Morus alba L. Cell Cultures as Sources of Antioxidant and Anti-Inflammatory Stilbenoids for Food Supplement Development
Abstract
1. Introduction
2. Results and Discussion
2.1. Calli and Suspensions Under Dark Conditions
2.2. Total Stilbenoid Content Expressed as Trans-Resveratrol Equivalents
2.3. Identification of Non-Toxic Concentrations of Juices
2.4. Juices from Suspension Cultures Slightly Reduced Intracellular ROS Generation
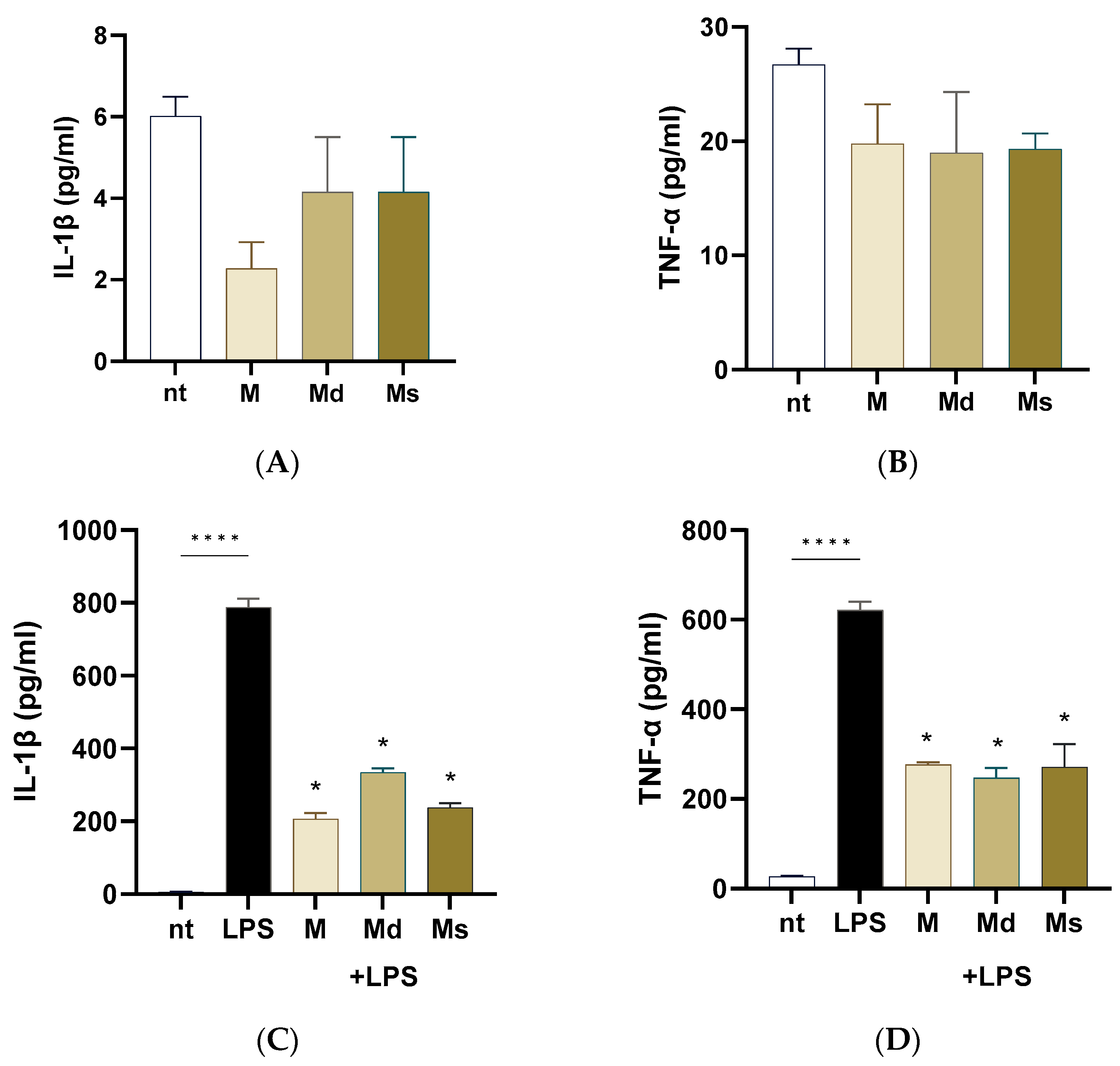
2.5. Effects on Lipopolysaccharide-Induced Inflammation
3. Materials and Methods
3.1. In Vitro Callus and Suspension Cultures
3.2. Juice Preparation
3.3. Chemicals
3.4. Quantitative Analysis of Stilbenoids Expressed as Trans-Resveratrol Equivalents
3.5. Cell Culture and Cell Viability
3.6. Intracellular Reactive Oxygen Species Measurement
3.7. Enzyme-Linked Immunosorbent Assay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohaddab, M.; El Goumi, Y.; Gallo, M.; Montesano, D.; Zengin, G.; Bouyahya, A.; Fakiri, M. Biotechnology and in vitro culture as an alternative system for secondary metabolite production. Molecules 2022, 27, 8093. [Google Scholar] [CrossRef] [PubMed]
- Shruti; Bharadvaja, N. Biotechnology based strategies for secondary metabolites enhancement: A review. Vegetos 2024, 37, 1211–1220. [Google Scholar] [CrossRef]
- Zouine, N.; El Ghachtouli, N.; El Abed, S.; Ibnsouda Koraichi, S. A comprehensive review on medicinal plant extracts as antibacterial agents: Factors, mechanism insights and future prospects. Sci. Afr. 2024, 26, e02395. [Google Scholar] [CrossRef]
- Rutkowska, J.; Pasqualone, A. Plant extracts as functional food ingredients. Foods 2025, 14, 374. [Google Scholar] [CrossRef]
- Dadhwal, R.; Banerjee, R. Ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological profile of Morus alba L.: A comprehensive review. S. Afr. J. Bot. 2023, 158, 98–117. [Google Scholar] [CrossRef]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Umar Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef]
- Singh, R.; Bagachi, A.; Semwal, A.; Kaur, S.; Bharadwaj, A. Traditional uses, phytochemistry and pharmacology of Morus alba Linn.: A review. J. Med. Plants Res. 2013, 7, 461–469. [Google Scholar]
- Chen, C.; Mohamad Razali, U.H.; Saikim, F.H.; Mahyudin, A.; Mohd Noor, N.Q.I. Morus alba L. plant: Bioactive compounds and potential as a functional food ingredient. Foods 2021, 10, 689. [Google Scholar] [CrossRef]
- Wang, Y.; Ai, Q.; Gu, M.; Guan, H.; Yang, W.; Zhang, M.; Mao, J.; Lin, Z.; Liu, Q.; Liu, J. Comprehensive overview of different medicinal parts from Morus alba L.: Chemical compositions and pharmacological activities. Front. Pharmacol. 2024, 15, 1364948. [Google Scholar] [CrossRef]
- Porasar, P.; Gibo, R.; Gogoi, B.; Sharma, D.; Bharadwaj, A.; Gam, S.; Hazarika, D.; Dutta, K.N. A systematic review on the phytochemistry, isolated compounds, nutritional benefits, pharmacology and toxicology of the plant species Morus alba L. Discov. Plants 2025, 2, 140–160. [Google Scholar] [CrossRef]
- Ishrat, Y.; Anab, F.; Syed, M.A.; Saman, U.; Zubia, B.; Sehrish, B.; Rabia, A. Review of ethnobotany, phytochemistry, antiviral and cytotoxic/anticancer of Morus alba Linn. Int. J. Adv. Res. 2016, 1, 84–96. [Google Scholar]
- Vuscan, A.N.; Miere, F.; Venter, A.C.; Vicas, S.I. Phytochemical composition of different botanical parts of Morus species, health benefits and application in food industry. Plants 2022, 11, 152. [Google Scholar] [CrossRef]
- Fatima, M.; Dar, M.A.; Dhanavade, M.J.; Abbas, S.Z.; Bukhari, M.N.; Arsalan, A.; Liao, Y.; Wan, J.; Shah Syed Bukhari, J.; Ouyang, Z. Biosynthesis and pharmacological activities of the bioactive compounds of white mulberry (Morus alba): Current paradigms and future challenges. Biology 2024, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Čulenová, M.; Sychrová, A.; Hassan, S.T.S.; Berchová-Bímová, K.; Svobodová, P.; Helclová, A.; Michnová, H.; Hošek, J.; Vasilev, H.; Suchý, P.; et al. Multiple in vitro biological effects of phenolic compounds from Morus alba root bark. J. Ethnopharm. 2020, 248, 112296. [Google Scholar] [CrossRef]
- Chaita, E.; Lambrinidis, G.; Cheimonidi, C.; Agalou, A.; Beis, D.; Trougakos, I.; Mikros, E.; Skaltsounis, A.L.; Aligiannis, N. Anti-melanogenic properties of greek plants. A novel depigmenting agent from Morus alba wood. Molecules 2017, 22, 514. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.H.; Yang, C.S.; Chen, J.J. Antioxidant, anti-α-glucosidase, antityrosinase, and anti-inflammatory activities of Bioactive Components from Morus alba. Antioxidants 2022, 11, 2222. [Google Scholar] [CrossRef]
- Batiha, G.E.; Al-Snafi, A.E.; Thuwaini, M.M.; Teibo, J.O.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; et al. Morus alba: A comprehensive phytochemical and pharmacological review. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.-C.; Lye, P.-Y.; Wong, S.-K. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin. J. Nat. Med. 2016, 14, 17–30. [Google Scholar]
- Morante-Carriel, J.; Živković, S.; Nájera, H.; Sellés-Marchart, S.; Martínez-Márquez, A.; Martínez-Esteso, M.J.; Obrebska, A.; Samper-Herrero, A.; Bru-Martínez, R. Prenylated Flavonoids of the Moraceae Family: A Comprehensive Review of Their Bio-logical Activities. Plants 2024, 13, 1211. [Google Scholar] [CrossRef]
- Bo, S.; Chang, S.C.; Shan, Y.; Chen, Y.; Liu, H.; Li, B.; Jiang, Y.; Zhu, H.; Yang, B. The bioactivity of prenylated stilbenoids and their structure-activity relationship. Food Res. Int. 2022, 157, 111275. [Google Scholar] [CrossRef]
- Tian, L.-L.; Bi, Y.-X.; Zhang, H. Structurally Diverse Stilbenoids as Potent α-Glucosidase Inhibitors with Antidiabetic Effect from Morus alba. J. Agric. Food Chem. 2024, 72, 17938–17952. [Google Scholar] [CrossRef]
- Vijayan, K.; JayaramaRaju, P.; Tikader, A.; Saratchnadra, B. Biotechnology of mulberry (Morus L.)—A review. Emir. J. Food Agric. 2014, 26, 472–496. [Google Scholar] [CrossRef]
- Sabater-Jara, A.B.; Almagro, L.; Nicolás Sánchez, I.; Pedreño, M.Á. Biotechnological approach to increase oxyresveratrol production in mulberry in vitro plants under elicitation. Plants 2023, 12, 546. [Google Scholar] [CrossRef] [PubMed]
- Taha, H.; Ghazy, U.M.; Gabr, A.M.M.; EL-Kazzaz, A.A.A.; Ahmed, E.A.M.M.; Haggag, K.M. Optimization of in vitro culture conditions affecting propagation of mulberry plant. Bull. Natl. Res. Cent. 2020, 44, 60. [Google Scholar] [CrossRef]
- Rana, M.S.; Amin, M.N.; Azad, M.A.K. In vitro regeneration protocol for mulberry (Morus alba L.) through tissue culture techniques. Int. J. Res. Innov. Soc. Sci. 2022, 6, 124–186. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, D.E.; Lee, H.S.; Kim, S.K.; Lee, W.S.; Kim, S.H.; Kim, M.W. Influence of auxins, cytokinins, and nitrogen on production of rutin from callus and adventitious roots of the white mulberry tree (Morus alba L.). Plant Cell Tissue Organ Cult. 2011, 105, 9–19. [Google Scholar] [CrossRef]
- Inyai, C.; Yusakul, G.; Komaikul, J.; Kitisripanya, T.; Likhitwitayawuid, K.; Sritularak, B.; Putalun, W. Improvement of stilbene production by mulberry Morus alba root culture via precursor feeding and co-elicitation. Bioprocess Biosyst. Eng. 2021, 44, 653–660. [Google Scholar] [CrossRef]
- Komaikul, J.; Kitisripanya, T.; Tanaka, H.; Sritularak, B.; Putalun, W. Enhanced mulberroside A production from cell suspension and root cultures of Morus alba using elicitation. Nat. Prod. Commun. 2015, 10, 1253–1256. [Google Scholar] [CrossRef]
- Inyai, C.; Boonsnongcheep, P.; Komaikul, J.; Sritularak, B.; Tanaka, H.; Putalun, W. Alginate immobilization of Morus alba L. cell suspension cultures improved the accumulation and secretion of stilbenoids. Bioprocess Biosyst. Eng. 2019, 42, 131–141. [Google Scholar] [CrossRef]
- Thamrongwatwongsa, J.; Pattarapipatkul, N.; Jaithon, T.; Jindaruk, A.; Paemanee, A.; Thienprasert, N.P.; Phonphoem, W.P. Mulberroside F from in vitro culture of mulberry and the potential use of the root extracts in cosmeceutical applications. Plants 2023, 12, 146. [Google Scholar] [CrossRef]
- Dalla Costa, V.; Piovan, A.; Brun, P.; Filippini, R. Morus alba calli: A sustainable source of phytochemicals and nutritive supplements. Vitr. Cell. Dev. Biol. Plant 2025. submitted. [Google Scholar]
- Häkkinen, S.T.; Nygren, H.; Nohynek, L.; Puupponen-Pimiä, R.; Heiniö, R.L.; Maiorova, N.; Rischer, H.; Ritala, A. Plant cell cultures as food—Aspects of sustainability and safety. Plant Cell Rep. 2020, 39, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–885. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Farkya, S.; Srivastava, A.K.; Bisaria, V.S. Bioprocess Considerations for Production of Secondary Metabolites by Plant Cell Suspension Cultures. Biotechnol. Bioprocess Eng. 2002, 7, 138–149. [Google Scholar] [CrossRef]
- Mustafa, N.R.; de Winter, W.; van Iren, F.; Verpoorte, R. Initiation, growth and cryopreservation of plant cell suspension cultures. Nat. Protoc. 2011, 6, 715–742. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Vidal-Sánchez, F.J.; Matencio, A.; Albaladejo-Maricó, L.; García-Carmona, F.; López-Nicolás, J.M. Stilbenes: Characterization, bioactivity, encapsulation and structural modifications. A review of their current limitations and promising approaches. Crit. Rev. Food Sci. Nutr. 2022, 63, 7269–7287. [Google Scholar] [CrossRef]
- Pongkitwitoon, B.; Simpan, K.; Chobsri, T.; Sritularak, B.; Putalun, W. Combined UV-C irradiation and precursor feeding enhances mulberroside A production in Morus alba L. cell suspension cultures. Sci. Asia 2020, 46, 679–685. [Google Scholar] [CrossRef]
- Shimoda, K.; Kubota, N.; Uesugi, D.; Kobayashi, Y.; Hamada, H.; Hamada, H. Glycosylation of stilbene compounds by cultured plant cells. Molecules 2020, 25, 1437. [Google Scholar] [CrossRef]
- Komaikul, J.; Kitisripanya, T.; Likhitwitayawuid, K.; Sritularak, B.; Tanaka, H.; Putalun, W. Improvement of stilbenoid production by 2-hydroxypropyl-β-cyclodextrin in white mulberry (Morus alba L.) callus cultures. Nat. Prod. Res. 2018, 33, 2762–2769. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tien, Y.J.; Chen, C.H.; Beltran, F.N.; Amore, E.C.; Wang, R.-J.; Wu, D.-J.; Mettling, C.; Lin, Y.-L.; Yang, W.-C. Morus alba and active compound oxyresveratrol exert anti-inflammatory activity via inhibition of leukocyte migration involving MEK/ERK signaling. BMC Complement. Altern. Med. 2013, 13, 45. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.-G.; Linhardt, R.J.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamim, A.; Khan, M.M.U.; et al. Resveratrol from Dietary Supplement to a Drug Candidate: An Assessment of Potential. Pharmaceuticals 2022, 15, 957. [Google Scholar] [CrossRef] [PubMed]
- Bejenaru, L.E.; Biţă, A.; Belu, I.; Segneanu, A.-E.; Radu, A.; Dumitru, A.; Ciocilteu, M.V.; Mogoşanu, G.D.; Bejenaru, C. Resveratrol: A Review on the Biological Activity and Applications. Appl. Sci. 2024, 14, 4534. [Google Scholar] [CrossRef]
- Naik, R.; Harmalkar, D.S.; Xu, X.; Jan, K.; Lee, K. Bioactive benzofuran derivatives: Moracins A–Z in medicinal chemistry. Eur. J. Med. Chem. 2015, 90, 379–393. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, G.; Chen, J.; Zheng, Z.-P. Characterization of a New Flavone and Tyrosinase Inhibition Constituents from the Twigs of Morus alba L. Molecules 2016, 21, 1130. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Wu, S.J.; Hsu, F.W.; Fang, L.W.; Liou, C.J. Mulberroside F improves airway hyperresponsiveness and inflammation in asthmatic mice. Kaohsiung J. Med. Sci. 2023, 39, 1213–1221. [Google Scholar] [CrossRef]
- Verdú-Navarro, F.; Moreno-Cid, J.A.; Weiss, J.; Egea-Cortines, M. The advent of plant cells in bioreactors. Front. Plant Sci. 2023, 14, 1310405. [Google Scholar] [CrossRef]
- Ma, G.; Chai, X.; Hou, G.; Zhao, F.; Meng, Q. Phytochemistry, bioactivities and future prospects of mulberry leaves: A review. Food Chem. 2022, 372, 131335. [Google Scholar] [CrossRef]
- Kim, J.-S.; Tae-Ha, T.-Y.; Ahn, J.-Y.; Kim, H.-K.; Kim, S. Composition and Quantitative Analysis of Stilbenoids in Mulberry (Morus alba L.) Leaves and Fruits with DAD/UV HPLC. J. Korean Soc. Food Sci. Nutr. 2008, 37, 124–128. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.; Bao, T.; Gowd, V. Mulberry Fruit Extract Affords Protection against Ethyl Carbamate-Induced Cytotoxicity and Oxidative Stress. Oxid. Med. Cell. Longev. 2017, 2017, 1594963. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Mahmutović, L.; Sezer, A.; Bilajac, E.; Hromić-Jahjefendić, A.; Uversky, V.N.; Glamočlija, U. Polyphenol stability and bioa-vailability in cell culture medium: Challenges, limitations and future directions. Int. J. Biol. Macromol. 2024, 279, 135232. [Google Scholar] [CrossRef]
- Bao, T.; Li, Y.; Xie, J.; Jia, Z.; Chen, W. Systematic evaluation of polyphenols composition and antioxidant activity of mulberry cultivars subjected to gastrointestinal digestion and gut microbiota fermentation. J. Funct. Foods 2019, 58, 338–349. [Google Scholar] [CrossRef]
- Martins, M.S.; Rodrigues, M.; Flores-Félix, J.D.; Garcia-Viguera, C.; Moreno, D.A.; Alves, G.; Silva, L.R.; Gonçalves, A.C. The Effect of Phenolic-Rich Extracts of Rubus fruticosus, R. ulmifolius and Morus nigra on Oxidative Stress and Caco-2 Inhibition Growth. Nutrients 2024, 16, 1361. [Google Scholar] [CrossRef]
- Jiang, D.Q.; Guo, Y.; Xu, D.H.; Huang, Y.S.; Yuan, K.; Lv, Z.Q. Antioxidant and anti-fatigue effects of anthocyanins of mulberry juice purification (MJP) and mulberry marc purification (MMP) from different varieties mulberry fruit in China. Food Chem. Toxicol. 2013, 59, 1–7. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Li, M.; Peng, K.; Lin, X.; Feng, Y.; Zou, Y.; Wu, X. Study on chemical composition, anti-inflammatory activity and quality control of the branch bark of Morus alba L. Fitoterapia 2025, 181, 106383. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Dalla Costa, V.; Piovan, A.; Filippini, R.; Brun, P. From Ethnobotany to Biotechnology: Wound Healing and Anti-Inflammatory Properties of Sedum telephium L. in vitro Cultures. Molecules 2024, 29, 2472. [Google Scholar] [CrossRef]
- Mei, S.; Chen, X. Investigation into the anti-inflammatory mechanism of coffee leaf extract in LPS-induced Caco-2/U937 co-culture model through cytokines and NMR-based untargeted metabolomics analyses. Food Chem. 2023, 15, 134592. [Google Scholar] [CrossRef]
- Voltan, S.; Martines, D.; Elli, M.; Brun, P.; Longo, S.; Porzionato, A.; Macchi, V.; D’Incà, R.; Scarpa, M.; Palù, G.; et al. Lactobacillus crispatus M247-derived H2O2 acts as a signal transducing molecule activating pe-roxisome proliferator activated receptor-gamma in the intestinal mucosa. Gastroenterology 2008, 135, 1216–1227. [Google Scholar] [CrossRef]


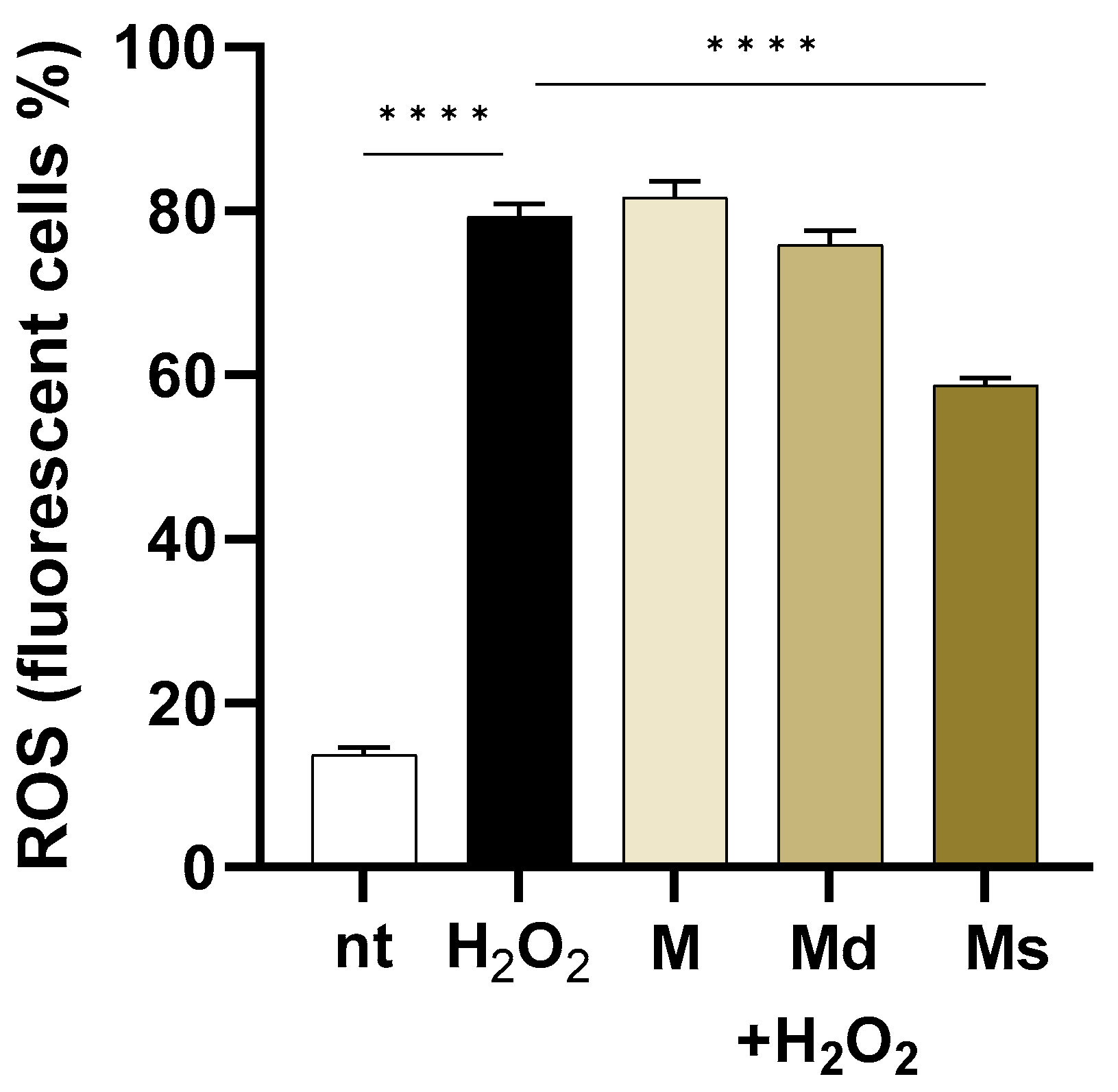

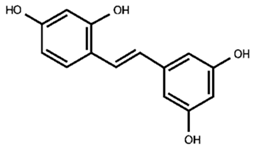
 resveratrol backbone |
| 2—trans-resveratrol derivative |
| 5—mulberroside E |
| 12—resveratrol glucoside |
| 14—piceid |
| 16—trans-resveratrol |
 oxyresveratrol backbone |
| 1—oxyresveratrol derivative |
| 3—oxyresveratrol triglucoside |
| 4—oxyresveratrol derivative |
| 8—mulberroside A |
| 10—oxyresveratrol glucoside |
| 11—oxyresveratrol glucoside isomer |
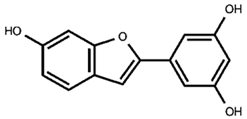 benzofuran stilbenoid backbone |
| 6—benzofuranic stilbenoid derivative |
| 7—mulberroside F |
| 9—benzofuranic stilbenoid derivative |
| 13—moracin M glucoside |
| 15—moracin M glucoside isomer |
| 17—moracin M |
| Cell Line | Growth Cycle (Days) | Total Stilbenoid Content (µg/mL of Juice) |
|---|---|---|
| M | 14 | 255.71 ± 2.22 b |
| 28 | 205.92 ± 3.26 c | |
| Md | 14 | 279.94 ± 7.47 b |
| 28 | 226.56 ± 2.19 c | |
| Ms | 13 | 331.55 ± 5.40 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalla Costa, V.; Piovan, A.; Brun, P.; Filippini, R. Morus alba L. Cell Cultures as Sources of Antioxidant and Anti-Inflammatory Stilbenoids for Food Supplement Development. Molecules 2025, 30, 2073. https://doi.org/10.3390/molecules30092073
Dalla Costa V, Piovan A, Brun P, Filippini R. Morus alba L. Cell Cultures as Sources of Antioxidant and Anti-Inflammatory Stilbenoids for Food Supplement Development. Molecules. 2025; 30(9):2073. https://doi.org/10.3390/molecules30092073
Chicago/Turabian StyleDalla Costa, Vanessa, Anna Piovan, Paola Brun, and Raffaella Filippini. 2025. "Morus alba L. Cell Cultures as Sources of Antioxidant and Anti-Inflammatory Stilbenoids for Food Supplement Development" Molecules 30, no. 9: 2073. https://doi.org/10.3390/molecules30092073
APA StyleDalla Costa, V., Piovan, A., Brun, P., & Filippini, R. (2025). Morus alba L. Cell Cultures as Sources of Antioxidant and Anti-Inflammatory Stilbenoids for Food Supplement Development. Molecules, 30(9), 2073. https://doi.org/10.3390/molecules30092073







