A Selective Assay to Detect Chitin and Biologically Active Nano-Machineries for Chitin-Biosynthesis with Their Intrinsic Chitin-Synthase Molecules
Abstract
:1. Introduction
2. Results and Discussion
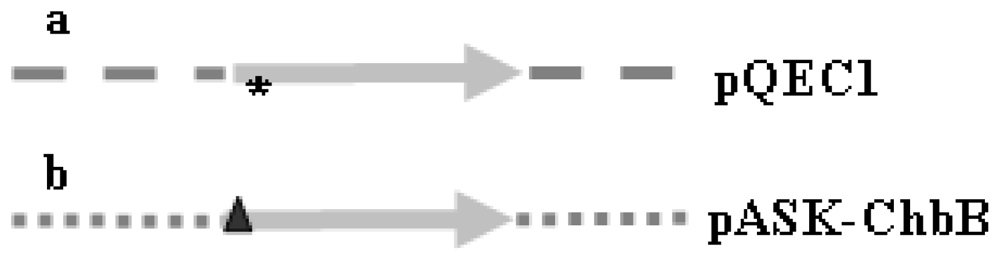
2.1. Features of the chbB Fusion Genes and Their Gene Products
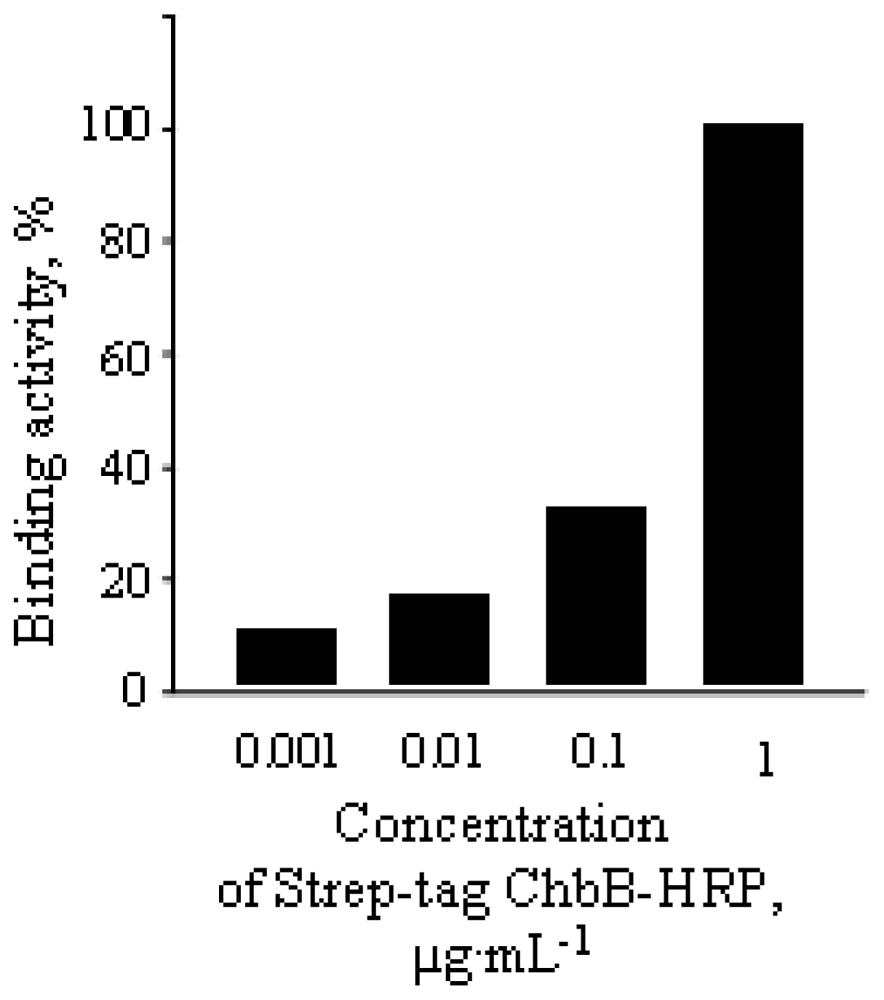
2.2. Characteristics of Strep-tag ChbB Coupled with Horseradish Peroxidase
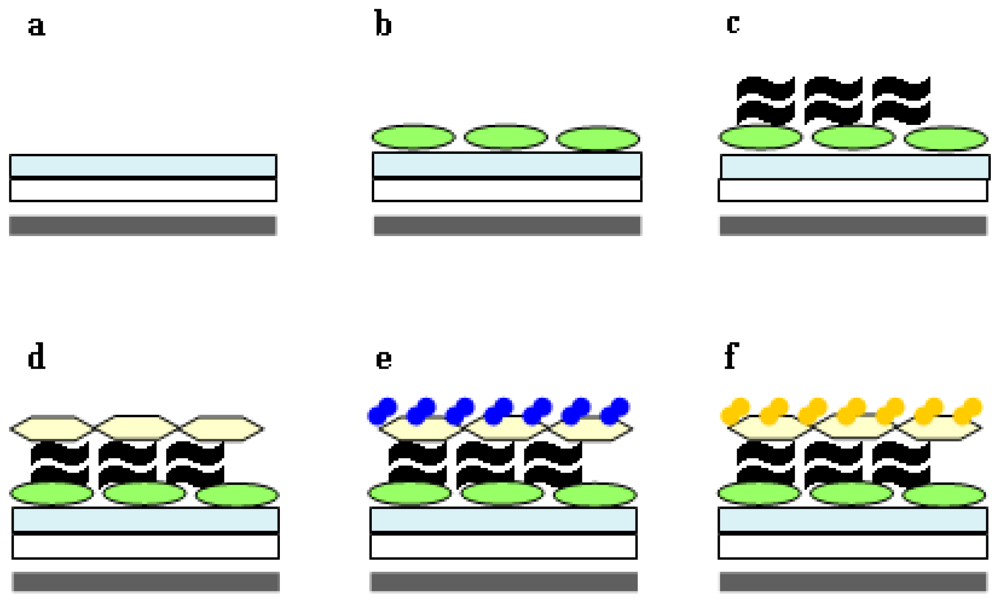
2.3. His-tag ChbB and Strep-tag ChbB Are Components of a Sensitive Chitin Synthase Assay
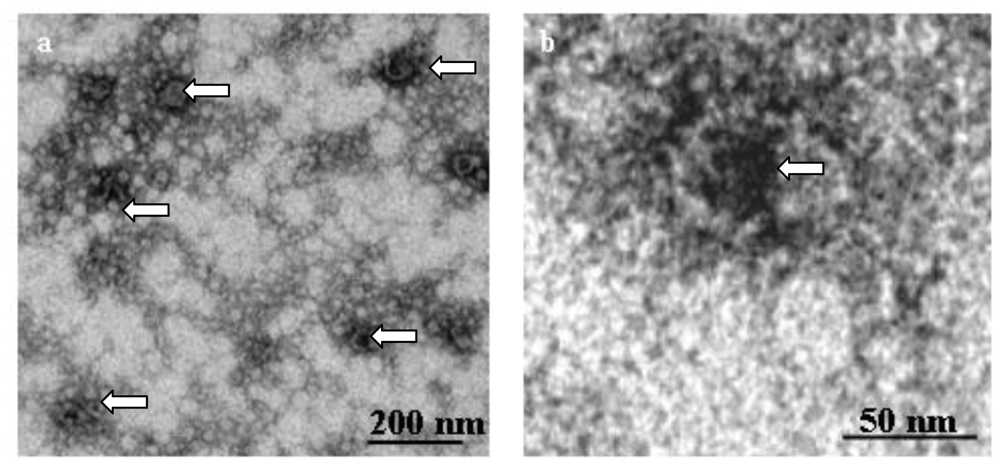
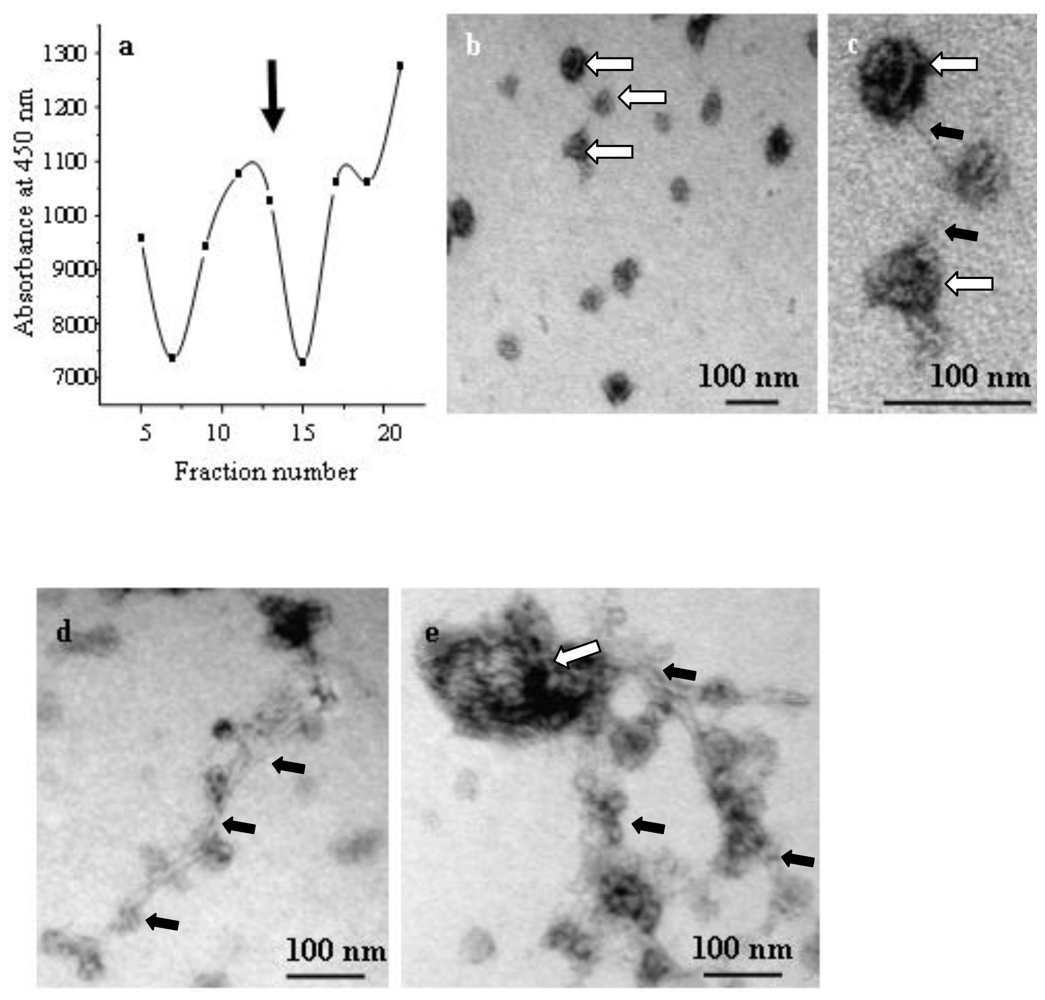
2.4. Application of the Chitin-assay to Refine the Isolation of Chitosomes
3. Experimental Section
3.1. Strains, Plasmids, Growth Conditions
3.2. Re-cloning of the chbB Gene and Its Analysis
3.3. Purification of the Strep-tagged ChbB Protein
3.4. Production and Purification of the His-tag ChbB Protein
3.5. Coupling of Strep-tag ChbB to the Horse Radish Peroxidase (HRP), Purification, and Identification of the Conjugate
3.6. Determination of Protein Concentration and the Quantity of Sulfhydryl Groups
3.7. Binding Test and Determination of the Sensitivity
3.8. Isolation of Chitosomes
3.9. Well-assay for Chitin Synthase Activity of Chitosomes, and Detection of Chitin Fibers
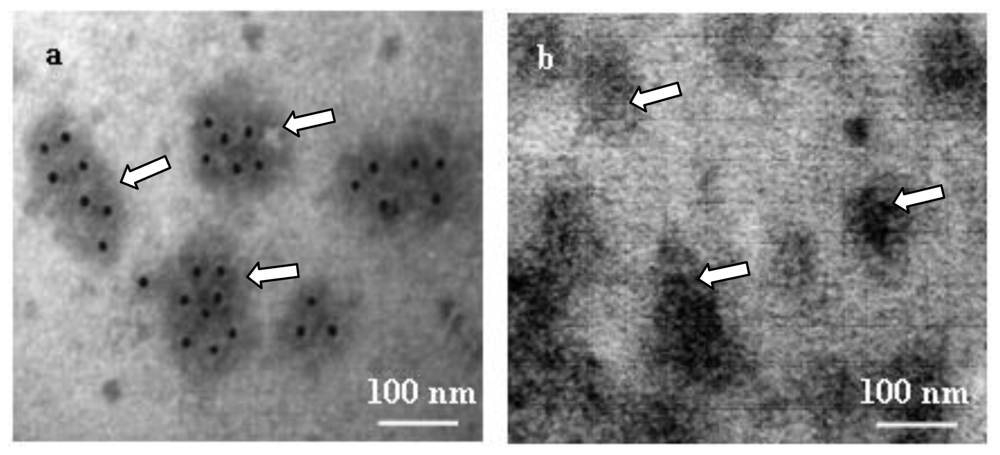
3.10. Electron Microscopy and Immuno-localization
4. Conclusions
Acknowledgements
References
- Schnellmann, J; Zeltins, A; Blaak, H; Schrempf, H. The novel lectin-like protein CHB1 is encoded by a chitin-inducible Streptomyces olivaceoviridis gene and binds specifically to crystalline α-chitin of fungi and other organisms. Mol. Microbiol 1994, 13, 807–819. [Google Scholar]
- Zeltins, A; Schrempf, H. Visualization of α-chitin with a specific chitin-binding protein (CHB1) from Streptomyces olivaceoviridis. Anal. Biochem 1995, 231, 287–294. [Google Scholar]
- Zeltins, A; Schrempf, H. Specific interaction of the Streptomyces chitin-binding protein CHB1 with α chitin: The role of individual tryptophan residues. Eur. J. Biochem 1997, 246, 557–564. [Google Scholar]
- Kolbe, S; Fischer, S; Becirevic, A; Hinz, P; Schrempf, H. The Streptomyces reticuli α-chitinbinding protein CHB2 and its gene. Microbiology 1998, 144, 1291–1297. [Google Scholar]
- Saito, A; Miyashita, K; Biukovic, G; Schrempf, H. Characteristics of an extracellular Streptomyces coelicolor A3(2) protein targeting chitin and chitosan. Appl. Environ. Microbiol 2001, 67, 1268–1273. [Google Scholar]
- Chu, HH; Hoang, V; Hofemeister, J; Schrempf, H. A Bacillus amyloliquefaciens ChbB protein binds β- and α-chitin and has homologues in related strains. Microbiology 2001, 147, 1793–1803. [Google Scholar]
- Svergun, DI; Becirevic, A; Schrempf, H; Koch, MHJ; Grüber, G. Solution structure and conformational changes of the Streptomyces Chitin-Binding Protein (CHB1). Biochemistry 2000, 39, 10677–10683. [Google Scholar]
- Siemieniewicz, KW; Schrempf, H. Concerted responses between the chitin-binding protein secreting Streptomyces olivaceoviridis and Aspergillus proliferans. Microbiology 2007, 153, 593–600. [Google Scholar]
- Pringle, JR. Staining of bud scars and other cell wall chitin with calcofluor. Methods Enzymol 1991, 194, 732–735. [Google Scholar]
- Vannini, GL; Pancaldi, S; Poli, F; Dall’Olio, G. Exocytosis in Saccharomyces cerevisiae treated with congo red. Cytobios 1987, 49, 89–97. [Google Scholar]
- Schekman, R; Brawley, V. Localized deposition of chitin on the yeast cell surface in response to mating pheromone. Proc. Natl. Acad. Sci. USA 1979, 76, 645–649. [Google Scholar]
- Wright, HT; Sandrasegaram, G; Wright, CS. Evolution of a family of N-acetylglucosamine binding proteins containing the disulfide-rich domain of wheat germ agglutinin. J. Mol. Evol 1991, 33, 283–294. [Google Scholar]
- Bartnicki-Garcia, S. Chitosomes: Past, present and future. FEMS Yeast Res 2006, 7, 957–965. [Google Scholar]
- Siemieniewicz, KW; Kajla, MK; Schrempf, H. Elucidating the biosynthesis of chitin filaments and their configuration with specific proteins and electron microscopy. Macromol. Biosci 2007, 7, 40–47. [Google Scholar]
- Terpe, K. Overview of tag protein fusions: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol 2003, 60, 523–533. [Google Scholar]
- March, JC; Rao, G; Bentley, WE. Biotechnological applications of green fluorescent protein. Appl. Microbiol. Biotechnol 2003, 62, 303–315. [Google Scholar]
- Ryan, O; Smyth, MR; Fágáin, CO. Horseradish peroxidase: The analyst’s friend. Essays Biochem 1994, 28, 129–146. [Google Scholar]
- Duncan, RJ; Weston, PD; Wrigglesworth, R. A new reagent which may be used to introduce sulfhydryl groups into proteins, and its use in the preparation of conjugates for immunoassay. Anal. Biochem 1983, 132, 68–73. [Google Scholar]
- Ellman, GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys 1959, 82, 70–77. [Google Scholar]
- Leal-Morales, CA; Bracker, CE; Bartnicki-Garcia, S. Subcellular localization, abundance and stability of chitin synthetases 1 and 2 from Saccharomyces cerevisiae. Microbiology 1994, 140, 2207–2216. [Google Scholar]
- Lucero, HA; Kuranda, MJ; Bulik, DA. A nonradioactive, high throughput assay for chitin synthase activity. Anal. Biochem 2002, 305, 97–105. [Google Scholar]
- Sambrook, J; Fritsch, EF; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, New York, NY, USA, 1989. [Google Scholar]
- Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- Hegermann, J; Overbeck, J; Schrempf, H. In vivo monitoring of the potassium channel KcsA in Streptomyces lividans hyphae using immuno-electron microscopy and energy-filtering transmission electron microscopy. Microbiology 2006, 152, 2831–2841. [Google Scholar]
- Leal-Morales, CA; Bracker, CE; Bartnicki-Garcia, S. Localization of chitin synthetase in cell-free homogenates of Saccharomyces cerevisiae: Chitosomes and plasma membrane. Proc. Natl. Acad. Sci. USA 1988, 85, 8516–8520. [Google Scholar]
- Merz, RA; Horsch, M; Nyhlén, LE; Rast, DM. Jollès, P, Muzzarelli, RAA, Eds.; Chitin and Chitinases; Birkhäuser Verlag: Basel, Switzerland, 1999; pp. 9–37. [Google Scholar]
- Ruiz-Herrera, J; Martínez-Espinoza, AD. Jollès, P, Muzzarelli, RAA, Eds.; Chitin and Chitinases; Birkhäuser Verlag: Basel, Switzerland, 1999; pp. 39–53. [Google Scholar]
- Muzzarelli, RAA. Chitin; Pergamon Press: Oxford, UK, 1977. [Google Scholar]
- Roncero, C. The genetic complexity of chitin synthesis in fungi. Curr. Genet 2002, 41, 367–378. [Google Scholar]
- Ruiz-Herrera, J; Ortiz-Castellanos, L. Analysis of the phylogenetic relationships and evolution of the cell walls from yeasts and fungi. FEMS Yeast Res 2010, 10, 225–243. [Google Scholar]
 ).
).
 ).
).

| Polysaccharide | Chitin | Chitosan | Xylan | Cellulose microcrystalline |
|---|---|---|---|---|
| Binding activity, % | 100 | 13.9 | No binding | No binding |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Herasimenka, Y.; Kotasinska, M.; Walter, S.; Schrempf, H. A Selective Assay to Detect Chitin and Biologically Active Nano-Machineries for Chitin-Biosynthesis with Their Intrinsic Chitin-Synthase Molecules. Int. J. Mol. Sci. 2010, 11, 3122-3137. https://doi.org/10.3390/ijms11093122
Herasimenka Y, Kotasinska M, Walter S, Schrempf H. A Selective Assay to Detect Chitin and Biologically Active Nano-Machineries for Chitin-Biosynthesis with Their Intrinsic Chitin-Synthase Molecules. International Journal of Molecular Sciences. 2010; 11(9):3122-3137. https://doi.org/10.3390/ijms11093122
Chicago/Turabian StyleHerasimenka, Yury, Marta Kotasinska, Stefan Walter, and Hildgund Schrempf. 2010. "A Selective Assay to Detect Chitin and Biologically Active Nano-Machineries for Chitin-Biosynthesis with Their Intrinsic Chitin-Synthase Molecules" International Journal of Molecular Sciences 11, no. 9: 3122-3137. https://doi.org/10.3390/ijms11093122
APA StyleHerasimenka, Y., Kotasinska, M., Walter, S., & Schrempf, H. (2010). A Selective Assay to Detect Chitin and Biologically Active Nano-Machineries for Chitin-Biosynthesis with Their Intrinsic Chitin-Synthase Molecules. International Journal of Molecular Sciences, 11(9), 3122-3137. https://doi.org/10.3390/ijms11093122




