Geochemical Study of Bitumen Residues on Potsherds from the al-Qusur Monastery (7th–9th c. CE): Composition and Origin
Abstract
1. Introduction
2. Materials and Methods
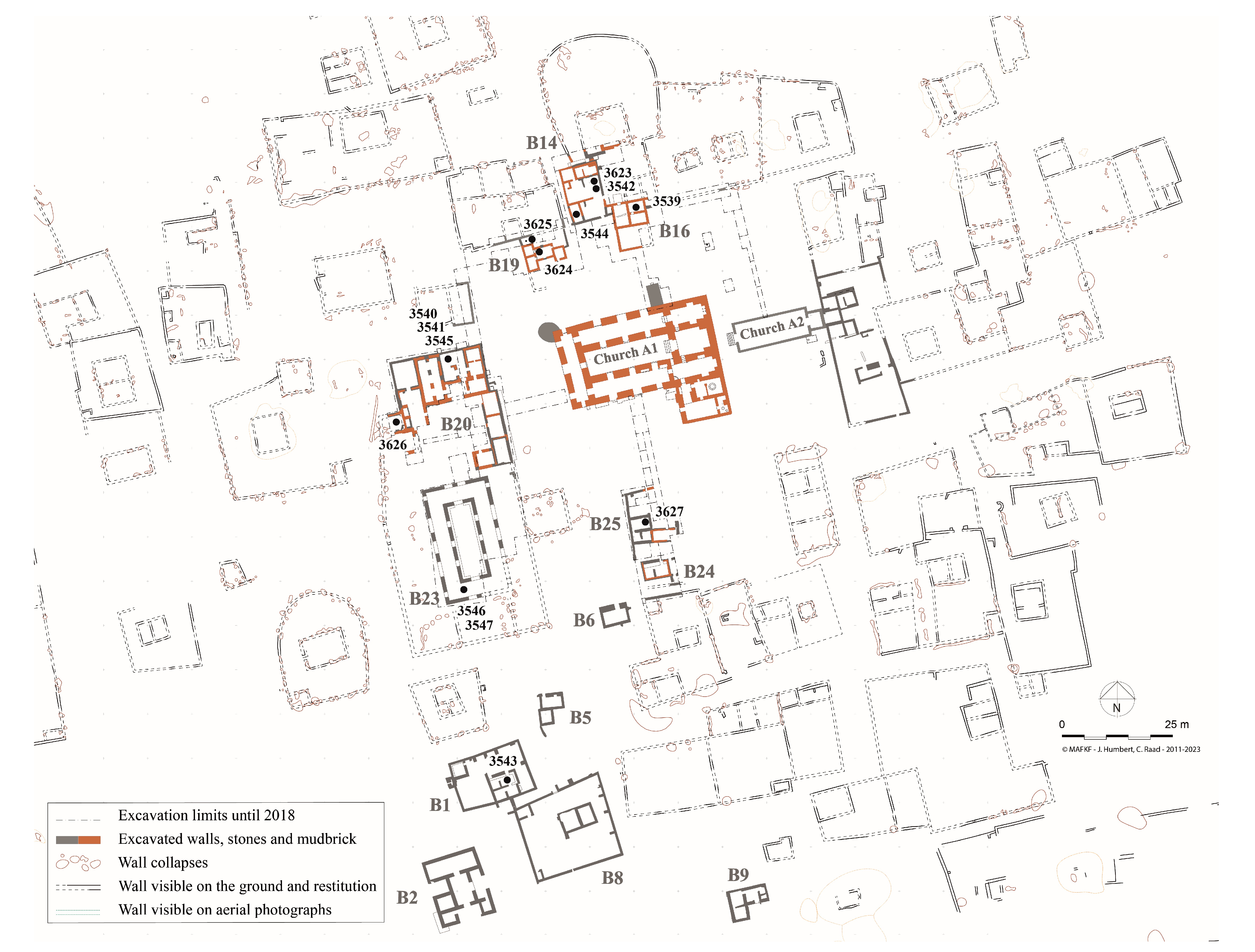
2.1. Bitumen at al-Qusur
2.2. Archaeological Samples Analyzed
2.3. Analytical Procedures
2.3.1. Bitumen Analysis
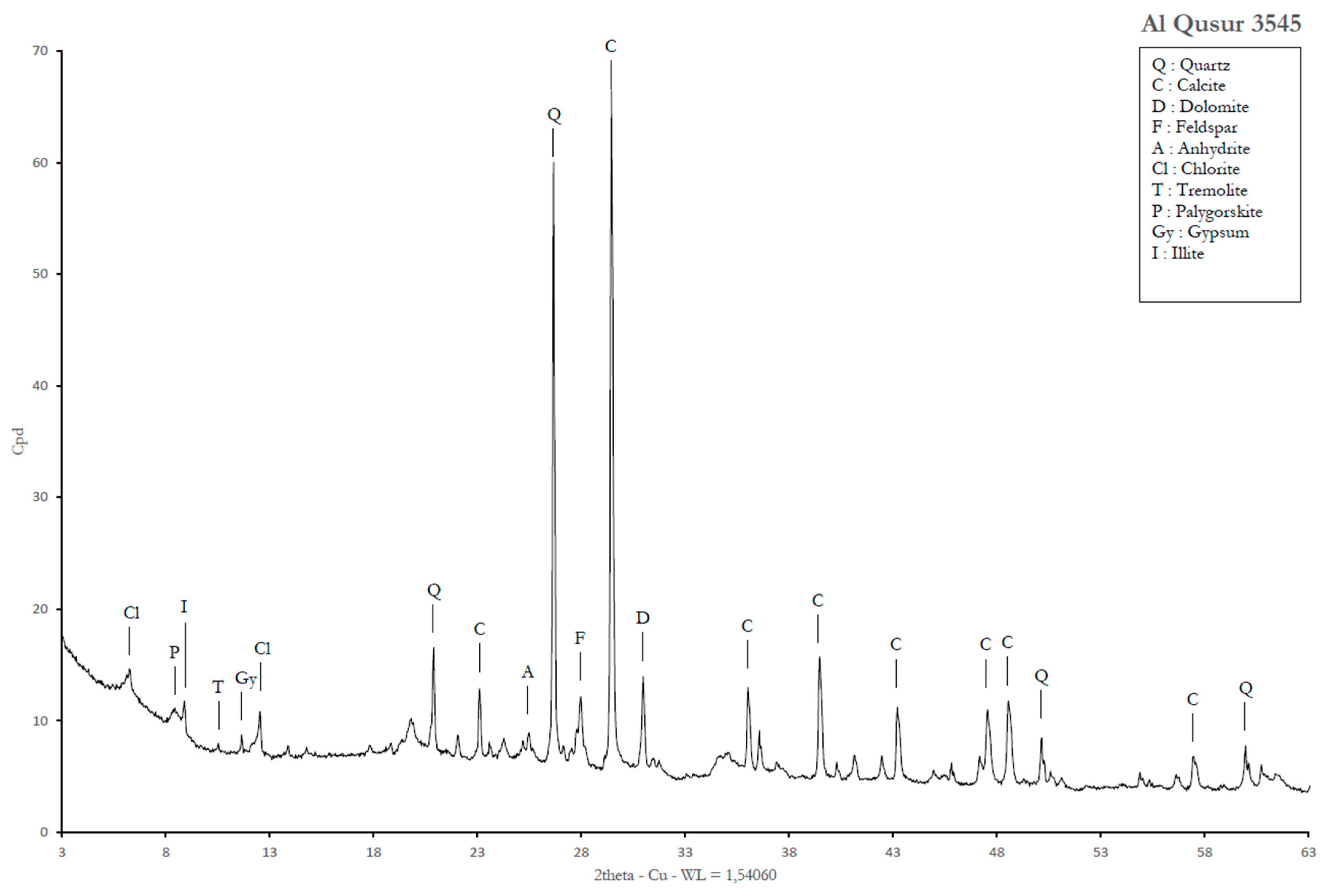
2.3.2. Mineralogical Analysis
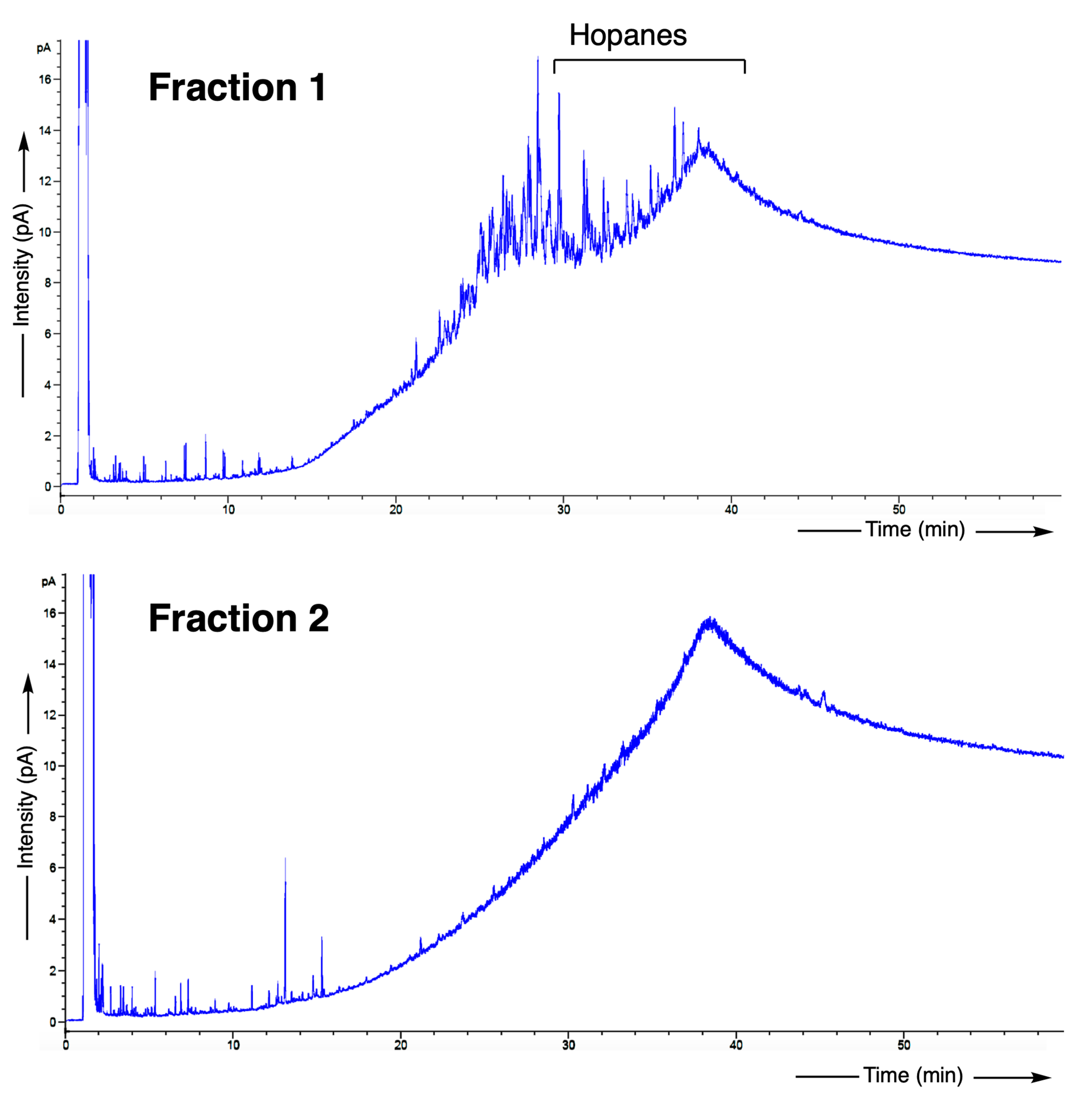
2.3.3. Complementary Organic Analyses
GC-FID
GC/MS
3. Results and Discussion
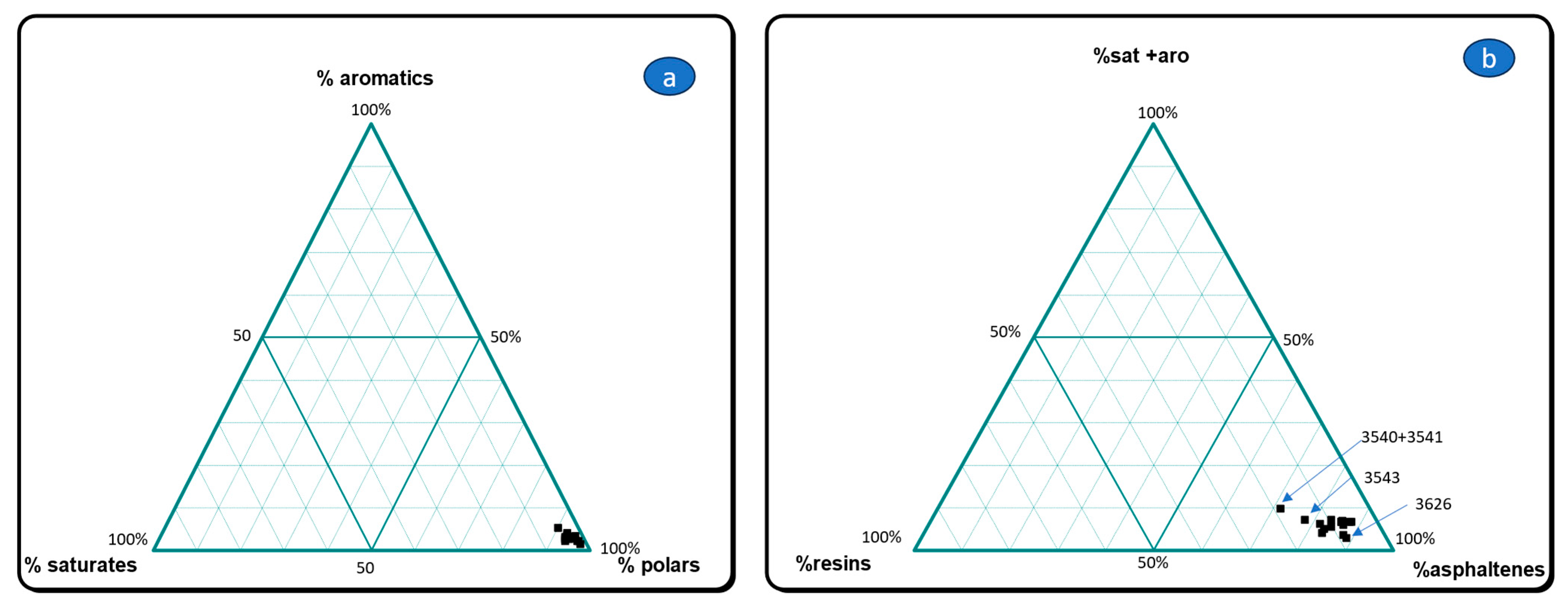
3.1. Gross Composition of Extractable Organic Matter
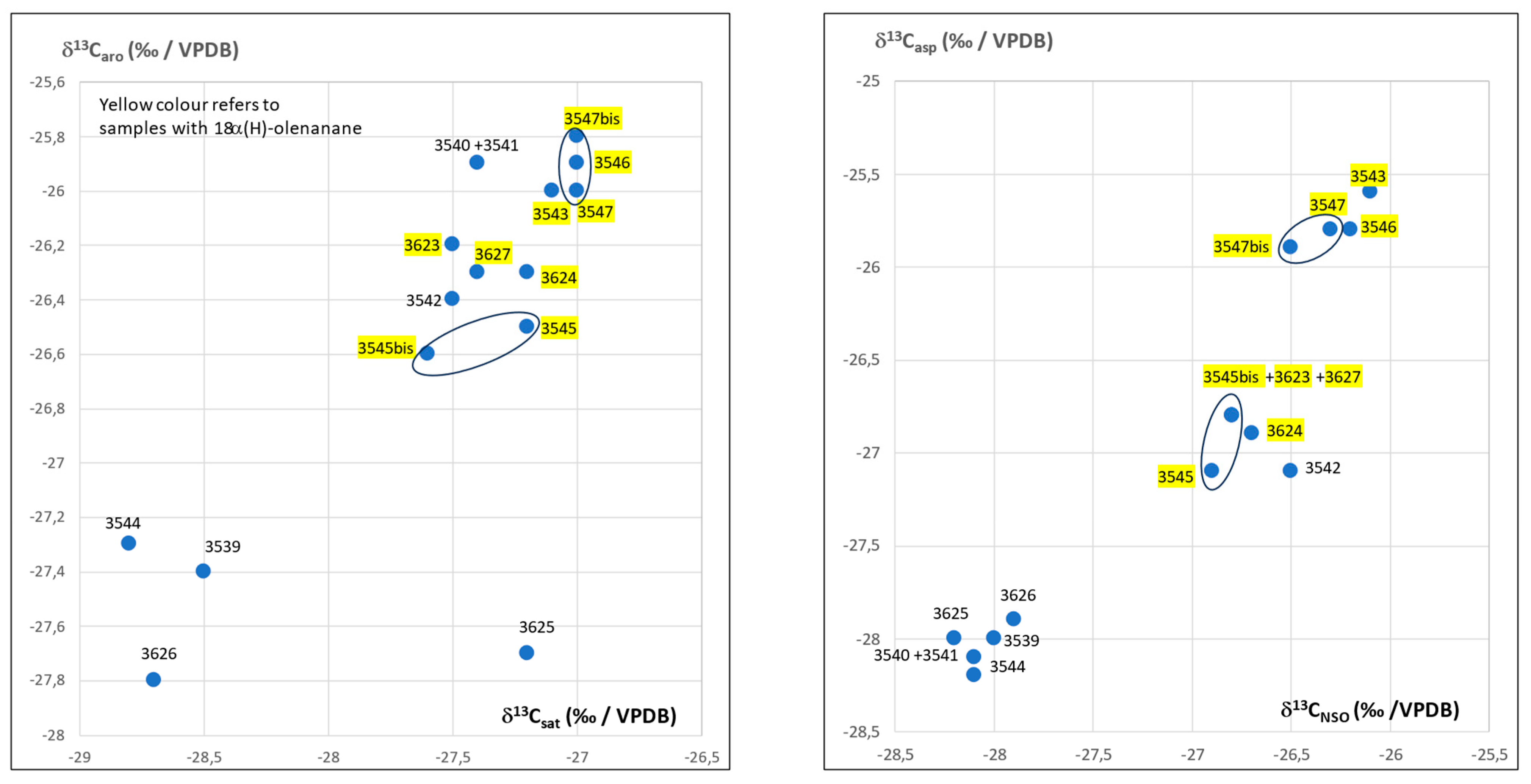
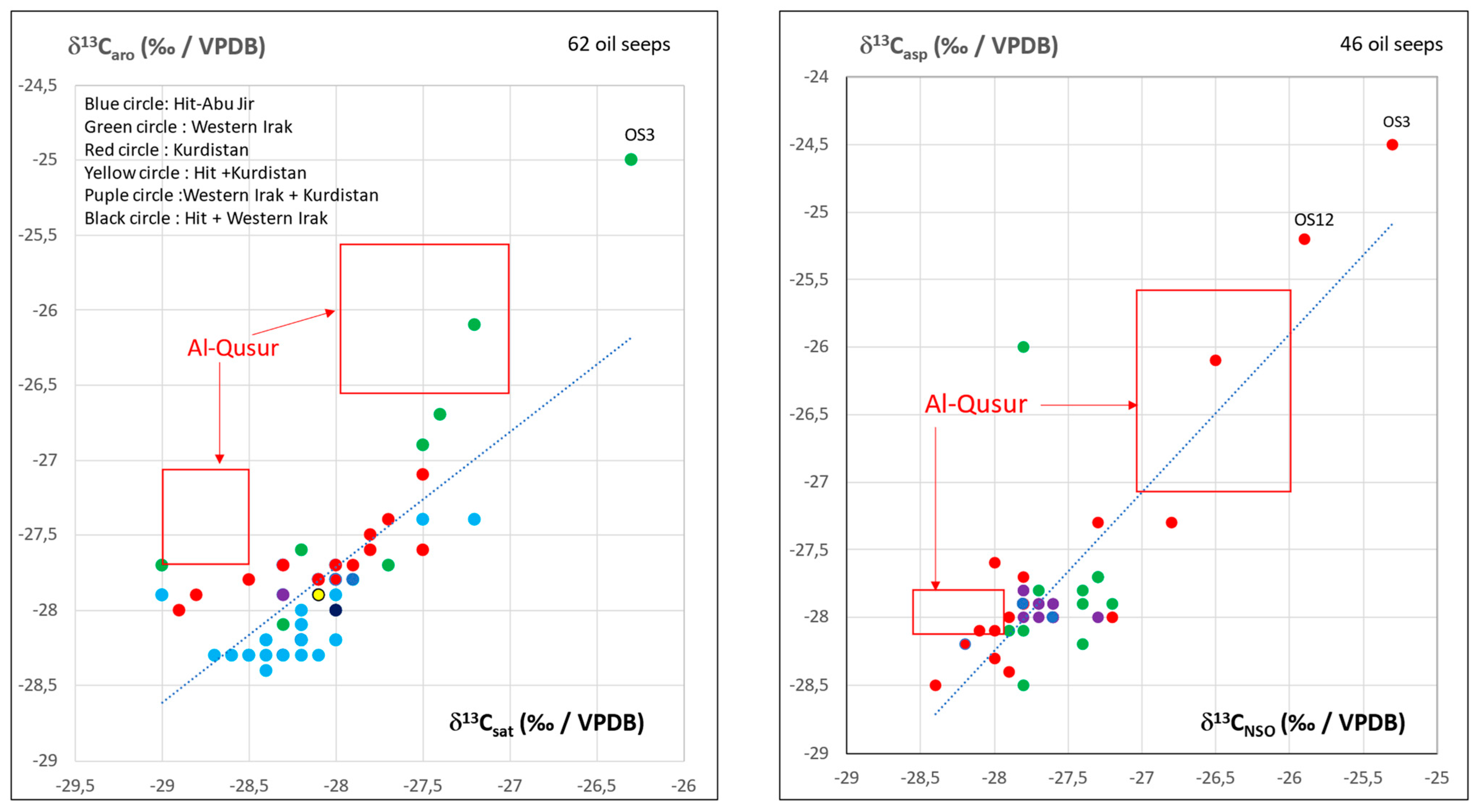
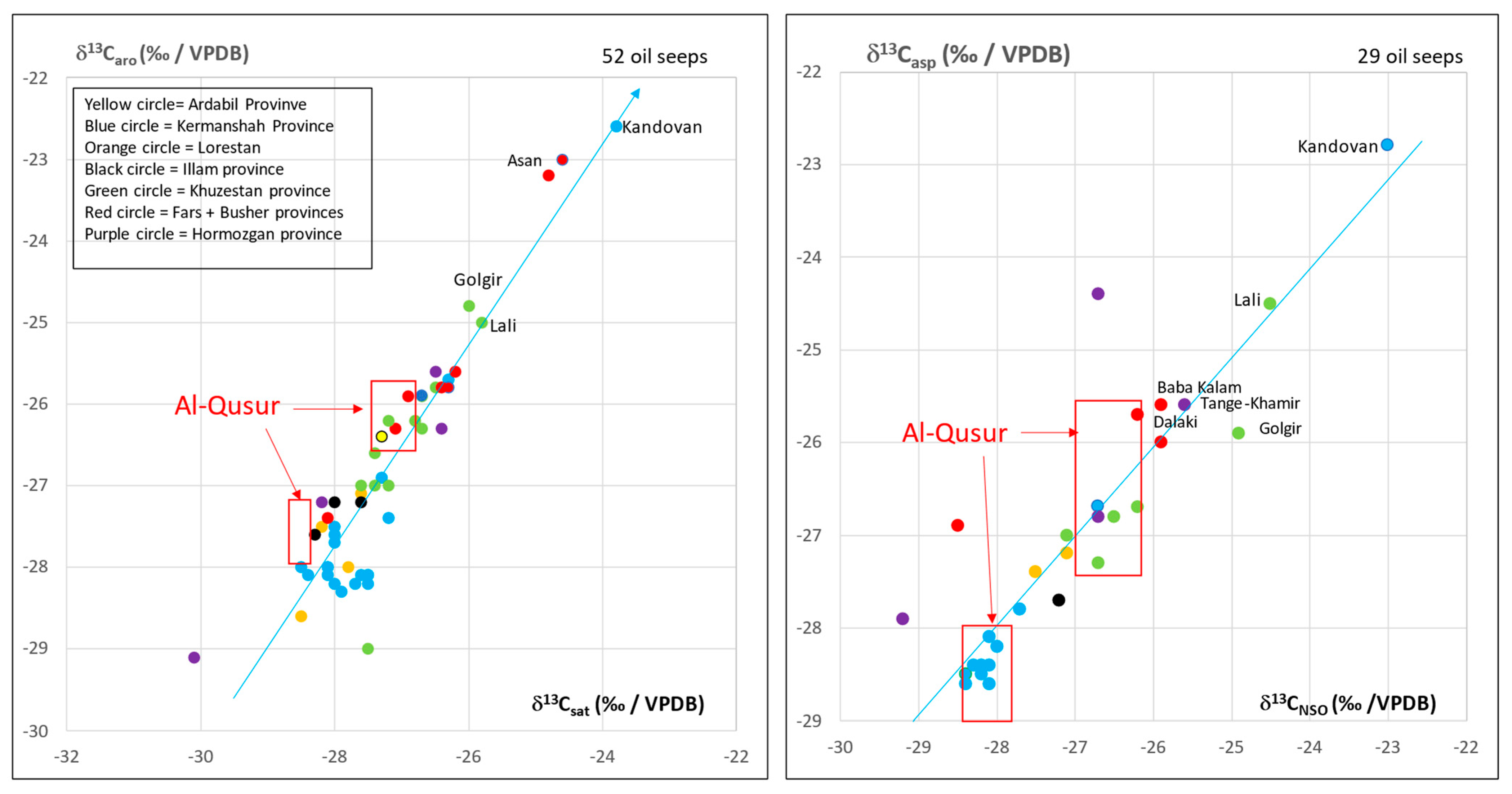
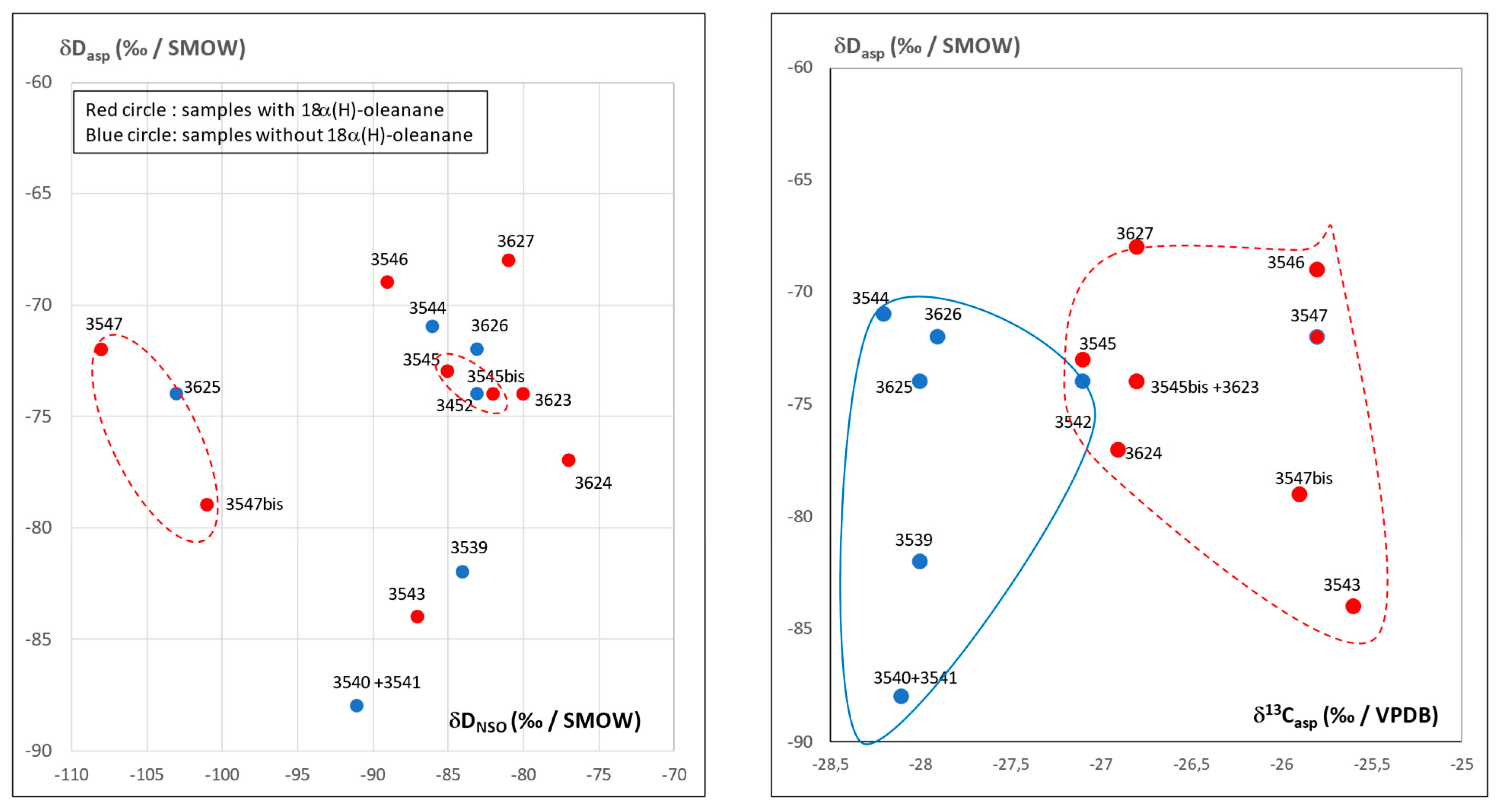
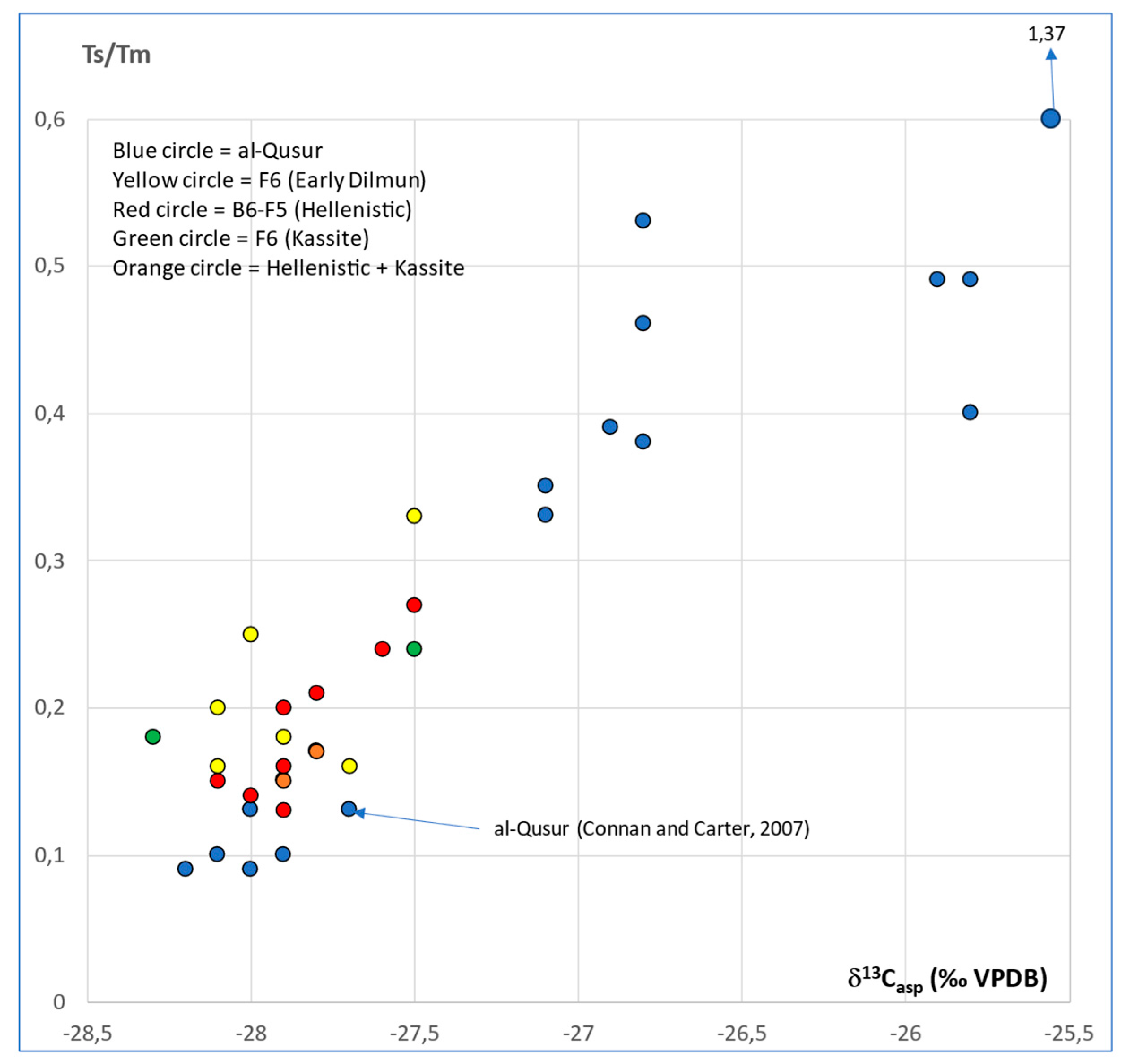
3.2. Isotopic Compositions
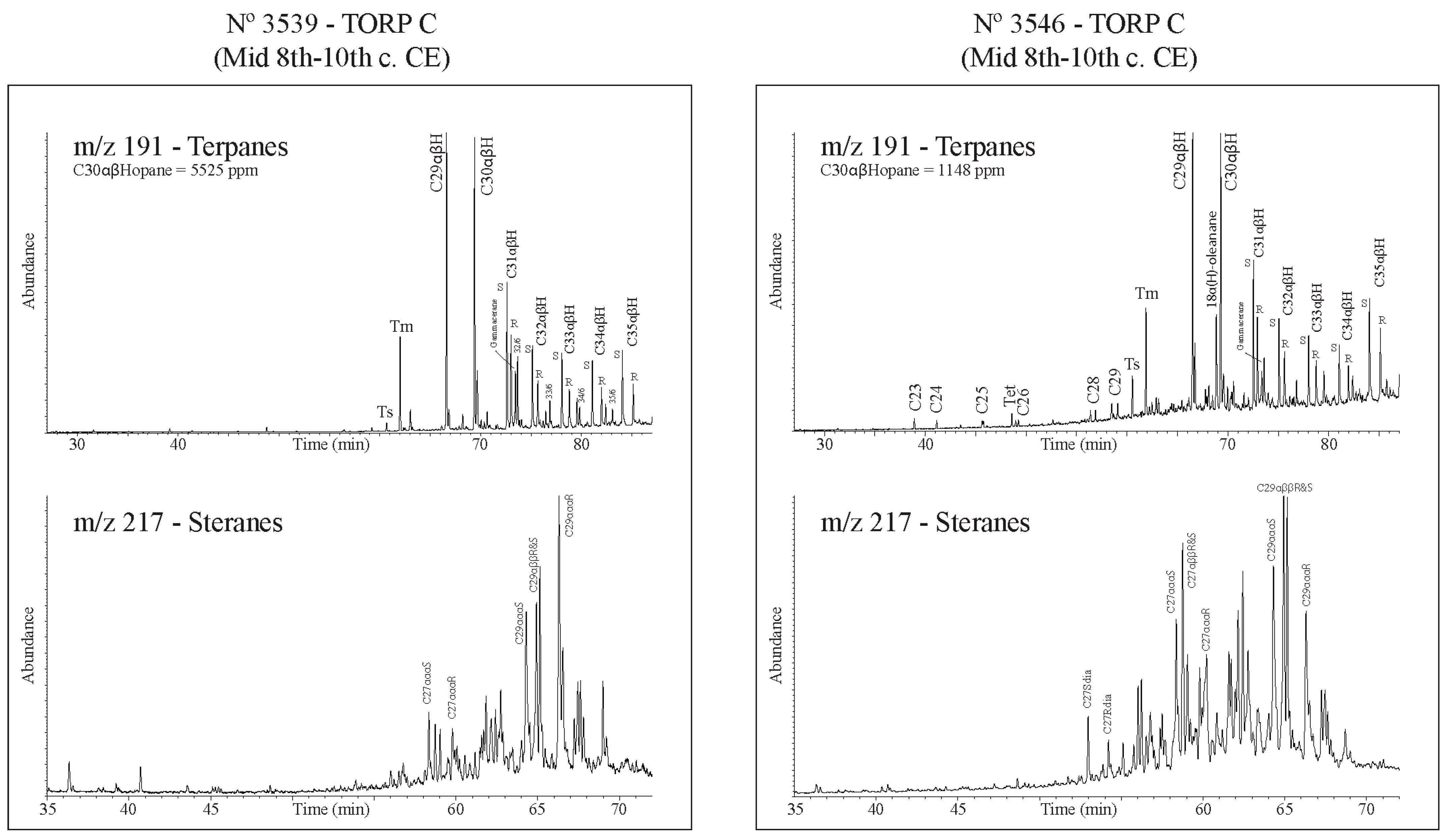
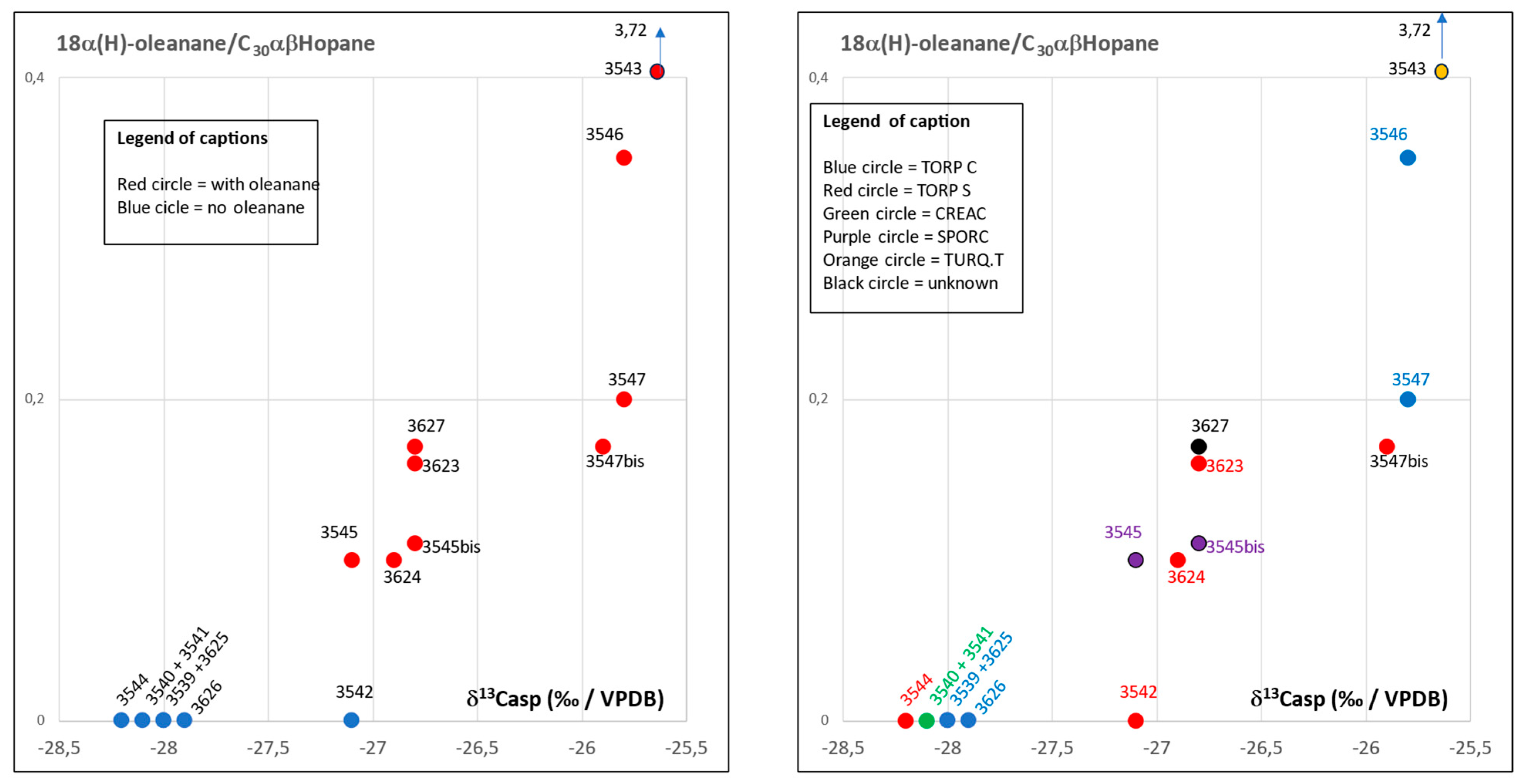
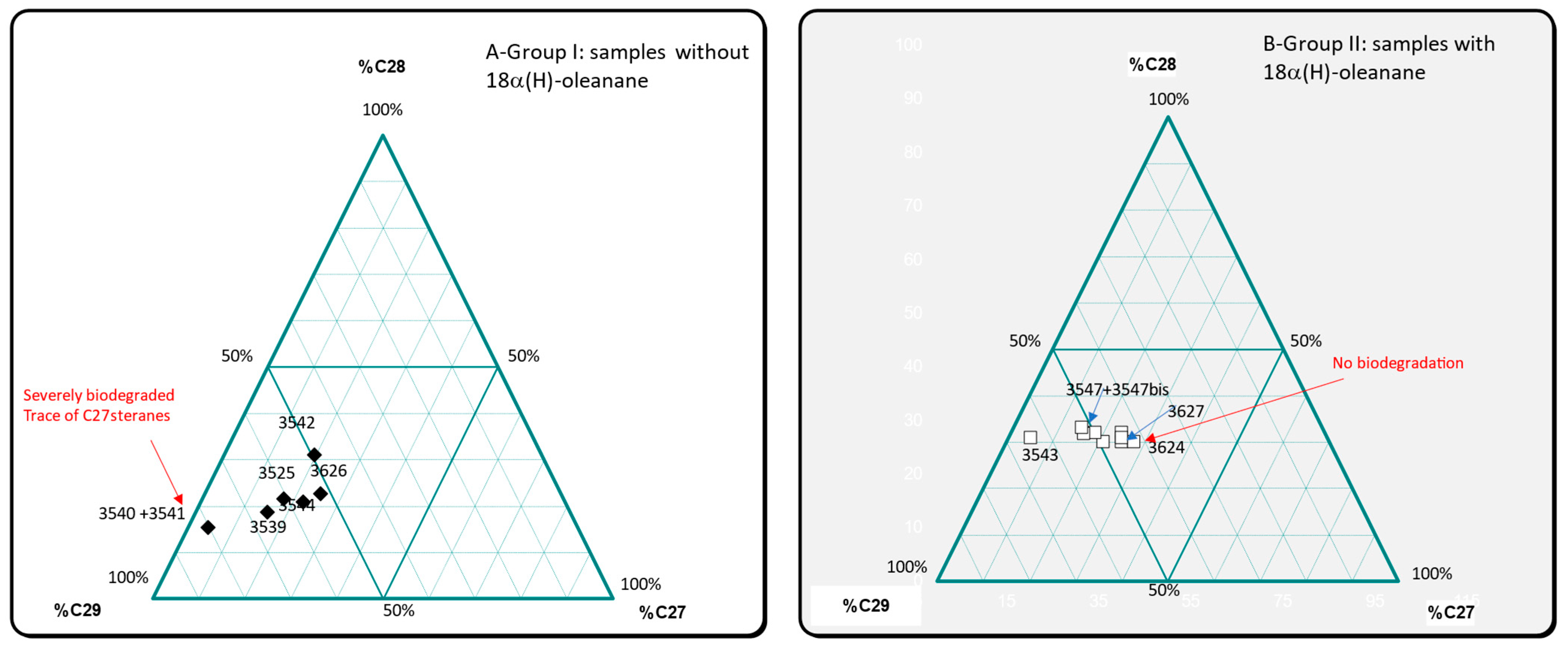
3.3. Biomarker Analysis
3.4. Detailed Geochemical Study of Samples No.3545 and 3545bis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonnéric, J. Mission archéologique franco-koweïtienne de Faïlaka: Le monastère sassanido-islamique d’al-Qusur. In Dossier «Prix Clio»; Centre Français de Recherche de la Péninsule Arabique: Kuwait City, Kuwait, 2018; pp. 1–19. Available online: https://ssl.clio.fr/securefilesystem/Bonneric.pdf (accessed on 20 April 2025).
- Bonnéric, J. Archaeological evidence of an early Islamic monastery in the centre of al-Qusur (Failaka Island, Kuwait). Arab. Arch. Epig. 2021, 32, 50–61. [Google Scholar] [CrossRef]
- Bonnéric, J. Why islands? Understanding the Insular Location of Late Sasanian and Early Islamic Christian Monasteries in the Arab-Persian Gulf. In Networked Spaces: The Spatiality of Networks in the Red Sea and Western Indian Ocean; Durand, C., Marchand, J., Redon, B., Schneider, P., Eds.; MOM Éditions: Lyon, France, 2022; pp. 193–210. [Google Scholar]
- Perrogon, R.; Bonnéric, J. A consideration on the interest of a pottery typology adapted to the late Sasanian and early Islamic monastery at Al-Qusur (Kuwait). Arab. Arch. Epig. 2021, 32, 70–82. [Google Scholar] [CrossRef]
- Di Miceli, A. The site of Al-Qurainiyah: Topography and phases of an early Islamic coastal settlement on Failaka Island. Arab. Arch. Epig. 2021, 32, 62–69. [Google Scholar] [CrossRef]
- Priestman, S. Bushehr, Dashtestan and Siraf: The Transformation of the Maritime Trade Network in the Middle Persian Gulf. In Sasanian Archaeology: Settlements, Environment and Material Culture; Simpson, S.-J., Ed.; Archaeopress Publishing Ltd.: Oxford, UK, 2022; pp. 153–177. [Google Scholar]
- Priestman, S. Ceramic Exchange and the Indian Ocean Economy (AD 400–1275). Volume 2: Indian Ocean Pottery Classification; The British Museum: London, UK, 2021. [Google Scholar] [CrossRef]
- Simpson, S.-J. Sasanian Pottery: Archaeological Evidence for Production, Circulation and Diachronic Change. In Sasanian Archaeology: Settlements, Environment and Material Culture; Simpson, S.-J., Ed.; Archaeopress Publishing Ltd.: Oxford, UK, 2022; pp. 255–374. [Google Scholar]
- Kawamata, M. Telūl Hamediyāt near Tells Gubba and Songor: Part III. Al-Rāfidān 1991, 12, 249–260. [Google Scholar]
- Carter, R.; Connan, J.; Priestman, S.; Tomber, R. Torpedo jars from Sir Bani Yas, Abu Dhabi. Tribulus J. Emir. Nat. Hist. Group 2011, 19, 162–163. [Google Scholar]
- Carter, R. Christianity in the Gulf during the first centuries of Islam. Arab. Arch. Epig 2008, 19, 71–108. [Google Scholar] [CrossRef]
- Priestman, S. Ceramic Exchange and the Indian Ocean Economy (AD 400–1275). Volume 1: Analysis; The British Museum: London, UK, 2021. [Google Scholar] [CrossRef]
- Carvajal López, J.; Guérin, A.; Georgakopoulou, M. Petrographic analysis of ceramics from Murwab, an early Islamic site in Qatar. Proc. Semin. Arab. Stud. 2022, 51, 55–70. [Google Scholar]
- West, N.; Alexander, R.; Kagi, R.I. The use of silicalite for rapid isolation of branched and cyclic fractions in petroleum. Org. Geochem. 1990, 15, 499–501. [Google Scholar] [CrossRef]
- Connan, J.; Engel, M.H.; Jackson, R.; Priestman, S.; Vosmer, T.; Zumberge, A. Geochemical analysis of two Samples of bitumen from jars discovered on Muhut and Masirah islands (Oman). Separations 2021, 8, 182. [Google Scholar] [CrossRef]
- Charrié-Duhaut, A.; Lemoine, S.; Adam, P.; Connan, J.; Albrecht, P. Abiotic oxidation of bitumens under natural conditions. Org. Geochem. 2000, 31, 977–1003. [Google Scholar] [CrossRef]
- Connan, J.; Borrell, F.; Vardi, J.; Wolff, S.; Ortiz, S.M.; Engel, M.; Gley, R.; Zumberge, A. Geochemical analysis of bituminous samples from the Pre-Pottery Neolithic B site of Nahal Efe (Northern Negev, Israel): Earliest evidence in the region and an example of alteration of the Dead Sea bitumen. Org. Geochem. 2024, 195, 104844. [Google Scholar] [CrossRef]
- Connan, J. Le Bitume dans l’Antiquité; Editions Errance: Arles, France, 2012; p. 272. [Google Scholar]
- Connan, J.; Carter, R. A geochemical study of bituminous mixtures from Failaka and Umm an-Namel (Kuwait), from the Early Dilmun to the Early Islamic period. Arab. Arch. Epig. 2007, 18, 139–181. [Google Scholar] [CrossRef]


| Sample Number | Country | Lab Number | Location | Building | Context | Archaeological Reference | Latitude | Longitude | Date Range | Pottery Type |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kuwait | 3539 | al-Qusur | building B16 (monk’s cell) | 5313 | QSR-12-A5/18 | 29°26′12” N | 48°20′52” E | Mid. 7th–early 9th CE | TORP-C |
| 2 | 3540 | building B20 (food processing), room R8 | 6067 | QSR-16-A4/25 | Late 8th–10th c. CE | CREAC (HAR-CR17) ?/BUFF ? | ||||
| 3 | 3541 | building B20, room R8 | 6067 | QSR-16-A4/25 | Late 8th–10th c. CE | CREAC (HAR-CR17) ./BUFF ? | ||||
| 4 | 3542 | building B14, courtyard | 7103 | QSR22-A5/0002 | Mid. 7th–early 9th c. CE | TORP-S | ||||
| 5 | 3543 | building B1 | 4 | QSR07/2100 | Mid. 7th–mid. 8th c. CE | TURQ.T (TUR-CR7) | ||||
| 6 | 3544 | building B14, room R1 | 7162 | QRS2022-A5/0001 | Mid. 7th–early 9th c. CE | TORP-S | ||||
| 7 | 3545 | building B20 (food processing), room R8 | 6067 | QRS16-A4/57 | Late 8th–10th c. CE | SPORC | ||||
| 8 | 3546 | building B23 (refectory) | 5635 | QSR15-A4/243 | Mid. 8th–10th c. CE | TORP-C | ||||
| 9 | 3547 | building B23 (refectory) | 5635 | QSR15-A4/243 | Mid. 8th–10th c. CE | TORP-C | ||||
| 26 | 3623 | building B14, courtyard | 7177 | QSR23-A5-B14-7177 | Mid. 7th–early 9th c. CE | TORP-S | ||||
| 27 | 3624 | building B19, room R2 | 6854 | QSR19-A5-U.97-6854 | Mid. 7th–early 9th c. CE | TORP-S | ||||
| 28 | 3625 | building B19, courtyard | 6862 | QSR19-A5-U.21-22-6862 | Mid. 8th–early 9th c. CE | TORP-C | ||||
| 29 | 3626 | building B20 (food processing), room R5 | / | QSR23-A4-B20-U.63 R15 | Mid. 8th–early 9th c. CE | TORP-C | ||||
| 30 | 3627 | building B25, room R4 | 7701 | QSR23-A6-B25-u.140-7701 | / | / |
| Sample Number | Country | Lab Number | Location | %EO | %Sat | %aro | %HC | %NSO | %ASP | %Pol | δ13Csat | δ13Caro | δ13CNSO | δ13Casp | δDNSO | δDasp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kuwait | 3539 | al-Qusur | 13.8 | 1.4 | 2 | 3.4 | 8.7 | 87.9 | 96.6 | −28.5 | −27.4 | −28 | −28 | −84 | −82 |
| 2 | 3540 + 3541 | 13.4 | 4.5 | 5.2 | 9.7 | 18.7 | 71.6 | 90.3 | −27.4 | −25.9 | −28.1 | −28.1 | −91 | −88 | ||
| 4 | 3542 | 53.5 | 3 | 4 | 7 | 9.5 | 83.5 | 93 | −27.5 | −26.4 | −26.5 | −27.1 | −83 | −74 | ||
| 5 | 3543 | 21 | 4 | 3.1 | 7.1 | 14.9 | 78 | 92.9 | −27.1 | −26 | −26.1 | −25.6 | −87 | −84 | ||
| 6 | 3544 | 15 | 1.7 | 3.2 | 4.9 | 12 | 83.1 | 95.1 | −28.8 | −27.3 | −28.1 | −28.2 | −86 | −71 | ||
| 7 | 3545 | 34.3 | 3.9 | 2.7 | 6.6 | 7.5 | 85.9 | 93.4 | −27.2 | −26.5 | −26.9 | −27.1 | −85 | −73 | ||
| 3545bis | 36.7 | 3.4 | 2.5 | 5.9 | 7.5 | 86.6 | 94.1 | −27.6 | −26.6 | −26.8 | −26.8 | −82 | −74 | |||
| 8 | 3546 | 22.1 | 3.3 | 3.5 | 6.8 | 7.1 | 86.1 | 93.2 | −27 | −25.9 | −26.2 | −25.8 | −89 | −69 | ||
| 9 | 3547 | 32.4 | 2.7 | 2.7 | 5.4 | 10.2 | 84.4 | 94.6 | −27 | −26 | −26.3 | −25.8 | −108 | −72 | ||
| 3547bis | 28.3 | 3.2 | 3 | 6.2 | 12.2 | 81.6 | 93.8 | −27 | −25.8 | −26.5 | −25.9 | −101 | −79 | |||
| 26 | Kuwait | 3623 | al-Qusur | 42.8 | 4.1 | 2.6 | 6.7 | 7.5 | 85.8 | 93.3 | −27.5 | −26.2 | −26.8 | −26.8 | −80 | −74 |
| 27 | 3624 | 41.5 | 4.5 | 2.2 | 6.7 | 5.3 | 88 | 93.3 | −27.2 | −26.3 | −26.7 | −26.9 | −77 | −77 | ||
| 28 | 3625 | 5.2 | 1.7 | 2.2 | 3.9 | 12.9 | 83.2 | 96.1 | −27.2 | −27.7 | −28.2 | −28 | −103 | −74 | ||
| 29 | 3626 | 1.7 | 1.4 | 1.4 | 2.8 | 8.4 | 88.8 | 97.2 | −28.7 | −27.8 | −27.9 | −27.9 | −83 | −72 | ||
| 30 | 3627 | 32.2 | 4.1 | 2.6 | 6.7 | 5.8 | 87.5 | 93.3 | −27.4 | −26.3 | −26.8 | −26.8 | −81 | −68 |
| Lab Number | C30αβHopane (ppm) | Tet/C23 | C29/H | Ol/H | C31R/H | GA/C31R | GA/C30H | C35S/C34S | Ster/Terp | Dia/Reg | %C27 | %C28 | %C29 | C2920S/20R | C29αββS/C29αααR | Ts/Tm | Tricyclics | Terpanes | Steranes | C27diasteranes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3539 | 5525 | 1.27 | 1.02 | 0 | 0.32 | 0.59 | 0.19 | 1.14 | 0.04 | 0.12 | 15.5 | 18.8 | 65.7 | 0.55 | 0.68 | 0.09 | almost absent | well preserved | biodegraded -C27 < C28 < C29 | |
| 3540 + 3541 | 13,768 | 3.41 | 1.19 | 0 | 0.23 | 0.56 | 0.13 | 0.87 | 0.08 | 0.08 | 4.3 | 15.5 | 80.2 | 0.38 | 0.81 | 0.1 | almost absent | preserved? | severely biodegraded-C27 | |
| 3542 | 3428 | 4.15 | 1.35 | 0 | 0.3 | 0.55 | 0.17 | 1.03 | 0.13 | 0.19 | 19.5 | 31.2 | 49.3 | 0.71 | 0.99 | 0.33 | almost absent | preserved? | biodegraded | |
| 3543 | 109 | 0.53 | 1.65 | 3.72 | 0.43 | 1.07 | 0.46 | 3.7 | 0.72 | 0.28 | 4.8 | 30.9 | 64.4 | 11.38 | 2.6 | 1.37 | absent | severely biodegraded | severely biodegraded- | |
| 3544 | 10,580 | 3.44 | 0.94 | 0 | 0.27 | 0.58 | 0.16 | 1.08 | 0.09 | 0.04 | 22.3 | 21 | 56.8 | 0.65 | 1.06 | 0.09 | absent | preserved | slightly biodegraded | |
| 3545 | 3563 | 1.15 | 0.98 | 0.1 | 0.31 | 0.36 | 0.11 | 1.15 | 0.36 | 0.69 | 25.1 | 29.9 | 45 | 0.76 | 1.37 | 0.35 | present low | preserved | preserved | present |
| 3545bis | 3426 | 1.79 | 1.01 | 0.11 | 0.33 | 0.35 | 0.12 | 1.25 | 0.33 | 0.86 | 21.1 | 30 | 48.9 | 1.05 | 1.52 | 0.38 | present low | preserved | preserved | present |
| 3546 | 1148 | 1.26 | 1.01 | 0.35 | 0.32 | 0.36 | 0.12 | 1.72 | 0.75 | 0.75 | 18.3 | 32 | 49.7 | 1.18 | 1.71 | 0.4 | present low | preserved | preserved | present |
| 3547 | 1569 | 1.24 | 0.94 | 0.2 | 0.38 | 0.29 | 0.11 | 1.26 | 0.36 | 0.92 | 15.9 | 31.8 | 52.2 | 1.19 | 1.71 | 0.49 | present low | preserved | slightly biodegraded-C29αββR -C27αββR biodegraded | present |
| 3447bis | 2027 | 1.85 | 0.94 | 0.17 | 0.36 | 0.3 | 0.11 | 1.19 | 0.34 | 0.87 | 14.8 | 33.1 | 52.1 | 1.16 | 1.79 | 0.49 | almost absent | preserved | slightly biodegraded-C29αββR -C27αββR biodegraded | present |
| 3623 | 1464 | 1.1 | 1.19 | 0.16 | 0.34 | 0.34 | 0.12 | 1.3 | 0.82 | 1.09 | 24.1 | 32 | 43.9 | 1.28 | 2.05 | 0.46 | almost absent | preserved | slightly biodegraded-C29αββR -C27αββR biodegraded | present |
| 3624 | 4313 | 1.46 | 1.06 | 0.1 | 0.32 | 0.31 | 0.1 | 1.05 | 0.33 | 0.81 | 27.8 | 30 | 42.2 | 0.88 | 1.42 | 0.39 | almost absent | preserved | preserved | abundant |
| 3625 | 2786 | 2.14 | 1.04 | 0 | 0.35 | 0.73 | 0.26 | 1.18 | 0.04 | 0.59 | 17.5 | 21.8 | 60.7 | 0.71 | 0.74 | 0.13 | absent | preserved | biodegraded-C27 < C28 < C29 | present low |
| 3626 | 21,510 | 3.62 | 1.13 | 0 | 0.25 | 0.54 | 0.14 | 0.9 | 0.08 | 0.05 | 24.9 | 22.9 | 52.2 | 0.47 | 0.77 | 0.1 | absent | preserved | biodegraded-C27 < C28 < C29 | absent |
| 3627 | 1244 | 0.58 | 1.23 | 0.17 | 0.32 | 0.42 | 0.13 | 1.55 | 0.7 | 1.09 | 24.6 | 30.8 | 44.6 | 1.18 | 1.44 | 0.53 | present | preserved? | preserved | abundant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Connan, J.; Bonnéric, J.; Perrogon, R.; Engel, M.H.; Gley, R.; Zumberge, A.; Schaeffer, P. Geochemical Study of Bitumen Residues on Potsherds from the al-Qusur Monastery (7th–9th c. CE): Composition and Origin. Molecules 2025, 30, 2006. https://doi.org/10.3390/molecules30092006
Connan J, Bonnéric J, Perrogon R, Engel MH, Gley R, Zumberge A, Schaeffer P. Geochemical Study of Bitumen Residues on Potsherds from the al-Qusur Monastery (7th–9th c. CE): Composition and Origin. Molecules. 2025; 30(9):2006. https://doi.org/10.3390/molecules30092006
Chicago/Turabian StyleConnan, Jacques, Julie Bonnéric, Rémi Perrogon, Michael H. Engel, Renaud Gley, Alex Zumberge, and Philippe Schaeffer. 2025. "Geochemical Study of Bitumen Residues on Potsherds from the al-Qusur Monastery (7th–9th c. CE): Composition and Origin" Molecules 30, no. 9: 2006. https://doi.org/10.3390/molecules30092006
APA StyleConnan, J., Bonnéric, J., Perrogon, R., Engel, M. H., Gley, R., Zumberge, A., & Schaeffer, P. (2025). Geochemical Study of Bitumen Residues on Potsherds from the al-Qusur Monastery (7th–9th c. CE): Composition and Origin. Molecules, 30(9), 2006. https://doi.org/10.3390/molecules30092006







