Effect of Auxins on the Accumulation of Alkaloids in Ungrafted Annona emarginata (Schltdl.) H. Rainer and Annona emarginata (Schltdl.) H. Rainer Grafted with Annona atemoya Mabb.
Abstract
1. Introduction
2. Results
2.1. Concentration of Total Alkaloids
2.2. Alkaloid Profile by DI-MS and Chemometric Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design
4.3. Application of Plant Growth Regulators
4.4. Quantitative and Qualitative Analyses of Alkaloids
4.4.1. Obtaining Total Alkaloid Extracts and Quantification
4.4.2. Analysis of the Alkaloid Profile by DI-MS
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IAA | indoleacetic acid |
| IBA | indolebutyric acid |
| NAA | naphthaleneacetic acid |
| DAT | days after treatments |
| APCI | atmospheric pressure chemical ionization |
| 2,4 D | 2,4-dichlorophenoxyacetic acid |
| BIAs | benzylisoquinolinic alkaloids |
| AAs | amaryllidaceae type alkaloids |
| PCA | principal component analysis |
| HCA | hierarchical cluster analysis |
References
- WFO. Annonaceae Juss. Available online: http://www.worldfloraonline.org/taxon/wfo-7000000031 (accessed on 18 April 2024).
- Chatrou, L.W.; Turner, I.M.; Klitgaard, B.B.; Maas, P.J.M.; Utteridge, T.M.A. A Linear Sequence to Facilitate Curation of Herbarium Specimens of Annonaceae. Kew Bull. 2018, 73, 39. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Silva, I.; Lopes, J.C.; Silva, L.V.; Bazante, M.L. Annona in Flora Do Brasil. 2020. Available online: http://floradobrasil2020.jbrj.gov.br/reflora/floradobrasil/FB110253 (accessed on 27 May 2024).
- de Lemos, E.E.P. A Produção de Anonáceas no Brasil. Rev. Bras. Frutic. 2014, 36, 77–85. [Google Scholar] [CrossRef]
- José, A.R.S.; Pires, M.d.M.; de Freitas, A.L.G.E.; Ribeiro, D.P.; Perez, L.A.A. Atualidades e Perspectivas Das Anonáceas no Mundo. Rev. Bras. Frutic. 2014, 36, 86–93. [Google Scholar] [CrossRef]
- De-la-Cruz-Chacón, I.; Riley-Saldaña, C.A.; Arrollo-Gómez, S.; Sancristóbal-Domínguez, T.J.; Castro-Moreno, M.; González-Esquinca, A.R. Spatio-Temporal Variation of Alkaloids in Annona purpurea and the Associated Influence on Their Antifungal Activity. Chem. Biodivers. 2019, 16, e1800284. [Google Scholar] [CrossRef]
- Mimi, C.O.; De-la-Cruz-Chacón, I.; Sousa, M.C.; Vieira, M.A.P.; Marques, M.O.M.; Ferreira, G.; Boaro, C.S.F. Chemophenetics as a Tool for Distinguishing Morphotypes of Annona emarginata (Schltdl.) H. Rainer. Chem. Biodivers. 2021, 18, e2100544. [Google Scholar] [CrossRef]
- Lúcio, A.S.S.C.; Almeida, J.R.G.d.S.; Da-Cunha, E.V.L.; Tavares, J.F.; Filho, J.M.B. Alkaloids of the Annonaceae: Occurrence and a Compilation of Their Biological Activities. Alkaloids Chem. Biol. 2015, 74, 233–409. [Google Scholar] [CrossRef]
- Rabêlo, S.V.; Costa, E.V.; Barison, A.; Dutra, L.M.; Nunes, X.P.; Tomaz, J.C.; Oliveira, G.G.; Lopes, N.P.; Santos, M.d.F.C.; da Almeida, J.R.S. Alkaloids Isolated from the Leaves of Atemoya (Annona cherimola × Annona squamosa). Rev. Bras. Farmacogn. 2015, 25, 419–421. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Plantas Medicinais: Fatores de Influência no Conteúdo de Metabólitos Secundários. Quím. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Kroymann, J. Natural Diversity and Adaptation in Plant Secondary Metabolism. Curr. Opin. Plant Biol. 2011, 14, 246–251. [Google Scholar] [CrossRef]
- Pichersky, E.; Lewinsohn, E. Convergent Evolution in Plant Specialized Metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef]
- Wink, M. Plant Secondary Metabolism: Diversity, Function and Its Evolution. Nat. Prod. Commun. 2008, 3, 1934578X0800300801. [Google Scholar] [CrossRef]
- Leite, D.O.D.; Nonato, C.d.F.A.; Camilo, C.J.; de Carvalho, N.K.G.; da Nóbrega, M.G.L.A.; Pereira, R.C.; da Costa, J.G.M. Annona Genus: Traditional Uses, Phytochemistry and Biological Activities. Curr. Pharm. Des. 2020, 26, 4056–4091. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; García-Magaña, M.d.L.; Domínguez-Ávila, J.A.; Yahia, E.M.; Salazar-López, N.J.; González-Aguilar, G.A.; Montalvo-González, E. Annonas: Underutilized Species as a Potential Source of Bioactive Compounds. Food Res. Int. 2020, 138, 109775. [Google Scholar] [CrossRef]
- Chowdhury, S.S.; Tareq, A.M.; Tareq, S.M.; Farhad, S.; Sayeed, M.A. Screening of Antidiabetic and Antioxidant Potential Along with Phytochemicals of Annona Genus: A Review. Futur. J. Pharm. Sci. 2021, 7, 144. [Google Scholar] [CrossRef]
- Li, H.-T.; Wu, H.-M.; Chen, H.-L.; Liu, C.-M.; Chen, C.-Y. The Pharmacological Activities of (−)-Anonaine. Molecules 2013, 18, 8257–8263. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hao, N.; Wang, Q.; Li, R.; Zhang, G.; Chen, G.; Liu, S.; Che, Z. Non-Food Bioactive Forest Product Liriodenine: Sources, Chemistry, and Bioactivities. Ind. Crops Prod. 2022, 187, 115447. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Sousa, M.C.; De-la-Cruz-Chacón, I.; Campos, F.G.; Vieira, M.A.R.; Corrêa, P.L.C.; Marques, M.O.M.; Boaro, C.S.F.; Ferreira, G. Plant Growth Regulators Induce Differential Responses on Primary and Specialized Metabolism of Annona emarginata (Annonaceae). Ind. Crops Prod. 2022, 189, 115789. [Google Scholar] [CrossRef]
- Muthulakshmi, S.; Pandiyarajan, V. Influence of IAA on the Vincristine Content of Catharanthus roseus (L.). G. Don. Asian J. Plant Sci. Res. 2013, 3, 81–87. [Google Scholar]
- Goddijn, O.J.M.; de Kam, R.J.; Zanetti, A.; Schilperoort, R.A.; Hoge, J.H.C. Auxin Rapidly Down-Regulates Transcription of the Tryptophan Decarboxylase Gene from Catharanthus roseus. Plant Mol. Biol. 1992, 18, 1113–1120. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yamada, Y. Alkaloid Biogenesis: Molecular Aspects. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 257–285. [Google Scholar] [CrossRef]
- Pasquali, G.; Goddijn, O.J.M.; de Waal, A.; Verpoorte, R.; Schilperoort, R.A.; Hoge, J.H.C.; Memelink, J. Coordinated Regulation of Two Indole Alkaloid Biosynthetic Genes from Catharanthus roseus by Auxin and Elicitors. Plant Mol. Biol. 1992, 18, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, M.R.; da Silveira, T.F.F.; Coutinho, J.P.; Souza, D.S.; Duarte, M.C.T.; Duarte, R.T.; Filho, J.T.; Godoy, H.T. Bioactivity of Atemoya Fruits and By-Products. Food Biosci. 2021, 41, 101036. [Google Scholar] [CrossRef]
- Pareek, S.; Yahia, E.M.; Pareek, O.P.; Kaushik, R.A. Postharvest Physiology and Technology of Annona Fruits. Food Res. Int. 2011, 44, 1741–1751. [Google Scholar] [CrossRef]
- dos Santos, W.N.L.; Sauthier, M.C.S.; Cavalcante, D.D.; Benevides, C.M.J.; Dias, F.S.; Santos, D.C.M.B. Mineral Composition, Nutritional Properties, Total Phenolics and Flavonoids Compounds of the Atemoya Fruit (Annona squamosa L. × Annona cherimola Mill.) and Evaluation Using Multivariate Analysis Techniques. An. Acad. Bras. Cienc. 2016, 88, 1243–1252. [Google Scholar] [CrossRef]
- Junqueira, N.T.V.; Junqueira, K.P. Principais Doenças de Anonáceas no Brasil: Descrição e Controle. Rev. Bras. Frutic. 2014, 36, 55–64. [Google Scholar] [CrossRef]
- Ferreira, G.; De-La-Cruz-Chacón, I.; Boaro, C.S.F.; Baron, D.; de Lemos, E.E.P. Propagation of Annonaceous Plants. Rev. Bras. Frutic. 2019, 41, e-500. [Google Scholar] [CrossRef]
- Bettiol Neto, J.E.; Pio, R.; Bueno, S.C.S.; Bastos, D.C.; Scarpare Filho, J.A. Enraizamento de Estacas Dos Porta-Enxertos Araticum-de-Terra-Fria (Rollinia sp.) e Araticum-Mirim (Rollinia emarginata Schltdl.) Para Anonáceas. Ciênc. Agrotecnol. 2006, 30, 1077–1082. [Google Scholar] [CrossRef]
- Baron, D.; Amaro, A.C.E.; Campos, F.G.; Ferreira, G. Leaf Gas Exchanges Responses of Atemoya Scion Grafted onto Annona Rootstocks. Theor. Exp. Plant Physiol. 2018, 30, 203–213. [Google Scholar] [CrossRef]
- Honório, A.B.M.; De-la-Cruz-Chacón, I.; Silva, G.C.; Mimi, C.O.; Campos, F.G.; Silva, M.R.; Boaro, C.S.F.; Ferreira, G. Differential Tolerance of Primary Metabolism of Annona emarginata (Schltdl.) H. Rainer to Water Stress Modulates Alkaloid Production. Horticulturae 2024, 10, 220. [Google Scholar] [CrossRef]
- da Silva, G.C.; Dutra, L.M.; da Silva Almeida, J.R.G.; da Silva, F.M.A.; Harakava, R.; Honório, A.B.M.; de-la-Cruz-Chacón, I.; Martínez-Vázquez, M.; Ferreira, G. Alkaloid Screening of Annona emarginata (Schltdl.) H. Rainer Rootstocks to Increase Fungal Tolerance in Annona atemoya Mabb. Crops Revealed by MS and NMR Chemical Profiling. Ind. Crops Prod. 2024, 212, 118335. [Google Scholar] [CrossRef]
- Silva, F.M.A.; Filho, F.A.S.; Lima, B.R.; Almeida, R.A.; Soares, E.R.; Koolen, H.H.F.; Souza, A.D.L.; Pinheiro, M.L.B. Chemotaxonomy of the Amazonian Unonopsis Species Based on Leaf Alkaloid Fingerprint Direct Infusion ESI-MS and Chemometric Analysis. J. Braz. Chem. Soc. 2016, 27, 599–604. [Google Scholar] [CrossRef]
- de Lima, B.; da Silva, F.; Soares, E.; de Almeida, R.; da Silva-Filho, F.; Barison, A.; Costa, E.; Koolen, H.; de Souza, A.; Pinheiro, M.L. Integrative Approach Based on Leaf Spray Mass Spectrometry, HPLC-DAD-MS/MS, and NMR for Comprehensive Characterization of Isoquinoline-Derived Alkaloids in Leaves of Onychopetalum amazonicum R. E. Fr. J. Braz. Chem. Soc. 2020, 31, 79–89. [Google Scholar] [CrossRef]
- da Silva, F.M.A.; Bataglion, G.A.; de Almeida, R.A.; Heerdt, G.; Sousa, I.L.; da Silva Filho, F.A.; de Alencar, D.C.; Costa, E.V.; de Souza, A.D.L.; Pinheiro, M.L.B.; et al. Positive Electrospray Ionization Ion Trap Mass Spectrometry and Ab Initio Computational Studies of the Multi-Pathway Fragmentation of Oxoaporphine Alkaloids. Int. J. Mass. Spectrom. 2017, 418, 30–36. [Google Scholar] [CrossRef]
- Dourado, S.H.A.; Santos, C.S.; Ferreira, V.d.S.; Pontes-Pires, A.F.; Valladão, D.M.d.S.; Ribeiro, E.B.; de Souza, C.A.S.; da Silva, F.M.A.; Costa, E.V.; Andrighetti, C.R. Anti-Herpes Activity of the Total Alkaloid Fraction from the Branches of Fusaea longifolia (Annonaceae). Acta Amaz. 2023, 53, 158–165. [Google Scholar] [CrossRef]
- Chang, F.R.; Chen, C.Y.; Hsieh, T.J.; Cho, C.P.; Wu, Y.C. Chemical Constituents from Annona Glabra III. J. Chin. Chem. Soc. 2000, 47, 913–920. [Google Scholar] [CrossRef]
- Paz, W.H.P.; de Oliveira, R.N.; Heerdt, G.; Angolini, C.F.F.; de Medeiros, L.S.; Silva, V.R.; Santos, L.S.; Soares, M.B.P.; Bezerra, D.P.; Morgon, N.H.; et al. Structure-Based Molecular Networking for the Target Discovery of Oxahomoaporphine and 8-Oxohomoaporphine Alkaloids from Duguetia surinamensis. J. Nat. Prod. 2019, 82, 2220–2228. [Google Scholar] [CrossRef]
- Chaves, M.H.; Santos, L.d.A.; Lago, J.H.G.; Roque, N.F. Alkaloids from Porcelia macrocarpa. J. Nat. Prod. 2001, 64, 240–242. [Google Scholar] [CrossRef]
- Matsushige, A.; Kotake, Y.; Matsunami, K.; Otsuka, H.; Ohta, S.; Takeda, Y. Annonamine, a New Aporphine Alkaloid from the Leaves of Annona muricata. Chem. Pharm. Bull. 2012, 60, 257–259. [Google Scholar] [CrossRef]
- Katchborian-Neto, A.; Alves, M.F.; Bueno, P.C.P.; de Jesus Nicácio, K.; Ferreira, M.S.; Oliveira, T.B.; Barbosa, H.; Murgu, M.; de Paula Ladvocat, A.C.C.; Dias, D.F.; et al. Integrative Open Workflow for Confident Annotation and Molecular Networking of Metabolomics MSE/DIA Data. Brief. Bioinform. 2024, 25, bbae013. [Google Scholar] [CrossRef]
- Costa, E.V.; Marques, F.d.A.; Pinheiro, M.L.B.; Braga, R.M.; Delarmelina, C.; Duarte, M.C.T.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Maia, B.H.L.N.S. Chemical Constituents Isolated from the Bark of Guatteria blepharophylla (Annonaceae) and Their Antiproliferative and Antimicrobial Activities. J. Braz. Chem. Soc. 2011, 22, 1111–1117. [Google Scholar] [CrossRef]
- Menezes, L.; Costa, C.; Rodrigues, A.; Santo, F.; Nepel, A.; Dutra, L.; Silva, F.; Soares, M.; Barison, A.; Costa, E.; et al. Cytotoxic Alkaloids from the Stem of Xylopia laevigata. Molecules 2016, 21, 890. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.A.; da Silva, G.A.; Marques, M.J.; Bastos, R.G.; da Silva, A.F.; Rosa, C.P.; Espuri, P.F. Triagem Fitoquímica, Atividade Antioxidante e Leishmanicida Do Extrato Hidroetanólico 70% (v/v) e Das Frações Obtidas de (Annona crassiflora Mart.). Rev. Fitos 2017, 10, 505–517. [Google Scholar] [CrossRef][Green Version]
- Martin, B.C.; De-la-Cruz-Chacón, I.; Mimi, C.O.; Boaro, C.S.F.; Campos, F.G.; Moreira-Coneglian, I.R.; Ferreira, G. Impact of External Sources of Indole Acetic Acid and 2,3,5-Triiodobenzoic Acid on Alkaloid Production and Their Relationships with Primary Metabolism and Antioxidant Activity in Annona emarginata (Schltdl.) H. Rainer. Plants 2024, 13, 2637. [Google Scholar] [CrossRef]
- Endara, M.; Coley, P.D. The Resource Availability Hypothesis Revisited: A Meta-analysis. Funct. Ecol. 2011, 25, 389–398. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Saxena, P. Auxin Driven Indoleamine Biosynthesis and the Role of Tryptophan as an Inductive Signal in Hypericum perforatum (L.). PLoS ONE 2019, 14, e0223878. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Morffy, N.; Strader, L.C. Old Town Roads: Routes of Auxin Biosynthesis across Kingdoms. Curr. Opin. Plant Biol. 2020, 55, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Facchini, P.J.; Huber-Allanach, K.L.; Tari, L.W. Plant Aromatic L-Amino Acid Decarboxylases: Evolution, Biochemistry, Regulation, and Metabolic Engineering Applications. Phytochemistry 2000, 54, 121–138. [Google Scholar] [CrossRef]
- Koirala, M.; dos Santos, K.C.G.; Gélinas, S.-E.; Ricard, S.; Karimzadegan, V.; Lamichhane, B.; Liyanage, N.S.; Merindol, N.; Desgagné-Penix, I. Auxin and Light-Mediated Regulation of Growth, Morphogenesis, and Alkaloid Biosynthesis in Crinum × powellii ‘Album’ Callus. Phytochemistry 2023, 216, 113883. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakagawa, K.; Fukui, H.; Tabata, M. Alkaloid Production in Cell Suspension Cultures of Thalictrum flavum and T. dipterocarpum. Plant Cell Rep. 1988, 7, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Ptak, A.; El Tahchy, A.; Skrzypek, E.; Wójtowicz, T.; Laurain-Mattar, D. Influence of Auxins on Somatic Embryogenesis and Alkaloid Accumulation in Leucojum aestivum Callus. Cent. Eur. J. Biol. 2013, 8, 591–599. [Google Scholar] [CrossRef]
- Ju, Y.W.; Kim, C.; Byun, S.Y. Phytohormone Effects with Elicitation on Cell Growth and Alkaloid Production in Suspension Cultures of Eschscholtzia californica. J. Microbiol. Biotechnol. 1993, 3, 238–243. [Google Scholar]
- Mekky, H.; Al-Sabahi, J.; Abdel-Kreem, M.F.M. Potentiating Biosynthesis of the Anticancer Alkaloids Vincristine and Vinblastine in Callus Cultures of Catharanthus roseus. S. Afr. J. Bot. 2018, 114, 29–31. [Google Scholar] [CrossRef]
- De la Cruz-Chacón, I.; González-Esquinca, A.R.; Fefer, P.G.; Garcia, L.F.J. Liriodenine, Early Antimicrobial Defence in Annona diversifolia. Z. Naturforsch. C 2011, 66, 377–384. [Google Scholar] [CrossRef]
- Vinche, A.D.L.; La-Cruz-Chacón, I.D.; González-Esquinca, A.R.; Silva, J.d.F.d.; Ferreira, G.; dos Santos, D.C.; Garces, H.G.; de Oliveira, D.V.M.; Marçon, C.; Cavalcante, R.d.S.; et al. Antifungal Activity of Liriodenine on Agents of Systemic Mycoses, with Emphasis on the Genus Paracoccidioides. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20200023. [Google Scholar] [CrossRef]
- Ge, X.; Zhong, Q.; Tan, X.; Wang, J.; Cao, L.; Zhou, Y.; Zou, Y.; Yuan, Y.; Wan, X.; Yan, C.; et al. Integrated Multi-Omics Analysis to Elucidate the Role of Shikimic Acid and Phenethylamine in the Effect of Scions on Rootstocks of Camellia oleifera. Ind. Crops Prod. 2023, 203, 117222. [Google Scholar] [CrossRef]
- Xu, D.; Yuan, H.; Tong, Y.; Zhao, L.; Qiu, L.; Guo, W.; Shen, C.; Liu, H.; Yan, D.; Zheng, B. Comparative Proteomic Analysis of the Graft Unions in Hickory (Carya cathayensis) Provides Insights into Response Mechanisms to Grafting Process. Front. Plant Sci. 2017, 8, 676. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, H.; Zhou, X.; Pan, M.; Xu, J.; Liu, M.; Wang, M.; Liu, G.; Xu, T.; Wang, Y.; et al. Grafting with Rootstocks Promotes Phenolic Compound Accumulation in Grape Berry Skin During Development Based on Integrative Multi-Omics Analysis. Hortic. Res. 2022, 9, uhac055. [Google Scholar] [CrossRef]
- Tarakemeh, A.; Azizi, M.; Rowshan, V.; Salehi, H.; Spina, R.; Dupire, F.; Arouie, H.; Laurain-Mattar, D. Screening of Amaryllidaceae Alkaloids in Bulbs and Tissue Cultures of Narcissus papyraceus and Four Varieties of N. tazetta. J. Pharm. Biomed. Anal. 2019, 172, 230–237. [Google Scholar] [CrossRef]
- Singh, A.; Desgagné-Penix, I. Transcriptome and Metabolome Profiling of Narcissus pseudonarcissus ‘King Alfred’ Reveal Components of Amaryllidaceae Alkaloid Metabolism. Sci. Rep. 2017, 7, 17356. [Google Scholar] [CrossRef] [PubMed]
- Radford, A.E.; Dickson, W.C.; Massey, J.R.; Bell, C.R. Vascular Plant Systematic; Harper and Row Publishers: New York, NY, USA, 1974; p. 891. [Google Scholar]
- Kessler, P.J.A. Annonaceae. In Flowering Plants · Dicotyledons. The Families and Genera of Vascular Plants; Kubitzki, K., Rohwer, J.G., Bittrich, V., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 2, pp. 93–129. [Google Scholar]
- De-La-Cruz-Chacón, I.; González-Esquinca, A.R. Liriodenine Alkaloid in Annona diversifolia During Early Development. Nat. Prod. Res. 2012, 26, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Février, A.; Ferreira, M.E.; Fournet, A.; Yaluff, G.; Inchausti, A.; de Arias, A.R.; Hocquemiller, R.; Waechter, A.-I. Acetogenins and Other Compounds from Rollinia emarginata and Their Antiprotozoal Activities. Planta Med. 1999, 65, 047–049. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 178. [Google Scholar]

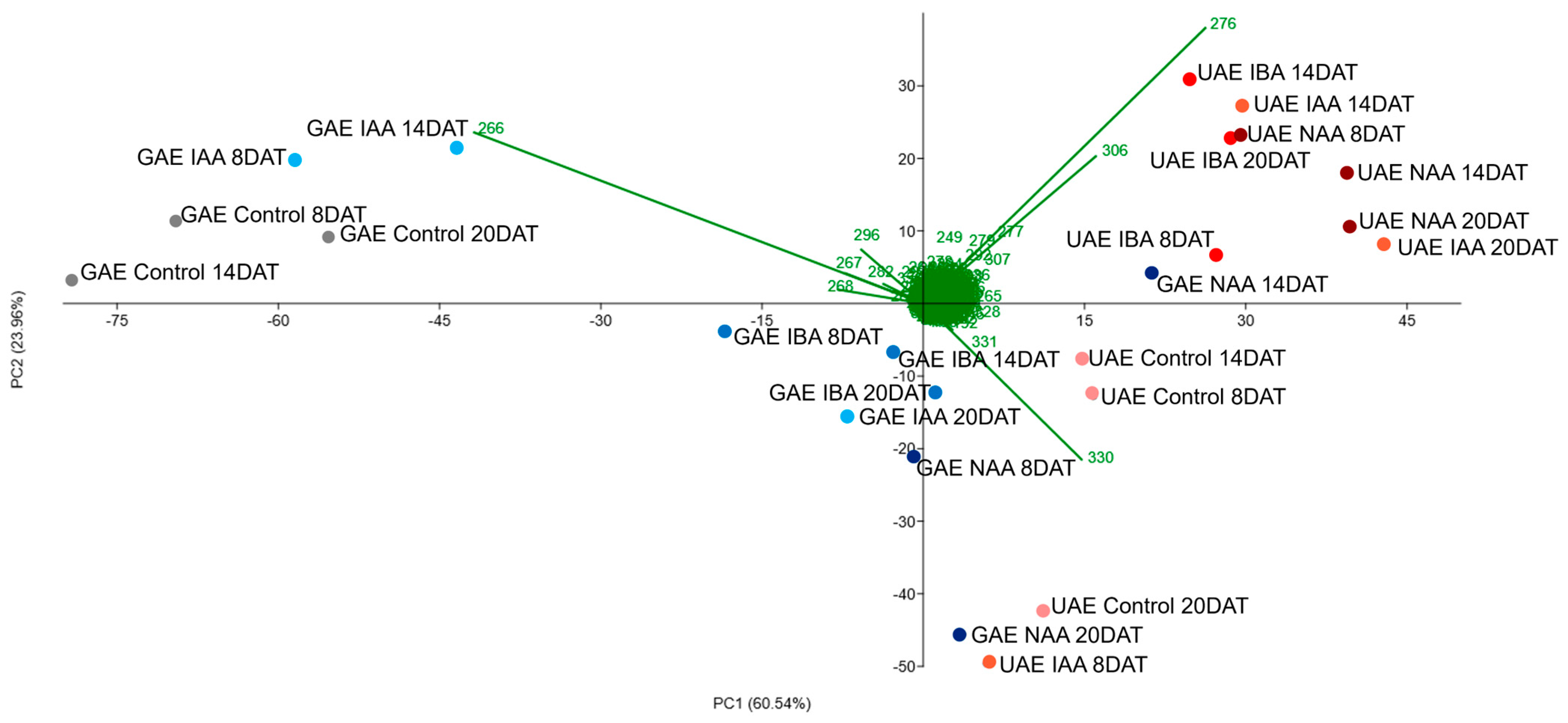
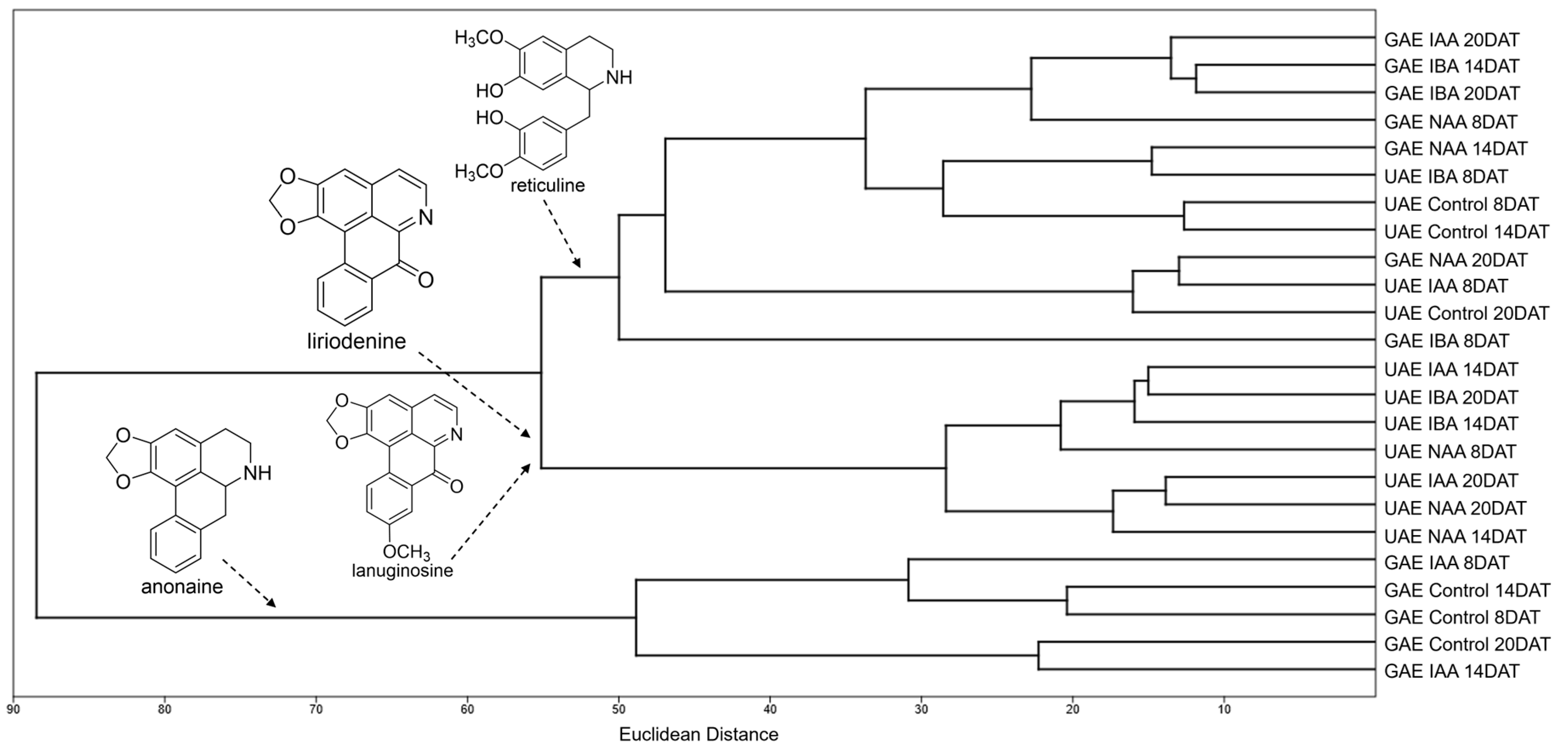
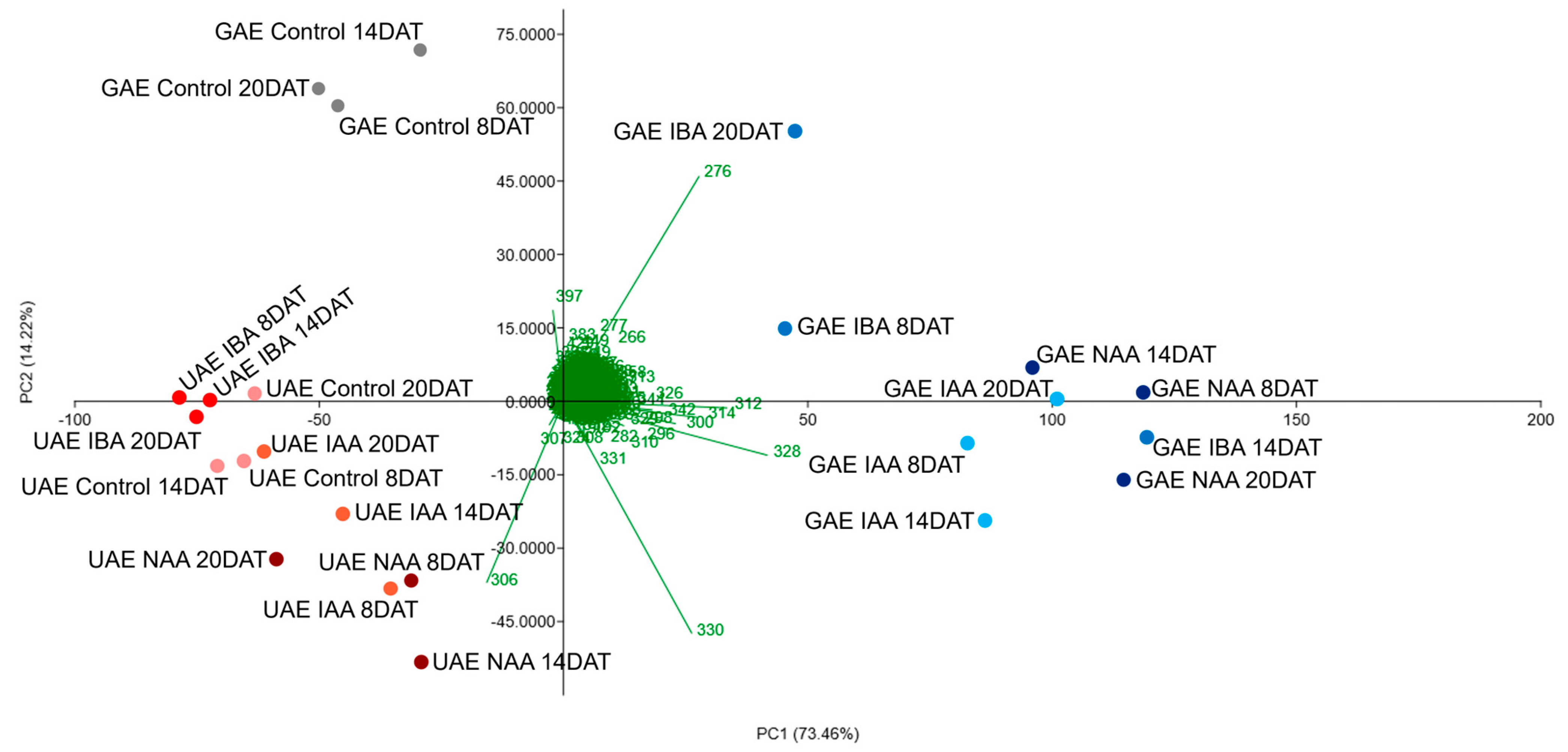

| Total Alkaloids in Roots (µg g−1 Dry Mass) | ||
|---|---|---|
| 8 DAT | ||
| Ungrafted A. emarginata | Grafted A. emarginata | |
| Control | 201.322 ± 6.72 Bb | 287.144 ± 40.5 BCa 1 |
| IAA | 297.661 ± 8.47 Aa | 230.714 ± 3.51 Cb |
| IBA | 197.750 ± 12.05 Bb | 370.268 ± 7.2 Aa |
| NAA | 310.033 ± 17.25 Aa | 344.812 ± 7.06 ABa |
| Treatment p < 0.001; F: 8.74 Species p < 0.001; F: 22.10 Treatment × Species p < 0.001; F: 17.30 | ||
| 14 DAT | ||
| Ungrafted A. emarginata | Grafted A. emarginata | |
| Control | 138.432 ± 15.16 Cb | 230.212 ± 5.95 Ba |
| IAA | 277.114 ± 18.83 ABa | 272.171 ± 18.35 ABa |
| IBA | 230.310 ± 7.39 Bb | 332.696 ± 43.96 Aa |
| NAA | 389.102 ± 42.53 Aa | 236.860 ± 10.57 ABb |
| Treatment p < 0.001; F: 13.38 Species p: 0.260; F: 1.31 Treatment × Species p < 0.001; F: 11.12 | ||
| 20 DAT | ||
| Ungrafted A. emarginata | Grafted A. emarginata | |
| Control | 185.863 ± 7.37 Ca | 226.580 ± 4.71 Ba |
| IAA | 311.020 ± 19.48 ABb | 378.382 ± 36.37 Aa |
| IBA | 245.452 ± 15.86 BCb | 330.315 ± 19.72 Aa |
| NAA | 380.146 ± 24.82 Aa | 364.690 ± 14.42 Aa |
| Treatment p < 0.001; F: 26.55 Species p: 0.003; F: 9.71 Treatment × Species p: 0.089; F: 2.37 | ||
| Total Alkaloids in Leaves (µg g−¹ Dry Mass) | ||
|---|---|---|
| 8 DAT | ||
| Ungrafted A. emarginata | Grafted A. atemoya | |
| Control | 32.487 ± 1.30 ABa | 20.511 ± 0.17 Db 1 |
| IAA | 34.572 ± 0.57 Ab | 49.509 ± 2.38 Aa |
| IBA | 29.168 ± 0.51 Ba | 26.486 ± 2.11 Ca |
| NAA | 35.963 ± 0.24 Aa | 33.535 ± 1.94 Ba |
| Treatment p < 0.001; F: 50.05 Species p: 0.140; F: 2.29 Treatment × Species p < 0.001; F: 24.58 | ||
| 14 DAT | ||
| Ungrafted A. emarginata | Grafted A. atemoya | |
| Control | 42.428 ± 0.94 Aa | 43.996 ± 1.25 Ca |
| IAA | 46.812 ± 1.03 Ab | 66.666 ± 2.31 Aa |
| IBA | 27.310 ± 1.90 Bb | 51.495 ± 0.60 Ba |
| NAA | 42.302 ± 0.81 Ab | 47.579 ± 4.20 BCa |
| Treatment p < 0.001; F: 20.40 Species p < 0.001; F: 77.48 Treatment × Species p < 0.001; F: 11.49 | ||
| 20 DAT | ||
| Ungrafted A. emarginata | Grafted A. atemoya | |
| Control | 33.079 ± 0.59 Ba | 23.237 ± 3.36 Cb |
| IAA | 45.663 ± 0.23 Aa | 46.039 ± 2.58 Aa |
| IBA | 24.938 ± 0.39 Cb | 35.293 ± 0.74 Ba |
| NAA | 34.236 ± 2.33 Ba | 34.847 ± 1.44 Ba |
| Treatment p < 0.001; F: 37.55 Species p: 0.773; F: 0.08 Treatment × Species p < 0.001; F: 10.18 | ||
| Alkaloid | [M+H]+ (m/z) | MS/MS (m/z) | Class * | References | Figures ** |
|---|---|---|---|---|---|
| Anonaine (1) | 266 | 249, 219, 191 | A | [33,35] | Figure S1 |
| Asimilobine (2) | 268 | 251, 219 | A | [33,34,35] | Figure S2 |
| Liriodenine (3) | 276 | 248, 220 | O | [33,35,36,37] | Figure S3 |
| N-methylanonaine (4) | 280 | 249, 219, 191 | A | [35] | Figure S4 |
| Nornuciferine (5) | 282 | 265, 250, 234 | A | [33,34,35] | Figure S5 |
| Lysicamine (6) | 292 | 277, 249 | O | [35] | Figure S6 |
| N-formyl-anonaine (7) | 294 | 249, 219, 191 | A | [38] | Figure S7 |
| Xylopine (8) | 296 | 279, 249, 221 | A | [33,35] | Figure S8 |
| Stepharine (9) | 298 | 281, 266, 250 | P | [39] | Figure S9 |
| 4′-O-methylcoclaurine (10) | 300 | 283 | B | [40,41] | Figure S10 |
| Lanuginosine (oxoxylopine) (11) | 306 | 291 | O | [33,36] | Figure S11 |
| N,O-dimethylcoclaurine (12) | 314 | 283 | B | [39] | Figure S12 |
| 7-hydroxy-7-methyl-N-formyl-anonaine (13) | 324 | 279, 249, 221 | A | - | Figure S13 |
| Nornantenine (14) | 326 | 309, 294, 278 | A | [42] | Figure S14 |
| Boldine (15) | 328 | 297, 265 | A | [37] | Figure S15 |
| Stepholidine (16) | 328 | 297, 265 | T | [35] | Figure S15 |
| Reticuline (17) | 330 | 192 | B | [33,35] | Figure S16 |
| Subsessiline (18) | 338 | 323 | O | [39,43] | Figure S17 |
| Xylopinine (19) | 356 | 192, 165 | A | [44] | Figure S18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mimi, C.O.; De-la-Cruz-Chacón, I.; da Silva, F.M.A.; Roberto, V.C.R.; Ferreira, G. Effect of Auxins on the Accumulation of Alkaloids in Ungrafted Annona emarginata (Schltdl.) H. Rainer and Annona emarginata (Schltdl.) H. Rainer Grafted with Annona atemoya Mabb. Molecules 2025, 30, 2070. https://doi.org/10.3390/molecules30092070
Mimi CO, De-la-Cruz-Chacón I, da Silva FMA, Roberto VCR, Ferreira G. Effect of Auxins on the Accumulation of Alkaloids in Ungrafted Annona emarginata (Schltdl.) H. Rainer and Annona emarginata (Schltdl.) H. Rainer Grafted with Annona atemoya Mabb. Molecules. 2025; 30(9):2070. https://doi.org/10.3390/molecules30092070
Chicago/Turabian StyleMimi, Carolina Ovile, Iván De-la-Cruz-Chacón, Felipe Moura Araujo da Silva, Victor Cauan Rocha Roberto, and Gisela Ferreira. 2025. "Effect of Auxins on the Accumulation of Alkaloids in Ungrafted Annona emarginata (Schltdl.) H. Rainer and Annona emarginata (Schltdl.) H. Rainer Grafted with Annona atemoya Mabb." Molecules 30, no. 9: 2070. https://doi.org/10.3390/molecules30092070
APA StyleMimi, C. O., De-la-Cruz-Chacón, I., da Silva, F. M. A., Roberto, V. C. R., & Ferreira, G. (2025). Effect of Auxins on the Accumulation of Alkaloids in Ungrafted Annona emarginata (Schltdl.) H. Rainer and Annona emarginata (Schltdl.) H. Rainer Grafted with Annona atemoya Mabb. Molecules, 30(9), 2070. https://doi.org/10.3390/molecules30092070








