Can Provence Flora Offer Effective Alternatives to Widely Used Medicinal Plants? A Comparative Study of Antioxidant Activity and Chemical Composition Using Molecular Networking
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Extracts
2.1.1. Chemical Classification Predictions
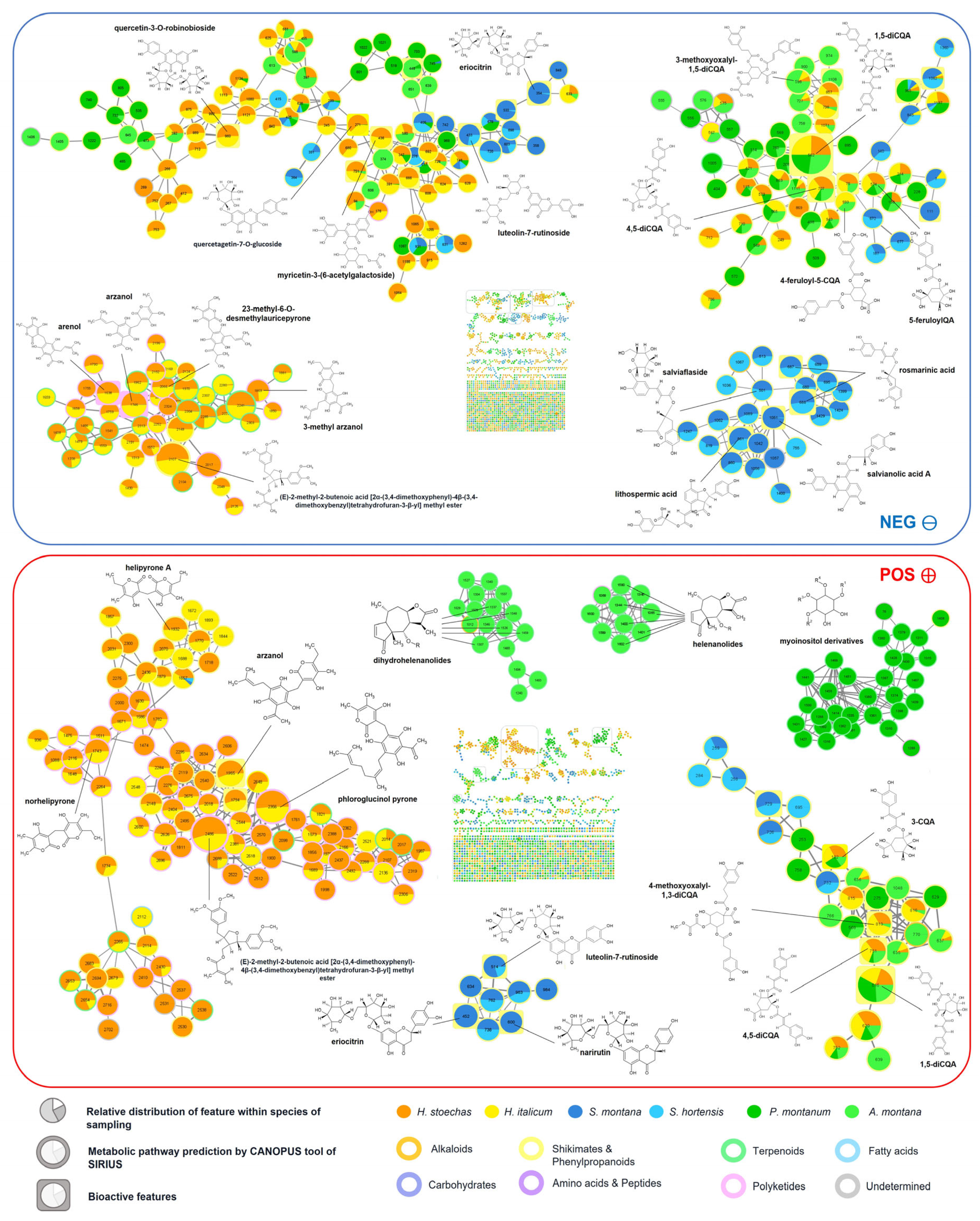
2.1.2. Molecular Networking
2.1.3. Arnica Pair
2.1.4. Helichrysum Pair
2.1.5. Satureja Pair
2.2. Antioxidant Activity Assessment
2.2.1. Total Phenolic and Flavonoid Contents
2.2.2. DPPH and ABTS Well-Plate Assays
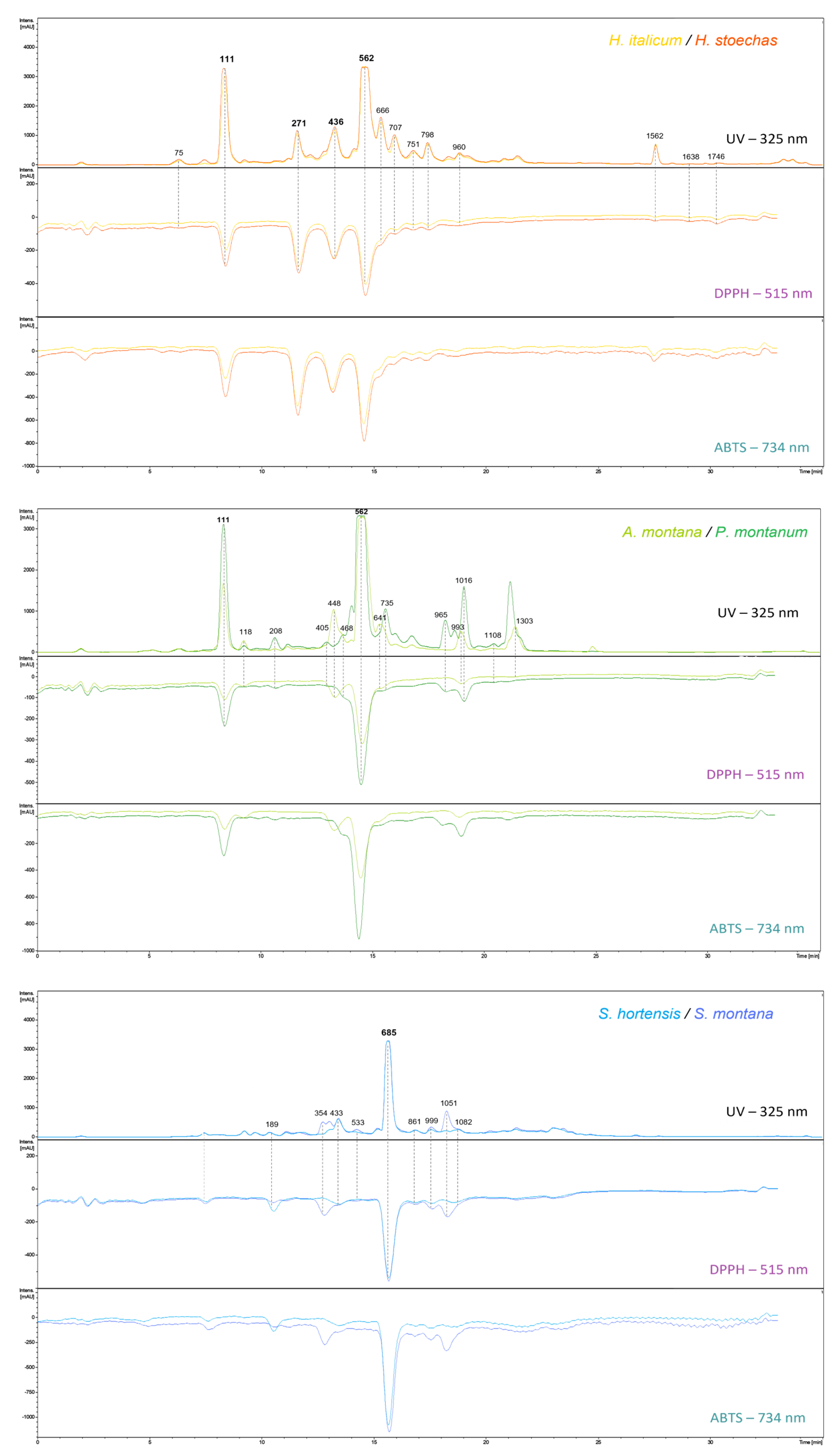
2.2.3. DPPH/ABTS-On-Line-UHPLC Assays
| Peak Area (mAU) | Rt (min) | Formula | Annotation | CL | ID− | [M−H]− | [M−H]− Fragments (Relative Intensities in %) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | PM | HI | HS | SH | SM | ||||||||
| 1954 | - | 3565 | 4634 | - | - | 1.65 | C16H18O9 | 3-caffeoylquinic acid | L2a | 75 | 353.0877 (−0.3) | 191.0556 (100), 179.0346 (64), 135.0446 (64), 85.0290 (7) | [92] |
| 32,896 | 62,763 | 59,859 | 74,503 | - | - | 3.58 | C16H18O9 | 5-caffeoylquinic acid | L1 | 111 | 353.0879 (+0.3) | 191.0571 (100), 353.0894 (73), 179.0360 (65), 135.0459 (27), 161.0252 (6), 173.0465 (4), 155.0361 (1) | [92] |
| 4298 | 1863 | 1259 | 2011 | 2589 | 3148 | 3.70 | C9H8O4 | caffeic acid | L1 | 118 | 179.0349 (−0.5) | 135.0454 (100), 89.0403 (12), 107.0505 (5), 117.0343 (4) | [113] |
| - | - | - | - | 1929 | 2186 | 6.58 | C18H16O8 | rosmarinic acid derivative | L3 | 189 | 359.0772 (+3.1) | 161.0241 (100), 174.9554 (63), 197.0452 (57), 135.0453 (50) | [114] |
| 944 | 5088 | - | - | - | - | 6.89 | C25H24O12 | 1,3-dicaffeoylquinic acid | L2b | 208 | 515.1188 (−1.4) | 191.0565 (100), 353.0878 (96), 179.0355 (86), 135.0457 (26), 515.1187 (15), 335.0780 (12), 161.0239 (9) | [93] |
| - | - | 18,988 | 19,462 | - | - | 8.14 | C21H20O13 | quercetagetin-7-O-glucoside | L1 | 271 | 479.0829 (−0.4) | 317.0311 (100), 479.0847 (27), 165.9914 (5), 139.0044 (3) | [115] |
| - | - | - | - | - | 6796 | 9.29 | C27H32O15 | eriodictyol-7-rutinoside | L2a | 354 | 595.1671 (+0.4) | 287.0570 (100), 151.0045 (61), 595.1673 (49), 506.1708 (23), 135.0458 (17), 459.1150 (7) | [116] |
| - | 2311 | - | - | - | - | 9.72 | C21H18O12 | luteolin-7-glucuronide | L2a | 405 | 461.0727 (+0.3) | 285.0417 (100), 461.0743 (11), 300.0283 (3) | [117] |
| - | - | 26,450 | 27,237 | - | - | 9.90 | C23H22O14 | myricetin-acetylhexoside | L2a | 436 | 521.0938 (+0.2) | 317.0290 (100), 521.0952 (45), 329.1405 (10), 165.9915 (7), 463.0835 (6) | [118] |
| - | - | - | - | 11,630 | 12,262 | 9.91 | C27H30O16 | luteolin-7-rutinoside | L2a | 433 | 593.1513 (+0.2) | 285.0412 (100), 593.1519 (99) | [119] |
| 17,590 | - | - | - | - | - | 10.02 | C22H20O14 | patuletin-3-glucuronide | L3 | 448 | 507.0779 (−0.3) | 331.0447 (100), 316.0210 (60), 507.0768 (19), 287.0187 (17), 270.0166 (9) | [120] |
| 5062 | 4828 | - | - | - | - | 10.19 | C22H22O13 | methoxy-myricetin-3-O-hexoside | L2a | 468 | 493.0988 (+0.1) | 330.0392 (100), 493.0995 (91), 315.0157 (89), 287.0206 (25) | [121] |
| - | - | - | - | - | 4209 | 10.63 | C27H32O14 | naringenin-7-rutinoside | L2a | 533 | 579.1724 (+0.8) | 271.0622 (100), 579.1726 (29), 151.0042 (13), 313.0730 (3) | [122] |
| 111,632 | 103,103 | 102,694 | 102,494 | - | - | 10.82 | C25H24O12 | 1,5-dicaffeoylquinic acid | L1 | 562 | 515.1197 (+0.4) | 191.0571 (100), 353.0894 (73), 179.0360 (65), 135.0459 (27), 161.0252 (6) | [93] |
| 14,299 | - | - | - | - | - | 11.38 | C22H22O12 | isorhamnetin-3-O-glucoside | L2a | 641 | 477.1037 (−0.3) | 477.1024 (100), 314.0420 (81), 299.0184 (58), 271.0238 (36), 243.0287 (17) | [123] |
| - | - | 28,068 | 29,179 | - | - | 11.48 | C23H22O13 | quercetin-3-O-glucosyl-6′-acetate | L2a | 666 | 505.0988 (+2.2) | 301.0355 (100), 505.1006 (2) | [46] |
| - | - | - | - | 70,991 | 70,405 | 11.63 | C18H16O8 | rosmarinic acid | L2a | 685 | 359.0778 (+1.6) | 161.0252 (100), 197.0464 (38), 135.0458 (31), 179.0358 (21), 72.9937 (21), 123.0458 (16) | [119] |
| - | - | 15,354 | 17,140 | - | - | 11.79 | C25H24O12 | 3,5-dicaffeoylquinic acid | L2a | 707 | 515.1194 (−0.2) | 353.0881 (100), 173.0455 (61), 179.0349 (49), 191.0562 (25), 135.0449 (16), 515.1205 (9) | [93] |
| 1368 | 17376 | - | - | - | - | 11.91 | C22H22O11 | hispidulin-4-glucoside | L2a | 735 | 461.1088 (−0.3) | 461.1108 (100), 283.0261 (93), 297.0416 (15) | [124] |
| - | - | 5353 | 5577 | - | - | 12.08 | C21H20O12 | quercetin-hexoside | L2b | 751 | 463.0882 (+0) | 301.0362 (100), 463.0897 (32), 151.0044 (24), 178.9994 (16) | [58] |
| - | - | 11,015 | 11,382 | - | - | 12.41 | C28H26O15 | 1,3-dicaffeoyl-4-methoxy-oxaloyl-quinic acid | L2a | 798 | 601.1199 (+0) | 395.0989 (100), 233.0671 (66), 353.0882 (44), 173.0459 (40), 191.0566 (28), 179.0353 (26), 439.0883 (9) | [59] |
| - | - | - | - | 2335 | 2671 | 12.94 | C27H22O12 | lithospermic acid | L2a | 861 | 537.1040 (+0.3) | 135.0460 (100), 295.0620 (97), 161.0253 (79), 359.0782 (75), 179.0360 (35), 197.0466 (33) | [125] |
| - | - | 5247 | 5667 | - | - | 13.81 | C30H26O14 | quercetin-3-O-robinobioside | L2a | 960 | 609.1251 (+0.2) | 300.0282 (100), 609.1265 (92), 463.0894 (46) | [126] |
| - | 12,944 | - | - | - | - | 13.87 | C15H10O6 | luteolin | L1 | 965 | 285.0405 (+0.1) | 285.0415 (100), 133.0301 (45), 151.0044 (6), 107.0144 (4) | [58] |
| 9640 | - | - | - | - | - | 14.18 | C16H12O8 | patuletin | L2a | 993 | 331.0457 (−0.7) | 316.0231 (100), 165.9919 (39), 110.0018 (34), 331.0477 (24), 139.0039 (22), 181.0157 (10), 121.0308 (9) | [127] |
| - | - | - | - | 2524 | 4667 | 14.21 | C27H22O12 | lithospermic acid derivative | L3 | 999 | 537.1047 (+1.6) | 339.0518 (100), 357.0623 (60), 519.0936 (52), 283.0619 (20), 197.0462 (17), 295.0617 (15) | [128] |
| - | 26204 | - | - | - | - | 14.39 | C16H12O7 | nepetin | L1 | 1016 | 315.0510 (−0.1) | 300.0283 (100), 315.0516 (14), 136.9886 (10), 201.0201 (6), 133.0299 (5), 65.0039 (4) | [129] |
| - | - | - | - | 1974 | 15905 | 14.62 | C26H22O10 | salvianolic acid A | L2a | 1051 | 493.1140 (+0) | 161.0246 (100), 135.0453 (75), 359.0777 (56), 197.0459 (29), 179.0352 (27), 295.0613 (13) | [130] |
| - | - | - | - | 3699 | 3566 | 15.14 | C15H12O5 | naringenin | L1 | 1082 | 271.0612 (+0) | 119.0505 (100), 151.0043 (68), 107.0143 (26), 271.0617 (25), 83.0143 (20), 65.0039 (19), 93.0351 (17) | [58] |
| - | 2702 | - | - | - | - | 15.41 | C37H32O18 | 1,4,5-tricaffeoyl-3-methoxy- oxaloylquinic acid | L2a | 1108 | 763.1509 (−0.9) | 395.0978 (100), 353.0874 (23), 233.0661 (18), 557.1305 (17), 515.1189 (16), 677.1512 (15), 763.1509 (12), 179.0346 (12), 601.1195 (11) | [59] |
| 16,527 | 5714 | - | - | - | - | 19.06 | C46H50N4O8 | N1,N5,N10,N14-tetra-trans-p- coumaroylspermine | L2a | 1303 | 785.3553 (−0.4) | 785.3547 (100), 545.2404 (81), 665.2977 (55), 145.0296 (9) | [131] |
| - | - | 9396 | 8821 | - | - | 23.54 | C18H16O7 | 3,5-dihydroxy-6,7,8-trimethoxy-flavone | L2a | 1562 | 343.0824 (+0.2) | 313.0352 (100), 270.0179 (63), 328.0588 (34), 186.0321 (31), 285.0409 (21), 242.0218 (20), 298.0126 (16) | [132] |
| - | - | 379 | 479 | - | - | 24.48 | C21H24O7 | arenol | L2a | 1638 | 387.1451 (+0.4) | 235.0982 (100), 247.0980 (88), 191.1080 (17), 139.0403 (15), 95.0504 (14) | [133] |
| - | - | 1295 | 1230 | - | - | 25.67 | C22H26O7 | arzanol | L2a | 1746 | 401.1606 (+0.1) | 235.0972 (100), 247.0973 (91), 191.1074 (23), 109.0655 (20), 153.0553 (18), 205.0866 (11) | [58] |
3. Materials and Methods
3.1. Plant Material
3.2. Chemical Supplies
3.3. Extraction and Sample Preparation
3.4. Chemical Composition Determination
3.4.1. UHPLC-MS/MS Analysis
3.4.2. Data Processing
3.4.3. Molecular Networking with GNPS
3.4.4. Features Annotation
3.5. Evaluation of Antioxidant Activity
3.5.1. DPPH/ABTS Well-Plate Assays
Sample Preparation and Plate Layout
DPPH Assay
ABTS Assay
EC50 Determination
3.5.2. On-Line RP-UHPLC-DPPH/ABTS-MS/MS Assay
3.6. Total Phenolic and Flavonoid Contents
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hepel, M.; Andreescu, S. Oxidative Stress and Human Health. In Oxidative Stress: Diagnostics, Prevention, and Therapy Volume 2; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2015; Volume 1200, pp. 1–33. ISBN 978-0-8412-3100-9. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, R. The Role of Antioxidants in Human Health. In Oxidative Stress: Diagnostics, Prevention, and Therapy; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Volume 1083, pp. 1–37. ISBN 978-0-8412-2683-8. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant Activity of Phenolic Compounds in 32 Selected Herbs. Food Chem. 2001, 105, 940–949. [Google Scholar] [CrossRef]
- Kusumawati, I.; Indrayanto, G. Chapter 15—Natural Antioxidants in Cosmetics. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 40, pp. 485–505. [Google Scholar]
- Sasounian, R.; Martinez, R.M.; Lopes, A.M.; Giarolla, J.; Rosado, C.; Magalhães, W.V.; Velasco, M.V.R.; Baby, A.R. Innovative Approaches to an Eco-Friendly Cosmetic Industry: A Review of Sustainable Ingredients. Clean Technol. 2024, 6, 176–198. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and Sustainable Use of Medicinal Plants: Problems, Progress, and Prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef]
- Bodeker, G.; Burford, G.; Kronenberg, F. Traditional, Complementary and Alternative Medicine: Policy and Public Health Perspectives; Imperial College Press: London, UK, 2006. [Google Scholar] [CrossRef]
- Heywood, V.H.; Synge, H.; Akerele, O. The Conservation of Medicinal Plants. In Proceedings of the International Consultation, Chiang Mai, Thailand, 21–27 March 1988; Cambridge University Press: Cambridge, UK, 1988. ISBN 0-521-39206-3. [Google Scholar]
- Lange, D. Europe’s Medicinal and Aromatic Plants: Their Use, Trade and Conservation; Trade Records and Analysis of Flora and Fauna in Commerce (TRAFFIC)-International: Cambridge, UK, 1998. [Google Scholar]
- Cui, N.; Chen, T.; Liao, B.; Xu, J.; Li, X. The Biology of Medicinal Resource Substitution in Salvia. Chin. Med. 2021, 16, 141. [Google Scholar] [CrossRef] [PubMed]
- Dal Cero, M.; Saller, R.; Weckerle, C.S. The Use of the Local Flora in Switzerland: A Comparison of Past and Recent Medicinal Plant Knowledge. J. Ethnopharmacol. 2014, 151, 253–264. [Google Scholar] [CrossRef]
- Jurkiewicz, A.; Ryszka, P.; Anielska, T.; Waligórski, P.; Białońska, D.; Góralska, K.; Tsimilli-Michael, M.; Turnau, K. Optimization of Culture Conditions of Arnica montana L.: Effects of Mycorrhizal Fungi and Competing Plants. Mycorrhiza 2010, 20, 293–306. [Google Scholar] [CrossRef]
- Stoll, U. Das Lorscher Arzneibuch. Ein Medizinisches Kompendium Des 8. Jahrhunderts—Codex Bambergensis Medicinalis 1. Franz Steiner Verlag: Stuttgart, Germany, 1992. [Google Scholar]
- Barbosa, W.L.R.; Nascimento, M.S.; Pinto, L.N.; Maia, F.L.C.; Sousa, A.A.; Silva, J.O.C.; Monteiro, M.M.; Oliveira, D.R. Selecting Medicinal Plants for Development of Phytomedicine and Use in Primary Health Care; InTech Open: Rijeka, Croatia, 2012; ISBN 978-953-307-805-2. [Google Scholar]
- Garnatje, T.; Peñuelas, J.; Vallès, J. Ethnobotany, Phylogeny, and ‘Omics’ for Human Health and Food Security. Trends Plant Sci. 2017, 22, 187–191. [Google Scholar] [CrossRef]
- Gras, A.; Hidalgo, O.; D’ambrosio, U.; Parada, M.; Garnatje, T.; Valles, J. The Role of Botanical Families in Medicinal Ethnobotany: A Phylogenetic Perspective. Plants 2021, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Saslis-Lagoudakis, C.H.; Klitgaard, B.B.; Forest, F.; Francis, L.; Savolainen, V.; Williamson, E.M.; Hawkins, J.A. The Use of Phylogeny to Interpret Cross-Cultural Patterns in Plant Use and Guide Medicinal Plant Discovery: An Example from Pterocarpus (Leguminosae). PLoS ONE 2011, 6, e22275. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Saslis-Lagoudakis, C.H.; Grace, O.M.; Nilsson, N.; Simonsen, H.T.; Horn, J.W.; Rønsted, N. Evolutionary Prediction of Medicinal Properties in the Genus Euphorbia L. Sci. Rep. 2016, 6, 30531. [Google Scholar] [CrossRef]
- Saslis-Lagoudakis, C.H.; Savolainen, V.; Williamson, E.M.; Forest, F.; Wagstaff, S.J.; Baral, S.R.; Watson, M.F.; Pendry, C.A.; Hawkins, J.A. Phylogenies Reveal Predictive Power of Traditional Medicine in Bioprospecting. Proc. Natl. Acad. Sci. USA 2012, 109, 15835–15840. [Google Scholar] [CrossRef]
- Yessoufou, K.; Daru, B.; Muasya, A. Phylogenetic Exploration of Commonly Used Medicinal Plants in South Africa. Mol. Ecol. Resour. 2014, 15, 405–413. [Google Scholar] [CrossRef]
- Cuttelod, A.; García, N.; Malak, D.A.; Temple, H.J.; Katariya, V. The Mediterranean: A Biodiversity Hotspot under Threat; IUCN: Gland, Switzerland, 2009; pp. 1–4. [Google Scholar]
- GIZ Practical Market Insights into the Product Group of Medicinal and Aromatic Plants Medicinal and Aromatic Plants, Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ). 2021. Available online: https://www.giz.de/en/downloads/giz-2021-en-practical-market-insights-into-the-product-group-of-medicinal-and-aromatic-plants.pdf (accessed on 6 April 2025).
- Grigoriadou, K.; Krigas, N.; Lazari, D.; Maloupa, E. Chapter 4—Sustainable Use of Mediterranean Medicinal-Aromatic Plants. In Feed Additives; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 57–74. ISBN 978-0-12-814700-9. [Google Scholar]
- Garayev, E.; Herbette, G.; Mabrouki, F.; Chiffolleau, P.; Roux, D.; Ollivier, E.; Elias, R.; Baghdikian, B. Chemical Constituents of Inula montana Flowers and Leaves. Chem. Nat. Compd. 2018, 54, 755–756. [Google Scholar] [CrossRef]
- Obon, C.; Rivera, D.; Verde, A.; Fajardo, J.; Valdes, A.; Alcaraz, F.; Carvalho, A.M. Arnica: A Multivariate Analysis of the Botany and Ethnopharmacology of a Medicinal Plant Complex in the Iberian Peninsula and the Balearic Islands. J. Ethnopharmacol. 2012, 144, 44–56. [Google Scholar] [CrossRef]
- Teixidor-Toneu, I.; Martin, G.J.; Ouhammou, A.; Puri, R.K.; Hawkins, J.A. An Ethnomedicinal Survey of a Tashelhit-Speaking Community in the High Atlas, Morocco. J. Ethnopharmacol. 2016, 188, 96–110. [Google Scholar] [CrossRef]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A Plant of Healing. J. Pharm. Pharmacol. 2017, 69, 925–945. [Google Scholar] [CrossRef]
- Antunes Viegas, D.; Palmeira-de-Oliveira, A.; Salgueiro, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Helichrysum italicum: From Traditional Use to Scientific Data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef]
- Boubeker, H.; Rebbas, K.; Belhattab, R. Activités Antioxydante et Antibactérienne Des Extraits d’Helichrysum stoechas (L.) Moench. Phytothérapie 2018, 16, 122. [Google Scholar] [CrossRef]
- Kramberger, K.; Kenig, S.; Jenko Pražnikar, Z.; Kočevar Glavač, N.; Barlič-Maganja, D. A Review and Evaluation of the Data Supporting Internal Use of Helichrysum italicum. Plants 2021, 10, 1738. [Google Scholar] [CrossRef]
- Les, F.; Venditti, A.; Cásedas, G.; Frezza, C.; Guiso, M.; Sciubba, F.; Serafini, M.; Bianco, A.; Valero, M.S.; López, V. Everlasting Flower (Helichrysum stoechas Moench) as a Potential Source of Bioactive Molecules with Antiproliferative, Antioxidant, Antidiabetic and Neuroprotective Properties. Ind. Crops Prod. 2017, 108, 295–302. [Google Scholar] [CrossRef]
- Pereira, C.G.; Barreira, L.; Bijttebier, S.; Pieters, L.; Neves, V.; Rodrigues, M.J.; Rivas, R.; Varela, J.; Custódio, L. Chemical Profiling of Infusions and Decoctions of Helichrysum italicum subsp. picardii by UHPLC-PDA-MS and in Vitro Biological Activities Comparatively with Green Tea (Camellia sinensis) and Rooibos Tisane (Aspalathus linearis). J. Pharm. Biomed. Anal. 2017, 145, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Węglarz, Z.; Kosakowska, O.; Pióro-Jabrucka, E.; Przybył, J.L.; Gniewosz, M.; Kraśniewska, K.; Szyndel, M.S.; Costa, R.; Bączek, K.B. Antioxidant and Antibacterial Activity of Helichrysum italicum (Roth) G. Don. from Central Europe. Pharmaceuticals 2022, 15, 735. [Google Scholar] [CrossRef]
- Ejaz, A.; Waliat, S.; Arshad, M.S.; Khalid, W.; Khalid, M.Z.; Rasul Suleria, H.A.; Luca, M.-I.; Mironeasa, C.; Batariuc, A.; Ungureanu-Iuga, M.; et al. A Comprehensive Review of Summer Savory (Satureja hortensis L.): Promising Ingredient for Production of Functional Foods. Front. Pharmacol. 2023, 14, 1198970. [Google Scholar] [CrossRef]
- Gomes, F.; Dias, M.I.; Lima, Â.; Barros, L.; Rodrigues, M.E.; Ferreira, I.C.F.R.; Henriques, M. Satureja montana L. and Origanum majorana L. Decoctions: Antimicrobial Activity, Mode of Action and Phenolic Characterization. Antibiotics 2020, 9, 294. [Google Scholar] [CrossRef]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M.; Sohraby, M. Summer Savory: From the Selection of Traditional Applications to the Novel Effect in Relief, Prevention, and Treatment of a Number of Serious Illnesses Such as Diabetes, Cardiovascular Disease, Alzheimer’s Disease, and Cancer. J. Tradit. Complement. Med. 2014, 4, 140–144. [Google Scholar] [CrossRef]
- Appendino, G.; Fontana, G.; Pollastro, F. Natural Products Drug Discovery. In Comprehensive Natural Products II—Chemistry and Biology; Elsevier: Asterdam, The Netherlands, 2010; Volume 3, pp. 205–236. [Google Scholar]
- Allard, P.-M.; Péresse, T.; Bisson, J.; Gindro, K.; Marcourt, L.; Pham, V.C.; Roussi, F.; Litaudon, M.; Wolfender, J.-L. Integration of Molecular Networking and In-Silico MS/MS Fragmentation for Natural Products Dereplication. Anal. Chem. 2016, 88, 3317–3323. [Google Scholar] [CrossRef]
- Ebbels, T.M.D.; van der Hooft, J.J.J.; Chatelaine, H.; Broeckling, C.; Zamboni, N.; Hassoun, S.; Mathé, E.A. Recent Advances in Mass Spectrometry-Based Computational Metabolomics. Curr. Opin. Chem. Biol. 2023, 74, 102288. [Google Scholar] [CrossRef]
- Kim, H.W.; Wang, M.; Leber, C.A.; Nothias, L.-F.; Reher, R.; Kang, K.B.; van der Hooft, J.J.J.; Dorrestein, P.C.; Gerwick, W.H.; Cottrell, G.W. NPClassifier: A Deep Neural Network-Based Structural Classification Tool for Natural Products. J. Nat. Prod. 2021, 84, 2795–2807. [Google Scholar] [CrossRef] [PubMed]
- Garayev, E. Heatmaps Generator Script. 2025. Available online: https://github.com/elnurgar/heatmaps_gen (accessed on 6 March 2025).
- Akaberi, M.; Sahebkar, A.; Azizi, N.; Emami, S.A. Everlasting Flowers: Phytochemistry and Pharmacology of the Genus Helichrysum. Ind. Crops Prod. 2019, 138, 111471. [Google Scholar] [CrossRef]
- Lephatsi, M.M.; Choene, M.S.; Kappo, A.P.; Madala, N.E.; Tugizimana, F. An Integrated Molecular Networking and Docking Approach to Characterize the Metabolome of Helichrysum splendidum and Its Pharmaceutical Potentials. Metabolites 2023, 13, 1104. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Buommino, E.; D’Angelo, G.; Coretti, L.; Scognamiglio, M.; Severino, V.; Pacifico, S.; Donnarumma, G.; Fiorentino, A. Spectroscopic Identification and Anti-Biofilm Properties of Polar Metabolites from the Medicinal Plant Helichrysum italicum against Pseudomonas aeruginosa. Bioorg. Med. Chem. 2013, 21, 7038–7046. [Google Scholar] [CrossRef] [PubMed]
- Timilsina, A.P.; Raut, B.K.; Huo, C.; Khadayat, K.; Budhathoki, P.; Ghimire, M.; Budhathoki, R.; Aryal, N.; Kim, K.H.; Parajuli, N. Metabolomics and Molecular Networking Approach for Exploring the Anti-Diabetic Activity of Medicinal Plants. RSC Adv. 2023, 13, 30665–30679. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Wang; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J.-L. Caffeoyl Derivatives: Major Antioxidant Compounds of Some Wild Herbs of the Asteraceae Family. Food Nutr. Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Innocenti, G.; Ferretti, V.; Aiello, N.; Scartezzini, F.; Vender, C. Quali-Quantitative Analysis of Arnica montana Wild Accessions Compared in Field-Results of the Second Year. In Proceedings of the I International Symposium on Medicinal Aromatic and Nutraceutical Plants from Mountainous Areas (MAP-Mountain 2011) 955, Saas-Fee, Switzerland, 6 July 2011; pp. 325–327. [Google Scholar]
- Ganzera, M.; Egger, C.; Zidorn, C.; Stuppner, H. Quantitative Analysis of Flavonoids and Phenolic Acids in Arnica montana L. by Micellar Electrokinetic Capillary Chromatography. Anal. Chim. Acta 2008, 614, 196–200. [Google Scholar] [CrossRef]
- Spitaler, R.; Schlorhaufer, P.D.; Ellmerer, E.P.; Merfort, I.; Bortenschlager, S.; Stuppner, H.; Zidorn, C. Altitudinal Variation of Secondary Metabolite Profiles in Flowering Heads of Arnica montana Cv. ARBO. Phytochemistry 2006, 67, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Duthen, S.; Gadéa, A.; Trempat, P.; Boujedaini, N.; Fabre, N. Comparison of the Phytochemical Variation of Non-Volatile Metabolites within Mother Tinctures of Arnica montana Prepared from Fresh and Dried Whole Plant Using UHPLC-HRMS Fingerprinting and Chemometric Analysis. Molecules 2022, 27, 2737. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Kuhnert, N. Identification and Characterization of Two New Derivatives of Chlorogenic Acids in Arnica (Arnica montana L.) Flowers by High-Performance Liquid Chromatography/Tandem Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 4033–4039. [Google Scholar] [CrossRef]
- Kramberger, K.; Barlič-Maganja, D.; Bandelj, D.; Baruca Arbeiter, A.; Peeters, K.; Miklavčič Višnjevec, A.; Jenko Pražnikar, Z. HPLC-DAD-ESI-QTOF-MS Determination of Bioactive Compounds and Antioxidant Activity Comparison of the Hydroalcoholic and Water Extracts from Two Helichrysum italicum Species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. Identification of Hydroxycinnamoylquinic Acids of Arnica Flowers and Burdock Roots Using a Standardized LC-DAD-ESI/MS Profiling Method. J. Agric. Food Chem. 2008, 56, 10105–10114. [Google Scholar] [CrossRef]
- Kłeczek, N.; Malarz, J.; Gierlikowska, B.; Kiss, A.K.; Stojakowska, A. Constituents of Xerolekia speciosissima (L.) Anderb. (Inuleae), and Anti-Inflammatory Activity of 7,10-Diisobutyryloxy-8, 9-Epoxythymyl Isobutyrate. Molecules 2020, 25, 4913. [Google Scholar] [CrossRef] [PubMed]
- Rechek, H.; Haouat, A.; Hamaidia, K.; Pinto, D.C.G.A.; Boudiar, T.; Válega, M.S.G.A.; Pereira, D.M.; Pereira, R.B.; Silva, A.M.S. Inula viscosa (L.) Aiton Ethanolic Extract Inhibits the Growth of Human AGS and A549 Cancer Cell Lines. Chem. Biodivers. 2023, 20, e202200890. [Google Scholar] [CrossRef]
- Stefanova, A.; Gevrenova, R.; Balabanova, V.; Lozanova, V.; Alexova, R.; Zheleva-Dimitrova, D. Caffeoylhexaric Acids in Inuleae: A Case Study of Geigeria alata, Inula helenium, and Telekia speciosa. Biochem. Syst. Ecol. 2024, 116, 104873. [Google Scholar] [CrossRef]
- Da Costa, F.B.; Terfloth, L.; Gasteiger, J. Sesquiterpene Lactone-Based Classification of Three Asteraceae Tribes: A Study Based on Self-Organizing Neural Networks Applied to Chemosystematics. Phytochemistry 2005, 66, 345–353. [Google Scholar] [CrossRef]
- Seaman, F.C. Sesquiterpene Lactones as Taxonomic Characters in the Asteraceae. Bot. Rev. 1982, 48, 121–594. [Google Scholar] [CrossRef]
- Moujir, L.; Callies, O.; Sousa, P.M.; Sharopov, F.; Seca, A.M. Applications of Sesquiterpene Lactones: A Review of Some Potential Success Cases. Appl. Sci. 2020, 10, 3001. [Google Scholar] [CrossRef]
- Gyawali, N.; Rayamajhi, A.; Karki, D.; Pokhrel, T.; Adhikari, A. Arnica montana L.: Traditional Uses, Bioactive Chemical Constituents, and Pharmacological Activities. In Medicinal Plants of the Asteraceae Family: Traditional Uses, Phytochemistry and Pharmacological Activities; Devkota, H.P., Aftab, T., Eds.; Springer Nature: Singapore, 2022; pp. 61–75. ISBN 978-981-19608-0-2. [Google Scholar]
- Perry, N.; Burgess, E.; Rodríguez Guitián, M.; Romero Franco, R.; López Mosquera, E.; Smallfield, B.; Joyce, N.; Littlejohn, R. Sesquiterpene Lactones in Arnica montana: Helenalin and Dihydrohelenalin Chemotypes in Spain. Planta Med. 2009, 75, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Seemann, A.; Wallner, T.; Poschlod, P.; Heilmann, J. Variation of Sesquiterpene Lactone Contents in Different Arnica montana Populations: Influence of Ecological Parameters. Planta Med. 2010, 76, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Staneva, J.; Denkova, P.; Todorova, M.; Evstatieva, L. Quantitative Analysis of Sesquiterpene Lactones in Extract of Arnica montana L. by 1H NMR Spectroscopy. J. Pharm. Biomed. Anal. 2011, 54, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Stefanache, C.P.; Silion, M.; Bujor, O.C.; Nicolescu, A.F.; Schiopu, R.A.; Deleanu, C.; Mardari, C.; Tănase, C.; Danila, D. Sesquiterpen-Lactone Profile for Arnica montana L. Species in Natural Growing Sites from the Romanian Eastern Carpathians. In Proceedings of the Conservation of plant diversity; 2017. [Google Scholar]
- Barbero, M.; Prandi, C. Pseudoguaianolides: Recent Advances in Synthesis and Applications. Nat. Prod. Commun. 2018, 13, 1934578X1801300303. [Google Scholar] [CrossRef]
- Paço, A.; Brás, T.; Santos, J.O.; Sampaio, P.; Gomes, A.C.; Duarte, M.F. Anti-Inflammatory and Immunoregulatory Action of Sesquiterpene Lactones. Molecules 2022, 27, 1142. [Google Scholar] [CrossRef]
- Garayev, E.; Herbette, G.; Di Giorgio, C.; Chiffolleau, P.; Roux, D.; Sallanon, H.; Ollivier, E.; Elias, R.; Baghdikian, B. New Sesquiterpene Acid and Inositol Derivatives from Inula montana L. Fitoterapia 2017, 120, 79–84. [Google Scholar] [CrossRef]
- González-Romero, M.A.; Villaescusa-Castillo, L.; Díaz-Lanza, A.M. Sesquiterpene Lactones from Inula montana L. Z. für Naturforschung C 2000, 55, 697–700. [Google Scholar] [CrossRef]
- Gevrenova, R.; Kostadinova, I.; Stefanova, A.; Balabanova, V.; Zengin, G.; Zheleva-Dimitrova, D.; Momekov, G. Phytochemical Profiling, Antioxidant and Cognitive-Enhancing Effect of Helichrysum italicum ssp. italicum (Roth) G. Don (Asteraceae). Plants 2023, 12, 2755. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Tešić, Ž.; Stupar, A.; Bulut, G.; Sinan, K.I.; Uysal, S.; Picot-Allain, M.C.N.; Mahomoodally, M.F. A Comparative Exploration of the Phytochemical Profiles and Bio-Pharmaceutical Potential of Helichrysum stoechas subsp. barrelieri Extracts Obtained via Five Extraction Techniques. Process Biochem. 2020, 91, 113–125. [Google Scholar] [CrossRef]
- Appendino, G.; Ottino, M.; Marquez, N.; Bianchi, F.; Giana, A.; Ballero, M.; Sterner, O.; Fiebich, B.L.; Munoz, E. Arzanol, an Anti-Inflammatory and Anti-HIV-1 Phloroglucinol α-Pyrone from Helichrysum italicum ssp. microphyllum. J. Nat. Prod. 2007, 70, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Deiana, M.; Atzeri, A.; Corona, G.; Incani, A.; Melis, M.P.; Appendino, G.; Dessì, M.A. Evaluation of the Antioxidant and Cytotoxic Activity of Arzanol, a Prenylated α-Pyrone–Phloroglucinol Etherodimer from Helichrysum italicum subsp. microphyllum. Chem. Biol. Interact. 2007, 165, 117–126. [Google Scholar] [CrossRef]
- Silva, L.; Rodrigues, A.M.; Ciriani, M.; Falé, P.L.V.; Teixeira, V.; Madeira, P.; Machuqueiro, M.; Pacheco, R.; Florêncio, M.H.; Ascensão, L.; et al. Antiacetylcholinesterase Activity and Docking Studies with Chlorogenic Acid, Cynarin and Arzanol from Helichrysum stoechas (Lamiaceae). Med. Chem. Res. 2017, 26, 2942–2950. [Google Scholar] [CrossRef]
- Taglialatela-Scafati, O.; Pollastro, F.; Chianese, G.; Minassi, A.; Gibbons, S.; Arunotayanun, W.; Mabebie, B.; Ballero, M.; Appendino, G. Antimicrobial Phenolics and Unusual Glycerides from Helichrysum italicum subsp. Microphyllum. J. Nat. Prod. 2013, 76, 346–353. [Google Scholar] [CrossRef]
- Boroja, T.; Katanić, J.; Rosić, G.; Selaković, D.; Joksimović, J.; Mišić, D.; Stanković, V.; Jovičić, N.; Mihailović, V. Summer Savory (Satureja hortensis L.) Extract: Phytochemical Profile and Modulation of Cisplatin-Induced Liver, Renal and Testicular Toxicity. Food Chem. Toxicol. 2018, 118, 252–263. [Google Scholar] [CrossRef]
- Chkhikvishvili, I.; Sanikidze, T.; Gogia, N.; Mchedlishvili, T.; Enukidze, M.; Machavariani, M.; Vinokur, Y.; Rodov, V. Rosmarinic Acid-Rich Extracts of Summer Savory (Satureja hortensis L.) Protect Jurkat T Cells against Oxidative Stress. Oxidative Med. Cell. Longev. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gião, M.S.; Pereira, C.I.; Fonseca, S.C.; Pintado, M.E.; Malcata, F.X. Effect of Particle Size upon the Extent of Extraction of Antioxidant Power from the Plants Agrimonia eupatoria, Salvia sp. and Satureja montana. Food Chem. 2009, 117, 412–416. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Švarc-Gajić, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of Phenolic Compounds and Antioxidant Activity of a Mediterranean Plant: The Case of Satureja montana subsp. kitaibelii. J. Funct. Foods 2015, 18, 1167–1178. [Google Scholar] [CrossRef]
- Lung, I.; Soran, M.; Tudoran, C.; Măruţoiu, C. Effect of Microwave Irradiation on Polyphenolic Compounds from Satureja hortensis L. Open Chem. 2013, 11, 535–541. [Google Scholar] [CrossRef]
- Habtemariam, S. Molecular Pharmacology of Rosmarinic and Salvianolic Acids: Potential Seeds for Alzheimer’s and Vascular Dementia Drugs. Int. J. Mol. Sci. 2018, 19, 458. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic Profiles of Cultivated, in Vitro Cultured and Commercial Samples of Melissa officinalis L. Infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.G. LC-MS Identification and Quantification of Phenolic Compounds in Solid Residues from the Essential Oil Industry. Antioxidants 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and Antimicrobial Activities of Cinnamic Acid Derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. J. Agric. food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Altintas, A.; Goger, F.; Duymus Agalar, H.; Kırımer, N.; Baser, K.H.C. Quantitative Analysis of Rosmarinic Acid in Rosmarinus officinalis Growing in Turkey by LC-MS/MS. Planta Medica 2011, 77, 1394. [Google Scholar] [CrossRef]
- Suwanchaikasem, P.; Chaichantipayuth, C.; Sukrong, S. Antioxidant-Guided Isolation of Rosmarinic Acid, a Major Constituent from Thunbergia Laurifolia, and Its Use as a Bioactive Marker for Standardization. Chiang Mai J. Sci. 2014, 41, 117–127. [Google Scholar]
- Biyik, B.; Yilmaz Sarialtin, S.; Gökbulut, A.; Çoban, T.; Coşkun, M. Trachystemon orientalis (L.) G. Don as a Valuable Source of Rosmarinic Acid: Biological Activities and HPLC Profiles. Turk. J. Pharm. Sci. 2023, 20, 141–148. [Google Scholar] [CrossRef]
- Hosseini, A.; Moein, M.; Sabahi, Z.; Moein, S.; Hafez Ghoran, S.; Naderian, M.; Zebarjad, Z. Antioxidant Potentials, Protease Inhibitory, and Cytotoxic Activities of Various Isolated Extracts from Salvia Aegyptiaca. Iran. Biomed. J. 2025, 29, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.C.J.M.D.S.; Cavaleiro, J.A.S.; Kamat, S.P.; Barros, C.M.R.F.; Domingues, M.R.M. Electrospray Tandem Mass Spectrometry Analysis of Methylenedioxy Chalcones, Flavanones and Flavones. Rapid Commun. Mass. Spectrom. 2013, 27, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Zhou, L.; Li, L.; Wang, L.; Gao, S.; Hu, M. Development and Validation of an LC-MS/MS Method for the Quantification of Flavonoid Glucuronides (Wogonoside, Baicalin, and Apigenin-Glucuronide) in the Bile and Blood Samples: Application to a Portal Vein Infusion Study. Anal. Biochem. 2020, 601, 113723. [Google Scholar] [CrossRef]
- Beszterda, M.; Frański, R. Comment on Tremmel et al. In Vitro Metabolism of Six C-Glycosidic Flavonoids from Passiflora Incarnata L. Int. J. Mol. Sci. 2021, 22, 6566. Int. J. Mol. Sci. 2022, 23, 4445. [Google Scholar] [CrossRef]
- Pandurangan, N.; Bose, C.; Banerji, A. Synthesis and Antioxygenic Activities of Seabuckthorn Flavone-3-Ols and Analogs. Bioorg. Med. Chem. Lett. 2011, 21, 5328–5330. [Google Scholar] [CrossRef]
- Yang, X.; Kang, S.-M.; Jeon, B.-T.; Kim, Y.-D.; Ha, J.-H.; Kim, Y.-T.; Jeon, Y.-J. Isolation and Identification of an Antioxidant Flavonoid Compound from Citrus-Processing by-Product. J. Sci. Food Agric. 2011, 91, 1925–1927. [Google Scholar] [CrossRef]
- Mavundza, E.J.; Tshikalange, T.E.; Lall, N.; Hussein, A.A.; Mudau, F.N.; Meyer, J.J.M. Antioxidant Activity and Cytotoxicity Effect of Flavonoids Isolated from Athrixia Phylicoides. J. Med. Plants Res. 2010, 2583–2586. [Google Scholar] [CrossRef]
- Qu, G.-Z.; Si, C.-L.; Wang, M.-H. Antioxidant Constituents from Leonurus japonicus. Nat. Prod. Sci. 2006, 12, 197–200. [Google Scholar]
- Sim, G.-S.; Lee, B.-C.; Cho, H.S.; Lee, J.W.; Kim, J.-H.; Lee, D.-H.; Kim, J.-H.; Pyo, H.-B.; Moon, D.C.; Oh, K.-W.; et al. Structure Activity Relationship of Antioxidative Property of Flavonoids and Inhibitory Effect on Matrix Metalloproteinase Activity in UVA-Irradiated Human Dermal Fibroblast. Arch. Pharmacal. Res. 2007, 30, 290–298. [Google Scholar] [CrossRef]
- Ohta, S.; Fujimaki, T.; Uy, M.M.; Yanai, M.; Yukiyoshi, A.; Hirata, T. Antioxidant Hydroxycinnamic Acid Derivatives Isolated from Brazilian Bee Pollen. Nat. Prod. Res. 2007, 21, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Litewski, S.; Mróz, M.; Bartoszek, A.; Kusznierewicz, B. Post-Chromatographic Derivatization Coupled with Mass Spectrometry as a Method of Profiling and Identification of Antioxidants; Ligustrum vulgare Phytocomplex as an Example. Molecules 2023, 28, 8000. [Google Scholar] [CrossRef] [PubMed]
- Burnaz, N.A.; Küçük, M.; Akar, Z. An On-Line HPLC System for Detection of Antioxidant Compounds in Some Plant Extracts by Comparing Three Different Methods. J. Chromatogr. B 2017, 1052, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, L.-D.; Li, B.-C.; Yang, J.; Yu, H.; Wan, J.-B.; Wang, Y.-T.; Li, P. Screening of Free Radical Scavengers from Erigeron breviscapus Using on-Line HPLC-ABTS/DPPH Based Assay and Mass Spectrometer Detection. Free Radic. Res. 2012, 46, 286–294. [Google Scholar] [CrossRef]
- Marksa, M.; Radušienė, J.; Jakštas, V.; Ivanauskas, L.; Marksienė, R. Development of an HPLC Post-Column Antioxidant Assay for Solidago canadensis Radical Scavengers. Nat. Prod. Res. 2016, 30, 536–543. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Ma, X.; Chu, Y.; Li, S.; Guo, J.; Jia, Y.; Zhou, S.; Zhu, Y.; Liu, C. Simultaneous Determination of Caffeic Acid and Its Major Pharmacologically Active Metabolites in Rat Plasma by LC-MS/MS and Its Application in Pharmacokinetic Study. Biomed. Chromatogr. 2015, 29, 552–559. [Google Scholar] [CrossRef]
- Petrakis, E.A.; Mikropoulou, E.V.; Mitakou, S.; Halabalaki, M.; Kalpoutzakis, E. A GC–MS and LC–HRMS Perspective on the Chemotaxonomic Investigation of the Natural Hybrid origanum × lirium and Its Parents, subsp. Hirtum and O. Scabrum. Phytochem. Anal. 2023, 34, 289–300. [Google Scholar] [CrossRef]
- Lavault, M.; Richomme, P. Constituents of Helichrysum stoechas Variety olonnense. Chem. Nat. Compd. 2004, 40, 118–121. [Google Scholar] [CrossRef]
- Li, L.; Feng, R.; Feng, X.; Chen, Y.; Liu, X.; Sun, W.; Zhang, L. The Development and Validation of an HPLC-MS/MS Method for the Determination of Eriocitrin in Rat Plasma and Its Application to a Pharmacokinetic Study. RSC Adv. 2020, 10, 10552–10558. [Google Scholar] [CrossRef]
- Ringl, A.; Prinz, S.; Huefner, A.; Kurzmann, M.; Kopp, B. Chemosystematic Value of Flavonoids from Crataegus × Macrocarpa (Rosaceae) with Special Emphasis on (R)- and (S)-Eriodictyol-7-O-glucuronide and Luteolin-7-O-glucuronide. Chem. Biodivers. 2007, 4, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ancillotti, C.; Ciofi, L.; Rossini, D.; Chiuminatto, U.; Stahl-Zeng, J.; Orlandini, S.; Furlanetto, S.; Del Bubba, M. Liquid Chromatographic/Electrospray Ionization Quadrupole/Time of Flight Tandem Mass Spectrometric Study of Polyphenolic Composition of Different Vaccinium Berry Species and Their Comparative Evaluation. Anal. Bioanal. Chem. 2017, 409, 1347–1368. [Google Scholar] [CrossRef] [PubMed]
- Velamuri, R.; Sharma, Y.; Fagan, J.; Schaefer, J. Application of UHPLC-ESI-QTOF-MS in Phytochemical Profiling of Sage (Salvia officinalis) and Rosemary (Rosmarinus officinalis). Planta Med. Int. Open 2020, 7, e133–e144. [Google Scholar] [CrossRef]
- Крoль, Т.А.; Зиннатшина, Л.В.; Гатиатулина, Е.Р.; Радимич, А.И.; Сайбель, О.Л.; Балеев, Д.Н.; Осипoв, В.И. СОСТАВ И СОДЕРЖАНИЕ ФЕНОЛЬНЫХ СОЕДИНЕНИЙ В РАЗЛИЧНЫХ ФРАКЦИЯХ ЭКСТРАКТА НАДЗЕМНОЙ ЧАСТИ ARNICA FOLIOSA NUTT. Химия растительнoгo сырья 2020, 4, 139–147. [Google Scholar] [CrossRef]
- CCMSLIB00004678828. Available online: https://mona.fiehnlab.ucdavis.edu/spectra/display/CCMSLIB00004678828 (accessed on 27 November 2024).
- Tsugawa, H.; Nakabayashi, R.; Mori, T.; Yamada, Y.; Takahashi, M.; Rai, A.; Sugiyama, R.; Yamamoto, H.; Nakaya, T.; Yamazaki, M. A Cheminformatics Approach to Characterize Metabolomes in Stable-Isotope-Labeled Organisms. Nat. Methods 2019, 16, 295–298. [Google Scholar] [CrossRef]
- MSBNK-RIKEN-PR040095. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-RIKEN-PR040095 (accessed on 27 November 2024).
- CCMSLIB00004717548 Spectrum: Hispidulin-4’-Glucoside, Splash10-01pk-0090700000-5f67cb4c81b0f3b44224. Available online: https://gnps.ucsd.edu/ProteoSAFe/gnpslibraryspectrum.jsp?SpectrumID=CCMSLIB00004717548 (accessed on 27 November 2024).
- Martins, N.; Barros, L.; Santos-Buelga, C.; Silva, S.; Henriques, M.; Ferreira, I.C. Decoction, Infusion and Hydroalcoholic Extract of Cultivated Thyme: Antioxidant and Antibacterial Activities, and Phenolic Characterisation. Food Chem. 2015, 167, 131–137. [Google Scholar] [CrossRef]
- Jang, D.; Jung, Y.S.; Kim, M.-S.; Oh, S.E.; Nam, T.G.; Kim, D.-O. Developing and Validating a Method for Separating Flavonoid Isomers in Common Buckwheat Sprouts Using HPLC-PDA. Foods 2019, 8, 549. [Google Scholar] [CrossRef]
- McNab, H.; Ferreira, E.; Hulme, A.; Quye, A. Negative Ion ESI-MS Analysis of Natural Yellow Dye Flavonoids-An Isotopic Labelling Study. Int. J. Mass. Spectrom. 2009, 284, 57–65. [Google Scholar] [CrossRef]
- Zengin, G.; Nilofar; Yildiztugay, E.; Bouyahya, A.; Cavusoglu, H.; Gevrenova, R.; Zheleva-Dimitrova, D.A. Comparative Study on UHPLC-HRMS Profiles and Biological Activities of Inula Sarana Different Extracts and Its Beta-Cyclodextrin Complex: Effective Insights for Novel Applications. Antioxidants 2023, 12, 1842. [Google Scholar] [CrossRef]
- MSBNK-BS-BS003294. Available online: https://massbank.eu/MassBank/RecordDisplay?id=MSBNK-BS-BS003294 (accessed on 27 November 2024).
- Yıldız, G.; İlgün, S.; Şeker Karatoprak, G.; Köse, Y.B.; Göger, F.; Temel, H.E.; Demirci, B. Chemical Profile, in Vitro Pharmacological Activity and Satureja cuneifolia Ten. Evaluation of Essential Oil Based on Distillation Time. Int. J. Environ. Health Res. 2024, 34, 1944–1960. [Google Scholar] [CrossRef]
- CCMSLIB00000846994. Available online: https://mona.fiehnlab.ucdavis.edu/spectra/display/CCMSLIB00000846994 (accessed on 27 November 2024).
- CCMSLIB00004718251 Spectrum: 5,7-Dihydroxy-3,6,4’-Trimethoxyflavone, Splash10-0209-0069000000-D5c358229c1d2b7831d2. Available online: https://gnps.ucsd.edu/ProteoSAFe/gnpslibraryspectrum.jsp?SpectrumID=CCMSLIB00004718251 (accessed on 27 November 2024).
- Zanganeh, F.; Tayarani-Najaran, Z.; Nesměrák, K.; Štícha, M.; Emami, S.A.; Akaberi, M. Dereplication of Natural Cytotoxic Products from Helichrysum oligocephalum Using Ultra-Performance Liquid Chromatography–Quadrupole Time of Flight-Mass Spectrometry. Sep. Sci. PLUS 2024, 7, 2300150. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Breaud, C.; Lallemand, L.; Mares, G.; Mabrouki, F.; Bertolotti, M.; Simmler, C.; Greff, S.; Mauduit, M.; Herbette, G.; Garayev, E.; et al. LC-MS Based Phytochemical Profiling towards the Identification of Antioxidant Markers in Some Endemic Aloe Species from Mascarene Islands. Antioxidants 2022, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Garayev, E. Precursor Value Corrector Script. 2024. Available online: https://github.com/elnurgar/dataanalysis (accessed on 7 September 2024).
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative Analysis of Multimodal Mass Spectrometry Data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef]
- Du, X.; Smirnov, A.; Pluskal, T.; Jia, W.; Sumner, S. Metabolomics Data Preprocessing Using ADAP and MZmine 2. In Computational Methods and Data Analysis for Metabolomics; Li, S., Ed.; Methods in Molecular Biology; Springer US: New York, NY, USA, 2020; Volume 2104, pp. 25–48. ISBN 978-1-07-160238-6. [Google Scholar]
- Karaman, I.; Pinto, R.C.; Graça, G. Metabolomics Data Preprocessing: From Raw Data to Features for Statistical Analysis. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 82, pp. 197–225. [Google Scholar]
- Pluskal, T.; Korf, A.; Smirnov, A.; Schmid, R.; Fallon, T.R.; Du, X.; Weng, J.-K. Metabolomics Data Analysis Using MZmine. In Processing Metabolomics and Proteomics Data with Open Software; Royal Society of Chemistry: London, UK, 2020; pp. 232–254. [Google Scholar]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A Public Repository for Sharing Mass Spectral Data for Life Sciences. J. Mass. Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Dührkop, K.; Nothias, L.-F.; Fleischauer, M.; Reher, R.; Ludwig, M.; Hoffmann, M.A.; Petras, D.; Gerwick, W.H.; Rousu, J.; Dorrestein, P.C.; et al. Systematic Classification of Unknown Metabolites Using High-Resolution Fragmentation Mass Spectra. Nat. Biotechnol. 2021, 39, 462–471. [Google Scholar] [CrossRef]
- Rutz, A.; Dounoue-Kubo, M.; Ollivier, S.; Bisson, J.; Bagheri, M.; Saesong, T.; Ebrahimi, S.N.; Ingkaninan, K.; Wolfender, J.-L.; Allard, P.-M. Taxonomically Informed Scoring Enhances Confidence in Natural Products Annotation. Front. Plant Sci. 2019, 10, 1329. [Google Scholar] [CrossRef]
- European Pharmacopoeia Monographs: Violae Herba Cum Flore Monograph (01/2008:1855). 2023. Available online: https://www.escop.com/downloads/wild-pansy-2/ (accessed on 28 April 2025).

| Species | DPPH EC50 (µg/mL) | ABTS EC50 (µg/mL) | TPC (%) | TFC (%) | TFC/TPC (%) |
|---|---|---|---|---|---|
| A. montana | 45.71 ± 3.05 **** | 15.59± 2.51 *** | 12.64 ± 3.13 | 5.88 ± 0.05 ** | 46.52 |
| P. montanum | 23.66 ± 0.75 **** | 9.23 ± 0.70 *** | 15.47 ± 0.52 | 7.96 ± 0.18 ** | 51.45 |
| H. italicum | 17.21 ± 2.38 | 5.53 ± 0.04 | 18.15 ± 2.59 | 12.93 ± 0.42 ** | 71.24 |
| H. stoechas | 17.58 ± 0.28 | 3.81 ± 0.75 | 21.44 ± 1.65 | 15.01 ± 1.32 ** | 70.01 |
| S. hortensis | 20.59 ± 0.39 | 5.88 ± 2.44 | 16.87 ± 0.50 | 4.62 ± 0.44 | 27.39 |
| S. montana | 19.66 ± 1.53 | 5.33 ± 1.15 | 17.13 ± 2.87 | 4.87 ± 0.51 | 28.43 |
| Gallic acid | 1.72 ± 0.25 | 0.35 ± 0.06 | - | - | - |
| Pair | Arnica Pair | Helichrysum Pair | Satureja Pair | |||
|---|---|---|---|---|---|---|
| Phytotherapy | Provence | Phytotherapy | Provence | Phytotherapy | Provence | |
| Species | Arnica montana L. | Pentanema montanum (L.) D.Gut.Larr., Santos-Vicente, Anderb., E.Rico & M.M.Mart.Ort. | Helichrysum italicum (Roth) G. Don | Helichrysum stoechas (L.) Moench | Satureja hortensis L. | Satureja montana L. |
| Sample name | AM | PM | HI | HS | SH | SM |
| Plant part | flower heads | flower heads | flower heads | flower heads | leaves | leaves |
| Source | Père Blaize Supplier | wild (Provence) | “Moulin Bonaventure” Supplier | wild (Provence) | PMA28 Supplier | Cailleau Supplier |
| Voucher | AMFl.FR.1 | IMFl.FR.20.1 | HIFl.FR.21.1 | HSFl.FR.18.1 | SHF.FR.22.1 | SMF.FR.19.1 |
| Harvest | 2022 | 2020 | 2021 | 2018 | 2022 | 2019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achard-Baccati, C.; Garayev, E.; Saïd Hassane, C.; Breaud, C.; Garaev, E.; Bertolotti, M.; Mabrouki, F.; Bun-Llopet, S.-S.; Baghdikian, B. Can Provence Flora Offer Effective Alternatives to Widely Used Medicinal Plants? A Comparative Study of Antioxidant Activity and Chemical Composition Using Molecular Networking. Molecules 2025, 30, 2072. https://doi.org/10.3390/molecules30092072
Achard-Baccati C, Garayev E, Saïd Hassane C, Breaud C, Garaev E, Bertolotti M, Mabrouki F, Bun-Llopet S-S, Baghdikian B. Can Provence Flora Offer Effective Alternatives to Widely Used Medicinal Plants? A Comparative Study of Antioxidant Activity and Chemical Composition Using Molecular Networking. Molecules. 2025; 30(9):2072. https://doi.org/10.3390/molecules30092072
Chicago/Turabian StyleAchard-Baccati, Clémentine, Elnur Garayev, Charifat Saïd Hassane, Célia Breaud, Eldar Garaev, Myriam Bertolotti, Fathi Mabrouki, Sok-Siya Bun-Llopet, and Béatrice Baghdikian. 2025. "Can Provence Flora Offer Effective Alternatives to Widely Used Medicinal Plants? A Comparative Study of Antioxidant Activity and Chemical Composition Using Molecular Networking" Molecules 30, no. 9: 2072. https://doi.org/10.3390/molecules30092072
APA StyleAchard-Baccati, C., Garayev, E., Saïd Hassane, C., Breaud, C., Garaev, E., Bertolotti, M., Mabrouki, F., Bun-Llopet, S.-S., & Baghdikian, B. (2025). Can Provence Flora Offer Effective Alternatives to Widely Used Medicinal Plants? A Comparative Study of Antioxidant Activity and Chemical Composition Using Molecular Networking. Molecules, 30(9), 2072. https://doi.org/10.3390/molecules30092072







