Recoverable and Sensitive Pressure-Induced Mechanochromic Photoluminescence of a Au-P Complex
Abstract
1. Introduction
2. Results and Discussion
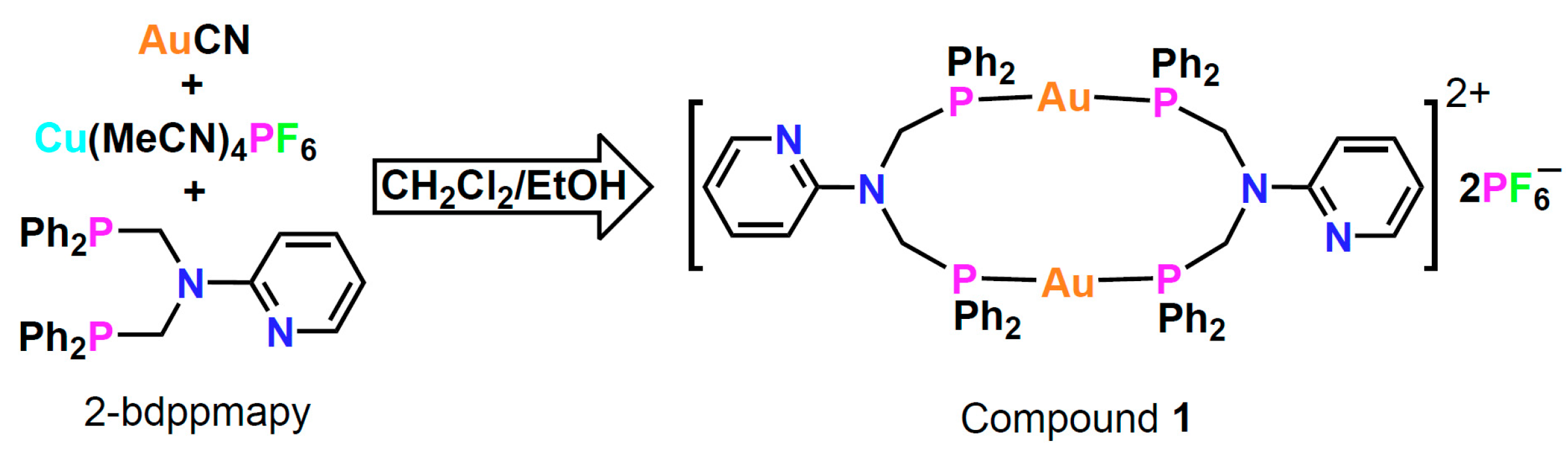
2.1. Synthesis and Characterisation
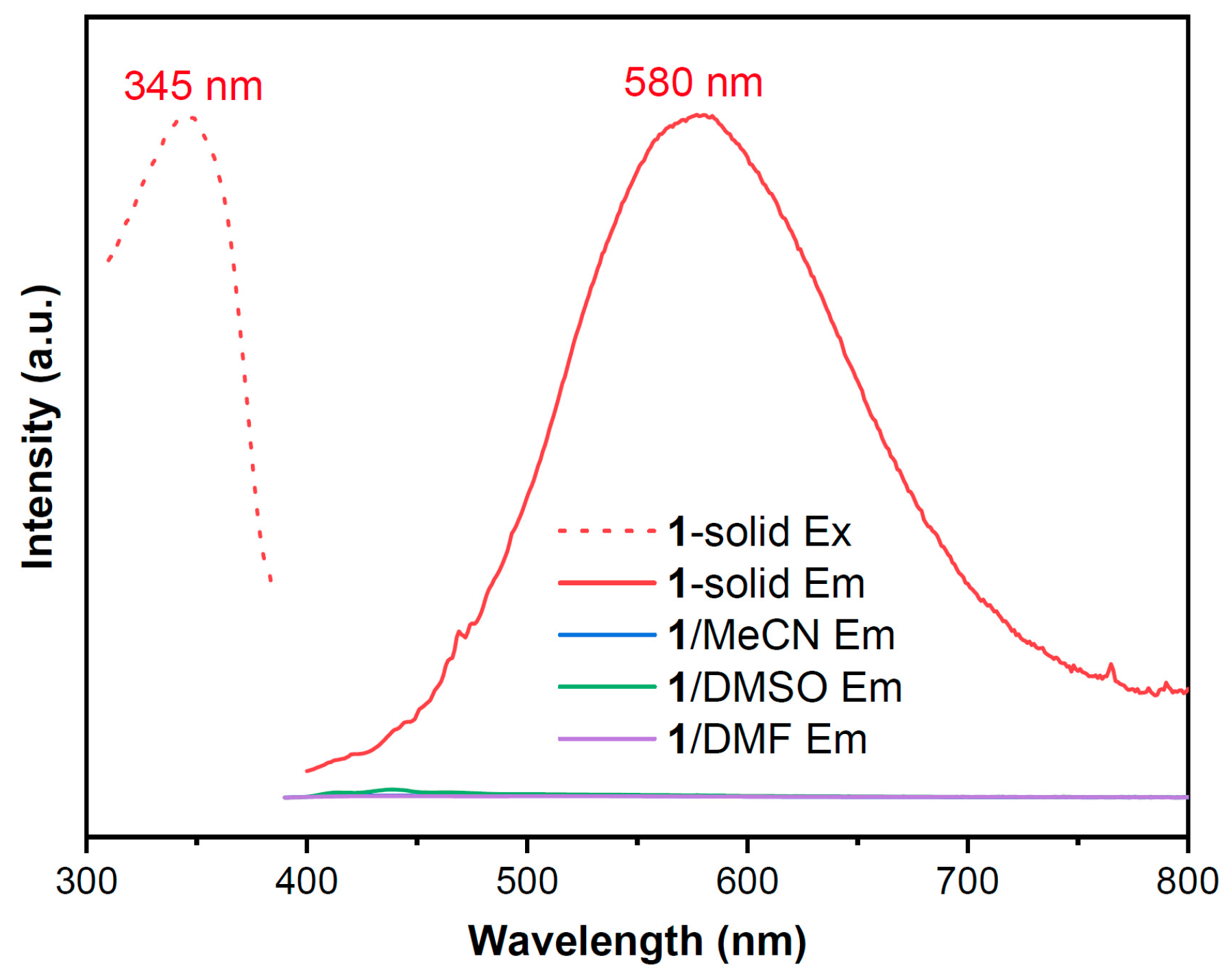
2.2. Photophysical Properties
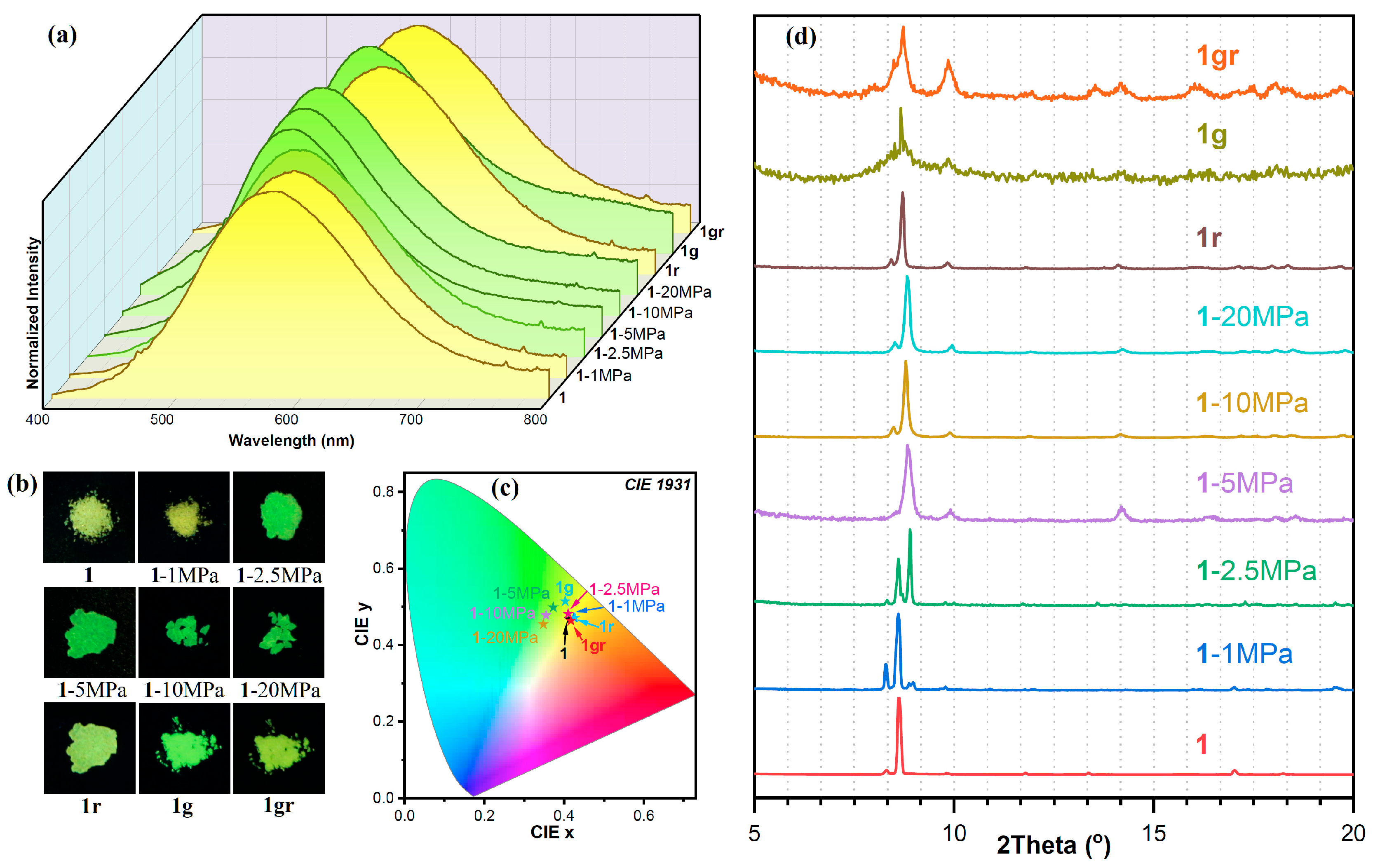
2.3. Pressure-Induced Mechanochromic Photoluminescence
2.4. Application to Encrypted Information Transfer
3. Experimental Section
3.1. Materials, Characterisation, and Measurements
3.2. Synthesis of [Au2(2-Bdppmapy)2](PF6)2 (1)
3.3. Preparations of 1—1 MPa, 1—2.5 MPa, 1—5 MPa, 1—10 MPa, 1—20 MPa, and 1g
3.4. Preparations of 1r and 1gr
3.5. Preparation of ‘Secret Writing Paper’
3.6. Single-Crystal X-Ray Diffraction (SCXRD) Determination
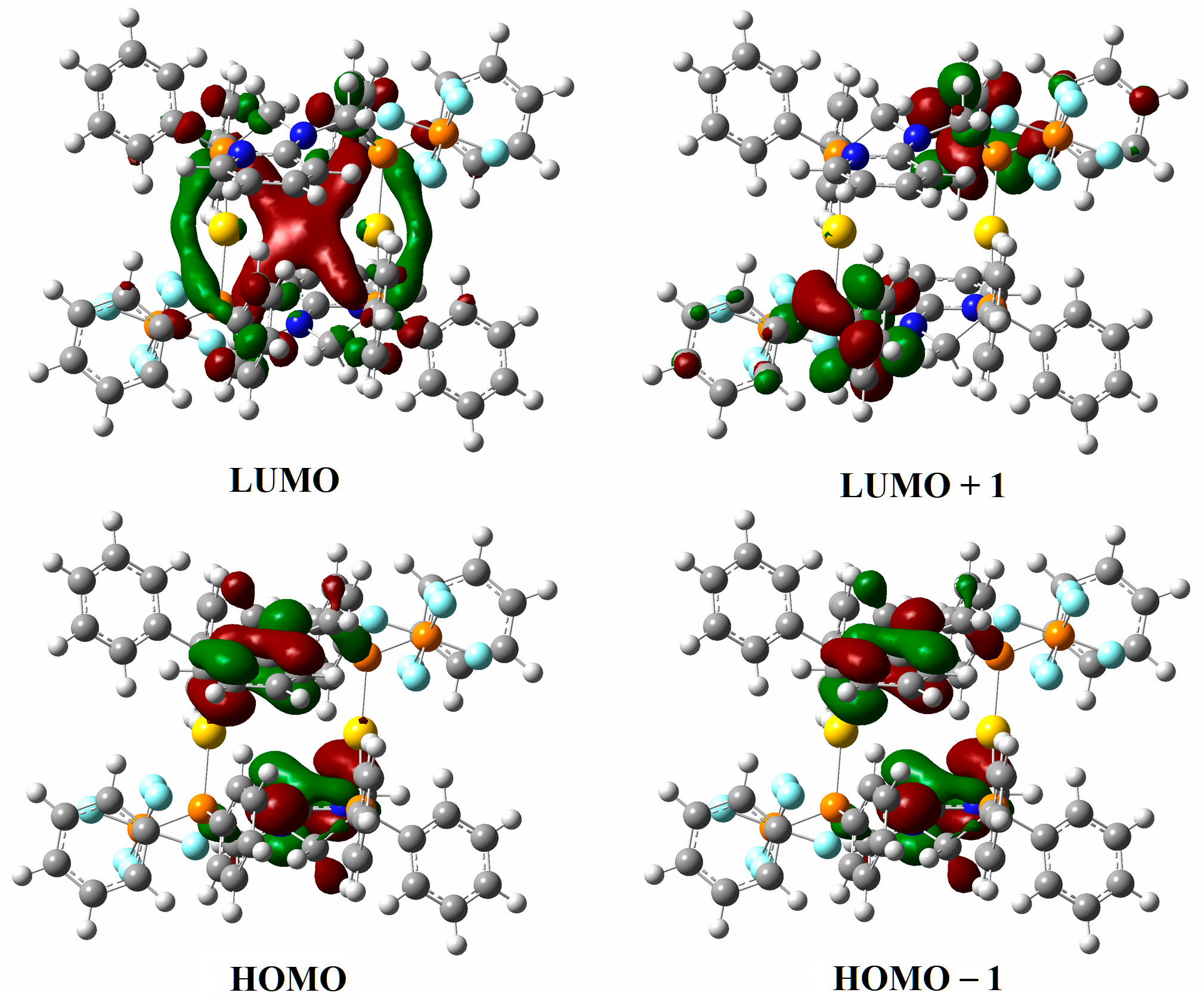
3.7. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, M.; Ito, H. Solid-state luminescence of Au(I) complexes with external stimuli-responsive properties. J. Photochem. Photobiol. C 2022, 51, 100478. [Google Scholar] [CrossRef]
- Tong, Y.; Chen, X.-W.; He, L.-H.; Chen, J.-L.; Liu, S.-J.; Wen, H.-R. Reversible stimuli-responsive luminescence of bimetallic cuprous complexes based on NH-deprotonated 3-(2′-pyridyl)pyrazole. J. Mater. Chem. C 2021, 9, 16664–16671. [Google Scholar] [CrossRef]
- Yang, D.-D.; Xiao, T.; Yang, Y.-Y.; Xue, J.-H.; Shi, Y.-S.; Ma, Q.; Zheng, X.-J. Two viologen-based complexes as persistent luminescent materials and their applications in inkless print and anticounterfeiting. Chem. Eng. J. 2024, 488, 151047. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, K.; Kim, C.K.; Tan, S.; Wang, S.; Wang, L.; Li, J.; Wang, Y. Stimuli-responsive cyclometalated platinum complex bearing bent molecular geometry for highly efficient solution-processable OLEDs. Chin. Chem. Lett. 2021, 32, 493–496. [Google Scholar] [CrossRef]
- Takeda, H.; Kobayashi, A.; Tsuge, K. Recent developments of photoactive Cu(I) and Ag(I) complexes with diphosphine and related ligands. Coord. Chem. Rev. 2022, 470, 214700. [Google Scholar] [CrossRef]
- Kumar, P.; Kaushik, R.; Ghosh, A.; Jose, D.A. Detection of Moisture by Fluorescent OFF-ON Sensor in Organic Solvents and Raw Food Products. Anal. Chem. 2016, 88, 11314–11318. [Google Scholar] [CrossRef]
- Lv, C.-L.; Yang, C.-H.; Liu, L.-Y.; Zhang, Z.-C. Organoimido functionalized trinuclear gold(I) clusters with fluorescent chromophore. Rare Met. 2020, 40, 1437–1442. [Google Scholar] [CrossRef]
- Lee, L.C.; Lo, K.K. Shining New Light on Biological Systems: Luminescent Transition Metal Complexes for Bioimaging and Biosensing Applications. Chem. Rev. 2024, 124, 8825–9014. [Google Scholar] [CrossRef]
- Seki, T.; Takamatsu, Y.; Ito, H. A Screening Approach for the Discovery of Mechanochromic Gold(I) Isocyanide Complexes with Crystal-to-Crystal Phase Transitions. J. Am. Chem. Soc. 2016, 138, 6252–6260. [Google Scholar] [CrossRef]
- Qin, Y.; She, P.; Huang, X.; Huang, W.; Zhao, Q. Luminescent manganese(II) complexes: Synthesis, properties and optoelectronic applications. Coord. Chem. Rev. 2020, 416, 213331. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Ai, Y.; Hong, E.Y.; Chan, A.K.; Yam, V.W. Supramolecular Self-Assembly and Dual-Switch Vapochromic, Vapoluminescent, and Resistive Memory Behaviors of Amphiphilic Platinum(II) Complexes. J. Am. Chem. Soc. 2017, 139, 13858–13866. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, J.; Peng, N.; Jiang, L.; Zhao, S.; Fu, D.-Y.; Li, G. Multi-stimuli responsive lanthanides-based luminescent hydrogels for advanced information encryption. J. Mol. Liq. 2022, 368, 120681. [Google Scholar] [CrossRef]
- Xiao, Z.-M.; Yang, J.-X.; Chen, X.; Tang, W.-J.; Peng, S.-K.; Hao, D.-B.; Zhao, Z.-P.; Zheng, J.; Li, D. A fluorescence–phosphorescence dual-emissive Cu3(pyrazolate)3 complex with highly tunable emission colours for anticounterfeiting and temperature sensing. Inorg. Chem. Front. 2024, 11, 1808–1818. [Google Scholar] [CrossRef]
- Yang, H.; Peng, S.K.; Zheng, J.; Luo, D.; Xie, M.; Huang, Y.L.; Cai, X.; Wang, J.; Zhou, X.P.; Li, D. Achiral Au(I) Cyclic Trinuclear Complexes with High-Efficiency Circularly Polarized Near-Infrared TADF. Angew. Chem. Int. Ed. 2023, 62, e202310495. [Google Scholar] [CrossRef]
- Wu, N.M.; Ng, M.; Yam, V.W. Photochromic Benzo[b]phosphole Alkynylgold(I) Complexes with Mechanochromic Property to Serve as Multistimuli-Responsive Materials. Angew. Chem. Int. Ed. 2019, 58, 3027–3031. [Google Scholar] [CrossRef]
- Wu, N.M.; Ng, M.; Yam, V.W. Photocontrolled multiple-state photochromic benzo[b]phosphole thieno[3,2-b]phosphole-containing alkynylgold(I) complex via selective light irradiation. Nat. Commun. 2022, 13, 33. [Google Scholar] [CrossRef]
- Rolz, M.; Butschke, B.; Breit, B. Azobenzene-Integrated NHC Ligands: A Versatile Platform for Visible-Light-Switchable Metal Catalysis. J. Am. Chem. Soc. 2024, 146, 13210–13225. [Google Scholar] [CrossRef]
- Pi, Q.; Bi, D.; Qiu, D.; Wang, H.; Cheng, X.; Feng, Y.; Zhao, Q.; Zhou, M. A dual-wavelength electrochromic film based on a Pt(II) complex for optical modulation at telecommunication wavelengths and dark solid-state display devices. J. Mater. Chem. C 2021, 9, 8994–9000. [Google Scholar] [CrossRef]
- Banasz, R.; Wałęsa-Chorab, M. Polymeric complexes of transition metal ions as electrochromic materials: Synthesis and properties. Coord. Chem. Rev. 2019, 389, 1–18. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, S.T.; Simon, F.; Steiner, A.M.; Schubert, J.; Du, Y.; Qiao, Z.; Fery, A.; Lissel, F. Poly(3-hexylthiophene)s Functionalized with N-Heterocyclic Carbenes as Robust and Conductive Ligands for the Stabilization of Gold Nanoparticles. Angew. Chem. Int. Ed. 2021, 60, 3912–3917. [Google Scholar] [CrossRef]
- Brown, C.M.; Carta, V.; Wolf, M.O. Thermochromic Solid-State Emission of Dipyridyl Sulfoxide Cu(I) Complexes. Chem. Mater. 2018, 30, 5786–5795. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Ryzhikov, M.R.; Berezin, A.S.; Kolesnikov, I.E.; Samsonenko, D.G.; Bagryanskaya, I.Y. Photoluminescence of Ag(I) complexes with a square-planar coordination geometry: The first observation. Inorg. Chem. Front. 2019, 6, 2855–2864. [Google Scholar] [CrossRef]
- Stal, S.; Huitorel, B.; Coustham, T.; Stephant, N.; Massuyeau, F.; Gacoin, T.; Bouteiller, L.; Perruchas, S. Photoactive CuI-Cross-Linked Polyurethane Materials. ACS Appl. Mater. Interfaces 2022, 14, 47931–47940. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Rogovoy, M.I.; Odud, I.M.; Davydova, M.P.; Rakhmanova, M.I.; Petrov, P.A.; Brel, V.K.; Artushin, O.I.; Brylev, K.A.; Samsonenko, D.G.; et al. Toward highly efficient TADF-active Cu(I), Ag(I) and Au(I) carbene complexes using symmetry-based design strategy. Inorg. Chem. Front. 2024, 11, 8778–8788. [Google Scholar] [CrossRef]
- Soto, M.A.; Carta, V.; Andrews, R.J.; Chaudhry, M.T.; MacLachlan, M.J. Structural Elucidation of Selective Solvatochromism in a Responsive-at-Metal Cyclometalated Platinum(II) Complex. Angew. Chem. Int. Ed. 2020, 59, 10348–10352. [Google Scholar] [CrossRef]
- Nagy, M.; Rácz, D.; Nagy, Z.L.; Fehér, P.P.; Kalmár, J.; Fábián, I.; Kiss, A.; Zsuga, M.; Kéki, S. Solvatochromic isocyanonaphthalene dyes as ligands for silver(I) complexes, their applicability in silver(I) detection and background reduction in biolabelling. Sens. Actuators B 2018, 255, 2555–2567. [Google Scholar] [CrossRef]
- Seki, T.; Ozaki, T.; Okura, T.; Asakura, K.; Sakon, A.; Uekusa, H.; Ito, H. Interconvertible multiple photoluminescence color of a gold(I) isocyanide complex in the solid state: Solvent-induced blue-shifted and mechano-responsive red-shifted photoluminescence. Chem. Sci. 2015, 6, 2187–2195. [Google Scholar] [CrossRef]
- Zhao, X.; Gong, J.; Li, Z.; Sung, H.H.Y.; Williams, I.D.; Lam, J.W.Y.; Zhao, Z.; Tang, B.Z.; Wong, W.Y.; Xu, L. Au···I coinage bonds: Boosting photoluminescence efficiency and solid-state molecular motion. Aggregate 2024, 6, e686. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Wang, Q.; Tong, H.; Zhu, J.; Liu, W.; Ouyang, G. Ligand Detachment—New Insight into the Mechanochromic Luminescence Mechanism of Copper Iodide Complexes with Thermally Activated Delayed Fluorescence. Adv. Opt. Mater. 2024, 12, 2400364. [Google Scholar] [CrossRef]
- Chen, X.-W.; He, L.-H.; Ju, P.; Chen, J.-L.; Liu, S.-J.; Wen, H.-R. Mechanochromic luminescent materials of bimetallic Cu(I) complexes showing thermally activated delayed fluorescence. J. Mater. Chem. C 2020, 8, 16160–16167. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Katagiri, Y.; Tanaka, Y.; Yoshizawa, M. Solution-state mechanochromic luminescence of Pt(II)-complexes displayed within micellar aromatic capsules. Chem. Sci. 2023, 14, 14211–14216. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.; Kim, J.; Lee, K.Y.; Kim, T.H. Non-Phase-Transition Luminescence Mechanochromism of a Copper(I) Coordination Polymer. Inorg. Chem. 2017, 56, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Deak, A.; Jobbagy, C.; Demeter, A.; Celko, L.; Cihlar, J.; Szabo, P.T.; Abranyi-Balogh, P.; Crawford, D.E.; Virieux, D.; Colacino, E. Mechanochemical synthesis of mononuclear gold(I) halide complexes of diphosphine ligands with tuneable luminescent properties. Dalton Trans. 2021, 50, 13337–13344. [Google Scholar] [CrossRef]
- Seki, T.; Sakurada, K.; Ito, H. Controlling mechano- and seeding-triggered single-crystal-to-single-crystal phase transition: Molecular domino with a disconnection of aurophilic bonds. Angew. Chem. Int. Ed. Engl. 2013, 52, 12828–12832. [Google Scholar] [CrossRef]
- Ito, H.; Muromoto, M.; Kurenuma, S.; Ishizaka, S.; Kitamura, N.; Sato, H.; Seki, T. Mechanical stimulation and solid seeding trigger single-crystal-to-single-crystal molecular domino transformations. Nat. Commun. 2013, 4, 2009. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.Y.; Ni, J.; Zhang, L.Y.; Chen, Z.N. Vapochromic and mechanochromic phosphorescence materials based on a platinum(II) complex with 4-trifluoromethylphenylacetylide. Inorg. Chem. 2012, 51, 5569–5579. [Google Scholar] [CrossRef]
- Eddingsaas, N.; Suslick, K. Intense Mechanoluminescence and Gas Phase Reactions from the Sonication of an Organic Slurry. J. Am. Chem. Soc. 2007, 129, 6718–6719. [Google Scholar] [CrossRef]
- Zhang, X.; Chi, Z.; Zhang, Y.; Liu, S.; Xu, J. Recent advances in mechanochromic luminescent metal complexes. J. Mater. Chem. C 2013, 1, 3376–3390. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, Z.; Yin, Y.; Sun, Y.; Liu, S. Progress in mechanochromic luminescence of gold(I) complexes. Chin. Chem. Lett. 2021, 32, 3718–3732. [Google Scholar] [CrossRef]
- Chen, W.-T.; Li, C.-H.; Liang, Z.-Y.; Zhang, Z.-L.; Liu, D.-Q.; Ye, J.-W.; Chen, L.; Chen, X.-M. Large and Tunable Wavelength Blue Shifts in Luminescent Piezochromism of Cu(I) Complexes via a Guest Encapsulation Strategy. ACS Mater. Lett. 2024, 6, 2077–2084. [Google Scholar] [CrossRef]
- Walters, D.T.; Aghakhanpour, R.B.; Powers, X.B.; Ghiassi, K.B.; Olmstead, M.M.; Balch, A.L. Utilization of a Nonemissive Triphosphine Ligand to Construct a Luminescent Gold(I)-Box That Undergoes Mechanochromic Collapse into a Helical Complex. J. Am. Chem. Soc. 2018, 140, 7533–7542. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-B.; Luo, W.-J.; Li, K.-X.; Yuan, W.-Z.; Zhang, Y.-M. Aggregation-induced phosphorescence and mechanochromic luminescence of a tetraphenylethene-based gold(I) isocyanide complex. Chin. Chem. Lett. 2017, 28, 1300–1305. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.-Y.; Wang, J.-Y.; Dai, F.-R.; Chen, Z.-N. Two-step phosphorescent mechanochromism due to intramolecular deformation. J. Mater. Chem. C 2020, 8, 715–720. [Google Scholar] [CrossRef]
- Zhang, J.; He, B.; Wu, W.; Alam, P.; Zhang, H.; Gong, J.; Song, F.; Wang, Z.; Sung, H.H.Y.; Williams, I.D.; et al. Molecular Motions in AIEgen Crystals: Turning on Photoluminescence by Force-Induced Filament Sliding. J. Am. Chem. Soc. 2020, 142, 14608–14618. [Google Scholar] [CrossRef]
- Ai, Y.; Li, Y.; Chan, M.H.; Xiao, G.; Zou, B.; Yam, V.W. Realization of Distinct Mechano- and Piezochromic Behaviors via Alkoxy Chain Length-Modulated Phosphorescent Properties and Multidimensional Self-Assembly Structures of Dinuclear Platinum(II) Complexes. J. Am. Chem. Soc. 2021, 143, 10659–10667. [Google Scholar] [CrossRef]
- Balch, A.L. Dynamic crystals: Visually detected mechanochemical changes in the luminescence of gold and other transition-metal complexes. Angew. Chem. Int. Ed. Engl. 2009, 48, 2641–2644. [Google Scholar] [CrossRef]
- Kuchison, A.M.; Wolf, M.O.; Patrick, B.O. Conjugated ligand-based tribochromic luminescence. Chem. Commun. 2009, 47, 7387–7389. [Google Scholar] [CrossRef]
- Lechner, A.; Gliemann, G. Pressure Effects on the Absorption and Emission of Tetracyanoplatinates(II) in Solution. J. Am. Chem. Soc. 1989, 111, 7469–7475. [Google Scholar] [CrossRef]
- Theoretical Studies of the Spectroscopic Properties of [Pt(trpy)C⋮CR]+ (trpy = 2,2′,6′,2′′-Terpyridine; R = H, CH2OH, and C6H5). J. Phys. Chem. A 2005, 109, 8809–8818. [CrossRef]
- Grey, J.K.; Butler, I.S.; Reber, C. Pressure-Induced Enhancements of Luminescence Intensities and Lifetimes Correlated with Emitting-State Distortions for Thiocyanate and Selenocyanate Complexes of Platinum(II) and Palladium(II). Inorg. Chem. 2003, 42, 6503–6518. [Google Scholar] [CrossRef]
- Rawashdeh-Omary, M.A.; Omary, M.A.; Patterson, H.H.; Fackler, J.P. Excited-State Interactions for [Au(CN)2−]n and [Ag(CN)2−]n Oligomers in Solution. Formation of Luminescent Gold-Gold Bonded Excimers and Exciplexes. J. Am. Chem. Soc. 2001, 123, 11237–11247. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Tokodai, N.; Omagari, S.; Nakanishi, T.; Hasegawa, Y.; Iwasa, T.; Taketsugu, T.; Ito, H. Luminescent Mechanochromic 9-Anthryl Gold(I) Isocyanide Complex with an Emission Maximum at 900 nm after Mechanical Stimulation. J. Am. Chem. Soc. 2017, 139, 6514–6517. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Chen, X.R.; Wu, K.; Lu, Z.; Wang, K.; Li, N.; Wei, R.J.; Zhan, S.Z.; Ning, G.H.; Zou, B.; et al. Pressure-induced phosphorescence enhancement and piezochromism of a carbazole-based cyclic trinuclear Cu(I) complex. Chem. Sci. 2021, 12, 4425–4431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, J.; Zhu, Y.; Zeng, Q.; Ai, Y.; Li, Y. Multi-stimuli responsive Pt(II) complexes for information storage and anti-counterfeiting. Chem. Eng. J. 2024, 498, 155049. [Google Scholar] [CrossRef]
- Ono, T.; Tsukiyama, Y.; Taema, A.; Sato, H.; Kiyooka, H.; Yamaguchi, Y.; Nagahashi, A.; Nishiyama, M.; Akahama, Y.; Ozawa, Y.; et al. Piezofluorochromism in Charge-Transfer Inclusion Crystals: The Influence of High Pressure versus Mechanical Grinding. ChemPhotoChem. 2018, 2, 416–420. [Google Scholar] [CrossRef]
- Ito, H.; Saito, T.; Oshima, N.; Kitamura, N.; Ishizaka, S.; Hinatsu, Y.; Wakeshima, M.; Kato, M.; Tsuge, K.; Sawamura, M. Reversible Mechanochromic Luminescence of [(C6F5Au)2(µ-1,4-Diisocyanobenzene)]. J. Am. Chem. Soc. 2008, 130, 10044–10045. [Google Scholar] [CrossRef]
- Lim, S.H.; Olmstead, M.M.; Balch, A.L. Molecular accordion: Vapoluminescence and molecular flexibility in the orange and green luminescent crystals of the dimer, Au2(µ-bis-(diphenylphosphino)ethane)2Br2. J. Am. Chem. Soc. 2011, 133, 10229–10238. [Google Scholar] [CrossRef]
- Lim, S.H.; Olmstead, M.M.; Balch, A.L. Inorganic topochemistry. Vapor-induced solid state transformations of luminescent, three-coordinate gold(I) complexes. Chem. Sci. 2013, 4, 311–318. [Google Scholar] [CrossRef]
- Deák, A.; Jobbagy, C.; Marsi, G.; Molnar, M.; Szakacs, Z.; Baranyai, P. Anion-, Solvent-, Temperature-, and Mechano-Responsive Photoluminescence in Gold(I) Diphosphine-Based Dimers. Chem. Eur. J. 2015, 21, 11495–11508. [Google Scholar] [CrossRef]
- Jobbágy, C.; Baranyai, P.; Marsi, G.; Rácz, B.; Li, L.; Naumov, P.; Deák, A. Novel gold(I) diphosphine-based dimers with aurophilicity triggered multistimuli light-emitting properties. J. Mater. Chem. C 2016, 4, 10253–10264. [Google Scholar] [CrossRef]
- Hu, S.; Yan, S.; Hu, Y.; Young, D.J.; Li, H.-X.; Lu, C.; He, J.-H.; Ren, Z.-G. A PN(Pz)P ligand protected Au2Cu2 complex for photoluminescent ultra-low humidity detection with reversible single-crystal-to-single-crystal transformations. Inorg. Chem. Front. 2023, 10, 3706–3713. [Google Scholar] [CrossRef]
- Huang, J.; Hu, Y.; Xu, W.; Yang, W.; Lu, C.; Young, D.J.; Ren, Z.-G. Switchable Fluorescence of a Mechanical Stimulus-Responsive Au-P-S Complex. Molecules 2024, 29, 5736. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, J.; Yan, S.; Yang, N.; Young, D.J.; Li, H.-X.; He, X.; Lu, Y.; Ren, Z.-G. A phosphorescent Au-P complex exhibiting ROS generation, enhanced emission in polymers and photodynamic inactivation of bacteria activities. J. Mol. Struc. 2025, 1337, 142254. [Google Scholar] [CrossRef]
- Pal, S.; Kathewad, N.; Pant, R.; Khan, S. Synthesis, Characterization, and Luminescence Studies of Gold(I) Complexes with PNP- and PNB-Based Ligand Systems. Inorg. Chem. 2015, 54, 10172–10183. [Google Scholar] [CrossRef]
- Kathewad, N.; Kumar, N.; Dasgupta, R.; Ghosh, M.; Pal, S.; Khan, S. The syntheses and photophysical properties of PNP-based Au(I) complexes with strong intramolecular Au⋯Au interactions. Dalton Trans. 2019, 48, 7274–7280. [Google Scholar] [CrossRef]
- Sathyanarayana, A.; Nakamura, S.-Y.; Hisano, K.; Tsutsumi, O.; Srinivas, K.; Prabusankar, G. Controlling the solid-state luminescence of gold(I) N-heterocyclic carbene complexes through changes in the structure of molecular aggregates. Sci. China Chem. 2018, 61, 957–965. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhang, J.; Dong, Y.-B.; Zhang, Y.; Yin, J.; Liu, S.H. Different structures modulated mechanochromism and aggregation-induced emission in a series of Gold(I) complexes. Dye. Pigment. 2018, 156, 74–81. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, G.; Pu, S.; Liu, S.H. Bipyridine-based aggregation-induced phosphorescent emission (AIPE)-active gold(I) complex with reversible phosphorescent mechanochromism and self-assembly characteristics. Dye. Pigment. 2018, 152, 54–59. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Tang, W.-J.; Yang, H.; Peng, S.-K.; Xiao, Z.-M.; Huang, G.-Q.; Zheng, J.; Li, D. Multistimuli-responsive behavior of a phosphorescent Cu3pyrazolate3complex for luminescent logic gates and encrypted information transformation. Inorg. Chem. Front. 2023, 10, 2594–2606. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Xin, G.; Fu, W.-F.; Xu, H.; Li, L. Interaction of free functional group with platinum(II) center in cyclometalated complexes: A structural and photophysical property investigation. Inorganica Chim. Acta 2010, 363, 338–345. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL-2016; Universität Göttingen: Göttingen, Germany, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]

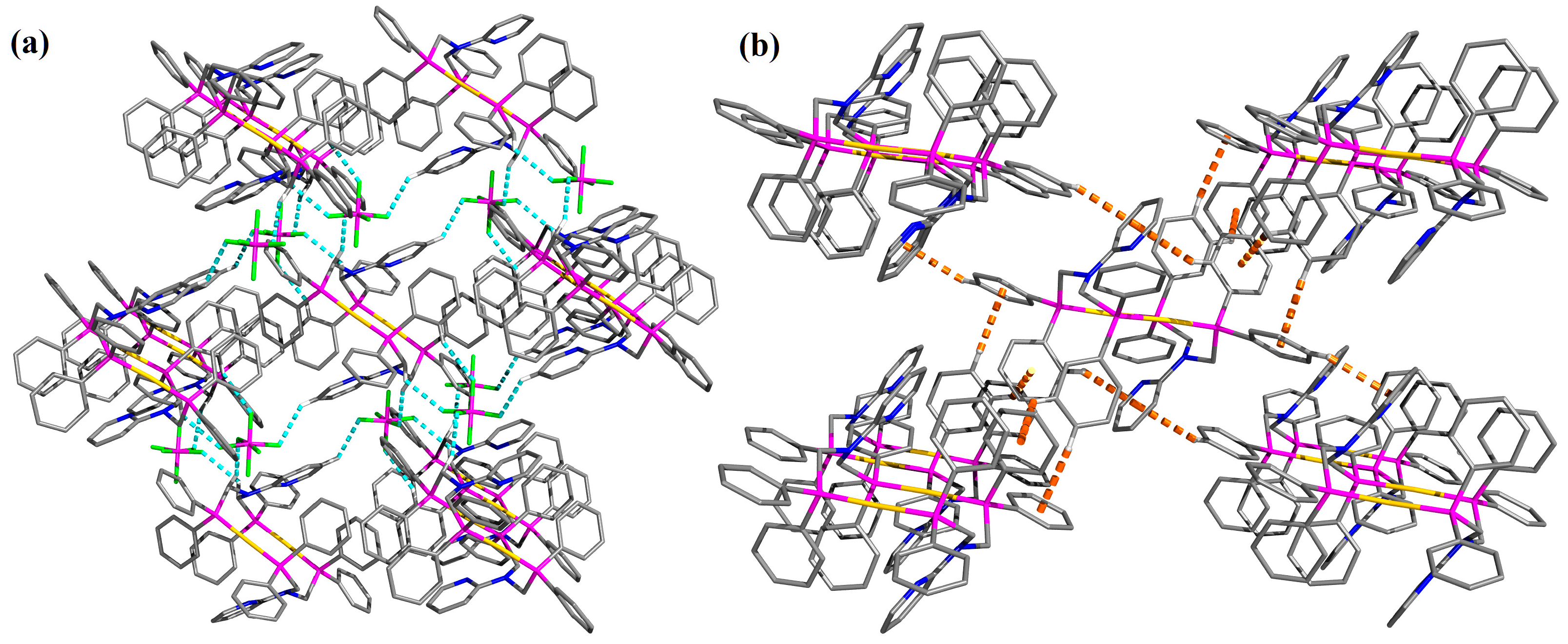
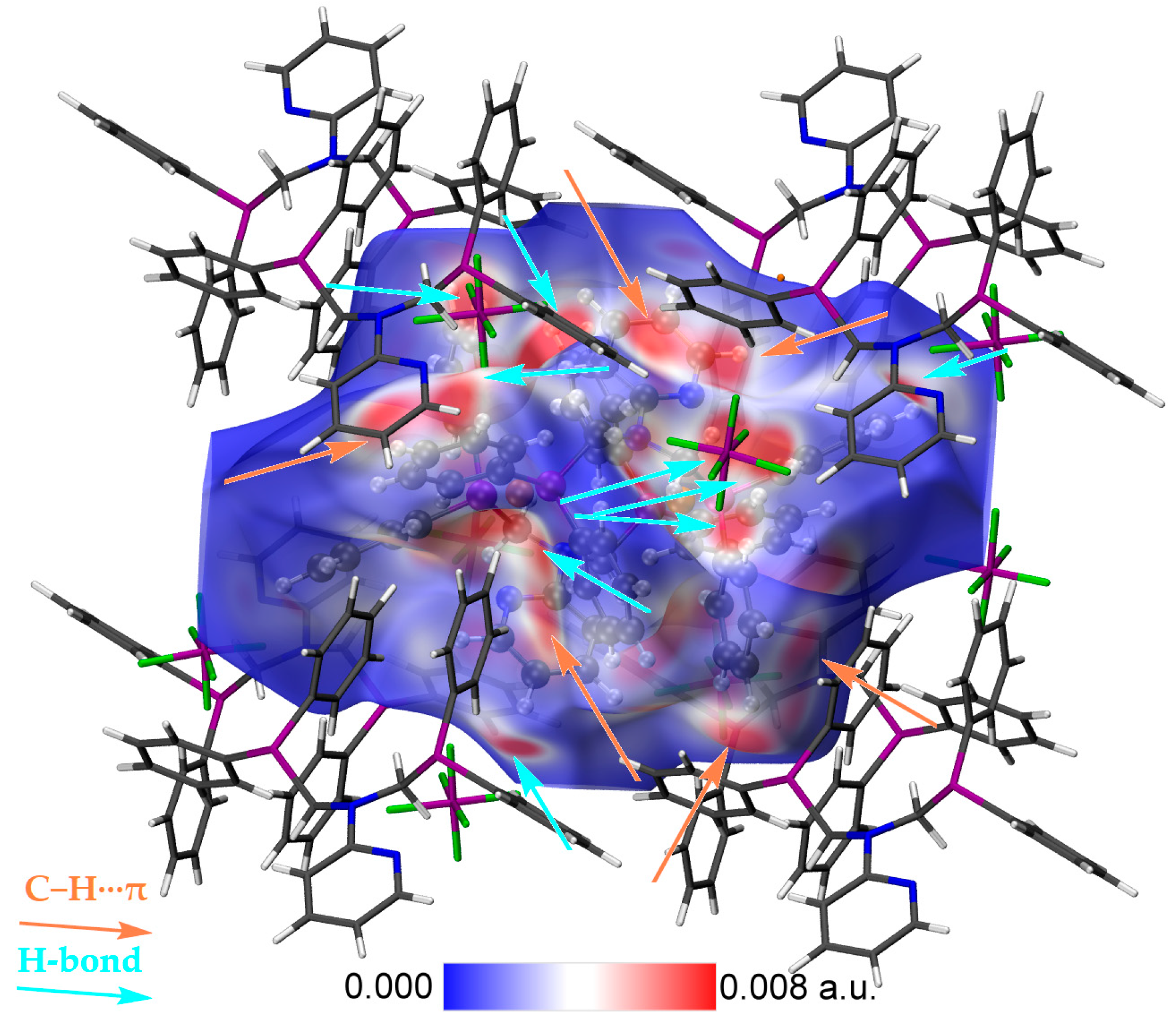


| D–H···A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
| C1–H1B···F3 | 0.98 | 2.42 | 3.35(2) | 157 |
| C2–H2A···F6 i | 0.98 | 2.48 | 3.09(2) | 120 |
| C6–H6···F4 ii | 0.94 | 2.43 | 3.21(2) | 141 |
| C21–H21···F2 i | 0.94 | 2.51 | 3.34(2) | 147 |
| D–H···Cg | H···Cg | γ |
|---|---|---|
| C9–H9···Cg1 i | 2.76 | 7.7 |
| C17–H17···Cg1 ii | 2.84 | 10.9 |
| C22–H22···Cg2 iii | 2.91 | 11.9 |
| C23–H23···Cg3 iii | 2.86 | 8.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Chang, Y.; Wang, J.; Young, D.J.; Li, H.-X.; Lu, Y.; Ren, Z.-G. Recoverable and Sensitive Pressure-Induced Mechanochromic Photoluminescence of a Au-P Complex. Molecules 2025, 30, 2011. https://doi.org/10.3390/molecules30092011
Yang N, Chang Y, Wang J, Young DJ, Li H-X, Lu Y, Ren Z-G. Recoverable and Sensitive Pressure-Induced Mechanochromic Photoluminescence of a Au-P Complex. Molecules. 2025; 30(9):2011. https://doi.org/10.3390/molecules30092011
Chicago/Turabian StyleYang, Ningwen, Yijia Chang, Jiangyue Wang, David James Young, Hong-Xi Li, Yuxin Lu, and Zhi-Gang Ren. 2025. "Recoverable and Sensitive Pressure-Induced Mechanochromic Photoluminescence of a Au-P Complex" Molecules 30, no. 9: 2011. https://doi.org/10.3390/molecules30092011
APA StyleYang, N., Chang, Y., Wang, J., Young, D. J., Li, H.-X., Lu, Y., & Ren, Z.-G. (2025). Recoverable and Sensitive Pressure-Induced Mechanochromic Photoluminescence of a Au-P Complex. Molecules, 30(9), 2011. https://doi.org/10.3390/molecules30092011








