Interfacial Synthesis of an Electro-Functional 2D Bis(terpyridine)copper(II) Polymer Nanosheet
Abstract
:1. Introduction
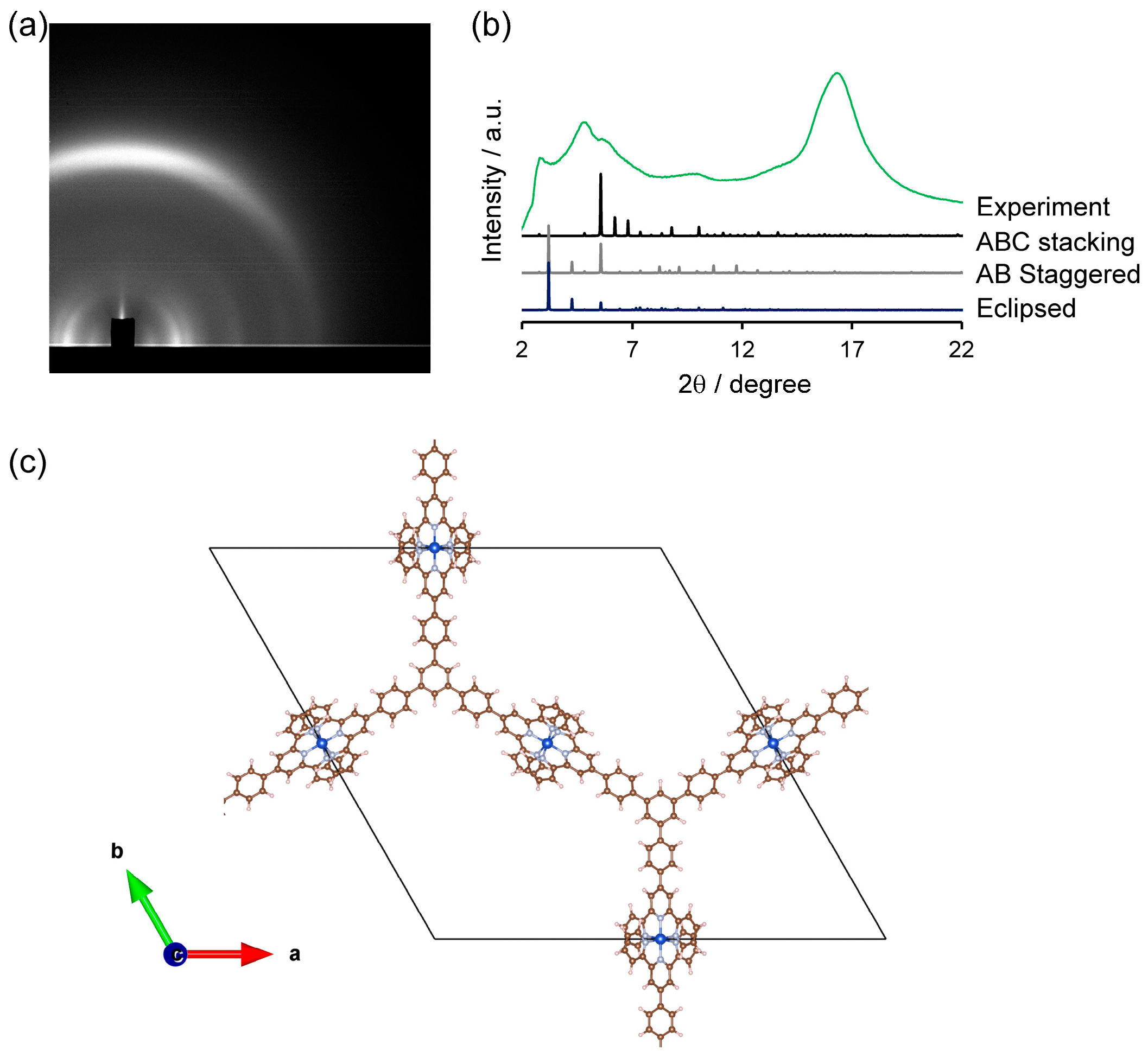
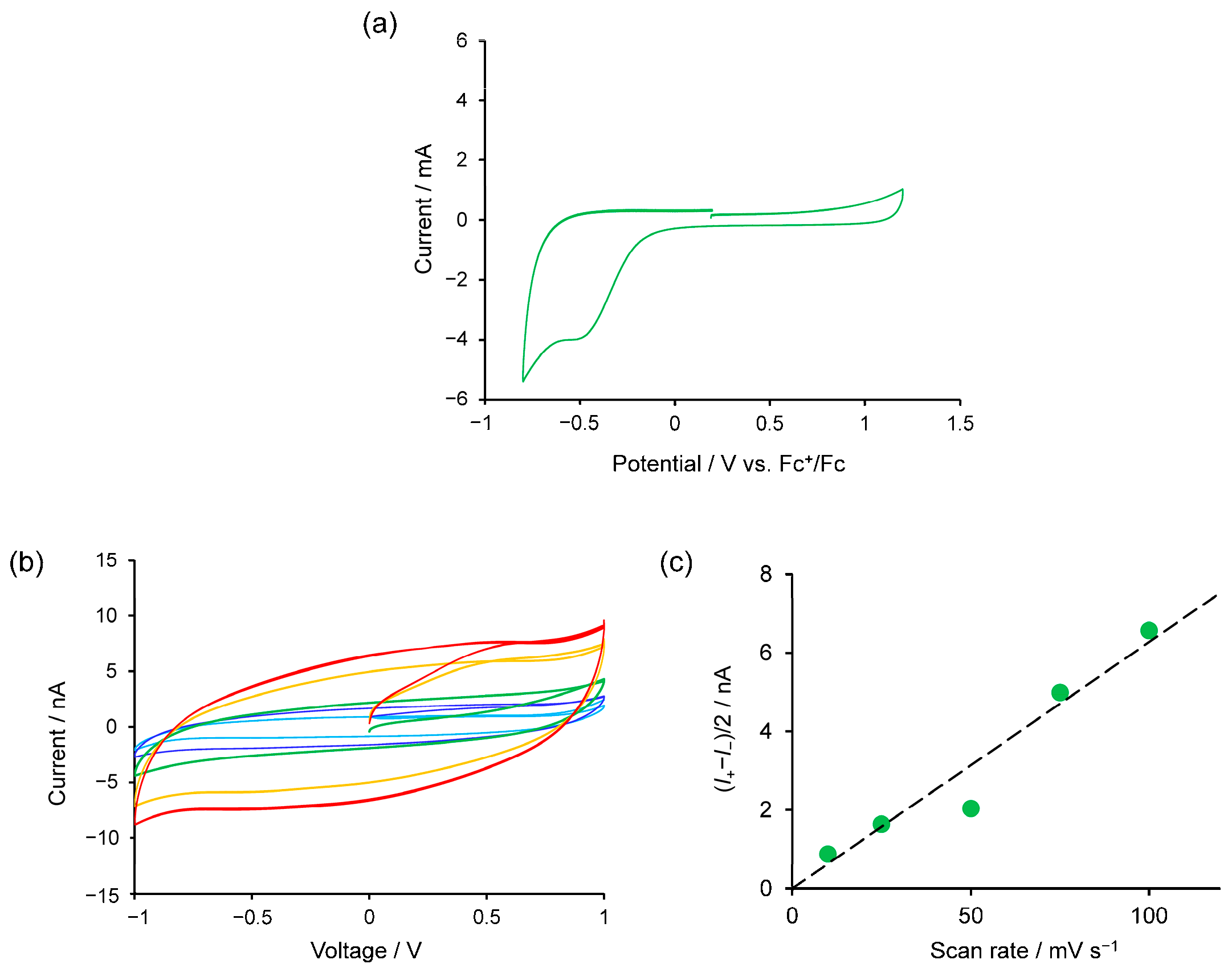
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Apparatus
3.3. Electrochemical Analysis
3.4. Preparation of Cu-tpy
3.5. I-V Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, H.; Park, G.; Jeon, J.; Park, S.S. Fabricating Large-Area Thin Films of 2D Conductive Metal–Organic Frameworks. Acc. Chem. Res. 2024, 57, 2336–2346. [Google Scholar] [CrossRef]
- Lu, Y.; Samorì, P.; Feng, X. Rational Construction of Two-Dimensional Conjugated Metal–Organic Frameworks (2D c-MOFs) for Electronics and Beyond. Acc. Chem. Res. 2024, 57, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, G.; Mirica, K.A. Conductive Framework Materials for Chemiresistive Detection and Differentiation of Toxic Gases. Acc. Chem. Res. 2024, 57, 2775–2789. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Park, I.-H.; Medishetty, R.; Vittal, J.J. Two-Dimensional Metal-Organic Framework Materials: Synthesis, Structures, Properties and Applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef] [PubMed]
- Bera, M.K.; Mohanty, S.; Kashyap, S.S.; Sarmah, S. Electrochromic coordination nanosheets: Achievements and future perspective. Coord. Chem. Rev. 2022, 454, 214353. [Google Scholar] [CrossRef]
- Sakamoto, R.; Hoshiko, K.; Liu, Q.; Yagi, T.; Nagayama, T.; Kusaka, S.; Tsuchiya, M.; Kitagawa, Y.; Wong, W.-Y.; Nishihara, H. A photofunctional bottom-up bis(dipyrrinato)zinc(II) complex nanosheet. Nat. Commun. 2015, 6, 6713. [Google Scholar] [CrossRef]
- Berry, T.; Morey, J.R.; Arpino, K.E.; Dou, J.-H.; Felser, C.; Dincǎ, M.; McQueen, T.M. Structural, Thermodynamic, and Transport Properties of the Small-Gap Two-Dimensional Metal–Organic Kagomé Materials Cu3(hexaiminobenzene)2 and Ni3(hexaiminobenzene)2. Inorg. Chem. 2022, 61, 6480–6487. [Google Scholar] [CrossRef]
- Estévez, S.M.; Wang, Z.; Liu, T.J.; Caballero, G.; Urbanos, F.J.; Figueruelo-Campanero, I.; García-Pérez, J.; Navío, C.; Polozij, M.; Zhang, J.; et al. Electrical Characterization of a Large-Area Single-Layer Cu3BHT 2D Conjugated Coordination Polymer. Adv. Funct. Mater. 2025, 35, 2416717. [Google Scholar] [CrossRef]
- Lu, Y.; Fu, Y.; Hu, Z.; Feng, S.; Torabi, M.; Gao, L.; Fu, S.; Wang, Z.; Huang, C.; Huang, X.; et al. Rational Construction of Layered Two-Dimensional Conjugated Metal–Organic Frameworks with Room-Temperature Quantum Coherence. J. Am. Chem. Soc. 2025, 147, 8778–8784. [Google Scholar] [CrossRef]
- Ohata, T.; Tachimoto, K.; Takeno, K.J.; Nomoto, A.; Watanabe, T.; Hirosawa, I.; Makiura, R. Influence of the Solvent on the Assembly of Ni3(hexaiminotriphenylene)2 Metal–Organic Framework Nanosheets at the Air/Liquid Interface. Bull. Chem. Soc. Jpn. 2023, 96, 274–282. [Google Scholar] [CrossRef]
- Zhu, X.; Miao, H.; Shan, Y.; Gao, G.; Gu, Q.; Xiao, Q.; He, X. Two-Dimensional Janus Film with Au Nanoparticles Assembled on Trinuclear Gold(I) Pyrazolate Coordination Nanosheets for Photocatalytic H2 Evolution. Inorg. Chem. 2022, 61, 13591–13599. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dong, J.; Liu, J.; Liang, Q.; Song, Y.; Li, W.; Lei, S.; Hu, W. High-Quality Two-Dimensional Metal-Organic Framework Nanofilms for Nonvolatile Memristive Switching. Small Struct. 2021, 2, 2000077. [Google Scholar] [CrossRef]
- Haraguchi, T.; Otsubo, K.; Sakata, O.; Fujiwara, A.; Kitagawa, H. Strain-Controlled Spin Transition in Heterostructured Metal–Organic Framework Thin Film. J. Am. Chem. Soc. 2021, 143, 16128–16135. [Google Scholar] [CrossRef]
- Bailmare, D.B.; Malozyomov, B.V.; Deshmukh, A.D. Electrodeposition of porous metal-organic frameworks for efficient charge storage. Commun. Chem. 2024, 7, 178. [Google Scholar] [CrossRef]
- Pilz, L.; Koenig, M.; Schwotzer, M.; Gliemann, H.; Wöll, C.; Tsotsalas, M. Enhancing the Quality of MOF Thin Films for Device Integration Through Machine Learning: A Case Study on HKUST-1 SURMOF Optimization. Adv. Funct. Mater. 2024, 34, 2404631. [Google Scholar] [CrossRef]
- Roy, B.C.; Ghosh, S.; Mahapatra, T.S.; Das, A. Ultrathin lanthanide-based 2D-coordination nanosheets: A versatile class of 2D materials. Coord. Chem. Rev. 2024, 518, 216058. [Google Scholar] [CrossRef]
- Yao, M.-S.; Otake, K.; Koganezawa, T.; Ogasawara, M.; Asakawa, H.; Tsujimoto, M.; Xue, Z.-Q.; Li, Y.-H.; Flanders, N.C.; Wang, P.; et al. Growth mechanisms and anisotropic softness–dependent conductivity of orientation-controllable metal–organic framework nanofilms. Proc. Natl. Acad. Sci. USA 2023, 120, e2305125120. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Sakamoto, R.; Yi, S.-T.; Katagiri, S.; Kambe, T.; Nishihara, H. Electrochromic Bis(terpyridine)metal Complex Nanosheets. J. Am. Chem. Soc. 2015, 137, 4681–4689. [Google Scholar] [CrossRef]
- Komeda, J.; Takada, K.; Maeda, H.; Fukui, N.; Tsuji, T.; Nishihara, H. Chemically laminated 2D bis(terpyridine)metal polymer films: Formation mechanism at the liquid-liquid interface and redox rectification. Chem. Eur. J. 2022, 28, e202201316. [Google Scholar] [CrossRef]
- Takada, K.; Ito, M.; Fukui, N.; Nishihara, H. Modulation between capacitor and conductor for a redox-active 2D bis(terpyridine)cobalt(II) nanosheet via anion-exchange. Commun. Chem. 2024, 7, 186. [Google Scholar] [CrossRef]
- Roy, S.; Chakraborty, C. Interfacial Coordination Nanosheet Based on Nonconjugated Three-Arm Terpyridine: A Highly Color-Efficient Electrochromic Material to Converge Fast Switching with Long Optical Memory. ACS Appl. Mater. Interfaces 2020, 12, 35181–35192. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cheng, X.; Chen, X.; Wang, H.; Zhao, Q.; Yang, C.; Du, X.; Xing, X.; Qiu, D. Interfacial self-assembly of a rigid-flexible terpyridine-Fe(ii) supramolecular film and the electrochromic performance of its solid-state devices. J. Mater. Chem. C 2024, 12, 18327–18333. [Google Scholar] [CrossRef]
- Chen, X.; Sun, X.; Dai, T.; Wang, H.; Zhao, Q.; Yang, C.; Du, X.; Xing, X.; Cheng, X.; Qiu, D. Novel Fe(II)-Based Supramolecular Film Prepared by Interfacial Self-Assembly of an Asymmetric Polypyridine Ligand and Its Electrochromic Performance. Molecules 2025, 30, 1376. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sakamoto, R.; Ho, C.-L.; Nishihara, H.; Wong, W.-Y. Electrochromic triphenylamine-based cobalt(II) complex nanosheets. J. Mater. Chem. C 2019, 7, 9159–9166. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, W.; Meng, Z.; Wong, W.-Y. A Tetrakis(terpyridine) Ligand–Based Cobalt(II) Complex Nanosheet as a Stable Dual-Ion Battery Cathode Material. Small 2020, 16, 1905204. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Han, C.-M.; Bai, C.; Ma, J.-J.; Yu, L.; Sun, L.-J.; Zhang, X.-Y.; Hu, H.-M. Supercapacitors Based on Mixed Nickel/Cobalt 2D MOF Coordination Nanosheets for Energy Storage. ACS Appl. Nano Mater. 2024, 7, 3897–3906. [Google Scholar] [CrossRef]
- Bera, M.K.; Sarmah, S.; Maity, A.; Higuchi, M. Construction of Heterometallic Coordination Nanosheets Comprising Both Inert and Labile Metal Ions Together via Metalloligand Approach. Inorg. Chem. 2025, in press. [CrossRef]
- Liu, J.-H.; Tu, T.; Shen, Y.-L.; Tu, B.; Qian, D.-J. Interfacial Self-Assembly of Organized Ultrathin Films of Tripodal Metal-Terpyridyl Coordination Polymers as Luminophores and Heterogeneous Catalysts for Photocatalytic CO2 Reduction. Langmuir 2023, 39, 4777–4788. [Google Scholar] [CrossRef]
- Li, X.-Y.; Zeng, H.; Hu, H.-M.; Sun, L.-J.; Zhang, J.-L.; Wang, X.-F. Multiterpyridyl Ligand/Cadmium(II) Coordination Polymer Nanosheets for Recoverable Luminescent Sensors. ACS Appl. Nano Mater. 2022, 5, 7113–7122. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C.; Zhan, X.; Yu, Y.; Zhang, K.; Luo, Q.; Gao, S.; Yang, J.; Xie, Y. Mechanistic insights into CO2 conversion chemistry of copper bis-(terpyridine) molecular electrocatalyst using accessible operando spectrochemistry. Nat. Commun. 2022, 13, 6029. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, J.; Wang, S. Interfacial Polymerization: From Chemistry to Functional Materials. Angew. Chem. Int. Ed. 2020, 59, 21840–21856. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, N.; Xu, Y.; Pang, H. Two-Dimensional MOF and COF Nanosheets: Synthesis and Applications in Electrochemistry. Chem. Eur. J. 2020, 26, 6402–6422. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, H.; Wang, Z.; Liu, R.; Luo, B.; Yang, D.; Chen, H.; Pan, L.; Ma, Z. Copper chloride complexes with substituted 4′-phenyl-terpyridine ligands: Synthesis, characterization, antiproliferative activities and DNA interactions. Dalton Trans. 2021, 50, 8243–8257. [Google Scholar] [CrossRef]
- Allmann, R.; Henke, W.; Reinen, D. Presence of a static Jahn-Teller distortion in copper(II) terpyridine complexes. 1. Crystal structure of diterpyridinecopper(II) nitrate. Inorg. Chem. 1978, 17, 378–382. [Google Scholar] [CrossRef]
- Meyer, A.; Schnakenburg, G.; Glaum, R.; Schiemann, O. (Bis(terpyridine))copper(II) Tetraphenylborate: A Complex Example for the Jahn–Teller Effect. Inorg. Chem. 2015, 54, 8456–8464. [Google Scholar] [CrossRef]
- Mondal, A.; Reddy, K.P.; Som, S.; Chopra, D.; Kundu, S. Nitrate and Nitrite Reductions at Copper(II) Sites: Role of Noncovalent Interactions from Second-Coordination-Sphere. Inorg. Chem. 2022, 61, 20337–20345. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.; Naisa, C.; Fu, Y.; Gali, S.M.; Paasch, S.; Wang, M.; Wittkaemper, H.; Papp, C.; Brunner, E.; et al. Poly(benzimidazobenzophenanthroline)-Ladder-TypeTwo-Dimensional Conjugated Covalent Organic Framework for Fast Proton Storage. Angew. Chem. Int. Ed. 2023, 62, e202310937. [Google Scholar] [CrossRef]
- Bauer, T.; Zheng, Z.; Renn, A.; Enning, R.; Stemmer, A.; Sakamoto, J.; Schlüter, A.D. Synthesis of Free-Standing, Monolayered Organometallic Sheets at the Air/Water Interface. Angew. Chem. Int. Ed. 2011, 50, 7879–7884. [Google Scholar] [CrossRef]
- Elgrishi, N.B.; Chambers, M.B.; Artero, V.; Fontecave, M. Terpyridine complexes of first row transition metals and electrochemical reduction of CO2 to CO. Phys. Chem. Chem. Phys. 2014, 16, 13635–13644. [Google Scholar] [CrossRef]
- Yamada, S. A Transient Supercapacitor with a Water-Dissolvable Ionic Gel for Sustainable Electronics. ACS Appl. Mater. Interfaces 2022, 14, 26595–26603. [Google Scholar] [CrossRef]
- Cavazzini, M.; Quici, S.; Scalera, C.; Puntoriero, F.; Ganga, G.L.; Campagna, S. Synthesis, Characterization, Absorption Spectra, and Luminescence Properties of Multinuclear Species Made of Ru(II) and Ir(III) Chromophores. Inorg. Chem. 2009, 48, 8578–8592. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takada, K.; Komeda, J.; Maeda, H.; Fukui, N.; Masunaga, H.; Sasaki, S.; Nishihara, H. Interfacial Synthesis of an Electro-Functional 2D Bis(terpyridine)copper(II) Polymer Nanosheet. Molecules 2025, 30, 2044. https://doi.org/10.3390/molecules30092044
Takada K, Komeda J, Maeda H, Fukui N, Masunaga H, Sasaki S, Nishihara H. Interfacial Synthesis of an Electro-Functional 2D Bis(terpyridine)copper(II) Polymer Nanosheet. Molecules. 2025; 30(9):2044. https://doi.org/10.3390/molecules30092044
Chicago/Turabian StyleTakada, Kenji, Joe Komeda, Hiroaki Maeda, Naoya Fukui, Hiroyasu Masunaga, Sono Sasaki, and Hiroshi Nishihara. 2025. "Interfacial Synthesis of an Electro-Functional 2D Bis(terpyridine)copper(II) Polymer Nanosheet" Molecules 30, no. 9: 2044. https://doi.org/10.3390/molecules30092044
APA StyleTakada, K., Komeda, J., Maeda, H., Fukui, N., Masunaga, H., Sasaki, S., & Nishihara, H. (2025). Interfacial Synthesis of an Electro-Functional 2D Bis(terpyridine)copper(II) Polymer Nanosheet. Molecules, 30(9), 2044. https://doi.org/10.3390/molecules30092044






