Voltametric Analysis of Ergosterol Isolated from Wild-Growing and Cultivated Edible Mushrooms from Serbia and Korea
Abstract
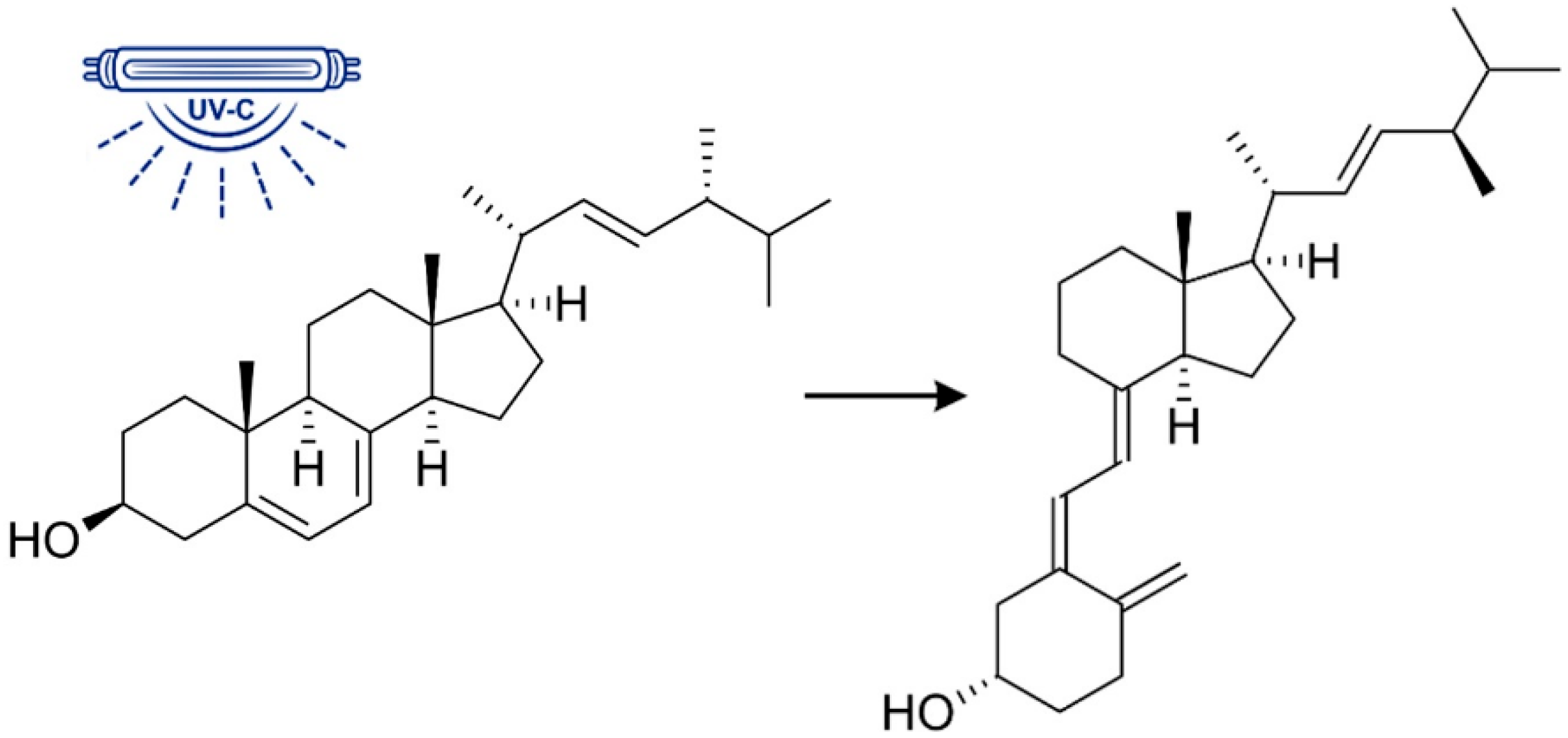
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Samples
3.2. Chemicals and Reagents
3.3. Ergosterol Extraction
3.4. Voltametric Analysis of Ergosterol
3.5. Sample Preparation for Elemental Analysis
3.6. Instrumental and Operating Parameters for ICP-OES Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taofiq, O.; Silva, A.R.; Costa, C.; Ferreira, I.; Nunes, J.; Prieto, M.A.; Simal-Gandara, J.; Barros, L.; Ferreira, I.C.F.R. Optimization of Ergosterol Extraction from Pleurotus Mushrooms Using Response Surface Methodology. Food Funct. 2020, 11, 5887–5897. [Google Scholar] [CrossRef] [PubMed]
- Umaña, M.; Eim, V.; Garau, C.; Rosselló, C.; Simal, S. Ultrasound-Assisted Extraction of Ergosterol and Antioxidant Components from Mushroom by-Products and the Attainment of a β-Glucan Rich Residue. Food Chem. 2020, 332, 127390. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; Bairwa, R.; Lal, P.; Pattanayak, S.; Chakrapani, K.; Poorvasandhya, R.; Kumar, A.; Altaf, M.A.; Tiwari, R.K.; Lal, M.K.; et al. Edible Mushrooms Trending in Food: Nutrigenomics, Bibliometric, from Bench to Valuable Applications. Heliyon 2024, 10, e36963. [Google Scholar] [CrossRef]
- Taofiq, O.; Corrêa, R.C.G.; Barros, L.; Prieto, M.A.; Bracht, A.; Peralta, R.M.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R. A Comparative Study between Conventional and Non-Conventional Extraction Techniques for the Recovery of Ergosterol from Agaricus Blazei Murrill. Food Res. Int. 2019, 125, 108541. [Google Scholar] [CrossRef]
- Rangsinth, P.; Sharika, R.; Pattarachotanant, N.; Duangjan, C.; Wongwan, C.; Sillapachaiyaporn, C.; Nilkhet, S.; Wongsirojkul, N.; Prasansuklab, A.; Tencomnao, T.; et al. Potential Beneficial Effects and Pharmacological Properties of Ergosterol, a Common Bioactive Compound in Edible Mushrooms. Foods 2023, 12, 2529. [Google Scholar] [CrossRef]
- Yongxia, Z.; Jian, X.; Suyuan, H.; Aixin, N.; Lihong, Z. Isolation and Characterization of Ergosterol from Monascus Anka for Anti-Lipid Peroxidation Properties. J. Mycol. Med. 2020, 30, 101038. [Google Scholar] [CrossRef]
- Hussein Zaki, A.; Haiying, B.; Mohany, M.; Al-Rejaie, S.S.; Abugammie, B. The Effect Mechanism of Ergosterol from the Nutritional Mushroom Leucocalocybe mongolica in Breast Cancer Cells: Protein Expression Modulation and Metabolomic Profiling Using UHPLC-ESI-Q. Saudi Pharm. J. 2024, 32, 102045. [Google Scholar] [CrossRef]
- Sun, Y.; Nzekoue, F.K.; Vittori, S.; Sagratini, G.; Caprioli, G. Conversion of Ergosterol into Vitamin D2 and Other Photoisomers in Agaricus bisporus Mushrooms under UV-C Irradiation. Food Biosci. 2022, 50, 102143. [Google Scholar] [CrossRef]
- Khare, L.; Karve, T.; Jain, R.; Dandekar, P. Menthol Based Hydrophobic Deep Eutectic Solvent for Extraction and Purification of Ergosterol Using Response Surface Methodology. Food Chem. 2021, 340, 127979. [Google Scholar] [CrossRef]
- Morales, D.; Gil-Ramirez, A.; Smiderle, F.R.; Piris, A.J.; Ruiz-Rodriguez, A.; Soler-Rivas, C. Vitamin D-Enriched Extracts Obtained from Shiitake Mushrooms (Lentinula edodes) by Supercritical Fluid Extraction and UV-Irradiation. Innov. Food Sci. Emerg. Technol. 2017, 41, 330–336. [Google Scholar] [CrossRef]
- Stefanović, V.; Trifković, J.; Mutić, J.; Tešić, Ž. Metal Accumulation Capacity of Parasol Mushroom (Macrolepiota procera) from Rasina Region (Serbia). Environ. Sci. Pollut. Res. 2016, 23, 13178–13190. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Lampi, A.-M.; Ronkainen, R.; Toivo, J.; Piironen, V. Sterol and Vitamin D2 Contents in Some Wild and Cultivated Mushrooms. Food Chem. 2002, 76, 293–298. [Google Scholar] [CrossRef]
- Jasinghe, V.J.; Perera, C.O. Distribution of Ergosterol in Different Tissues of Mushrooms and Its Effect on the Conversion of Ergosterol to Vitamin D2 by UV Irradiation. Food Chem. 2005, 92, 541–546. [Google Scholar] [CrossRef]
- Vukojević, V.; Djurdjić, S.; Švorc, Ľ.; Veličković, T.Ć.; Mutić, J.; Stanković, D.M. Analytical Approach for Detection of Ergosterol in Mushrooms Based on Modification Free Electrochemical Sensor in Organic Solvents. Food Anal. Methods 2018, 11, 2590–2596. [Google Scholar] [CrossRef]
- Falandysz, J.; Szymczyk, K.; Ichihashi, H.; Bielawski, L.; Gucia, M.; Frankowska, A.; Yamasaki, S.-I. ICP/MS and ICP/AES Elemental Analysis (38 Elements) of Edible Wild Mushrooms Growing in Poland. Food Addit. Contam. 2001, 18, 503–513. [Google Scholar] [CrossRef]
- Dimitrijevic, M.V.; Mitic, V.D.; Cvetkovic, J.S.; Stankov Jovanovic, V.P.; Mutic, J.J.; Nikolic Mandic, S.D. Update on Element Content Profiles in Eleven Wild Edible Mushrooms from Family Boletaceae. Eur. Food Res. Technol. 2016, 242, 1–10. [Google Scholar] [CrossRef]
- Haro, A.; Trescastro, A.; Lara, L.; Fernández-Fígares, I.; Nieto, R.; Seiquer, I. Mineral Elements Content of Wild Growing Edible Mushrooms from the Southeast of Spain. J. Food Compos. Anal. 2020, 91, 103504. [Google Scholar] [CrossRef]
- Xu, H.; Song, P.; Gu, W.; Yang, Z. Effects of Heavy Metals on Production of Thiol Compounds and Antioxidant Enzymes in Agaricus bisporus. Ecotoxicol. Environ. Saf. 2011, 74, 1685–1692. [Google Scholar] [CrossRef]
- Dowlati, M.; Sobhi, H.R.; Esrafili, A.; Kia, M.F.; Yeganeh, M. Heavy metals content in edible mushrooms: A systematic review, meta-analysis and health risk assessment. Trends Food Sci. Technol. 2021, 109, 527–535. [Google Scholar] [CrossRef]
- Scalese, G.; Mosquillo, M.F.; Pérez-Díaz, L.; Gambino, D. Biosynthesis of Ergosterol as a Relevant Molecular Target of Metal-Based Antiparasitic and Antifungal Compounds. Coord. Chem. Rev. 2024, 503, 215608. [Google Scholar] [CrossRef]
- Saini, R.K.; Rauf, A.; Khalil, A.A.; Ko, E.-Y.; Keum, Y.-S.; Anwar, S.; Alamri, A.; Rengasamy, K.R.R. Edible Mushrooms Show Significant Differences in Sterols and Fatty Acid Compositions. S. Afr. J. Bot. 2021, 141, 344–356. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Wang, J.-H.; Liu, X.; Kuang, H.-C.; Huang, X.-N. Determination of Ergosterol in Ganoderma Spore Lipid from the Germinating Spores of Ganoderma Lucidum by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2006, 54, 6172–6176. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-P.; Wang, J.-H.; Liu, X.; Kuang, H.-C.; Zhao, S.-Y. Simultaneous Determination of Free Ergosterol and Ergosteryl Esters in Cordyceps Sinensis by HPLC. Food Chem. 2007, 105, 1755–1759. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Kuang, H.-C.; Wang, J.-H.; Liu, X. Evaluation of Ergosterol and Its Esters in the Pileus, Gill, and Stipe Tissues of Agaric Fungi and Their Relative Changes in the Comminuted Fungal Tissues. Appl. Microbiol. Biotechnol. 2008, 80, 459–465. [Google Scholar] [CrossRef]
- Teichmann, A.; Dutta, P.C.; Staffas, A.; Jägerstad, M. Sterol and Vitamin D2 Concentrations in Cultivated and Wild Grown Mushrooms: Effects of UV Irradiation. LWT—Food Sci. Technol. 2007, 40, 815–822. [Google Scholar] [CrossRef]
- Thakur, A.; Pandey, K.K.; Kharka, K.; Borker, S.S.; Kumar, B.; Bhatt, A.; Kumar, R. Comparative Analysis of Substrate Components, Nutritive Value, Ergosterol Distribution, and Vitamin D2 Enrichment in Shiitake Mushrooms Cultivated on Wood Substrates. J. Food Compos. Anal. 2024, 134, 106508. [Google Scholar] [CrossRef]
- Xu, Z.; Meenu, M.; Xu, B. Effects of UV-C Treatment and Ultrafine-Grinding on the Biotransformation of Ergosterol to Vitamin D2, Physiochemical Properties, and Antioxidant Properties of Shiitake and Jew’s Ear. Food Chem. 2020, 309, 125738. [Google Scholar] [CrossRef]
- Jung, E.-B.; Choi, H.-J.; Lee, J.-Y.; Hwang, H.-J.; Chung, M.-S. Comparison between Intense Pulsed Light and Continuous Ultraviolet Treatment Processes for Enhancing the Vitamin D2 Content of Shiitake Mushroom (Lentinula edodes) Powder. Food Chem. 2025, 468, 142434. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Sun, Y.; Caprioli, G.; Vittori, S.; Sagratini, G. Effect of the Ultrasound-Assisted Extraction Parameters on the Determination of Ergosterol and Vitamin D2 in Agaricus bisporus, A. Bisporus Portobello, and Pleurotus ostreatus Mushrooms. J. Food Compos. Anal. 2022, 109, 104476. [Google Scholar] [CrossRef]
- Sławińska, A.; Fornal, E.; Radzki, W.; Skrzypczak, K.; Zalewska-Korona, M.; Michalak-Majewska, M.; Parfieniuk, E.; Stachniuk, A. Study on Vitamin D2 Stability in Dried Mushrooms during Drying and Storage. Food Chem. 2016, 199, 203–209. [Google Scholar] [CrossRef]
- Monika Gąsecka, M.; Magdziak, Z.; Siwulski, M.; Mleczek, M. Profile of phenolic and organic acids, antioxidant properties and ergosterol content in cultivated and wild growing species of Agaricus. Eur. Food Res. Technol. 2018, 244, 259–268. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, J.; Yan, R.; Shao, S.; Zhou, T.; Lei, J.; Wang, Z. Effects of UV-C Treatment and Cold Storage on Ergosterol and Vitamin D2 Contents in Different Parts of White and Brown Mushroom (Agaricus bisporus). Food Chem. 2016, 210, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Román-Hidalgo, C.; Villar-Navarro, M.; Falcón-García, G.E.; Carbonero-Aguilar, M.P.; Bautista-Palomas, J.D.; Bello-López, M.A.; Martín-Valero, M.J.; Fernández-Torres, R. Selective, Rapid and Simultaneous Determination of Ergosterol and Ergocalciferol in Mushrooms by UPLC-Q-TOF-MS. J. Pharm. Biomed. Anal. 2021, 194, 113748. [Google Scholar] [CrossRef]
- Sommer, K.; Vetter, W. Sterols in Fresh and Preserved Button Mushroom (Agaricus bisporus) Products. Eur. Food Res. Technol. 2025, 251, 613–624. [Google Scholar] [CrossRef]
- Guan, W.; Fan, X.; Yan, R. Effects of UV-C Treatment on Inactivation of Escherichia Coli O157:H7, Microbial Loads, and Quality of Button Mushrooms. Postharvest Biol. Technol. 2012, 64, 119–125. [Google Scholar] [CrossRef]
- Hu, D.; Yang, X.; Hu, C.; Feng, Z.; Chen, W.; Shi, H. Comparison of Ergosterol and Vitamin D 2 in Mushrooms Agaricus bisporus and Cordyceps Militaris Using Ultraviolet Irradiation Directly on Dry Powder or in Ethanol Suspension. ACS Omega 2021, 6, 29506–29515. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, M.; Mujumdar, A.S. UV Induced Conversion during Drying of Ergosterol to Vitamin D in Various Mushrooms: Effect of Different Drying Conditions. Trends Food Sci. Technol. 2020, 105, 200–210. [Google Scholar] [CrossRef]
- Kalyniukova, A.; Tomášková, I.; Pešková, V.; Pastierovič, F.; Samek, M.; Balogh, J. Development of a Novel Dispersive Liquid-Liquid Microextraction for the Determination of Ergosterol in Roots and Various Fungi Samples. Microchem. J. 2022, 174, 107095. [Google Scholar] [CrossRef]
- Jasinska, A.; Stoknes, K.; Niedzielski, P.; Budka, A.; Mleczek, M. Mushroom Production on Digestate: Mineral Composition of Cultivation Compost, Mushrooms, Spent Mushroom Compost and Spent Casing. J. Agric. Food Res. 2024, 18, 101518. [Google Scholar] [CrossRef]
- Isildak, O.; Turkekul, I.; Elmastas, M.; Aboul-Enein, H.Y. Bioaccumulation of Heavy Metals in Some Wild-Grown Edible Mushrooms. Anal. Lett. 2007, 40, 1099–1116. [Google Scholar] [CrossRef]
- Salihović, M.; Pazalja, M.; Šapčanin, A.; Dojčinović, B.P.; Špirtović-Halilović, S. Element contents and health risk assessment in wild edible mushrooms of Bosnia and Herzegovina. Plant Soil Environ 2021, 67, 668–677. [Google Scholar] [CrossRef]
- Borovička, J.; Řanda, Z. Distribution of Iron, Cobalt, Zinc and Selenium in Macrofungi. Mycol. Prog. 2007, 6, 249–259. [Google Scholar] [CrossRef]
- Falandysz, J.; Borovička, J. Macro and Trace Mineral Constituents and Radionuclides in Mushrooms: Health Benefits and Risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Drewnowska, M.; Falandysz, J. Investigation on Mineral Composition and Accumulation by Popular Edible Mushroom Common Chanterelle (Cantharellus cibarius). Ecotoxicol. Environ. Saf. 2015, 113, 9–17. [Google Scholar] [CrossRef]
- Tuzen, M.; Sesli, E.; Soylak, M. Trace Element Levels of Mushroom Species from East Black Sea Region of Turkey. Food Control 2007, 18, 806–810. [Google Scholar] [CrossRef]
- Mleczek, M.; Budka, A.; Siwulski, M.; Mleczek, P.; Budzyńska, S.; Proch, J.; Gąsecka, M.; Niedzielski, P.; Rzymski, P. A Comparison of Toxic and Essential Elements in Edible Wild and Cultivated Mushroom Species. Eur. Food Res. Technol. 2021, 247, 1249–1262. [Google Scholar] [CrossRef]
- Alonso, J.; García, M.A.; Pérez-López, M.; Melgar, M.J. The Concentrations and Bioconcentration Factors of Copper and Zinc in Edible Mushrooms. Arch. Environ. Contam. Toxicol. 2003, 44, 180–188. [Google Scholar] [CrossRef]
- Eliaš, D.; Tóth Hervay, N.; Gbelská, Y. Ergosterol Biosynthesis and Regulation Impact the Antifungal Resistance and Virulence of Candida spp. Stresses 2024, 4, 641–662. [Google Scholar] [CrossRef]
- Shanholtzer, C.N.; Rice, C.; Watson, K.; Carreon, H.; Long, T.E. Effect of Copper on the Antifungal Activity of Disulfiram (Antabuse®) in Fluconazole-Resistant Candida Strains. Med. Mycol. 2022, 60, myac016. [Google Scholar] [CrossRef]
- Vázquez-Blanco, R.; Arias-Estévez, M.; Bååth, E.; Fernández-Calviño, D. Comparison of Cu Salts and Commercial Cu Based Fungicides on Toxicity towards Microorganisms in Soil. Environ. Pollut. 2020, 257, 113585. [Google Scholar] [CrossRef]
- Kalač, P. Trace Element Contents in European Species of Wild Growing Edible Mushrooms: A Review for the Period 2000–2009. Food Chem. 2010, 122, 2–15. [Google Scholar] [CrossRef]
- Certified Reference Material ERM—CD281, Rye grass, Institute for Reference Materials and Measurements, Geel, Belgium. 2010. Available online: https://crm.jrc.ec.europa.eu/p/q/ERM-CD281/ERM-CD281-RYE-GRASS/ERM-CD281 (accessed on 15 February 2025).
| Origin | Family Name | Botanical Name | Common Name | Ergosterol | Fe | Zn | Cu |
|---|---|---|---|---|---|---|---|
| Wild-grown, Serbia | Boletaceae | Phylloporus rhodoxanthus | Gilled bolete | 0.13 | 10.55 | 43.51 | 5.46 |
| Boletus edulis | Penny bun | 0.71 | 45.83 | 17.64 | 7.91 | ||
| Boletus appendiculatus | Butter bolete | 0.01 | 44.98 | 49.92 | 5.74 | ||
| Boletus appendiculatus | Butter bolete | 0.13 | 55.99 | 103.4 | 93.03 | ||
| Phylloporus rhodoxanthus | Gilled bolete | ˂LOD | 102.1 | 51.31 | 33.06 | ||
| Leccinum crocipodium | Saffron bolete | 1.06 | 49.8 | 66.10 | 63.66 | ||
| Butyriboletus fechtneri | Pale bolete | 7.04 | 393 | 68.22 | 25.71 | ||
| Boletus impolitus | Iodine bolete | 0.35 | 26.76 | 149.7 | 5.02 | ||
| Boletus impolitus | Iodine bolete | ˂LOD | 16.54 | 13.71 | 2.91 | ||
| Boletus edulis | Penny bun | 0.61 | 8.50 | 27.12 | 33.70 | ||
| Cultivated, Serbia | Agaricaceae | Agaricus bisporus | White button | 1.43 | 210.3 | 52.32 | 26.03 |
| Agaricus bisporus | White button | 1.14 | 265.1 | 84.21 | 38.28 | ||
| Agaricus bisporus | White button | 1.69 | 3204 | 50.32 | 19.41 | ||
| Agaricus bisporus | White button | 3.92 | 325.2 | 140.2 | 65.19 | ||
| Agaricus bisporus | White button | 4.67 | 216.4 | 65.84 | 25.04 | ||
| Cultivated, Korea | Physalacriaceae | Flammulina velutiper | Enoki | 0.54 | 47.48 | 41.92 | 5.12 |
| Lyophyllaceae | Hypsizygus marmoreus | Shimeji | 1.46 | 44.33 | 44.12 | 6.90 | |
| Agaricaceae | Agaricus bisporus | White button | 1.90 | 24.35 | 37.63 | 4.44 | |
| Pleurotaceae | Pleurotus giganteus | Giant oyster | 1.83 | 61.34 | 77.31 | 21.74 | |
| Lyophyllaceae | Hypsizygus marmoreus | Shimeji | 2.43 | 54.65 | 67.50 | 42.27 | |
| Omphalotaceae | Lentinula edodes | Shitake | 5.90 | 46.70 | 51.71 | 6.07 | |
| Agaricaceae | Agaricus bisporus | White button | 6.96 | 479.9 | 55.14 | 23.81 | |
| Auriculariaceae | Auricularia auricula-judae | Wood ear, Jew’s ear | 1.99 | 47.32 | 13.13 | 1.62 | |
| Pleurotaceae | Pleurotus ostreatus | Oyster | 4.48 | 10.24 | 60.91 | 5.67 | |
| Pleurotaceae | Pleurotus eryngii | King oyster | 3.16 | 76.61 | 32.52 | 5.29 | |
| Minimum | 0.01 | 8.50 | 13.12 | 1.62 | |||
| Maximum | 7.04 | 479.9 | 149.7 | 93.03 | |||
| Mean | 2.39 | 119.3 | 58.59 | 22.88 | |||
| Standard deviation | 2.16 | 135.6 | 33.64 | 23.29 |
| Botanical Name | Common Name | Storage Condition | Ergosterol | Country | Reference |
|---|---|---|---|---|---|
| Lentinula edodes | Shitake | Fresh | 1179.74 µg/g | China | [28] |
| Lentinula edodes | Shitake | Fresh | 6820 µg/g | India | [26] |
| Agaricus bisporus | White button | Fresh | 1.1–36.1 mg/100 g | Poland | [31] |
| Brown button | Fresh | 26.68 mg/100 g | |||
| Lentinula edodes | Shitake | Fresh | 4.59 mg/g | China | [27] |
| Auricularia auricula-judae | Jew’s ear | Fresh | 4.22 mg/g | ||
| Agaricus bisporus | White button (caps) | Fresh | 6.12 mg/g | Canada | [32] |
| White button (stems) | 5.20 mg/g | ||||
| Brown button (caps) | Fresh | 7.59 mg/g | |||
| Brown button (stems) | 7.56 mg/g | ||||
| Agaricus bisporus | Button | Fresh | 9.85 mg/g | Poland | [30] |
| Pleurotus ostreatus | Oyster | Fresh | 7.64 mg/g | ||
| Lentinula edodes | Shitake | Fresh | 9.33 mg/g | ||
| Agaricus bisporus | Button | Air-dried | 8.61 mg/g | ||
| Pleurotus ostreatus | Oyster | Air-dried | 7.54 mg/g | ||
| Lentinula edodes | Shitake | Air-dried | 8.59 mg/g | ||
| Agaricus bisporus | Button | Freeze-dried | 9.88 mg/g | ||
| Pleurotus ostreatus | Oyster | Freeze-dried | 8.79 mg/g | ||
| Lentinula edodes | Shitake | Freeze-dried | 8.94 mg/g | ||
| Agaricus bisporus | Button | 6.9 mg/g | Italy | [29] | |
| Agaricus bisporus | Portobello | Fresh | 7.3 mg/g | ||
| Pleurotus ostreatus | Oyster | 4.9 mg/g | |||
| Agaricus bisporus | White button | Fresh | 2429.68–6995.30 µg/g | India | [9] |
| Agaricus bisporus | White button | Fresh | 3.8 mg/g | Spain | [33] |
| Pleurotus ostreatus | Oyster | Fresh | 774.2–762.4 mg/100 g | Korea | [21] |
| Lentinula edodes | Shitake | 515.8 mg/100 g |
| Button Mushroom (Stem) | Button Mushroom (Cap) | |
|---|---|---|
| Ergosterol (mg/g) | 3.058 | 0.634 |
| 3.981 | 0.434 | |
| 1.655 | 0.729 | |
| 1.662 | 1.057 | |
| 2.19 | 1.603 | |
| 2.041 | 1.338 | |
| 8.441 | 1.451 | |
| 4.255 | 1.131 | |
| 2.883 | 1.614 | |
| 1.717 | 0.705 | |
| 7.001 | 1.149 | |
| Minimum | 1.655 | 0.434 |
| Maximum | 8.441 | 1.614 |
| Mean | 3.53 | 1.08 |
| Standard deviation | 0.686 | 0.122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đogo Mračević, S.; Mutić, J.; Stanković, V.; Ražić, S. Voltametric Analysis of Ergosterol Isolated from Wild-Growing and Cultivated Edible Mushrooms from Serbia and Korea. Molecules 2025, 30, 2010. https://doi.org/10.3390/molecules30092010
Đogo Mračević S, Mutić J, Stanković V, Ražić S. Voltametric Analysis of Ergosterol Isolated from Wild-Growing and Cultivated Edible Mushrooms from Serbia and Korea. Molecules. 2025; 30(9):2010. https://doi.org/10.3390/molecules30092010
Chicago/Turabian StyleĐogo Mračević, Svetlana, Jelena Mutić, Vesna Stanković, and Slavica Ražić. 2025. "Voltametric Analysis of Ergosterol Isolated from Wild-Growing and Cultivated Edible Mushrooms from Serbia and Korea" Molecules 30, no. 9: 2010. https://doi.org/10.3390/molecules30092010
APA StyleĐogo Mračević, S., Mutić, J., Stanković, V., & Ražić, S. (2025). Voltametric Analysis of Ergosterol Isolated from Wild-Growing and Cultivated Edible Mushrooms from Serbia and Korea. Molecules, 30(9), 2010. https://doi.org/10.3390/molecules30092010








