Enantioselective Total Synthesis of Daedaleanol B from (+)-Sclareolide
Abstract
1. Introduction
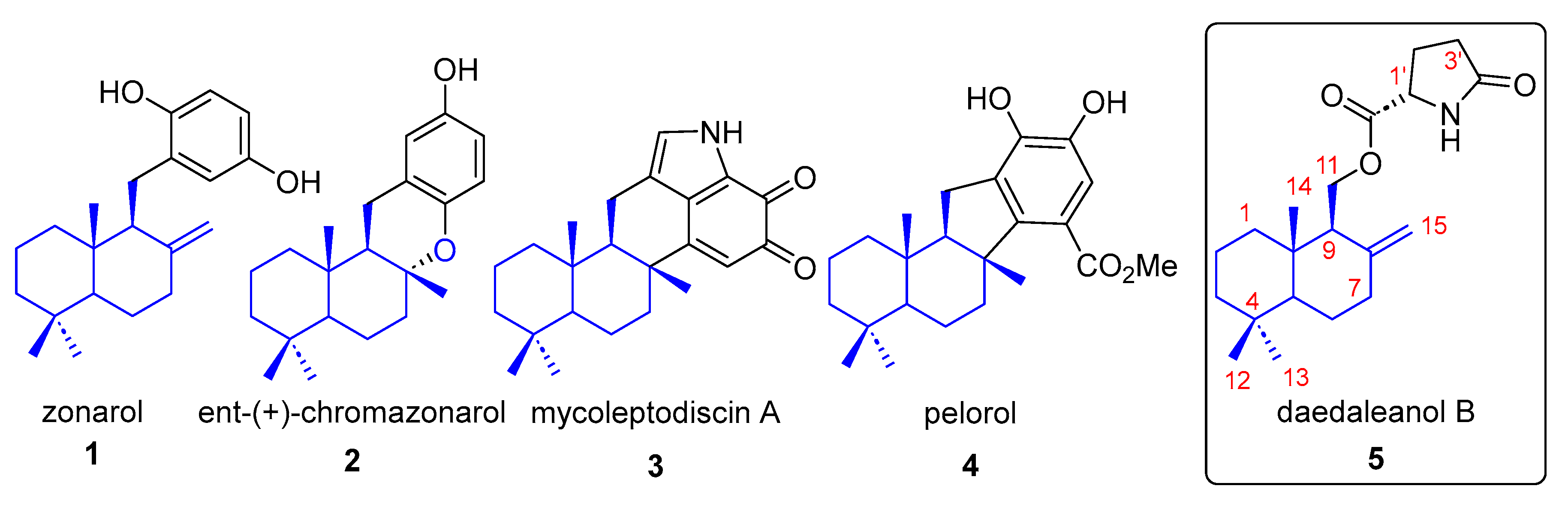
2. Results and Discussion
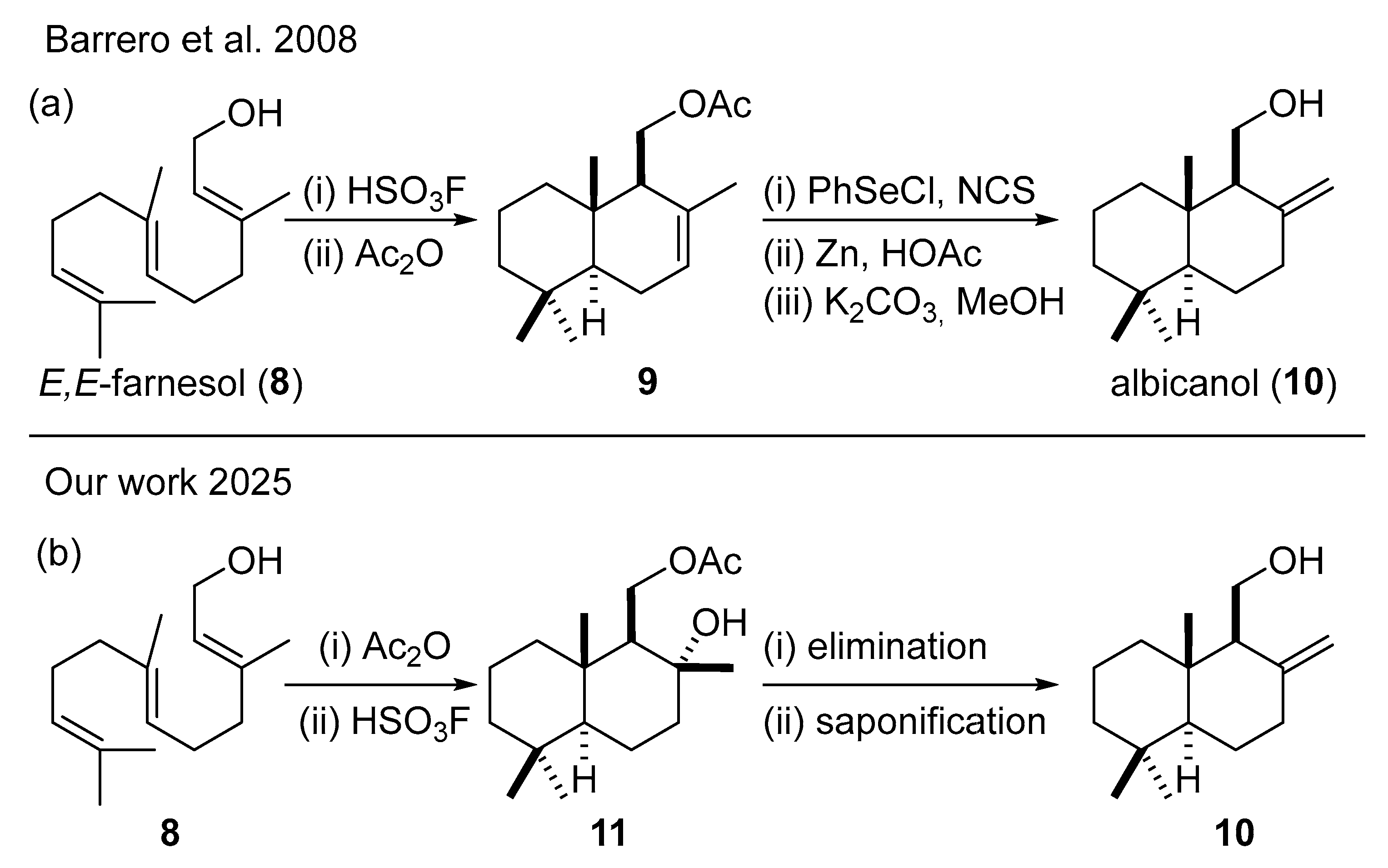
2.1. Racemic Synthesis from (E,E)-Farnesol
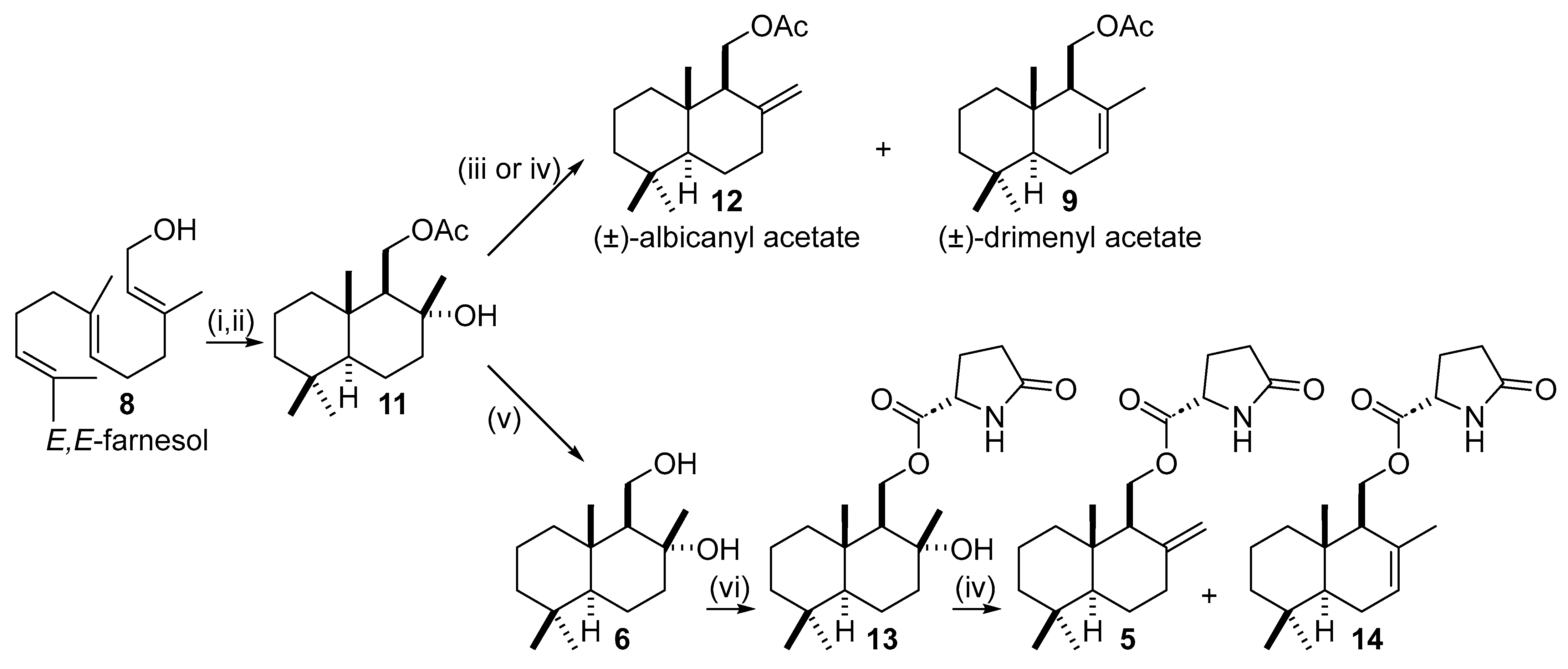
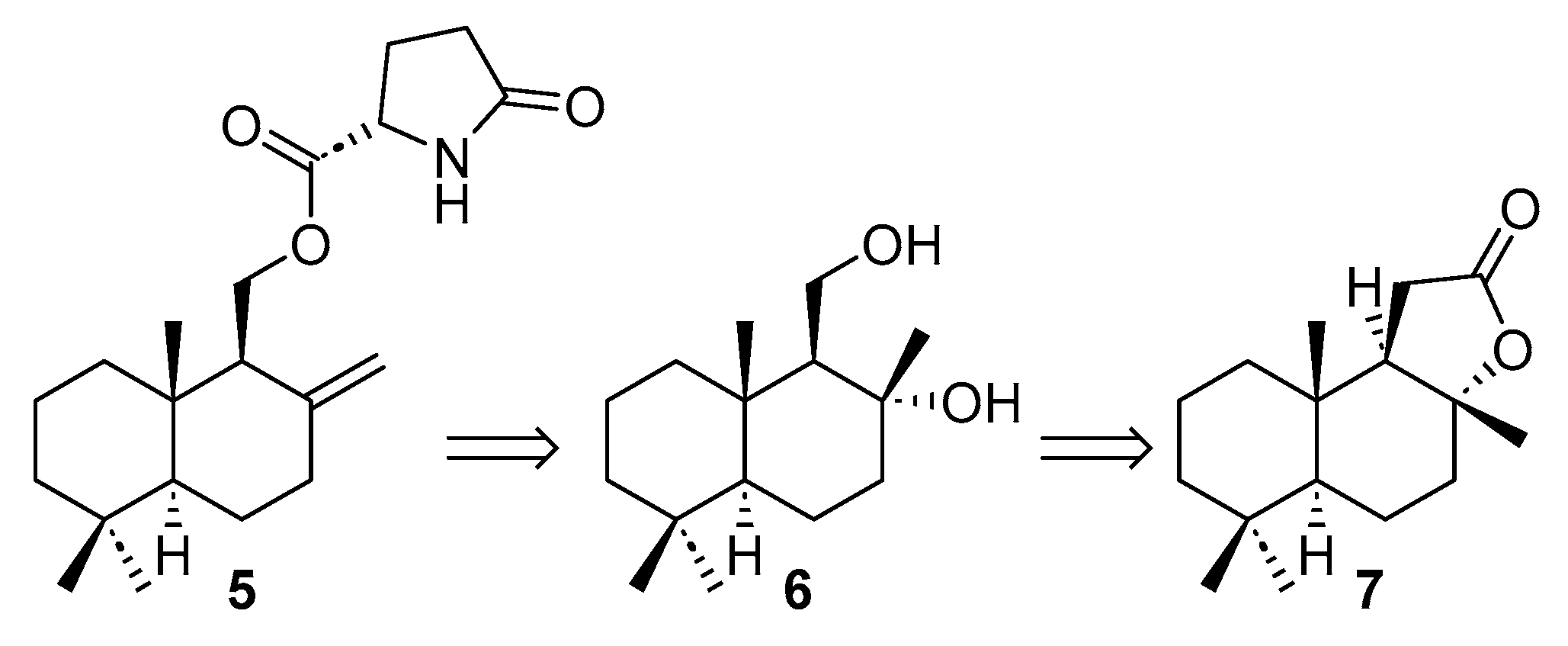
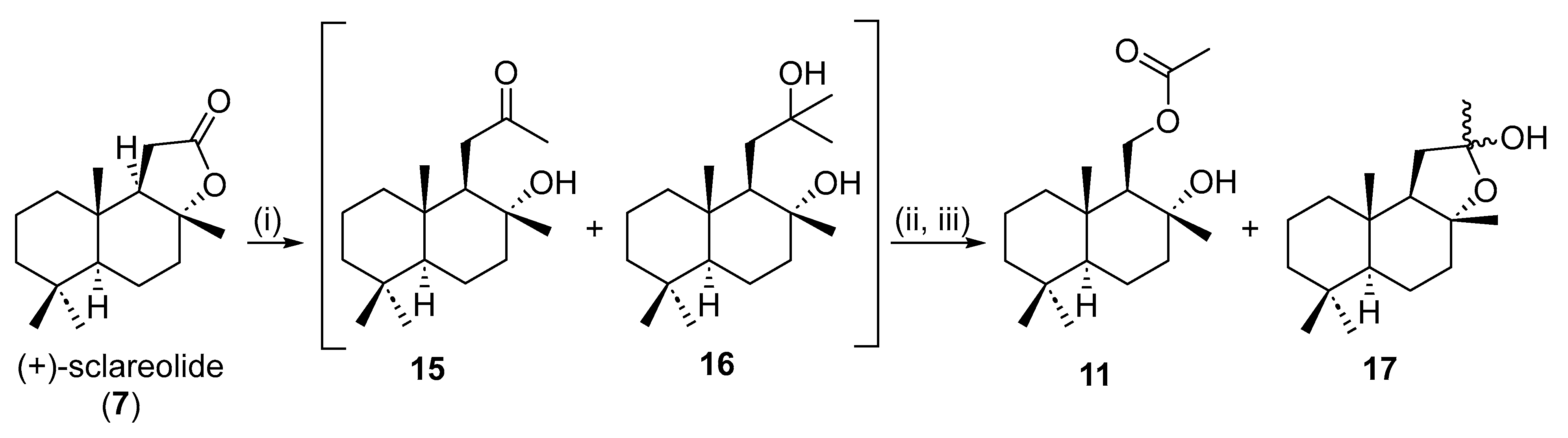
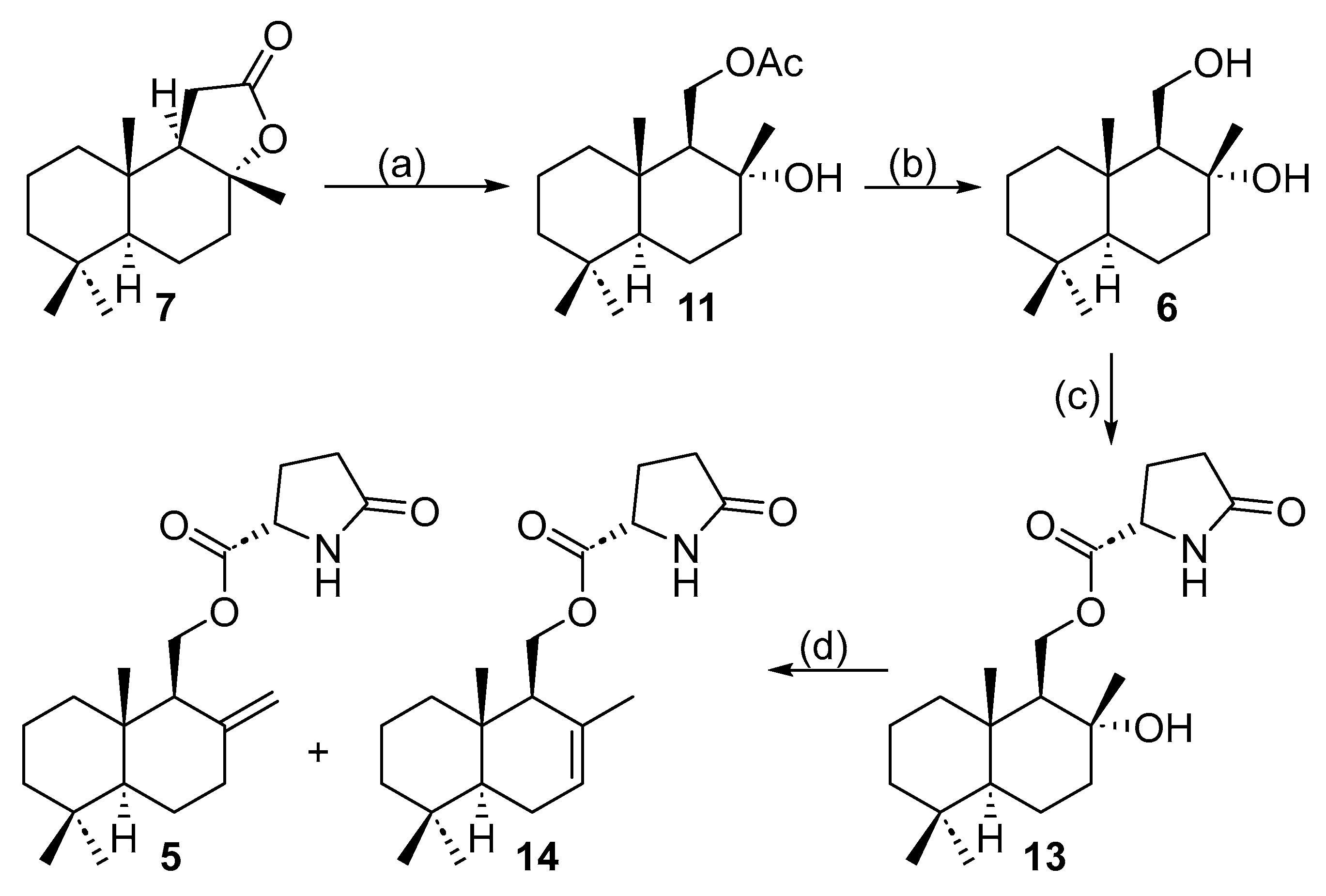
2.2. Enantioselective Synthesis from (+)-Sclareolide
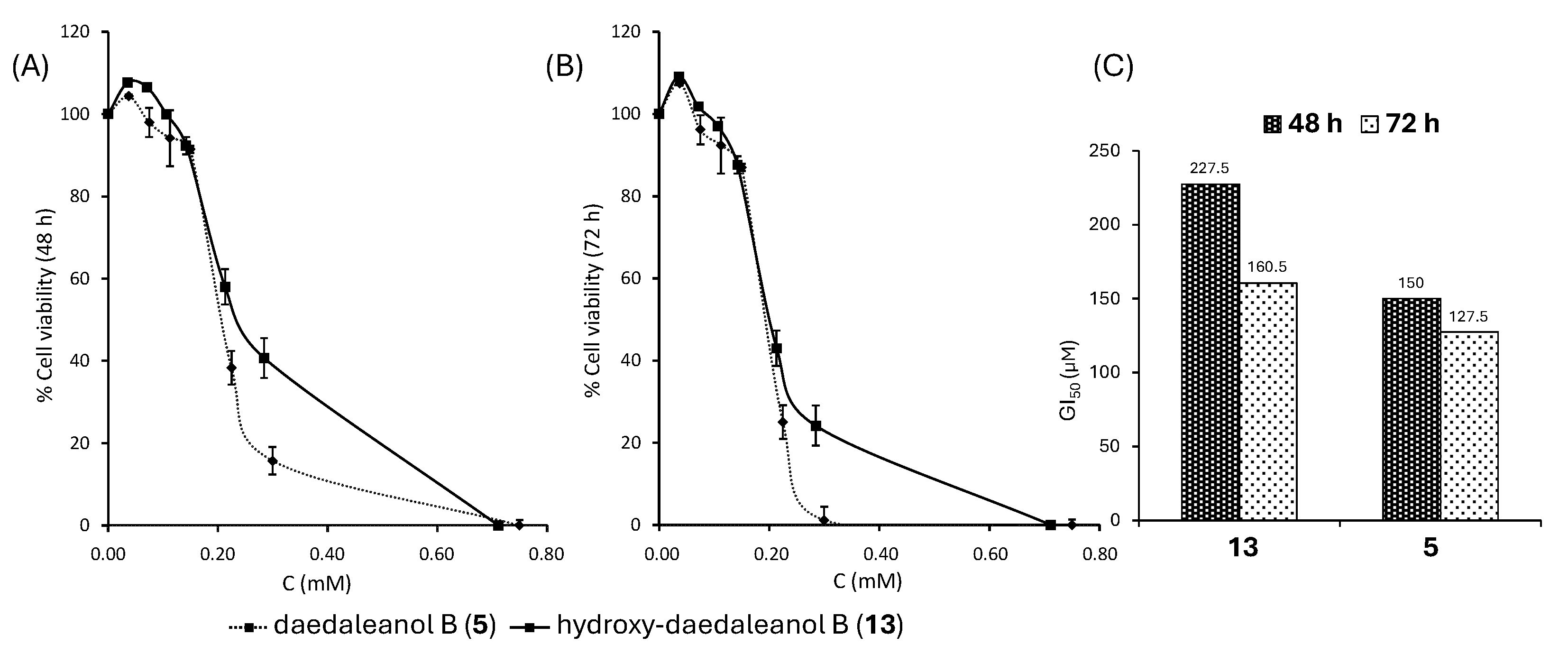
2.3. Antiproliferative Activity of 5 and 13 on HT-29 Cells
3. Materials and Methods
3.1. General Experimental Details
3.2. Synthetic Methodology
3.2.1. Synthesis of (E,E)-Farnesyl Acetate
3.2.2. Synthesis of 11-Acetoxy-Driman-8α-ol (11) from (E,E)-Farnesyl Acetate
3.2.3. Synthesis of (±)-Albicanyl Acetate and (±)-Drimenyl Acetate (9 and 12)
3.2.4. Synthesis of 11-Acetoxy-Driman-8α-ol (11) from Sclareolide (7)
3.2.5. Synthesis of Drimane-8 α,11-Diol (6)
3.2.6. Synthesis of Hydroxydaedaelanol (13)
3.2.7. Synthesis of Daedaleanol B (5) from Hydroxydaedaleanol (13)
3.3. Antiproliferative Activity of 5 and 13 on HT-29 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shan, W.-G.; Ying, Y.-M.; Ma, L.-F.; Zhan, Z.-J. Drimane-related merosesquiterpenoids, a promising library of metabolites for drug development. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 45, pp. 147–215. [Google Scholar] [CrossRef]
- Matsuda, Y.; Abe, I. Biosynthesis of fungal meroterpenoids. Nat. Prod. Rep. 2016, 33, 26–53. [Google Scholar] [CrossRef]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, N.; Kong, W.; Song, B.; Li, S. Facile and divergent optimization of chromazonarol enabled the identification of simplified drimane meroterpenoids as novel pharmaceutical leads. Eur. J. Med. Chem. 2022, 227, 113912. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Sun, S.; Wang, X.; Li, S. Modular Synthesis and Antimicrobial Investigation of Mycoleptodiscin A and Simplified Indolosesquiterpenoids. Org. Lett. 2024, 26, 5764–5769. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; He, X.; Yang, J.; Wang, X.; Li, S. Facile Synthesis and First Antifungal Exploration of Tetracyclic Meroterpenoids: (+)-Aureol, (−)-Pelorol, and Its Analogs. J. Nat. Prod. 2024, 87, 1092–1102. [Google Scholar] [CrossRef]
- Du, W.; Cheng, Z.; Pan, X.; Liu, C.; Yue, M.; Li, T.; Xiao, Z.; Li, L.-L.; Zeng, X.; Lin, X.; et al. Microbe engineering to provide drimane-type building blocks for chiral pool synthesis of meroterpenoids. Angew. Chem. Int. Ed. 2025, 64, e202419463. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu-Sen, B.; Ying-Da, W.; Jia-Jia, C.; Fang, W.; Hong-Gao, L.; Guang-Yu, Z.; Dai, Y.-C. Species diversity of pathogenic wood-rotting fungi (Agaricomycetes, Basidiomycota) in China. Mycology 2023, 14, 204–226. [Google Scholar] [CrossRef]
- Dai, Y.C.; Cui, B.K.; Yuan, H.S.; Li, B.D. Pathogenic wood-decaying fungi in China. For. Path. 2007, 37, 105–120. [Google Scholar] [CrossRef]
- Goodell, B. Brown-rot fungal degradation of wood: Our evolving view. ACS Symp. Ser. 2003, 845, 97–118. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef]
- del Cerro, C.; Erickson, E.; Dong, T.; Wong, A.R.; Eder, E.K.; Purvine, S.O.; Mitchell, H.D.; Weitz, K.K.; Markillie, L.M.; Burnet, M.C.; et al. Intracellular pathways for lignin catabolism in white-rot fungi. Proc. Natl. Acad. Sci. USA 2021, 118, e2017381118. [Google Scholar] [CrossRef]
- Gebhardt, P.; Dornberger, K.; Gollmick, F.A.; Graefe, U.; Haertl, A.; Goerls, H.; Schlegel, B.; Hertweck, C. Quercinol, an anti-inflammatory chromene from the wood-rotting fungus Daedalea quercina (Oak Mazegill). Bioorg. Med. Chem. Lett. 2007, 17, 2558–2560. [Google Scholar] [CrossRef]
- Rösecke, J.; König, W.A. Constituents of the fungi Daedalea quercina and Daedaleopsis confragosa var. tricolor. Phytochemistry 2000, 54, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, S.-B.; Chen, H.-P.; Zhao, Z.-Z.; Li, Z.-H.; Feng, T.; Liu, J.-K. Daedaleanols A and B, two new sesquiterpenes from cultures of the basidiomycete Daedalea incana. Nat. Prod. Res. 2018, 33, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Lindner, D.L.; Ryvarden, L.; Baroni, T.J. A new species of Daedalea (Basidiomycota) and a synopsis of core species in Daedalea sensu stricto. N. Am. Fungi 2011, 6, 1–12. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- de Castria, T.B.; Lenz, G.; Valagni, G.; Kim, R.D. Role of neoadjuvant therapies in locally advanced colon cancer. Chin. Med. J. 2025, 138, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Armstrong, V.; Cortes, M.; Barrero, A.F. Synthesis of racemic and chiral albicanol, albicanyl acetate and cyclozonarone: Cytotoxic activity of ent-cyclozonarone. J. Braz. Chem. Soc. 2008, 19, 1258–1263. [Google Scholar] [CrossRef]
- Polovinka, M.P.; Korchagina, D.V.; Gatilov, Y.V.; Bagrianskaya, I.Y.; Barkhash, V.A.; Perutskii, V.B.; Ungur, N.D.; Vlad, P.F.; Shcherbukhin, V.V.; Zefirov, N.S. Cyclization and rearrangements of farnesol and nerolidol stereoisomers in superacids. J. Org. Chem. 1994, 59, 1509–1517. [Google Scholar] [CrossRef]
- Kulcitki, V. A biomimetic approach to some specifically functionalized cyclic terpenoids. Acta Biochim. Pol. 2007, 54, 679–693. [Google Scholar] [CrossRef]
- Kuchkova, K.I.; Chumakov, Y.M.; Simonov, Y.A.; Bocelli, G.; Panasenko, A.A.; Vlad, P.F. A short efficient synthesis of 11-monoacetate of drimane-8α,11-diol from norambreinolide. Synthesis 1997, 1997, 1045–1048. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Song, Z.; Li, W.; Zhu, F.; Zhang, J.; Li, S. Synthesis and bio-inspired optimization of drimenal: Discovery of chiral drimane fused oxazinones as promising antifungal and antibacterial candidates. Eur. J. Med. Chem. 2018, 143, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Daub, M.E.; Prudhomme, J.; Ben Mamoun, C.; Le Roch, K.G.; Vanderwal, C.D. Antimalarial Properties of Simplified Kalihinol Analogues. ACS Med. Chem. Lett. 2017, 8, 355–360. [Google Scholar] [CrossRef]
- Yanagihara, M.; Murai, K.; Kishimoto, N.; Abe, T.; Misumi, S.; Arisawa, M. Total Synthesis and Biological Evaluation of the Potent HIV Latency-Reversing Agent Ansellone A and its Analogues. Org. Lett. 2021, 23, 1720–1725. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 113–124. [Google Scholar] [CrossRef]
- Fogh, J.; Trempe, G. New Human Tumor Cell Lines. In Human Tumor Cells In Vitro; Fogh, J., Ed.; Springer: Boston, MA, USA, 1975; pp. 115–159. [Google Scholar] [CrossRef]
- Ortea, I.; González-Fernández, M.J.; Ramos-Bueno, R.P.; Guil-Guerrero, J.L. Proteomics Study Reveals That Docosahexaenoic and Arachidonic Acids Exert Different In Vitro Anticancer Activities in Colorectal Cancer Cells. J. Agric. Food Chem. 2018, 66, 6003–6012. [Google Scholar] [CrossRef] [PubMed]
| Natural δH (ppm), J (Hz) | Synthetic δH (ppm), J (Hz) | |
|---|---|---|
| H-2 | 1.61 (1H, m) 1.52 (1H, m) | 1.62 (1H, dt, J = 13.4, 3.2 Hz) 1.54–1.50 (1H, m) |
| H-5 | 1.20 (1H, dd, J = 12.6, 2.6 Hz) | 1.23 (1H, dd, J = 12.5, 2.6 Hz) |
| H-6 | 1.75 (1H, m) 1.35 (1H, m) | 1.76–1.74 (1H, m) 1.39 (1H, dd J = 12.7, J = 4.5) |
| H-11 | 4.43 (1H, dd, J = 11.1, 3.9 Hz) 4.32 (1H, dd, J = 11.1, 9.1 Hz) | 4.46 (1H, dd, J = 11.2, 3.9 Hz) 4.35 (1H, dd, J = 11.2, 9.0 Hz) |
| H-12 | 0.90 (3H, s) | 0.92 (3H, s) |
| H-13 | 0.85 (3H, s) | 0.87 (3H, s) |
| H-14 | 0.81 (3H, s) | 0.83 (3H, s) |
| H-15 | 4.86 (1H, s) 4.53 (1H, s) | 4.89 (1H, q, J = 1.3 Hz) 4.55 (1H, q, J = 1.3 Hz) |
| H-1′ | 4.23 (1H, dd, J = 9.1, 4.1 Hz) | 4.25 (1H, dd, J = 9.1, 4.1 Hz) |
| Natural δC (ppm) | Synthetic δC (ppm) | |
|---|---|---|
| C-1 | 40.2 | 40.3 |
| C-2 | 20.2 | 20.2 |
| C-3 | 43.1 | 43.0 |
| C-4 | 34.4 | 34.4 |
| C-5 | 56.3 | 56.4 |
| C-6 | 25.1 | 25.1 |
| C-7 | 38.7 | 38.7 |
| C-8 | 148.2 | 148.2 |
| C-9 | 56.2 | 56.2 |
| C-10 | 40.1 | 40.1 |
| C-11 | 63.5 | 63.4 |
| C-12 | 34.1 | 34.1 |
| C-13 | 22.2 | 22.2 |
| C-14 | 15.6 | 15.7 |
| C-15 | 107.6 | 107.6 |
| CO | 174.1 | 174.1 |
| C-1′ | 57.2 | 57.2 |
| C-2′ | 25.9 | 25.9 |
| C-3′ | 30.2 | 30.3 |
| COO | 180.9 | 181.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Moreno-Gutiérrez, I.; Berenguel-Gómez, S.; Cánovas-Aragón, M.J.; Guil-Guerrero, J.L.; Chileh-Chelh, T.; Muñoz-Dorado, M.; Álvarez-Corral, M.; Rodríguez-García, I. Enantioselective Total Synthesis of Daedaleanol B from (+)-Sclareolide. Molecules 2026, 31, 185. https://doi.org/10.3390/molecules31010185
Moreno-Gutiérrez I, Berenguel-Gómez S, Cánovas-Aragón MJ, Guil-Guerrero JL, Chileh-Chelh T, Muñoz-Dorado M, Álvarez-Corral M, Rodríguez-García I. Enantioselective Total Synthesis of Daedaleanol B from (+)-Sclareolide. Molecules. 2026; 31(1):185. https://doi.org/10.3390/molecules31010185
Chicago/Turabian StyleMoreno-Gutiérrez, Irene, Sonia Berenguel-Gómez, María José Cánovas-Aragón, José Luis Guil-Guerrero, Tarik Chileh-Chelh, Manuel Muñoz-Dorado, Miriam Álvarez-Corral, and Ignacio Rodríguez-García. 2026. "Enantioselective Total Synthesis of Daedaleanol B from (+)-Sclareolide" Molecules 31, no. 1: 185. https://doi.org/10.3390/molecules31010185
APA StyleMoreno-Gutiérrez, I., Berenguel-Gómez, S., Cánovas-Aragón, M. J., Guil-Guerrero, J. L., Chileh-Chelh, T., Muñoz-Dorado, M., Álvarez-Corral, M., & Rodríguez-García, I. (2026). Enantioselective Total Synthesis of Daedaleanol B from (+)-Sclareolide. Molecules, 31(1), 185. https://doi.org/10.3390/molecules31010185







