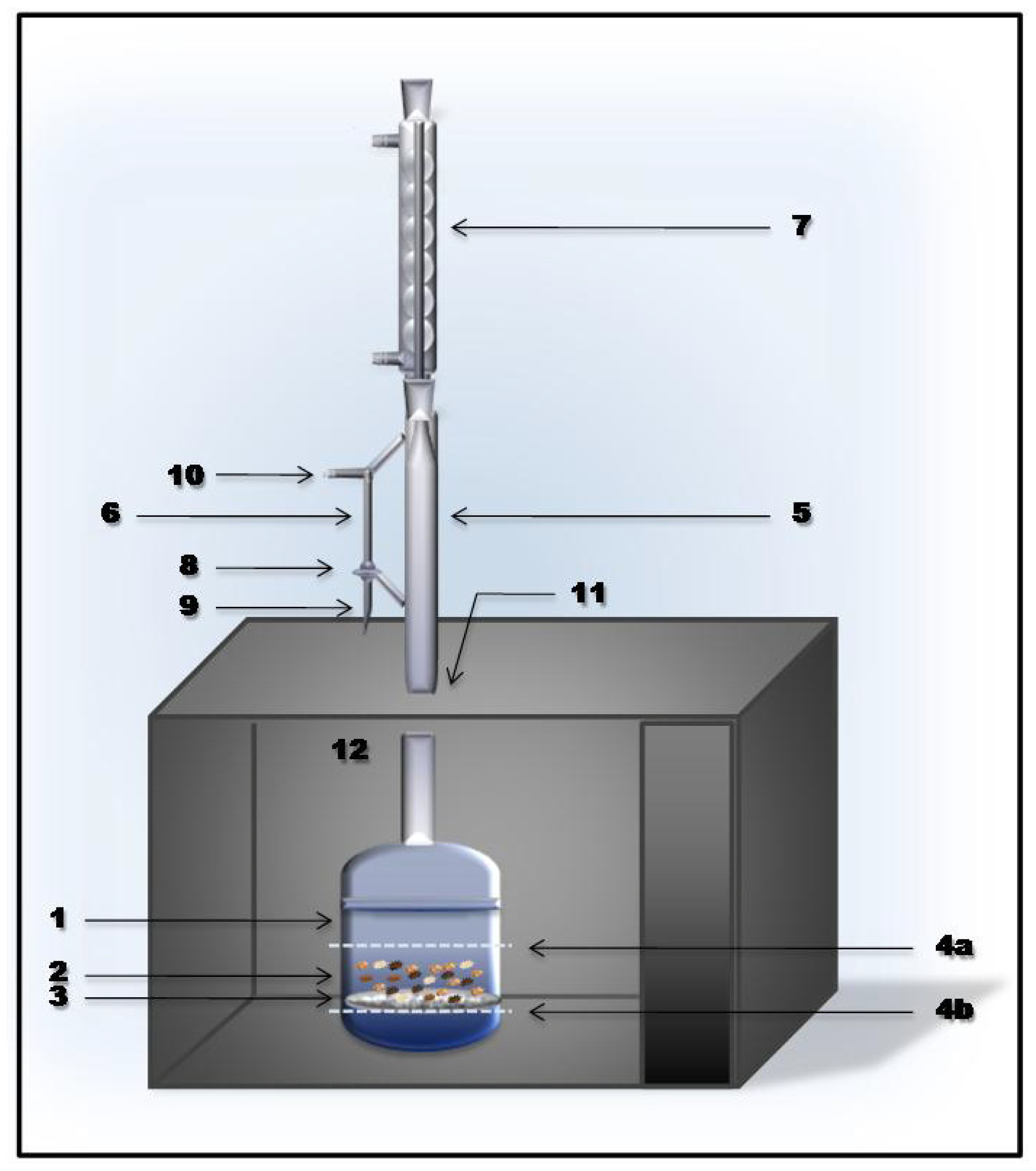
2.1. Apparatus
2.2. Reagents and Solutions
2.3. Sample Preparation
2.4. MIEL Procedure
2.5. Soxhlet Extraction Procedure
2.6. Analytical Procedure
2.7. Data Treatment
3. Results and Discussion
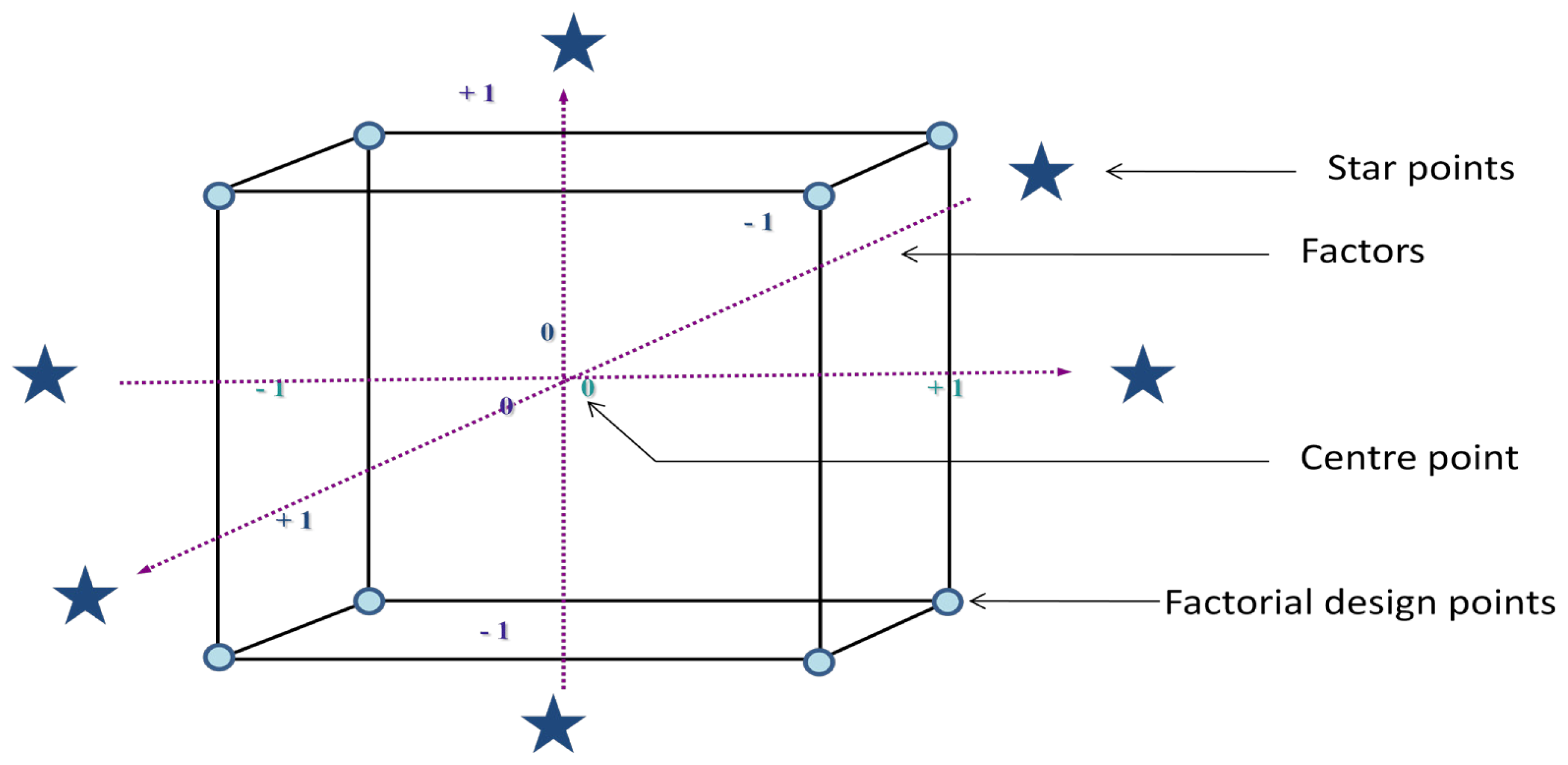
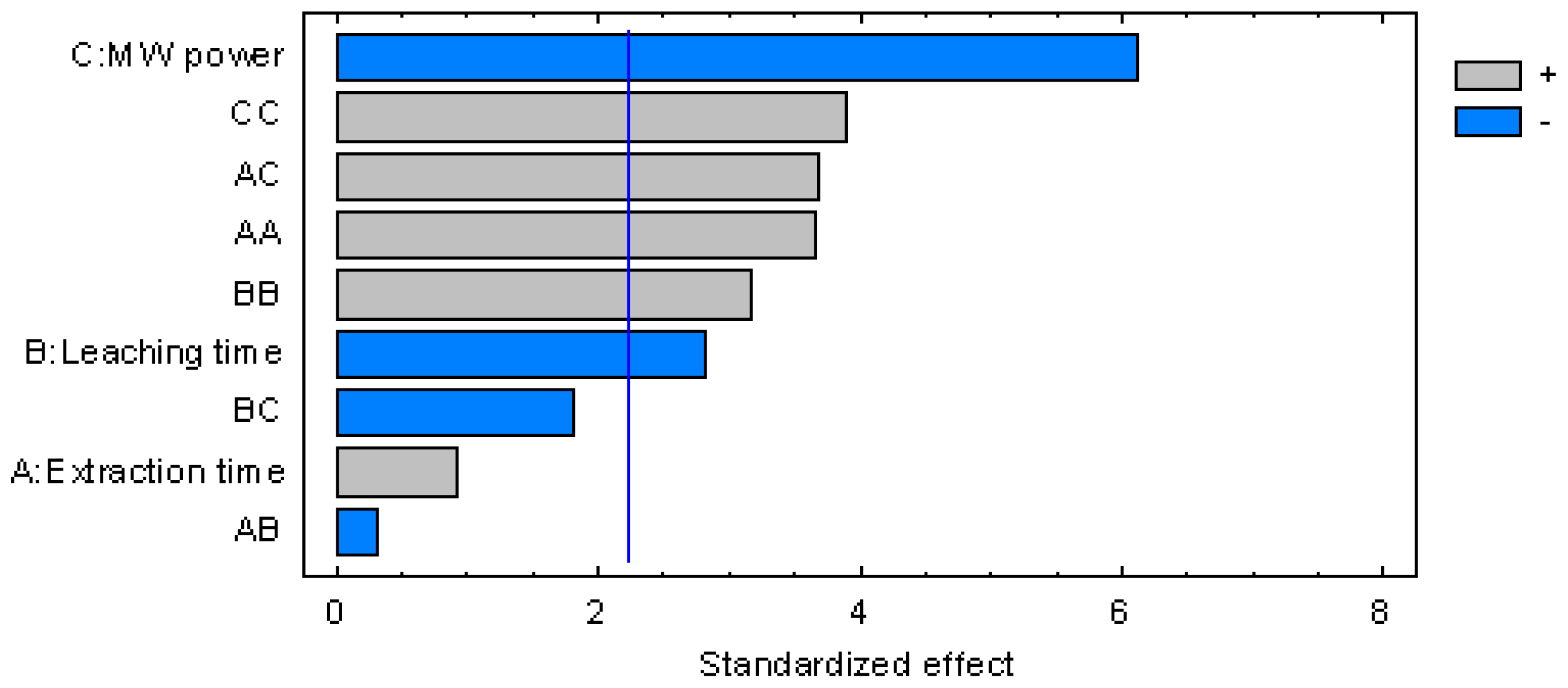
3.1. Central Composite Design Results
3.2. Optimal Conditions
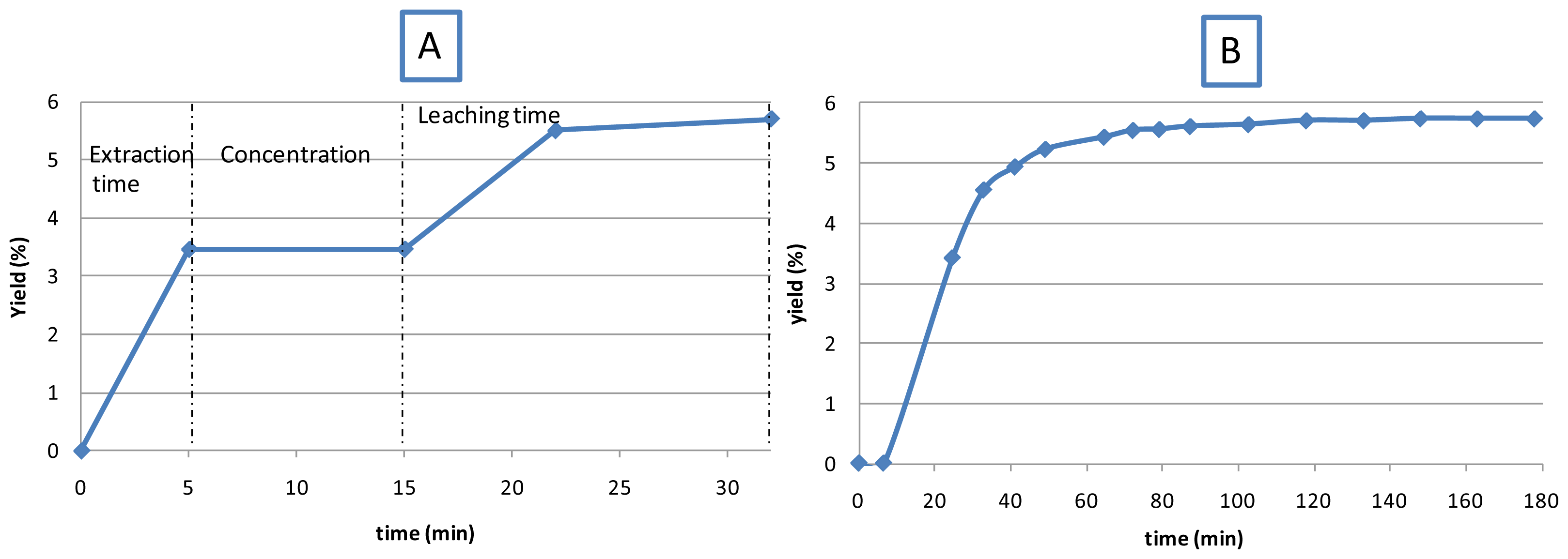
3.3. Kinetics of Extraction: Comparison of MIEL vs. Soxhlet
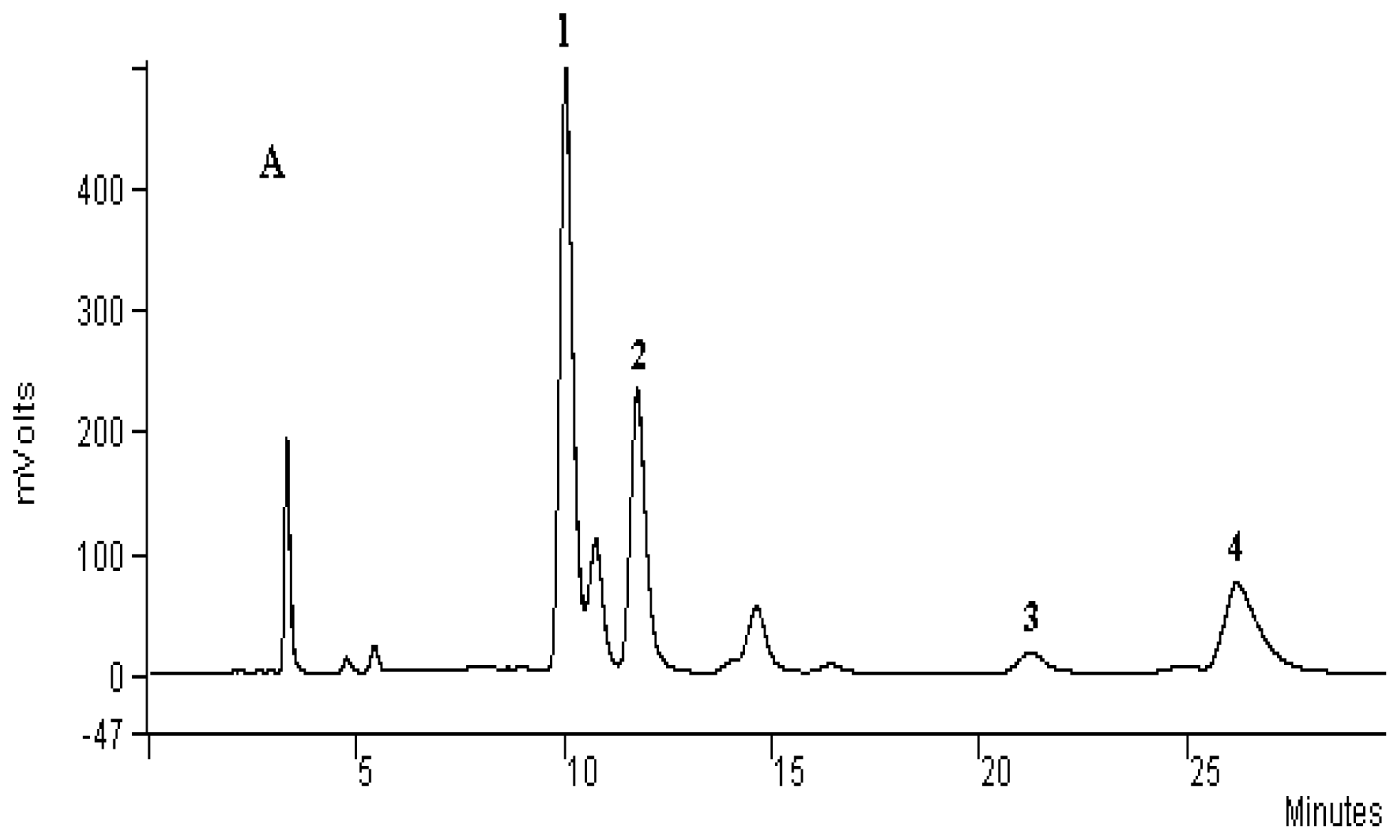
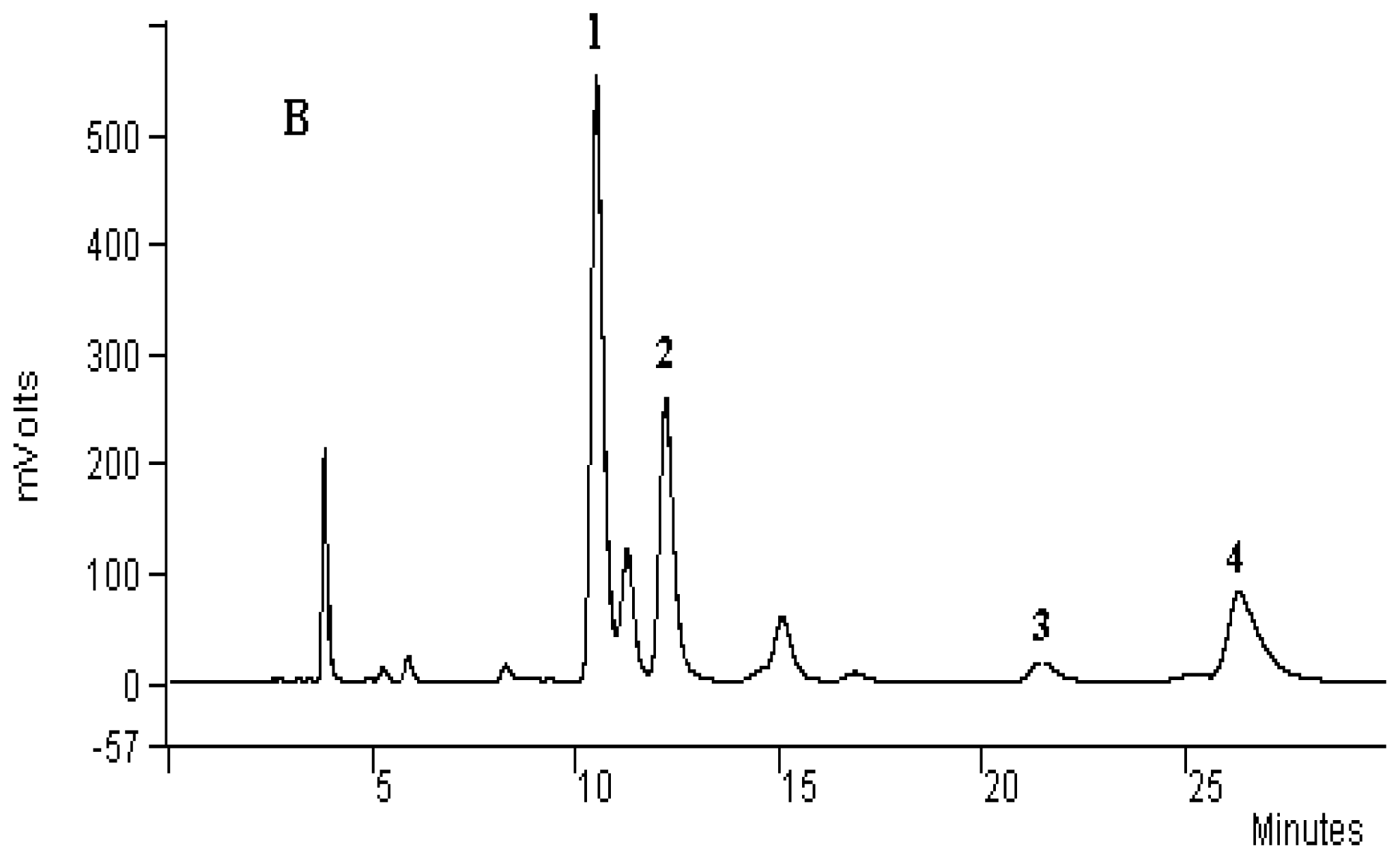
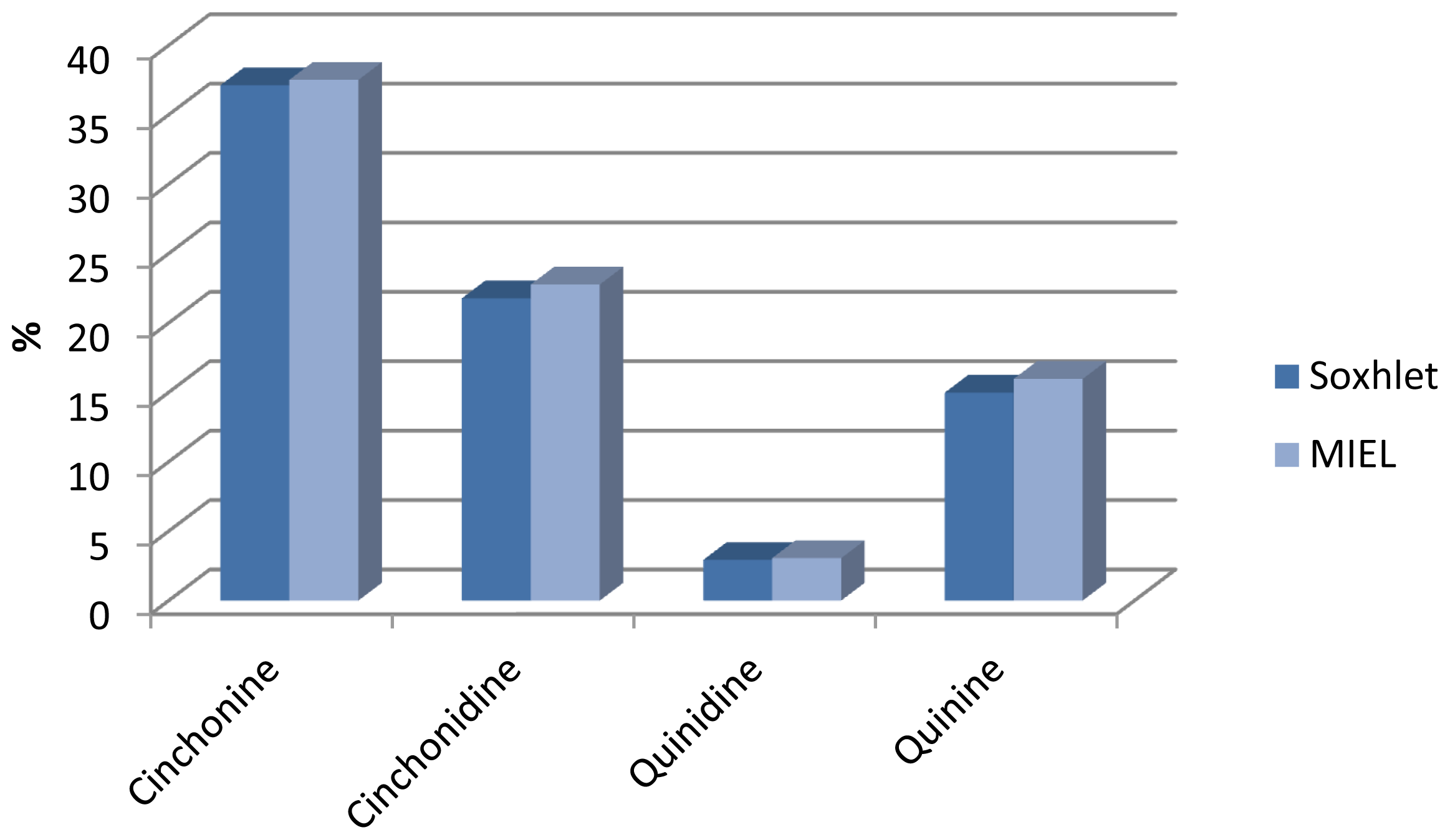
3.4. Analysis of Principal Alkaloids
3.5. Cost, Energy, and Environmental Ecology
4. Conclusions
Acknowledgments
References
- Bruneton, J. Pharmacognosie, Phytochimie, Plantes Médicinales, 4th ed; Tec & Doc: Paris, France, 2009. [Google Scholar]
- Shah, B.H.; Nawaz, Z.; Virani, S.S.; Ali, I.Q.; Saeed, S.A.; Gilani, A.H. The inhibitory effect of cinchonine on human platelet aggregation due to blockade of calcium influx. Biochem. Pharmacol 1998, 56, 955–960. [Google Scholar]
- McCalley, D.V. Analysis of the Cinchona alkaloids by high-performance liquid chromatography and other separation techniques. J. Chromatogr. A 2002, 967, 1–19. [Google Scholar]
- Council of Europe, European Pharmacopeia, 6th ed; Council of Europe: Strasbourg, France, 2008; p. 3008.
- Gatti, R.; Gioia, M.G.; Cavrini, V. Determination of Cinchona alkaloids and Vitamin B6 by high-performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 2004, 512, 85–91. [Google Scholar]
- Soxhlet, F. Die gewichtsanalytische bestimmung des milchfettes. Dingler’s Polytech. J 1879, 232, 461–465. [Google Scholar]
- Pointet, K.; Milliet, A. PAHs analysis of fish whole gall bladders and livers from the natural reserve of camargue by GC/MS. Chemosphere 2000, 40, 293–299. [Google Scholar]
- Tanabe, S.; Watanabe, M.; Minh, T.B.; Kunisue, T.; Nakanishi, S.; Ono, H.; Tanaka, H. PCDDs, PCDFs and coplanar PCBs inalbatross from the North Pacific and Southern Oceans: Levels, patterns and toxicological implications. Envrion. Sci. Technol 2004, 38, 403–413. [Google Scholar]
- Schmarr, H.G.; Gross, H.B.; Shibamoto, T. Analysis of polar cholesterol oxidation products: Evaluation of a new method involving transesterification, solid phase extraction, and gas chromatography. J. Agric. Food Chem 1996, 44, 512–517. [Google Scholar]
- Mulinacci, N.; Innocenti, M.; La Marca, G.; Mercalli, E.; Giaccherini, C.; Romani, A.; Erica, S.; Vincieri, F.F. Solid olive residues: Insight into their phenolic composition. J. Agric. Food Chem 2005, 53, 8963–8969. [Google Scholar]
- Singh, G.; Marimuthu, P.; de Heluani, C.S.; Catalan, C.A.N. Antioxidant and biocidal activities of Carum nigrum (seed) essential oil, oleoresin, and their selected components. J. Agric. Food Chem 2006, 54, 174–181. [Google Scholar]
- Rao, N.V.; Nagaratna, P.K.M.; Satyanarayana, S.; Hemamalini, K.; Kumar, S.M.S. Antiulcer, anti-inflammatory and analgesic activities of leaf extracts of Pongania Pinnata linn (FABACEAE). Pharmacologyonline 2007, 1, 529–538. [Google Scholar]
- Devine, D.M.; Devery, S.M.; Lyons, J.G.; Geever, L.M.; Kennedy, J.E.; Higginbotham, C.L. Multifunctional polyvinylpyrrolidinone-polyacrylic acid copolymer hydrogels for biomedical applications. Int. J. Pharm 2006, 326, 50–59. [Google Scholar]
- Clark, R.E.; Margraf, H.W.; Beauchamp, R.A. Fat and solid filtration in clinical perfusion. Surgery 1975, 77, 216–224. [Google Scholar]
- ISO 659-1988 (E); International Organization for Standardization (ISO): Geneva, Switzerland, 1988. Available online: http://www.fritsch.de/uploads/media/lit_ballmill_oilseeds_determination_of_oil_content_reference_method_1_pdf accessed on 10 November 2011.
- Luque de Castro, M.D.; García-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 36, 1–10. [Google Scholar]
- Paré, J.R.J.; Bélanger, J.M.R. Instrumental Methods in Food Analysis; Elsevier Science: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Kingston, H.M.; Jassie, L.B. Introduction to Microwave Sample Preparation; American Chemical Society: Washington, DC, USA, 1988. [Google Scholar]
- Kingston, H.M.; Haswell, S.J. Microwave Enhanced Chemistry: Fundamentals, Sample Preparation and Application; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Zlotorzynski, A. The application of microwave radiation to analytical and environmental chemistry. Crit. Rev. Anal. Chem 1995, 25, 43–76. [Google Scholar]
- Pan, X.; Niu, G.; Liu, H. Comparison of microwave-assisted extraction and conventional extraction techniques for the extraction of tanshinones from Salvia miltiorrhiza bunge. Biochem. Eng. J 2002, 12, 71–77. [Google Scholar]
- Zhu, X.; Su, Q.; Cai, J.; Yang, J. Optimization of microwave-assisted solvent extraction for volatile organic acids in tobacco and its comparison with conventional extraction methods. Anal. Chim. Acta 2006, 579, 88–94. [Google Scholar]
- Barriada-Pereira, M.; Concha-Grana, E.; Gonzalez-Castro, M.J.; Muniategui-Lorenzo, S.; Lopez-Mahia, P.; Prada-Rodriguez, D.; Fernandez-Fernandez, E. Microwave-assisted extraction versus Soxhlet extraction in the analysis of 21 organochlorine pesticides in plants. J. Chromatogr. A 2003, 1008, 115–122. [Google Scholar]
- Hemwimon, S.; Pavasant, P.; Shotipruk, A. Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia. Sep. Purif. Technol 2007, 54, 44–50. [Google Scholar]
- García-Ayuso, L.E.; Sánchez, M.; Fernández De Alba, A.; Luque De Castro, M.D. Focused microwave-assisted soxhlet: An advantageous tool for sample extraction. Anal. Chem 1998, 70, 2426–2431. [Google Scholar]
- Luque-García, J.L.; Luque De Castro, M.D. Focused microwave-assisted Soxhlet extraction: Devices and applications. Talanta 2004, 64, 571–577. [Google Scholar]
- García-Ayuso, L.E.; Velasco, J.; Dobarganes, M.C.; Luque De Castro, M.D. Determination of the oil content of seeds by focused microwave-assisted Soxhlet extraction. Chromatographia 2000, 52, 103–108. [Google Scholar]
- Priego-López, E.; Velasco, J.; Dobarganes, M.C.; Ramis-Ramos, G.; Luque De Castro, M.D. Focused microwave-assisted Soxhlet extraction: An expeditive approach for the isolation of lipids from sausage products. Food Chem 2003, 83, 143–149. [Google Scholar]
- García-Ayuso, L.E.; Velasco, J.; Dobarganes, M.C.; Luque De Castro, M.D. Accelerated extraction of the fat content in cheese using a focused microwave-assisted Soxhlet device. J. Agric. Food Chem 1999, 47, 2308–2315. [Google Scholar]
- Priego-Capote, F.; Ruiz-Jiménez, J.; Luque de Castro, M.D.L. Identification and quantification of trans fatty acids in bakery products by gas chromatography-mass spectrometry after focused microwave Soxhlet extraction. Food Chem 2007, 100, 859–867. [Google Scholar]
- Paré, J.R.J.; Bélanger, J.M.R. Microwave-Assisted Process: Principles and Applications. In Instrumental Methods in Food Analysis; Paré, J.R.J., Bélanger, J.M.R., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1997; Volume 18, pp. 395–420. [Google Scholar]
- Bélanger, J.M.R.; Paré, J.R.J. Microwave-Assisted Processes in Food Analysis. In Handbook of Food Analysis Instruments; Ötles, S., Ed.; Taylor & Francis–CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Bousbia, N.; Vian, M.A.; Ferhat, M.A.; Petitcolas, E.; Meklati, B.Y.; Chemat, F. Comparison of two isolation methods for essential oil from rosemary leaves: Hydrodistillation and microwave hydrodiffusion and gravity. Food Chem 2009, 114, 355–362. [Google Scholar]
- Cañizares-Macías, M.P.; García-Mesa, J.A.; Luque de Castro, M.D. Determination of the oxidative stability of olive oil, using focused-microwave energy to accelerate the oxidation process. Anal. Bioanal. Chem. 2004, 378, 479–483. [Google Scholar]
- Chemat, F.; Esveld, E. Microwave superheated boiling of organic liquids: Origin, effect and application. Chem. Eng. Technol 2001, 24, 735–744. [Google Scholar]
- Bernard, J. Le gaz naturel remonte le courant. Sci. Vie 2001, 214, 68–73. [Google Scholar]
| Level | Extraction Time (min) | Leaching Time (min) | MW Power (W) |
|---|---|---|---|
| −α (−1.6818) | 5 | 5 | 60 |
| −1 | 8 | 8 | 100 |
| 0 | 12 | 12 | 150 |
| +1 | 16 | 16 | 200 |
| +α (1.6818) | 19 | 19 | 240 |
| Run Order | Extraction Time (min) | Leaching Time (min) | MW Power (W) | Response (%) |
|---|---|---|---|---|
| 1 | 0 (12) | 1.6818 (19) | 0 (150) | 3.03 |
| 1 | 0 (12) | 1.6818 (19) | 0 (150) | 3.03 |
| 2 | 0 (12) | 0 (12) | 0 (150) | 2.72 |
| 3 | 0 (12) | 0 (12) | 0 (150) | 2.71 |
| 4 | 1 (16) | 1 (16) | 1 (200) | 2.85 |
| 5 | −1 (8) | 1 (16) | 1 (200) | 2.42 |
| 6 | −1.6818 (5) | 0 (12) | 0 (150) | 2.91 |
| 7 | 0 (12) | 0 (12) | 0 (150) | 2.74 |
| 8 | 0 (12) | 0 (12) | 1.6818 (240) | 2.84 |
| 9 | −1 (8) | −1 (8) | 1 (200) | 3.1 |
| 10 | −1 (8) | 1 (16) | −1 (100) | 4.04 |
| 11 | 1.6818 (19) | 0 (12) | 0 (150) | 3.73 |
| 12 | −1 (8) | −1 (8) | −1 (100) | 3.97 |
| 13 | 0 (12) | 0 (12) | −1.6818 (60) | 3.87 |
| 14 | 0 (12) | 0 (12) | 0 (150) | 2.71 |
| 15 | 1 (16) | −1 (8) | −1 (100) | 3.43 |
| 16 | 0 (12) | 0 (12) | 0 (150) | 2.8 |
| 17 | 0 (12) | 0 (12) | 0 (150) | 2.75 |
| 18 | 1 (16) | 1 (16) | −1 (100) | 3.18 |
| 19 | 1 (16) | −1 (8) | 1 (200) | 3.39 |
| 20 | 0 (12) | −1.6818 (5) | 0 (150) | 3.46 |
| Effect | Sum of Squares | Df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A:Extraction time | 0.0357836 | 1 | 0.0357836 | 0.87 | 0.3737 |
| B:Leaching time | 0.330082 | 1 | 0.330082 | 8.00 | 0.0179 |
| C:MW power | 1.54419 | 1 | 1.54419 | 37.42 | 0.0001 |
| AA | 0.556121 | 1 | 0.556121 | 13.47 | 0.0043 |
| AB | 0.00405 | 1 | 0.00405 | 0.10 | 0.7605 |
| AC | 0.5618 | 1 | 0.5618 | 13.61 | 0.0042 |
| BB | 0.416118 | 1 | 0.416118 | 10.08 | 0.0099 |
| BC | 0.1352 | 1 | 0.1352 | 3.28 | 0.1004 |
| CC | 0.628391 | 1 | 0.628391 | 15.23 | 0.0030 |
| Total error | 0.412711 | 10 | 0.0412711 | ||
| Total (corr.) | 4.36238 | 19 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fabiano-Tixier, A.-S.; Elomri, A.; Blanckaert, A.; Seguin, E.; Petitcolas, E.; Chemat, F. Rapid and Green Analytical Method for the Determination of Quinoline Alkaloids from Cinchona succirubra Based on Microwave-Integrated Extraction and Leaching (MIEL) Prior to High Performance Liquid Chromatography. Int. J. Mol. Sci. 2011, 12, 7846-7860. https://doi.org/10.3390/ijms12117846
Fabiano-Tixier A-S, Elomri A, Blanckaert A, Seguin E, Petitcolas E, Chemat F. Rapid and Green Analytical Method for the Determination of Quinoline Alkaloids from Cinchona succirubra Based on Microwave-Integrated Extraction and Leaching (MIEL) Prior to High Performance Liquid Chromatography. International Journal of Molecular Sciences. 2011; 12(11):7846-7860. https://doi.org/10.3390/ijms12117846
Chicago/Turabian StyleFabiano-Tixier, Anne-Sylvie, Abdelhakim Elomri, Axelle Blanckaert, Elisabeth Seguin, Emmanuel Petitcolas, and Farid Chemat. 2011. "Rapid and Green Analytical Method for the Determination of Quinoline Alkaloids from Cinchona succirubra Based on Microwave-Integrated Extraction and Leaching (MIEL) Prior to High Performance Liquid Chromatography" International Journal of Molecular Sciences 12, no. 11: 7846-7860. https://doi.org/10.3390/ijms12117846
APA StyleFabiano-Tixier, A.-S., Elomri, A., Blanckaert, A., Seguin, E., Petitcolas, E., & Chemat, F. (2011). Rapid and Green Analytical Method for the Determination of Quinoline Alkaloids from Cinchona succirubra Based on Microwave-Integrated Extraction and Leaching (MIEL) Prior to High Performance Liquid Chromatography. International Journal of Molecular Sciences, 12(11), 7846-7860. https://doi.org/10.3390/ijms12117846