Abstract
Mitochondria are involved either directly or indirectly in oncogenesis and the alteration of metabolism in cancer cells. Cancer cells contain large numbers of abnormal mitochondria and produce large amounts of reactive oxygen species (ROS). Oxidative stress is caused by an imbalance between the production of ROS and the antioxidant capacity of the cell. Several cancer therapies, such as chemotherapeutic drugs and radiation, disrupt mitochondrial homeostasis and release cytochrome c, leading to apoptosome formation, which activates the intrinsic pathway. This is modulated by the extent of mitochondrial oxidative stress. The peroxiredoxin (Prx) system is a cellular defense system against oxidative stress, and mitochondria in cancer cells are known to contain high levels of Prx III. Here, we review accumulating evidence suggesting that mitochondrial oxidative stress is involved in cancer, and discuss the role of the mitochondrial Prx III antioxidant system as a potential target for cancer therapy. We hope that this review will provide the basis for new strategic approaches in the development of effective cancer treatments.
1. Mitochondria and Cancer
As the main energy producers in cells, mitochondria subject substrates to oxidative phosphorylation, thereby generating the energy molecule ATP. During this process, mitochondria inevitably generate reactive oxygen species (ROS). ROS are involved in the regulation of many physiological processes, including cell signaling, but are harmful to cells if produced in excessive amounts. Furthermore, mitochondria, which are crucial regulators of the intrinsic pathway of apoptosis, perform vital and lethal functions in physiological and pathological contexts [1,2]. Mitochondria control the activation of apoptotic effector mechanisms by regulating the translocation of pro-apoptotic proteins from the mitochondrial intermembrane space to the cytosol. In addition, they play a major role in multiple forms of non-apoptotic cell death [3]. In this context, mitochondrial abnormalities occur in various diseases, including cardiovascular, neurodegenerative, metabolic diseases, and cancer.
In cancer cells, key mitochondrial regulators of cell death and other processes are often altered [4]. Cancer-cell mitochondria differ structurally and functionally from their normal-cell counterparts [4,5]. Rapidly growing tumors readily become hypoxic due to the inability of the local vasculature to supply an adequate amount of oxygen. Furthermore, mutations in mitochondrial and nuclear DNA that affect components of the mitochondrial respiratory chain result in inefficient ATP production, ROS overproduction, and oxidative damage to mitochondria and macromolecules [5]. Over 70 years ago, Warburg pioneered research into alterations in mitochondrial respiration in the context of cancer and proposed a mechanism to explain how they evolve during the carcinogenic process [6]. This process differs from that in normal cells, which utilize oxidative phosphorylation primarily for growth and survival. Although the observation of high rates of aerobic glycolysis in tumor cells has been corroborated, the role of mitochondria in tumor cells has been contentious [7]. The major role of aerobic glycolysis in cancer cells is likely to be the generation of glycolytic intermediates for the pentose phosphate pathway in nucleotide and phospholipid synthesis, while glycolytic ATP generation is likely to be important for survival under hypoxic conditions [8]. The glutamine-fueled TCA cycle generates ATP, ROS, nicotinamide adenine dinucleotide phosphate (NADPH), amino acids, and lipids. The synthesis of ATP requires large amounts of oxygen, which routinely leads to the generation of ROS such as hydrogen peroxide, the superoxide anion, and organic peroxide [9]. These ROS can cause cellular damage if they are not detoxified by antioxidant systems. Increased mitochondrial ROS generation and the disturbance of peroxiredoxin (Prx) production in cancer cells may lead to oxidative stress and the induction of apoptosis. The Prx system is a cellular defense system against oxidative stress. Mitochondria in cancer cells are known to contain high levels of Prx III and Prx V [10–14]. However, Prx V founds in various compartments in the cell, including mitochondria, peroxisome and nucleus [15–17]. Moreover, mitochondria are a major site of hydrogen peroxide generation in cells [18]. Prx III prefers to scavenge hydrogen peroxide, which will be the target for up to 90% of H2O2. In contrast, Prx V behaves more effectively as a scavenger of peroxynitrite [19–22]. Here, we discuss the role of the mitochondrial Prx III antioxidant system being exclusively present in mitochondria as a potential target for cancer therapy, and examine the effects of antioxidant proteins on ROS in mitochondria. We hope that this review article will advance our understanding of mitochondrial biology in cancer, and provide a basis for designing new strategies to achieve effective cancer treatment.
2. Mitochondrion-Targeting Cancer Therapy
Mitochondria are known to play a key role in apoptosis and to trigger cell death via several mechanisms, including the disruption of electron transport and energy metabolism, the release or activation of proteins that mediate apoptosis, and the alteration of the cellular redox potential [23–25]. Apoptotic cell death is characterized by a host of morphological and biochemical features, including mitochondrial outer membrane permeabilization (MOMP) and the release of pro-apoptotic proteins [26]. In response to pro-apoptotic stimuli, including ROS and Ca2+ overload, the permeability transition pore complex (PTPC) assumes a high-conductance state that deregulates the entry of small solutes into the mitochondrial matrix along their electrochemical gradients[1]. This mitochondrial permeability transition (MPT) results in immediate dissipation of the mitochondrial membrane potential and osmotic swelling of the mitochondrial matrix. As the surface area of the inner membrane considerably exceeds that of the outer membrane, the MPT eventually leads to MOMP (Figure 1). The MPT can be triggered by agents that increase cytosolic Ca2+ concentrations or stimulate ROS generation. The mitochondrial pore, a putative multimeric complex situated at mitochondrial contact sites, mediates the MPT. Based on biochemical evidence, the standard model for the PT pore consists of a voltage-dependent anion channel (VDAC) in the outer membrane, adenine nucleotide translocase (ANT), and cyclophilin D (CypD) in the matrix (Figure1A; [27,28]. As shown in Figure 1A, the VDAC has always been considered a key component of the PTPC. However, considerable recent evidence suggests that the conclusions of standard model studies were incorrect (Figure 1B). Closure of the VDAC has been shown to increase the influx of Ca2+ into mitochondria [29], which has the net effect of inducing, rather than inhibiting, the MPT. Moreover, recent genetic studies have confirmed a regulatory role for CypD in the MPT [30,31]. Mice lacking ANT or the VDAC still exhibit a classical MPT response that is inhibited by cyclosporine A [32,33]. Thus, current genetic strategies indicate that only CypD functions as a necessary effector of the MPT, and suggest that alternative proteins and/or mechanisms must play roles in mitochondrial-dependent cell mortality via the PT pore. Because two proposed models for the PT pore have not yet been fully elucidated, the PT pore is not sufficiently well characterized to be a target for anticancer drugs. Although the exact molecular identity of the effectors of the MPT is under debate, it is agreed to be a crucial step in cell death.
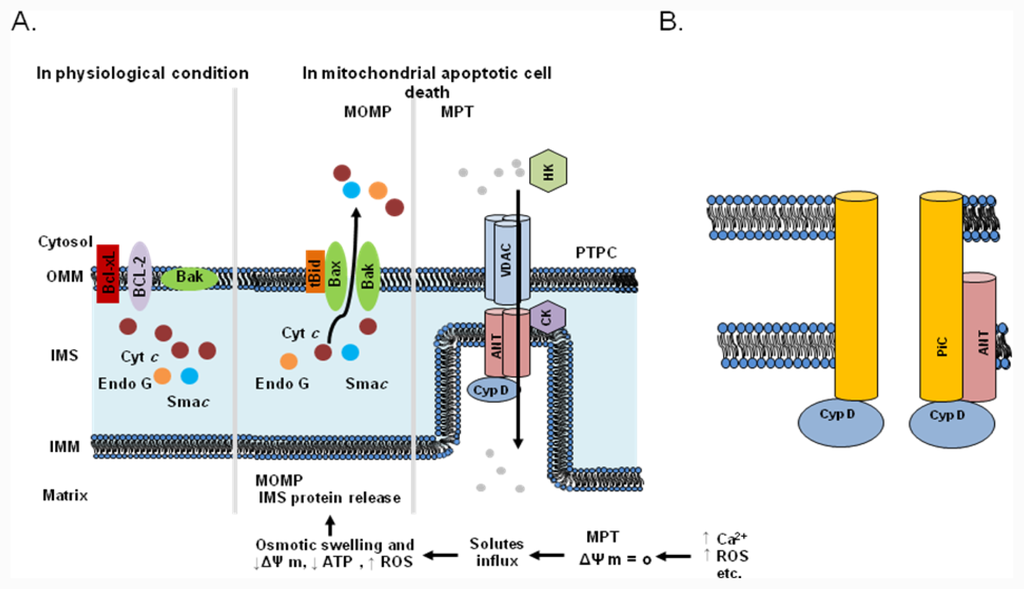
Figure 1.
Molecular mechanisms of the mitochondrial permeability transition (MPT) and mitochondrial apoptotic cell death. (a) Mitochondrial outer membrane permeabilization (MOMP) leads to apoptogenic protein release. Bax or Bak forms a pore in the OM after activation by a BH3-only protein such as Bid (after the truncation of Bid by caspase-8). The opening of the PT pore allows an influx of water and ions into the matrix, causing matrix swelling. This leads to rupture of the OM and the release of intermembrane space (IMS) proteins. The permeability transition pore complex (PTPC) is a highly dynamic supramolecular entity that can comprise a voltage-dependent anion channel (VDAC), adenine nucleotide translocase (ANT), and cyclophilin D (CypD). Other proteins, including the peripheral benzodiazepine receptor (PBR), hexokinase (HK), and creatine kinase (CK), may also be associated with the PTPC. It is not clear whether the PTPC has a role under physiological conditions. Mitochondria exhibit a high mitochondrial transmembrane potential, which is generated by the respiratory chain and exploited for ATP generation. It has been proposed that under these conditions the PTPC exists in a low-conductance state, thereby contributing to the exchange of small metabolites between the cytosol and the mitochondrial matrix, a process that is predominantly mediated by mitochondrial solute carriers. However, under pathological conditions characterized by a high Ca2+ concentration, increased oxidative stress, low levels of ATP, and mitochondrial depolarization, the complex forms an open pore between the inner and outer membranes, allowing the free diffusion of solutes across the membranes. The opening of the PTPC results in mitochondrial swelling, mitochondrial Ca2+ efflux, and the release of apoptogenic proteins such as cytochrome c and Smac from the IMS. (b) Alternative models proposed in light of recent findings in gene-targeted mice. A VDAC is no longer part of the model and it appears that an OM component may not be necessary for this process. ANT now appears to be more of a regulatory protein, and only CypD remains as an established component. In contrast, the mitochondrial phosphate carrier (PiC) has been added to the model as a candidate component of the pore-forming unit of the MPT pore.
Because most cancer cells have increased resistance to the activation of MOMP and escape apoptosis as a result of various modifications in apoptosis regulators, including Bcl-2 family members, p53, and caspases [34], various mitochondrion-targeted cancer treatment strategies have been developed in the last decade [35,36]. These strategies focused mainly on the development of compounds that regulate mitochondrial Bcl-2 family proteins, modulate MOMP and hyperpolarized mitochondria inner membrane potential sensing, or target high levels of ROS and overexpressed receptors in cancer cells [35]. An excellent previous review by Fulda et al. summarized examples of mitochondrion-targeted compounds (Table 1); [36]. Numerous molecules that are currently in use or being tested in clinical trials act on mitochondria [37]. Clinically approved anticancer drugs such as etoposide [38], paclitaxel [39], and vinorelbine [40], as well as an increasing number of experimental anticancer drugs, including ceramide [41], MKT077 [42], and CD437 [43], have been found to act directly on mitochondria to trigger apoptosis. Several classes of compounds with distinct mechanisms of action can stimulate the MPT and mitochondrial apoptosis in cancer cells, pointing to some functional redundancy and suggesting the likely existence of alternative biochemical cascades leading to mitochondrial membrane permeabilization. Thus, the selective targeting of cancer cells using mitochondrial-targeted agents is likely to attract great interest. A better understanding of the key pathophysiological differences between mitochondria in cancer cells and their counterparts in non-cancerous cells will undoubtedly be instrumental in increasing the level of selectivity of mitochondrion-targeted anticancer agents. Nevertheless, a limited number of studies have evaluated agents targeting the mitochondrial ROS regulatory system.

Table 1.
Examples of mitochondrion-targeted compounds.
3. Regulation of the Mitochondrial Antioxidant System
Since the discovery that electron leakage and incomplete reduction of oxygen occur in the respiration chain [44], mitochondria have been considered a major contributor to cellular oxidative damage due to their generation of ROS. Moreover, mitochondria possess a multilevel network of enzymatic and non-enzymatic antioxidant systems for the detoxification of H2O2 (Figure 2). The biological significance of mitochondrial ROS has been highlighted by the targeted deletion or overexpression of antioxidant proteins. For example, superoxide dismutase (SOD) 2, thioredoxin (Trx) 2, Prx III and Prx V have been reported to constitute a novel antioxidant defense system that detoxifies ROS generated in mitochondria [45,46]. Prx3-knockout (KO) mice were showed aberrant regulation of oxidative stress. Proteomic analysis and gene expression analysis in adipocytes from Prx3-KO mice also showed defect in mitochondria biogenesis along with enzymes involved in glucose/lipid metabolism and oxidative phosphorylation [47]. Trx2-KO mice have an embryonic lethal phenotype [48]. SOD2-KO mice typically die within 3 weeks of birth as a result of severe neurodegeneration and mitochondrial oxidative damage [49,50]. Prx V was associated with the mitochondrial pathway of apoptosis and calcium loading capacity of mitochondria, as well as changes in mitochondrial morphology [14]. The homozygous glutathione peroxidase (GPx)1-KO mice appeared healthy and manifested no increased sensitivity to hyperoxia or increased levels of protein carbonyl groups or lipid peroxides [51]. However, a protective role for GPx1 became apparent, when the GPx1 KO and control mice were subjected to extreme oxidative stress such as that associated with ischemia-reperfusion injury or treatment with paraquat or a bolus of H2O2 [52,53]. In mammalian cells, GPx1 is the major isoform and is expressed in all tissues; it is localized predominantly in the cytosol, but a small proportion (10%) of GPx1 molecules is also present in the mitochondrial matrix [51,54,55]. Thereby, it remains unclear whether the effect of GPx1-KO under these conditions was attributable to the absence of the enzyme form the cytosol or from mitochondria, or from both. To date, the multiplelevel network of antioxidant system in mitochondria has been extensively discussed in a number of recent publications. Based on these studies, mitochondrial-targeted agents emerge as a means to selectively target tumors. Here, we provide a comprehensive compendium on the mitochondrial-targeted compounds for the treatment of human cancer.
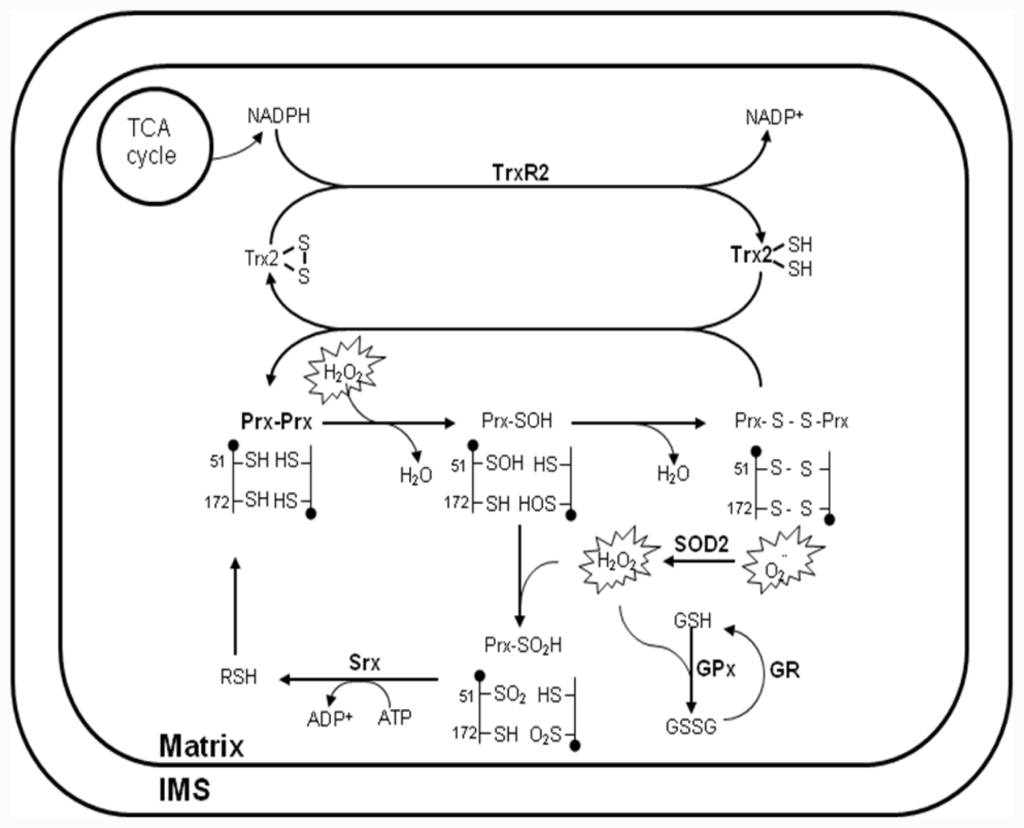
Figure 2.
Antioxidant system for H2O2 removal in mitochondria. Reactive oxygen species (ROS), in the form of O2− and H2O2, have multiple intra- and extramitochondrial sources. O2− is converted to H2O2 through the action of superoxide dismutase (SOD) 2 and /or spontaneous dismutation. H2O2 can diffuse into the mitochondrial matrix, where it is removed via three systems/mechanisms: 1) peroxiredoxin (Prx) III coupled to thioredoxin (Trx) 2 and Trx reductase (TrxR) 2; 2) glutathione peroxidase (GPx) coupled to glutathione (GSH) and GSH reductase (GR); and 3) non-enzymatic scavenging by redox compounds. The peroxidatic cysteine Cys-SH is selectively oxidized by H2O2 to Cys-SOH, which then reacts with the resolving cysteine Cys-SH of the other subunit in the homodimer to form an intermolecular disulfide bond. Subsequently, the disulfide bond is specifically reduced by Trx2, which in turn receives reducing equivalents from nicotinamide adenine dinucleotide phosphate (NADPH) via TrxR2. The Cys-SOH generated is oxidized to Cys-SO2H, leading to peroxidase inactivation. Reactivation of the enzyme is achieved by reduction of the Cys-SO2H moiety in a reaction that requires ATP hydrolysis and is catalyzed by sulfiredoxin (Srx), with reducing equivalents provided by physiological thiols (RSH) such as GSH and Trx. The respiration substrates malate/glutamate and succinate provide energy in the form of reducing covalents (NADPH), which are maintained by ΔΨm-dependent transhydrogenase and tricarboxylic acid (TCA) cycle enzymes. NADPH is utilized by the reductases in the peroxidase system (TrxR and GR) to reduce disulfide bonds formed in proteins during the detoxification of H2O2.
Multiple compounds act on components of the antioxidant system to induce ROS generation and apoptosis. Reportedly, the increase in intrinsic ΔΨm correlates with increased malignancy (apoptosis resistance and tumor progression) [56], suggesting that cytotoxic agents that permeabilize the mitochondrial membrane, such as compounds that induce the overproduction of ROS, are effective anticancer drugs in cancer cells. The inhibition of antioxidant systems is an alternative way to induce ROS accumulation. Compounds that inhibit antioxidant systems include the SOD inhibitors 2-methoxyestradiol, choline tetrathiomolybdate (ATN-224), and mangafodipir; buthionine sulfoximine, imexon, and phenylethyl isothiocyanate (PEITC), which cause glutathione (GSH) inhibition or depletion; and menadione, motexafin gadolinium, β-lapachone, elesclomol (STA-4783), arsenic trioxide, parthenolide, dimethylamino-parthenolide (DMAPT), and bistetrahydrofuranic acetogenins, which induce ROS production (Table 2).

Table 2.
Development and clinical status of anti-cancer drugs targeting the mitochondrial oxidative system.
2-methoxyoestradiol inhibits angiogenesis by reducing endothelial cell proliferation and inducing endothelial cell apoptosis, and selectively kills human leukemia cells by inhibiting SOD, thereby causing superoxide accumulation [57]. Several Phase I/II trials in patients with solid malignancies or multiple myeloma have demonstrated that 2-methoxyoestradiol is well tolerated and causes disease stabilization [58–60]. Similar effects are produced by the intracellular copper-chelating agent ATN-224 [61]. ATN-224 is an orally bioavailable, second-generation tetrathiomolybdate analog with potential antiangiogenic and antineoplastic activities. Mangafodipir is a SOD mimic with catalase and GSH reductase activities. Consisting of manganese ions chelated to fodipir (dipyridoxyl diphosphate; DPDP), it scavenges oxygen free radicals such as the superoxide anion, hydrogen peroxide, and the hydroxyl radical, potentially preventing oxygen free radical damage to macromolecules such as DNA and minimizing oxygen free radical–related chemotoxicity in normal tissues. In cancer cells, it has been shown to increase H2O2 levels and to potentiate the antitumor activity of paclitaxel in a mouse xenotransplant colon cancer model [62]. Moreover, it is being tested in a Phase II trial in patients with colon cancer.
Buthionine sulfoximine irreversibly inhibits γ-glutamylcysteine synthetase. It increases ROS levels by inhibiting the synthesis of reduced GSH [63]. Imexon depletes the GSH pool due to its thiol-binding activity [64]. Buthionine sulfoximine and the alkylating agent melphalan are being evaluated in Phase II clinical trials in patients with melanoma or relapsed/refractory ovarian cancer. PEITC, which is thiol modifier, preferentially causes ROS overproduction, mitochondrial oxidative damage, MOMP, and apoptosis in cancer cells, presumably due to their increased ROS levels [65,66]. The compound is known effects on the selenoprotein thioredoxin reductase, glutathione reductase and intracellular GSH levels. Moreover, Prx III is early oxidized after exposure of this compound [67].
Menadione binds to and inhibits the activity of the PTPs that dephosphorylate and inactivate epidermal growth factor receptor (EGFR) and erythroblastic leukemia viral oncogene homolog 2 (ErbB2) in human keratinocytes. Local reversal of EGFR and ErbB2 inhibition associated with the systemic administration of EGFR inhibitors may help alleviate EGFR inhibitor–mediated skin toxicity. Menadione undergoes futile redox cycles in the respiratory chain. Thiol cross-linking agents, such as diamide, bismaleimido-hexane, and dithiodipyridine, cause ANT thiol oxidation and can bypass B-cell lymphoma 2 (BCL-2)–mediated cytoprotection [78,79]. β-lapachone is bioactivated by NAD(P)H:quinone oxidoreductase-1 (NQO1), causing futile oxidoreduction that generates high levels of superoxide, and is currently under clinical investigation, as a monotherapy or in combination with gemcitabine, in patients with pancreatic and head-and-neck cancer. STA-4783 induces oxidative stress, increasing levels of ROS such as hydrogen peroxide in both cancer cells and normal cells. Because tumor cells have elevated levels of ROS compared with normal cells, the increase in oxidative stress beyond baseline levels elevates ROS levels beyond sustainable levels, exhausting tumor cell antioxidant capacity. This may result in the activation of the mitochondrial apoptosis pathway [72]. Arsenic trioxide is a small-molecule arsenic compound with antineoplastic activity. Although the mechanism of action of arsenic trioxide is not completely understood, it causes damage to or degradation of the promyelocytic leukemia protein/retinoic acid receptor-α (PML/RARα) fusion protein; induces apoptosis in acute promyelocytic leukemia cells and many other tumor cell types; promotes cell differentiation and suppresses cell proliferation in many different tumor cell types; and is pro-angiogenic. Parthenolide is a sesquiterpene lactone that can cause allergic reactions. It has anti-inflammatory, antimicrobial, and anticancer properties, activates the tumor suppressor p53, and inhibits nuclear factor-kappa B (NF-κB) and the signal transducer and activator of transcription 3 (STAT-3; [80]. It also induces intracellular oxidative stress, which is manifested by increased ROS levels and activation of c-Jun N-terminal kinase (JNK). The water-soluble parthenolide analog DMAPT, which swiftly kills leukemic stem cells from both myeloid and lymphoid leukemias, is also highly cytotoxic to bulk leukemic cell populations. Molecular studies have found that the key activities of DMAPT include the induction of oxidative stress responses, the inhibition of NF-κB, and the activation of p53 [75]. Natural bistetrahydrofuranic acetogenins show growth inhibitory activity against human breast, lung, liver, and colon cell lines [77]. Recently, structure–activity relationship (SAR) analysis has led to the synthesis of promising new derivatives with improved antitumor properties. However, trials of numerous ROS-regulating compounds, including menadione and STA-4783, have been discontinued due to safety concerns.
4. Peroxiredoxin III: A Potential Mitochondrial Target for Cancer Therapy
Peroxiredoxins are a family of enzymes that catalyze the reduction of hydrogen peroxide and hydroperoxides to water and alcohol, respectively [81,82]. The six isoforms of mammalian Prx (I–VI) are classified into three subfamilies (2-Cys, atypical 2-Cys, and 1-Cys) based on the number and position of the cysteine (Cys) residues that participate in catalysis. Also, the Prxs can be categorized by their subcellular localization; Prx I, II and VI found in the cytoplasm, Prx IV in the endoplasmic reticulum, Prx III in the mitochondria, and Prx V found in various compartments in the cell, including peroxisomes and mitochondria. Prx I–IV (2-Cys Prx subfamily) have two conserved Cys residues. In the catalytic cycle of the 2-Cys Prxs, the conserved N-terminal Cys sulfhydryl (Cys-SH) is first oxidized by peroxides to Cys sulfenic acid (Cys-SOH), which then reacts with the conserved COOH-terminal Cys-SH of the other subunit in the homodimer to form a disulfide bond. The Prx V is an atypical 2-Cys Prx that becomes oxidized at the peroxidatic cysteine (Cys48) to a sulfenic acid, which condenses with a resolving cysteine (Cys152) within the same polypeptide to form an intramolecular disulfide linkage [16]. In contrast, Prx VI has only one Cys residue is involved in the peroxidase activity Prx VI (1-Cys), and unlike the other members, does not use thioredoxin as a reductant. The N-terminal Cys-SH of Prx VI is readily oxidized, but the resulting Cys-SOH does not form a disulfide because of the unavailability of another Cys-SH nearby [83,84]. As the physiological reductant, Prx VI utilizes GSH via the formation of disulfide with GSH mediated by πGST.
The hyper-proliferative property of cancer cells is known to be associated with increased production of intracellular ROS [85]. Moreover, many reports have claimed an association between alterations in the protein level of Prx isoforms. Such Prxs serve divergent functions, such as protecting cells against oxidative stress, regulating cell signaling associated with H2O2, and influencing cell differentiation and proliferation, immune responses, and apoptosis [82,86,87] Recent studies reported elevated expression of Prx I in several human cancers, including non-small cell lung cancer (NSCLC) [88,89], oral cancer [90], breast cancer [11], and liver cancer [91]. Prx II levels are increased in breast, mesothelioma, and head-and-neck cancers [10,92]. While increased Prx II expression rendered leukemia and stomach cancer cells resistant to various chemotherapeutic agents [93,94], downregulation of Prx II sensitized head-and-neck cancer cells to radiation and gastric carcinoma to cisplatin [95,96]. Moreover, downregulation of Prx II enhances apoptotic cell death induced by tumor necrosis factor (TNF)-α and TNF-related apoptosis-inducing ligand (TRAIL). Importantly, cytosolic Prx II regulates caspase-8 activation, but exerts no influence on sustained JNK activation [97]. Downregulation of Prx I was shown to sensitize lung cancer cells to radiation and reduce metastasis [98,99], and to increase the sensitivity of prostate cells to androgen ablation treatment [100]. Prx IV is decreased in stomach cancers [101]; may play an important role in protecting cells from ionizing radiation-induced apoptosis in head-and-neck squamous cell carcinoma [102]; in lung cancer cells, Prx IV interacts with surfiredoxin and the interaction axis leads to acceleration of tumor growth and metastasis formation in vivo [103]. Prx V represented antioxidant functions in the lung cartilage, and brain [104–106]. Overexpression of Prx V was reported to protect Chinese hamster ovary cells from oxidative stress; suppressed p53-dependent apoptosis [107]; promoted differerentiation, and reduced apoptosis in the mice muscle cells [108] and human tendon cells [109]. However, it still remains unknown whether the function of this protein is restricted to its antioxidant activity, and position of major compartments to protect cells from cell death. Prx VI is decreased in a mouse that is susceptible to experimental atherosclerosis [110] and is elevated in the spinal cord of mice expressing mutant superoxide dismutase1 [111]; in brains of patients with parkinsonian dementia [112], sporadic Creutzfeldt-Jacob disease [113], and Pick disease [114]; in the healing edge of skin wounds [115]; and in experimental cellular premature senescence [116]. Especially, it is elevated in lungs with malignant mesothelioma [10] or high grade squamous cell carcinoma [117].
Like cytosolic Prx I and Prx II, mitochondrial Prx III is overexpressed in hepatocellular carcinoma [12] and breast cancer [11]. The overexpression of Prx III can protect cells against oxidative injury [13,118], whereas the deletion of Prx III in HeLa cells can increase intracellular levels of H2O2 and sensitize cells to the induction of apoptosis by staurosporine and TNF-α [119]. Furthermore, the abundance of Prx III was found to be reduced in the brains of patients with Alzheimer’s disease and Down syndrome, possibly rendering the neuronal cells of these patients more vulnerable to cell death [120].
The role of Prx III in the scavenging of mitochondrial H2O2 has recently been emphasized. Originally cloned from murine erythroleukemia cells, Prx III has been identified as a gene induced by oncogenic c-Myc [121]. Its specific localization to mitochondria [122,123] suggests that Prx III, together with its mitochondrion-specific electron suppliers Trx2 and Trx reductase (TrxR) 2 [124,125], might provide a primary line of defense against H2O2 produced by the mitochondrial respiratory chain [126,127], as SOD2 does against the superoxide radical. In the presence of excess H2O2, Prx III is highly sensitive to oxidative inaction. Hyperoxidation of Prx III has been observed in cultured cells following prolonged exposure to high levels of H2O2 or drugs that generate H2O2 [128–130]. Moreover, hyperoxidized Prx III is reduced more slowly that hyperoxidized Prx I and II in the cytoplasm [129] and the slow reduction will enable the hyperoxidized form of Prx III to accumulate under certain conditions. Therefore, hyperoxidized Prx III formation by H2O2 leads to an increase in mitochondrial H2O2 and that this may influence the progression of apoptosis.
In addition, sulfiredoxin (Srx) plays a crucial role by reducing hyperoxidized Prx III via translocation into mitochondria. Noh et al. reported that the overexpression of mitochondrion-targeted Srx efficiently promotes the restoration of Prx III and results in cellular resistance to apoptosis, with enhanced elimination of mitochondrial H2O2 and decreased rates of ΔΨm collapse [131]. Thus, a Trx-related antioxidant system composed of Trx2, TrxR2, and Prx III has been closely associated with the regulation of apoptosis and the redox control of MPT pores for the release of cytochrome c [79,94,132]. However, rare attempts to characterize Prx III and its electron suppliers have produced intriguing results that demonstrate the removal of exogenous ROS by actively respiring mitochondria.
5. Outlook and Future Perspectives
Most of the currently used cytotoxic anticancer therapeutics have no clear-cut cell specificity, yet tend to kill tumor cells more efficiently than normal cells. With rare exceptions, single drugs at clinically tolerable doses have not been able to cure cancer. Prolonged drug exposure may result in cumulative toxicity. The clinical efficacy of chemotherapy must be enhanced, its attendant toxicity reduced, and resistance overcome. To overcome multidrug resistance in cancer cells, recent chemotherapeutics could be used in combination with other molecules. In the 1960s and early 1970s, drug combination regimens were developed based on the known biochemical actions of available anticancer drugs, rather than on their clinical efficacy. However, such regimens were largely ineffective [133,134]. The era of effective combination chemotherapy began when a number of active drugs of different classes became available for use in combination to treat acute leukemia and lymphomas. After this initial success with hematologic malignancies, combination chemotherapy was applied to the treatment of most solid tumors.
Structural and functional mitochondrial alterations associated with malignant transformation seem to be phenomena common to many types of cancer. Most classical anticancer agents engage signaling pathways that lie upstream of mitochondria and converge on mitochondria due to their role as integrators of pro-death and pro-survival signals. MOMP occurs as a consequence of upstream signaling events that are frequently deregulated in human cancers and that become resistant to a number of conventional therapeutic strategies targeting upstream MOMP regulators. Anticancer drugs that target mitochondria have the potential to bypass the resistance mechanisms that evolved in response to treatment with conventional chemotherapeutics. The combined use of mitochondriontargeted agents with conventional chemotherapeutics and other chemotherapeutic drugs, such as ROS scavenger inhibitors or ROS inducers, may be necessary to achieve maximum efficacy. The pharmacological depletion of ROS scavengers in cancer cells markedly reduces their clonogenicity and results in radiosensitization. As mentioned above, recent studies have shown that the overexpression of Prx III and its electron donors can protect cells, whereas their depletion induced cell death in cancer cells. Therefore, drugs targeting Prx III and the mitochondrion-specific electron suppliers Trx2, TrxR2, and Srx may potentially be administered in combination with various chemotherapeutic agents, including cisplatin, paclitaxel, and etoposide. However, caution must be exercised to prevent a potential increase in toxic side effects. A comprehensive understanding of mitochondrial biology in cancer cells and the interaction between cellular metabolism and drug action is essential in the development of mitochondrion-targeted agents for cancer treatment.
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health, Welfare and Family affairs, Republic of Korea (0920040) and the Priority Research Centers Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2010-0020224).
- Conflict of InterestThe authors declare no conflict of interest.
References
- Kroemer, G; Galluzzi, L; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev 2007, 87, 99–163. [Google Scholar]
- Galluzzi, L; Joza, N; Tasdemir, E; Maiuri, MC; Hengartner, M; Abrams, JM; Tavernarakis, N; Penninger, J; Madeo, F; Kroemer, G. No death without life: vital functions of apoptotic effectors. Cell Death Differ 2008, 15, 1113–1123. [Google Scholar]
- Galluzzi, L; Kroemer, G. Necroptosis: a specialized pathway of programmed necrosis. Cell 2008, 135, 1161–1163. [Google Scholar]
- Gogvadze, V; Orrenius, S; Zhivotovsky, B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol 2008, 18, 165–173. [Google Scholar]
- Modica-Napolitano, JS; Singh, KK. Mitochondrial dysfunction in cancer. Mitochondrion 2004, 4, 755–762. [Google Scholar]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar]
- Weinhouse, S. On respiratory impairment in cancer cells. Science 1956, 124, 267–269. [Google Scholar]
- Weinberg, F; Hamanaka, R; Wheaton, WW; Weinberg, S; Joseph, J; Lopez, M; Kalyanaraman, B; Mutlu, GM; Budinger, GR; Chandel, NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar]
- Reed, DJ. Glutathione: toxicological implications. Annu. Rev. Pharmacol. Toxicol 1990, 30, 603–631. [Google Scholar]
- Kinnula, VL; Lehtonen, S; Sormunen, R; Kaarteenaho-Wiik, R; Kang, SW; Rhee, SG; Soini, Y. Overexpression of peroxiredoxins I, II, III, V, and VI in malignant mesothelioma. J. Pathol 2002, 196, 316–323. [Google Scholar]
- Noh, DY; Ahn, SJ; Lee, RA; Kim, SW; Park, IA; Chae, HZ. Overexpression of peroxiredoxin in human breast cancer. Anticancer Res 2001, 21, 2085–2090. [Google Scholar]
- Choi, JH; Kim, TN; Kim, S; Baek, SH; Kim, JH; Lee, SR; Kim, JR. Overexpression of mitochondrial thioredoxin reductase and peroxiredoxin III in hepatocellular carcinomas. Anticancer Res 2002, 22, 3331–3335. [Google Scholar]
- Nonn, L; Berggren, M; Powis, G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol. Cancer Res 2003, 1, 682–689. [Google Scholar]
- Kropotov, A; Gogvadze, V; Shupliakov, O; Tomilin, N; Serikov, VB; Tomilin, NV; Zhivotovsky, B. Peroxiredoxin V is essential for protection against apoptosis in human lung carcinoma cells. Exp. Cell Res 2006, 312, 2806–2815. [Google Scholar]
- Knoops, B; Clippe, A; Bogard, C; Arsalane, K; Wattiez, R; Hermans, C; Duconseille, E; Falmagne, P; Bernard, A. Cloning and characterization of AOEB166, a novel mammalian antioxidant enzyme of the peroxiredoxin family. J. Biol. Chem 1999, 274, 30451–30458. [Google Scholar]
- Seo, MS; Kang, SW; Kim, K; Baines, IC; Lee, TH; Rhee, SG. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J. Biol. Chem 2000, 275, 20346–20354. [Google Scholar]
- Kropotov, A; Usmanova, N; Serikov, V; Zhivotovsky, B; Tomilin, N. Mitochondrial targeting of human peroxiredoxin V protein and regulation of PRDX5 gene expression by nuclear transcription factors controlling biogenesis of mitochondria. FEBS J 2007, 274, 5804–5814. [Google Scholar]
- Murphy, MP. How mitochondria produce reactive oxygen species. Biochem. J 2009, 417, 1–13. [Google Scholar]
- Cao, Z; Bhella, D; Lindsay, JG. Reconstitution of the mitochondrial PrxIII antioxidant defence pathway: general properties and factors affecting PrxIII activity and oligomeric state. J. Mol. Biol 2007, 372, 1022–1033. [Google Scholar]
- Manta, B; Hugo, M; Ortiz, C; Ferrer-Sueta, G; Trujillo, M; Denicola, A. The peroxidase and peroxynitrite reductase activity of human erythrocyte peroxiredoxin 2. Arch. Biochem. Biophys 2009, 484, 146–154. [Google Scholar]
- Trujillo, M; Clippe, A; Manta, B; Ferrer-Sueta, G; Smeets, A; Declercq, JP; Knoops, B; Radi, R. Pre-steady state kinetic characterization of human peroxiredoxin 5: taking advantage of Trp84 fluorescence increase upon oxidation. Arch. Biochem. Biophys 2007, 467, 95–106. [Google Scholar]
- Dubuisson, M; Vander Stricht, D; Clippe, A; Etienne, F; Nauser, T; Kissner, R; Koppenol, WH; Rees, JF; Knoops, B. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS Lett 2004, 571, 161–165. [Google Scholar]
- Gulbins, E; Dreschers, S; Bock, J. Role of mitochondria in apoptosis. Exp. Physiol 2003, 88, 85–90. [Google Scholar]
- Hiendleder, S; Schmutz, SM; Erhardt, G; Green, RD; Plante, Y. Transmitochondrial differences and varying levels of heteroplasmy in nuclear transfer cloned cattle. Mol. Reprod. Dev 1999, 54, 24–31. [Google Scholar]
- Waterhouse, NJ; Goldstein, JC; Kluck, RM; Newmeyer, DD; Green, DR. The (Holey) study of mitochondria in apoptosis. Methods Cell Biol 2001, 66, 365–391. [Google Scholar]
- Orrenius, S; Zhivotovsky, B; Nicotera, P. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol 2003, 4, 552–565. [Google Scholar]
- Zamzami, N; Kroemer, G. The mitochondrion in apoptosis: how Pandora's box opens. Nat. Rev. Mol. Cell Biol 2001, 2, 67–71. [Google Scholar]
- Murphy, E; Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemiareperfusion injury. Physiol. Rev 2008, 88, 581–609. [Google Scholar]
- Tan, W; Colombini, M. VDAC closure increases calcium ion flux. Biochim. Biophys. Acta 2007, 1768, 2510–2515. [Google Scholar]
- Baines, CP; Kaiser, RA; Purcell, NH; Blair, NS; Osinska, H; Hambleton, MA; Brunskill, EW; Sayen, MR; Gottlieb, RA; Dorn, GW; Robbins, J; Molkentin, JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005, 434, 658–662. [Google Scholar]
- Schinzel, AC; Takeuchi, O; Huang, Z; Fisher, JK; Zhou, Z; Rubens, J; Hetz, C; Danial, NN; Moskowitz, MA; Korsmeyer, SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. USA 2005, 102, 12005–12010. [Google Scholar]
- Kokoszka, JE; Waymire, KG; Levy, SE; Sligh, JE; Cai, J; Jones, DP; MacGregor, GR; Wallace, DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 2004, 427, 461–465. [Google Scholar]
- Baines, CP; Kaiser, RA; Sheiko, T; Craigen, WJ; Molkentin, JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol 2007, 9, 550–555. [Google Scholar]
- Costantini, P; Belzacq, AS; Vieira, HL; Larochette, N; de Pablo, MA; Zamzami, N; Susin, SA; Brenner, C; Kroemer, G. Oxidation of a critical thiol residue of the adenine nucleotide translocator enforces Bcl-2-independent permeability transition pore opening and apoptosis. Oncogene 2000, 19, 307–314. [Google Scholar]
- Fantin, VR; Leder, P. Mitochondriotoxic compounds for cancer therapy. Oncogene 2006, 25, 4787–4797. [Google Scholar]
- Fulda, S; Galluzzi, L; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov 2010, 9, 447–464. [Google Scholar]
- Pathania, D; Millard, M; Neamati, N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv. Drug Deliv. Rev 2009, 61, 1250–1275. [Google Scholar]
- Robertson, JD; Gogvadze, V; Zhivotovsky, B; Orrenius, S. Distinct pathways for stimulation of cytochrome c release by etoposide. J. Biol. Chem 2000, 275, 32438–32443. [Google Scholar]
- Kidd, JF; Pilkington, MF; Schell, MJ; Fogarty, KE; Skepper, JN; Taylor, CW; Thorn, P. Paclitaxel affects cytosolic calcium signals by opening the mitochondrial permeability transition pore. J. Biol. Chem 2002, 277, 6504–6510. [Google Scholar]
- Chinnery, PF; Taylor, GA; Howell, N; Andrews, RM; Morris, CM; Taylor, RW; McKeith, IG; Perry, RH; Edwardson, JA; Turnbull, DM. Mitochondrial DNA haplogroups and susceptibility to AD and dementia with Lewy bodies. Neurology 2000, 55, 302–304. [Google Scholar]
- Stover, TC; Sharma, A; Robertson, GP; Kester, M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin. Cancer Res 2005, 11, 3465–3474. [Google Scholar]
- Propper, DJ; Braybrooke, JP; Taylor, DJ; Lodi, R; Styles, P; Cramer, JA; Collins, WC; Levitt, NC; Talbot, DC; Ganesan, TS; Harris, AL. Phase I trial of the selective mitochondrial toxin MKT077 in chemo-resistant solid tumours. Ann. Oncol 1999, 10, 923–927. [Google Scholar]
- Holmes, WF; Soprano, DR; Soprano, KJ. Elucidation of molecular events mediating induction of apoptosis by synthetic retinoids using a CD437-resistant ovarian carcinoma cell line. J. Biol. Chem 2002, 277, 45408–45419. [Google Scholar]
- Loschen, G; Flohe, L; Chance, B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett 1971, 18, 261–264. [Google Scholar]
- Rabilloud, T; Heller, M; Rigobello, MP; Bindoli, A; Aebersold, R; Lunardi, J. The mitochondrial antioxidant defence system and its response to oxidative stress. Proteomics 2001, 1, 1105–1110. [Google Scholar]
- Banmeyer, I; Marchand, C; Clippe, A; Knoops, B. Human mitochondrial peroxiredoxin 5 protects from mitochondrial DNA damages induced by hydrogen peroxide. FEBS Lett 2005, 579, 2327–2333. [Google Scholar]
- Huh, JY; Kim, Y; Jeong, J; Park, J; Kim, I; Huh, KH; Kim, YS; Woo, HA; Rhee, SG; Lee, KJ; Ha, H. Peroxiredoxin 3 Is a Key Molecule Regulating Adipocyte Oxidative Stress, Mitochondrial Biogenesis, and Adipokine Expression. Antioxid Redox Signal 2011. In Press. [Google Scholar]
- Nonn, L; Williams, RR; Erickson, RP; Powis, G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol. Cell Biol 2003, 23, 916–922. [Google Scholar]
- Lebovitz, RM; Zhang, H; Vogel, H; Cartwright, J, Jr; Dionne, L; Lu, N; Huang, S; Matzuk, MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci USA 1996, 93, 9782–9787. [Google Scholar]
- Hinerfeld, D; Traini, MD; Weinberger, RP; Cochran, B; Doctrow, SR; Harry, J; Melov, S. Endogenous mitochondrial oxidative stress: neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. J. Neurochem 2004, 88, 657–667. [Google Scholar]
- Ho, YS; Magnenat, JL; Bronson, RT; Cao, J; Gargano, M; Sugawara, M; Funk, CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J. Biol. Chem 1997, 272, 16644–16651. [Google Scholar]
- de Haan, JB; Bladier, C; Griffiths, P; Kelner, M; O'Shea, RD; Cheung, NS; Bronson, RT; Silvestro, MJ; Wild, S; Zheng, SS; Beart, PM; Hertzog, PJ; Kola, I. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J. Biol. Chem 1998, 273, 22528–22536. [Google Scholar]
- Crack, PJ; Taylor, JM; Flentjar, NJ; de Haan, J; Hertzog, P; Iannello, RC; Kola, I. Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J. Neurochem 2001, 78, 1389–1399. [Google Scholar]
- Esworthy, RS; Ho, YS; Chu, FF. The Gpx1 gene encodes mitochondrial glutathione peroxidase in the mouse liver. Arch. Biochem. Biophys 1997, 340, 59–63. [Google Scholar]
- Panfili, E; Sandri, G; Ernster, L. Distribution of glutathione peroxidases and glutathione reductase in rat brain mitochondria. FEBS Lett 1991, 290, 35–37. [Google Scholar]
- Heerdt, BG; Houston, MA; Wilson, AJ; Augenlicht, LH. The intrinsic mitochondrial membrane potential (Deltapsim) is associated with steady-state mitochondrial activity and the extent to which colonic epithelial cells undergo butyrate-mediated growth arrest and apoptosis. Cancer Res 2003, 63, 6311–6319. [Google Scholar]
- Cleveland, JL; Kastan, MB. Cancer. A radical approach to treatment. Nature 2000, 407, 309–311. [Google Scholar]
- Matei, D; Schilder, J; Sutton, G; Perkins, S; Breen, T; Quon, C; Sidor, C. Activity of 2 methoxyestradiol (Panzem NCD) in advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis: a Hoosier Oncology Group trial. Gynecol. Oncol 2009, 115, 90–96. [Google Scholar]
- Tevaarwerk, AJ; Holen, KD; Alberti, DB; Sidor, C; Arnott, J; Quon, C; Wilding, G; Liu, G. Phase I trial of 2-methoxyestradiol NanoCrystal dispersion in advanced solid malignancies. Clin. Cancer Res 2009, 15, 1460–1465. [Google Scholar]
- Rajkumar, SV; Richardson, PG; Lacy, MQ; Dispenzieri, A; Greipp, PR; Witzig, TE; Schlossman, R; Sidor, CF; Anderson, KC; Gertz, MA. Novel therapy with 2- methoxyestradiol for the treatment of relapsed and plateau phase multiple myeloma. Clin. Cancer Res 2007, 13, 6162–6167. [Google Scholar]
- Juarez, JC; Manuia, M; Burnett, ME; Betancourt, O; Boivin, B; Shaw, DE; Tonks, NK; Mazar, AP; Donate, F. Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 7147–7152. [Google Scholar]
- Alexandre, J; Nicco, C; Chereau, C; Laurent, A; Weill, B; Goldwasser, F; Batteux, F. Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. J. Natl. Cancer. Inst 2006, 98, 236–244. [Google Scholar]
- Maeda, H; Hori, S; Ohizumi, H; Segawa, T; Kakehi, Y; Ogawa, O; Kakizuka, A. Effective treatment of advanced solid tumors by the combination of arsenic trioxide and l-buthioninesulfoximine. Cell Death Differ 2004, 11, 737–746. [Google Scholar]
- Dragovich, T; Gordon, M; Mendelson, D; Wong, L; Modiano, M; Chow, HH; Samulitis, B; O'Day, S; Grenier, K; Hersh, E; Dorr, R. Phase I trial of imexon in patients with advanced malignancy. J. Clin. Oncol 2007, 25, 1779–1784. [Google Scholar]
- Trachootham, D; Zhou, Y; Zhang, H; Demizu, Y; Chen, Z; Pelicano, H; Chiao, PJ; Achanta, G; Arlinghaus, RB; Liu, J; Huang, P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 2006, 10, 241–252. [Google Scholar]
- Xiao, D; Lew, KL; Zeng, Y; Xiao, H; Marynowski, SW; Dhir, R; Singh, SV. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis 2006, 27, 2223–2234. [Google Scholar]
- Brown, KK; Eriksson, SE; Arner, ES; Hampton, MB. Mitochondrial peroxiredoxin 3 is rapidly oxidized in cells treated with isothiocyanates. Free Radic. Biol. Med 2008, 45, 494–502. [Google Scholar]
- Huang, P; Feng, L; Oldham, EA; Keating, MJ; Plunkett, W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature 2000, 407, 390–395. [Google Scholar]
- Wood, L; Leese, MR; Leblond, B; Woo, LW; Ganeshapillai, D; Purohit, A; Reed, MJ; Potter, BV; Packham, G. Inhibition of superoxide dismutase by 2-methoxyoestradiol analogues and oestrogen derivatives: structure-activity relationships. Anticancer Drug Des 2001, 16, 209–215. [Google Scholar]
- Magda, D; Miller, RA. Motexafin gadolinium: a novel redox active drug for cancer therapy. Semin. Cancer Biol 2006, 16, 466–476. [Google Scholar]
- Bey, EA; Bentle, MS; Reinicke, KE; Dong, Y; Yang, CR; Girard, L; Minna, JD; Bornmann, WG; Gao, J; Boothman, DA. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proc. Natl. Acad. Sci. USA 2007, 104, 11832–11837. [Google Scholar]
- Tuma, RS. Reactive oxygen species may have antitumor activity in metastatic melanoma. J. Natl. Cancer Inst 2008, 100, 11–12. [Google Scholar]
- Kirshner, JR; He, S; Balasubramanyam, V; Kepros, J; Yang, CY; Zhang, M; Du, Z; Barsoum, J; Bertin, J. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol. Cancer Ther 2008, 7, 2319–2327. [Google Scholar]
- Belzacq, AS; El Hamel, C; Vieira, HL; Cohen, I; Haouzi, D; Metivier, D; Marchetti, P; Brenner, C; Kroemer, G. Adenine nucleotide translocator mediates the mitochondrial membrane permeabilization induced by lonidamine, arsenite and CD437. Oncogene 2001, 20, 7579–7587. [Google Scholar]
- Guzman, ML; Rossi, RM; Neelakantan, S; Li, X; Corbett, CA; Hassane, DC; Becker, MW; Bennett, JM; Sullivan, E; Lachowicz, JL; et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood 2007, 110, 4427–4435. [Google Scholar]
- Guzman, ML; Rossi, RM; Karnischky, L; Li, X; Peterson, DR; Howard, DS; Jordan, CT. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005, 105, 4163–4169. [Google Scholar]
- Chahboune, N; Barrachina, I; Royo, I; Romero, V; Saez, J; Tormo, JR; De Pedro, N; Estornell, E; Zafra-Polo, MC; Pelaez, F; Cortes, D. Guanaconetins, new antitumoral acetogenins, mitochondrial complex I and tumor cell growth inhibitors. Bioorg. Med. Chem 2006, 14, 1089–1094. [Google Scholar]
- Palmeira, CM; Wallace, KB. Benzoquinone inhibits the voltage-dependent induction of the mitochondrial permeability transition caused by redox-cycling naphthoquinones. Toxicol. Appl. Pharmacol 1997, 143, 338–347. [Google Scholar]
- Petronilli, V; Costantini, P; Scorrano, L; Colonna, R; Passamonti, S; Bernardi, P. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J. Biol. Chem 1994, 269, 16638–16642. [Google Scholar]
- Wang, W; Adachi, M; Kawamura, R; Sakamoto, H; Hayashi, T; Ishida, T; Imai, K; Shinomura, Y. Parthenolide-induced apoptosis in multiple myeloma cells involves reactive oxygen species generation and cell sensitivity depends on catalase activity. Apoptosis 2006, 11, 2225–2235. [Google Scholar]
- Chae, HZ; Robison, K; Poole, LB; Church, G; Storz, G; Rhee, SG. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Natl. Acad. Sci. USA 1994, 91, 7017–7021. [Google Scholar]
- Rhee, SG; Chae, HZ; Kim, K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med 2005, 38, 1543–1552. [Google Scholar]
- Choi, HJ; Kang, SW; Yang, CH; Rhee, SG; Ryu, SE. Crystal structure of a novel human peroxidase enzyme at 2.0 A resolution. Nat. Struct. Biol 1998, 5, 400–406. [Google Scholar]
- Kang, SW; Baines, IC; Rhee, SG. Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J. Biol. Chem 1998, 273, 6303–6311. [Google Scholar]
- Cerutti, PA. Oxy-radicals and cancer. Lancet 1994, 344, 862–863. [Google Scholar]
- Wood, ZA; Schroder, E; Robin Harris, J; Poole, LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci 2003, 28, 32–40. [Google Scholar]
- Fujii, J; Ikeda, Y. Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep 2002, 7, 123–130. [Google Scholar]
- Kim, JH; Bogner, PN; Baek, SH; Ramnath, N; Liang, P; Kim, HR; Andrews, C; Park, YM. Up-regulation of peroxiredoxin 1 in lung cancer and its implication as a prognostic and therapeutic target. Clin. Cancer Res 2008, 14, 2326–2333. [Google Scholar]
- Chang, JW; Lee, SH; Jeong, JY; Chae, HZ; Kim, YC; Park, ZY; Yoo, YJ. Peroxiredoxin-I is an autoimmunogenic tumor antigen in non-small cell lung cancer. FEBS Lett 2005, 579, 2873–2877. [Google Scholar]
- Yanagawa, T; Iwasa, S; Ishii, T; Tabuchi, K; Yusa, H; Onizawa, K; Omura, K; Harada, H; Suzuki, H; Yoshida, H. Peroxiredoxin I expression in oral cancer: a potential new tumor marker. Cancer Lett 2000, 156, 27–35. [Google Scholar]
- Song, IS; Kim, SU; Oh, NS; Kim, J; Yu, DY; Huang, SM; Kim, JM; Lee, DS; Kim, NS. Peroxiredoxin I contributes to TRAIL resistance through suppression of redox-sensitive caspase activation in human hepatoma cells. Carcinogenesis 2009, 30, 1106–1114. [Google Scholar]
- Karihtala, P; Mantyniemi, A; Kang, SW; Kinnula, VL; Soini, Y. Peroxiredoxins in breast carcinoma. Clin. Cancer Res 2003, 9, 3418–3424. [Google Scholar]
- Chung, YM; Yoo, YD; Park, JK; Kim, YT; Kim, HJ. Increased expression of peroxiredoxin II confers resistance to cisplatin. Anticancer Res 2001, 21, 1129–1133. [Google Scholar]
- Zhang, P; Liu, B; Kang, SW; Seo, MS; Rhee, SG; Obeid, LM. Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. J. Biol. Chem 1997, 272, 30615–30618. [Google Scholar]
- Park, SH; Chung, YM; Lee, YS; Kim, HJ; Kim, JS; Chae, HZ; Yoo, YD. Antisense of human peroxiredoxin II enhances radiation-induced cell death. Clin. Cancer Res 2000, 6, 4915–4920. [Google Scholar]
- Yo, YD; Chung, YM; Park, JK; Ahn, CM; Kim, SK; Kim, HJ. Synergistic effect of peroxiredoxin II antisense on cisplatin-induced cell death. Exp. Mol. Med 2002, 34, 273–277. [Google Scholar]
- Lee, JY; Jung, HJ; Song, IS; Williams, MS; Choi, C; Rhee, SG; Kim, J; Kang, SW. Protective role of cytosolic 2-cys peroxiredoxin in the TNF-alpha-induced apoptotic death of human cancer cells. Free Radic. Biol. Med 2009, 47, 1162–1171. [Google Scholar]
- Chen, MF; Chen, WC; Wu, CT; Lin, PY; Shau, H; Liao, SK; Yang, CT; Lee, KD. p53 status is a major determinant of effects of decreasing peroxiredoxin I expression on tumor growth and response of lung cancer cells to treatment. Int. J. Radiat. Oncol. Biol. Phys 2006, 66, 1461–1472. [Google Scholar]
- Chen, MF; Keng, PC; Shau, H; Wu, CT; Hu, YC; Liao, SK; Chen, WC. Inhibition of lung tumor growth and augmentation of radiosensitivity by decreasing peroxiredoxin I expression. Int. J. Radiat. Oncol. Biol. Phys 2006, 64, 581–591. [Google Scholar]
- Park, SY; Yu, X; Ip, C; Mohler, JL; Bogner, PN; Park, YM. Peroxiredoxin 1 interacts with androgen receptor and enhances its transactivation. Cancer Res 2007, 67, 9294–9303. [Google Scholar]
- Jang, JS; Cho, HY; Lee, YJ; Ha, WS; Kim, HW. The differential proteome profile of stomach cancer: identification of the biomarker candidates. Oncol. Res 2004, 14, 491–499. [Google Scholar]
- Park, JJ; Chang, HW; Jeong, EJ; Roh, JL; Choi, SH; Jeon, SY; Ko, GH; Kim, SY. Peroxiredoxin IV protects cells from radiation-induced apoptosis in head-and-neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys 2009, 73, 1196–1202. [Google Scholar]
- Wei, Q; Jiang, H; Xiao, Z; Baker, A; Young, MR; Veenstra, TD; Colburn, NH. Sulfiredoxin-Peroxiredoxin IV axis promotes human lung cancer progression through modulation of specific phosphokinase signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 7004–7009. [Google Scholar]
- Kinnula, VL; Lehtonen, S; Kaarteenaho-Wiik, R; Lakari, E; Paakko, P; Kang, SW; Rhee, SG; Soini, Y. Cell specific expression of peroxiredoxins in human lung and pulmonary sarcoidosis. Thorax 2002, 57, 157–164. [Google Scholar]
- Wang, MX; Wei, A; Yuan, J; Trickett, A; Knoops, B; Murrell, GA. Expression and regulation of peroxiredoxin 5 in human osteoarthritis. FEBS Lett 2002, 531, 359–362. [Google Scholar]
- Plaisant, F; Clippe, A; Vander Stricht, D; Knoops, B; Gressens, P. Recombinant peroxiredoxin 5 protects against excitotoxic brain lesions in newborn mice. Free Radic. Biol. Med 2003, 34, 862–872. [Google Scholar]
- Zhou, Y; Kok, KH; Chun, AC; Wong, CM; Wu, HW; Lin, MC; Fung, PC; Kung, H; Jin, DY. Mouse peroxiredoxin V is a thioredoxin peroxidase that inhibits p53-induced apoptosis. Biochem. Biophys. Res. Commun 2000, 268, 921–927. [Google Scholar]
- Mikhailov, VM; Kropotov, AV; Zelenin, AV; Krutilina, RI; Kolesnikov, VA; Zelenina, IA; Baranov, AN; Shtein, GI; Ostapenko, OV; Tomilin, NV; Baranov, VS. The BCL-xL and ACR-1 genes promote differentiation and reduce apoptosis in muscle fibers of mdx mice. Genetika 2002, 38, 1445–1450. [Google Scholar]
- Yuan, J; Murrell, GA; Trickett, A; Landtmeters, M; Knoops, B; Wang, MX. Overexpression of antioxidant enzyme peroxiredoxin 5 protects human tendon cells against apoptosis and loss of cellular function during oxidative stress. Biochim. Biophys. Acta 2004, 1693, 37–45. [Google Scholar]
- Wang, X; Phelan, SA; Petros, C; Taylor, EF; Ledinski, G; Jurgens, G; Forsman-Semb, K; Paigen, B. Peroxiredoxin 6 deficiency and atherosclerosis susceptibility in mice: significance of genetic background for assessing atherosclerosis. Atherosclerosis 2004, 177, 61–70. [Google Scholar]
- Strey, CW; Spellman, D; Stieber, A; Gonatas, JO; Wang, X; Lambris, JD; Gonatas, NK. Dysregulation of stathmin, a microtubule-destabilizing protein, and up-regulation of Hsp25, Hsp27, and the antioxidant peroxiredoxin 6 in a mouse model of familial amyotrophic lateral sclerosis. Am. J. Pathol 2004, 165, 1701–1718. [Google Scholar]
- Power, JH; Shannon, JM; Blumbergs, PC; Gai, WP. Nonselenium glutathione peroxidase in human brain: elevated levels in Parkinson's disease and dementia with lewy bodies. Am. J. Pathol 2002, 161, 885–894. [Google Scholar]
- Krapfenbauer, K; Yoo, BC; Fountoulakis, M; Mitrova, E; Lubec, G. Expression patterns of antioxidant proteins in brains of patients with sporadic Creutzfeldt-Jacob disease. Electrophoresis 2002, 23, 2541–2547. [Google Scholar]
- Krapfenbauer, K; Engidawork, E; Cairns, N; Fountoulakis, M; Lubec, G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res 2003, 967, 152–160. [Google Scholar]
- Munz, B; Frank, S; Hubner, G; Olsen, E; Werner, S. A novel type of glutathione peroxidase: expression and regulation during wound repair. Biochem. J 1997, 326, 579–585. [Google Scholar]
- Dierick, JF; Kalume, DE; Wenders, F; Salmon, M; Dieu, M; Raes, M; Roepstorff, P; Toussaint, O. Identification of 30 protein species involved in replicative senescence and stress-induced premature senescence. FEBS Lett 2002, 531, 499–504. [Google Scholar]
- Lehtonen, ST; Svensk, AM; Soini, Y; Paakko, P; Hirvikoski, P; Kang, SW; Saily, M; Kinnula, VL. Peroxiredoxins, a novel protein family in lung cancer. Int. J. Cancer 2004, 111, 514–521. [Google Scholar]
- Hattori, F; Murayama, N; Noshita, T; Oikawa, S. Mitochondrial peroxiredoxin-3 protects hippocampal neurons from excitotoxic injury in vivo. J. Neurochem 2003, 86, 860–868. [Google Scholar]
- Chang, TS; Cho, CS; Park, S; Yu, S; Kang, SW; Rhee, SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J. Biol. Chem 2004, 279, 41975–41984. [Google Scholar]
- Kim, SH; Fountoulakis, M; Cairns, N; Lubec, G. Protein levels of human peroxiredoxin subtypes in brains of patients with Alzheimer's disease and Down syndrome. J Neural Transm Suppl 2001, 223–235. [Google Scholar]
- Wonsey, DR; Zeller, KI; Dang, CV. The c-Myc target gene PRDX3 is required for mitochondrial homeostasis and neoplastic transformation. Proc. Natl. Acad. Sci. USA 2002, 99, 6649–6654. [Google Scholar]
- Chae, HZ; Kim, HJ; Kang, SW; Rhee, SG. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res. Clin. Pract 1999, 45, 101–112. [Google Scholar]
- Araki, M; Nanri, H; Ejima, K; Murasato, Y; Fujiwara, T; Nakashima, Y; Ikeda, M. Antioxidant function of the mitochondrial protein SP-22 in the cardiovascular system. J. Biol. Chem 1999, 274, 2271–2278. [Google Scholar]
- Spyrou, G; Enmark, E; Miranda-Vizuete, A; Gustafsson, J. Cloning and expression of a novel mammalian thioredoxin. J. Biol. Chem 1997, 272, 2936–2941. [Google Scholar]
- Lee, SR; Kim, JR; Kwon, KS; Yoon, HW; Levine, RL; Ginsburg, A; Rhee, SG. Molecular cloning and characterization of a mitochondrial selenocysteine-containing thioredoxin reductase from rat liver. J. Biol. Chem 1999, 274, 4722–4734. [Google Scholar]
- Miranda-Vizuete, A; Damdimopoulos, AE; Spyrou, G. The mitochondrial thioredoxin system. Antioxid. Redox Signal 2000, 2, 801–810. [Google Scholar]
- Pedrajas, JR; Miranda-Vizuete, A; Javanmardy, N; Gustafsson, JA; Spyrou, G. Mitochondria of Saccharomyces cerevisiae contain one-conserved cysteine type peroxiredoxin with thioredoxin peroxidase activity. J. Biol. Chem 2000, 275, 16296–16301. [Google Scholar]
- Rabilloud, T; Heller, M; Gasnier, F; Luche, S; Rey, C; Aebersold, R; Benahmed, M; Louisot, P; Lunardi, J. Proteomics analysis of cellular response to oxidative stress. Evidence for in vivo overoxidation of peroxiredoxins at their active site. J. Biol. Chem 2002, 277, 19396–19401. [Google Scholar]
- Woo, HA; Kang, SW; Kim, HK; Yang, KS; Chae, HZ; Rhee, SG. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J. Biol. Chem 2003, 278, 47361–47364. [Google Scholar]
- Saito, Y; Nishio, K; Ogawa, Y; Kinumi, T; Yoshida, Y; Masuo, Y; Niki, E. Molecular mechanisms of 6-hydroxydopamine-induced cytotoxicity in PC12 cells: involvement of hydrogen peroxide-dependent and -independent action. Free Radic. Biol. Med 2007, 42, 675–685. [Google Scholar]
- Noh, YH; Baek, JY; Jeong, W; Rhee, SG; Chang, TS. Sulfiredoxin Translocation into Mitochondria Plays a Crucial Role in Reducing Hyperoxidized Peroxiredoxin III. J. Biol. Chem 2009, 284, 8470–8477. [Google Scholar]
- Tanaka, T; Hosoi, F; Yamaguchi-Iwai, Y; Nakamura, H; Masutani, H; Ueda, S; Nishiyama, A; Takeda, S; Wada, H; Spyrou, G; Yodoi, J. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO J 2002, 21, 1695–1703. [Google Scholar]
- Nathanson, L; Hall, TC; Schilling, A; Miller, S. Concurrent combination chemotherapy of human solid tumors: experience with a three-drug regimen and review of the literature. Cancer Res 1969, 29, 419–425. [Google Scholar]
- DeVita, VT; Schein, PS. The use of drugs in combination for the treatment of cancer: rationale and results. N. Engl. J. Med 1973, 288, 998–1006. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).