Identification of Chemical Components in Three Types of Rose Essential Oils Based on Gas Chromatography-Mass Spectrometry (GC-MS) and Chemometric Methods
Abstract
1. Introduction
2. Results
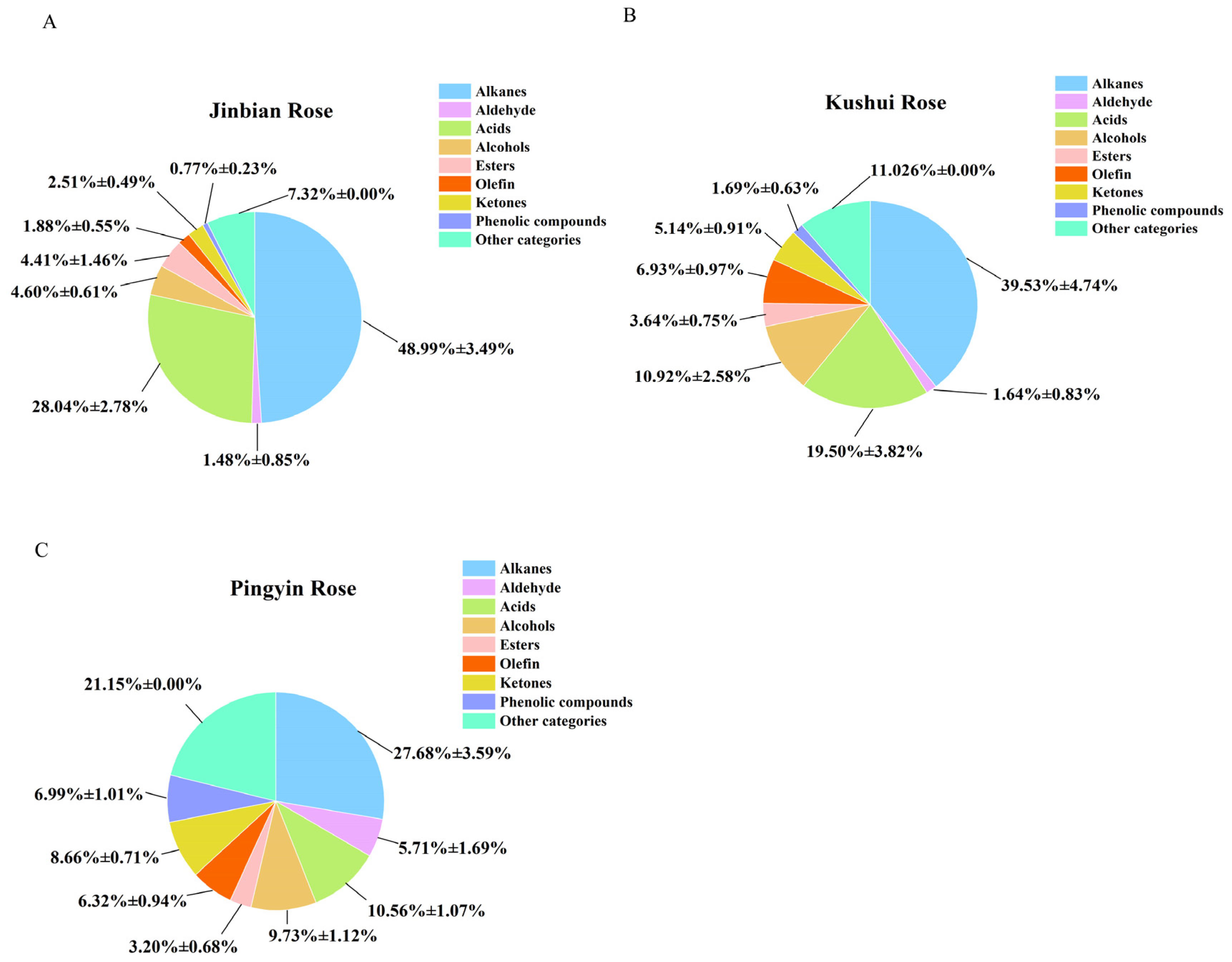
2.1. Analysis of the Main Major Compound Classes in the Essential Oils of the Three Types of Roses
2.2. Analysis of Specific Components in the Essential Oils of Three Types of Roses
2.3. Analysis of the Same Components in the Three Types of Dried Roses
2.3.1. Hierarchical Cluster Analysis (HCA)
2.3.2. Principal Component Analysis (PCA)
2.3.3. Partial Least Squares-Discriminant Analysis (PLS-DA)
2.3.4. Orthogonal Partial Least Squares-Discriminant Analysis (OPLS-DA)
3. Discussion
3.1. Analysis and Comparison of the Main Components in the Essential Oils of Three Types of Roses
3.2. Analysis of the Main Compounds in Three Types of Rose Essential Oils
3.3. Future Prospects
4. Materials and Methods
4.1. Materials
4.2. Extraction Method of Rose Essential Oil
4.3. Gas Chromatography-Mass Spectrometry (GC-MS)
4.4. Statistical Analyses and Software
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raka, R.N.; Zhiqian, D.; Yue, Y.; Luchang, Q.; Suyeon, P.; Junsong, X.; Hua, W. Pingyin rose essential oil alleviates LPS-Induced inflammation in RAW 264.7 cells via the NF-κB pathway: An integrated in vitro and network pharmacology analysis. BMC Complement. Med. Ther. 2022, 22, 272. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; People’s Medical Publishing House, Co. Ltd.: Beijing, China, 2020. [Google Scholar]
- Wang, H.Y.; Wang, J.T. Research and application status of rose ethereal oil. Cereals Oils 2015, 28, 5–9. [Google Scholar]
- Li, C.L.; Zhao, Y.M.; Yang, J.L. Research progress on extraction process, chemical composition and biological activity of roses. Anal. Test. Technol. Instrum. 2020, 26, 9. [Google Scholar]
- Zhang, Z.S.; Zhang, W.C. Research progress on extraction and daily application of rose essential oil. Food Nutr. Sci. 2024, 13, 212–216. [Google Scholar]
- Tohtiruzi, R.; Liu, C.; Abdukerem, D.; Abdukader, A. Study on the factors affecting the extraction rate of volatile oil from roses. Org. Chem. Res. 2023, 11, 165–170. [Google Scholar]
- Li, X.G.; Liu, Q.; Zhu, G.J.; Liu, Q.L.; Lv, W.C.; Li, J.F.; Qiao, Z. Analysis of the composition of Pingyin rose essential oil and its application in cigarettes. Gansu Sci. Technol. 2022, 38, 4. [Google Scholar]
- Zhang, H.X.; Wang, Z.Z.; Yang, Y.H.; Zheng, Z.F.; Du, Z.Z. Development of a blonde edge rose beverage and evaluation of its aroma and antioxidant activity. Food Ind. 2021, 42, 5. [Google Scholar]
- Chen, L.Y.; Wang, S.P.; Zhang, Y.L.; Jiang, B.X.; Zhang, J. Analysis of essential oil of bitter water rose and its pigment extraction. Chem. World 2024, 4, 1–6. [Google Scholar]
- Li, H.; Bai, H.T. Essential Oil Rose Resources. Life World 2020, 8, 44–45. [Google Scholar]
- Liang, X.P.; Xie, H.C. Research Progress of Rose Essential Oil. Anhui Agric. Sci. Bull. 2020, 26, 2. [Google Scholar]
- GB/T 43954-2024; State Administration for Market Regulation and National Standardization Administration Double Petaled Red Rose Essential Oil. China Standard Press: Beijing, China, 2024.
- Ma, J.P.; Shi, S.L.; Zhang, Y.X.; Guo, X.C.; Cai, Y.; Guo, P.H.; Yang, J.T.; Gao, D.D. Study on the extraction of essential oil and antibacterial activity of Rose Bitterwater in Lanzhou by ultrasonic and microwave synergistic extraction method. J. Chin. Cereals Oils Assoc. 2023, 38, 151–157. [Google Scholar]
- Wang, Q.M.; Tao, L.; Li, Z.K. Application of Jinbian Rose Extract in the Preparation of Drugs for Anti Chemotherapy Side Effects, Protection of Intestinal Mucosal Barrier, and/or Enhancement of Body Immunity; China Patent Database: Beijing, China, 2015. [Google Scholar]
- Zhang, L.; Kou, Y.P.; Duan, M.A.; Wang, X.F.; Jia, R.D.; Zhao, X.; Li, Q.X.; Ge, H.; Yang, S.H. Researcher Progress on Oil-bearing Roses. J. Plant Genet. Resour. 2024, 25, 777–789. [Google Scholar]
- Song, N.; Zhou, X.; Gong, B.; Feng, J. Exploration on Historical Tracing and Modern Clinical Application of TCM Aromatherapy. Flavour Fragr. Cosmet. 2021, 6, 94–98. [Google Scholar]
- An, B.F.; Chen, C.Y.; Qiao, G.F.; Wang, L.F.; Tao, Y.X.; Liu, T.Z. Research Progress on the Extraction and Application of Rose Essential Oil. J. Anhui Agric. Sci. 2024, 52, 6–10. [Google Scholar]
- Chen, Y.H.; Bi, J.H.; Xie, M.; Zhang, H.; Shi, Z.Q.; Guo, H.; Yin, H.B.; Zhang, J.N.; Xin, G.Z.; Song, H.P. Classification-based strategies to simplify complex traditional Chinese medicine (TCM) researches through liquid chromatography-mass spectrometry in the last decade (2011–2020): Theory, technical route and difficulty. J. Chromatogr. A 2021, 1651, 462307. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Q.; Wang, C.; Qiu, H.; Liu, B.; Jiang, Z.H.; Zhang, W. Comparison of two exploratory data analysis methods for classification of Phyllanthus chemical fingerprint: Unsupervised vs. supervised pattern recognition technologies. Anal. Bioanal. Chem. 2015, 407, 1389–1401. [Google Scholar] [CrossRef]
- Guo, L.; Duan, L.; Liu, K.; Liu, E.H.; Li, P. Chemical comparison of Tripterygium wilfordii and Tripterygium hypoglaucum based on quantitative analysis and chemometrics methods. J. Pharm. Biomed. Anal. 2014, 95, 220–228. [Google Scholar] [CrossRef]
- Li, P.; Zeng, S.L.; Duan, L.; Ma, X.D.; Dou, L.L.; Wang, L.J.; Li, P.; Bi, Z.M.; Liu, E.H. Comparison of Aurantii Fructus Immaturus and Aurantii Fructus based on multiple chromatographic analysis and chemometrics methods. J. Chromatogr. A 2016, 1469, 96–107. [Google Scholar] [CrossRef]
- Li, S.; Huang, Y.; Zhang, F.; Ao, H.; Chen, L. Comparison of Volatile Oil between the Ligusticum sinese Oliv. and Ligusticum jeholense Nakai et Kitag. Based on GC-MS and Chemical Pattern Recognition Analysis. Molecules 2022, 27, 5325. [Google Scholar] [CrossRef]
- Song, B.; Wang, W.; Liu, R.; Cai, J.; Jiang, Y.; Tang, X.; Wu, H.; Ao, H.; Chen, L. Geographic Differentiation of Essential Oil from Rhizome of Cultivated Atractylodes lancea by Using GC-MS and Chemical Pattern Recognition Analysis. Molecules 2023, 28, 2216. [Google Scholar] [CrossRef]
- Cao, X.; You, G.; Li, H.; Li, D.; Wang, M.; Ren, X. Comparative Investigation for Rotten Xylem (kuqin) and Strip Types (tiaoqin) of Scutellaria baicalensis Georgi Based on Fingerprinting and Chemical Pattern Recognition. Molecules 2019, 24, 2431. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.; Wang, J.; Chen, L.; Li, S.; Dai, C. Comparison of Volatile Oil between the Fruits of Amomum villosum Lour. and Amomum villosum Lour. var. xanthioides T. L. Wu et Senjen Based on GC-MS and Chemometric Techniques. Molecules 2019, 24, 1663. [Google Scholar] [CrossRef]
- Wu, H.M.; Huang, X.L.; Hao, J.J.; Kong, J.; Xu, F.; Wang, X.P. Study on the Anti-inflammatory Mechanism of Rose Based on Network Pharmacology. Chin. J. Mod. Appl. Pharm. 2020, 37, 7. [Google Scholar]
- He, L.Y.; Yang, Z.L.; Fan, D.M. Research Progress on Functional Componentsand Product Development of Rose. Sci. Technol. Food Ind. 2021, 42, 6. [Google Scholar]
- World Health Organization. Guidelines for the Assessment of Herbal Medicines; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Feng, W.Q.; Zhou, L.L.; Han, Y.; Zhang, T.T.; Wen, J.W.; Chen, C.; Wang, Y.; He, Y. Combing chemical composition profiling with machine learning for geographical origins identification of Nardostachys jatamansi DC. Microchem. J. 2024, 207, 112087. [Google Scholar] [CrossRef]
- Cebi, N.; Arici, M.; Sagdic, O. The famous Turkish rose essential oil: Characterization and authenticity monitoring by FTIR, Raman and GC-MS techniques combined with chemometrics. Food Chem. 2021, 354, 129495. [Google Scholar] [CrossRef]
- Lei, K.; Yuan, M.; Li, S.; Zhou, Q.; Li, M.; Zeng, D.; Guo, Y.; Guo, L. Performance evaluation of E-nose and E-tongue combined with machine learning for qualitative and quantitative assessment of bear bile powder. Anal. Bioanal. Chem. 2023, 415, 3503–3513. [Google Scholar] [CrossRef]
- Wang, X.X.; Wei, J.; Liu, J.Y.; Yin, G.Y.; Xu, S.T. Chemical component analysis of essential oil of Rosa gallica Linn in Yunnan by GC/MS and application research. J. Yunnan Univ. (Nat. Sci. Ed.) 2011, 33, 414–417. [Google Scholar]
- Zhou, W.; Zhou, X.P.; Zhao, G.H.; Liu, H.W.; Ding, L.; Chen, L.R. Studies of aroma components on essential oil of Chinese kushui rose. Se Pu Chin. J. Chromatogr. 2002, 20, 560–564. [Google Scholar]
- Ameena, M.; Arumugham, M.; Ramalingam, K.; Shanmugam, R. Biomedical Applications of Lauric Acid: A Narrative Review. Cureus 2024, 16, e62770. [Google Scholar]
- Vanitha, V.; Vijayakumar, S.; Nilavukkarasi, M.; Punitha, V.N.; Vidhya, E.; Praseetha, P.K. Heneicosane—A novel microbicidal bioactive alkane identified from Plumbago zeylanica L. Ind. Crops Prod. 2020, 154, 112748. [Google Scholar] [CrossRef]
- Liang, S.; Li, Z.; Bao, C.; Liu, B.; Zhang, H.; Yuan, Y.; Yan, H.; Chen, S.; Zhang, H.; Shi, W.; et al. Non-Cardiotoxic Tetradecanoic Acid-2,4-Dinitrophenol Ester Nanomicelles in Microneedles Exert Potent Anti-Obesity Effect by Regulating Adipocyte Browning and Lipogenesis. Small 2023, 19, e2301751. [Google Scholar] [CrossRef] [PubMed]
- Sokmen, B.B.; Hasdemir, B.; Yusufoglu, A.; Yanardag, R. Some monohydroxy tetradecanoic acid isomers as novel urease and elastase inhibitors and as new antioxidants. Appl. Biochem. Biotechnol. 2014, 172, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Riaz, M.; Munir, N.; Akhter, N.; Zafar, S.; Jabeen, F.; Ali Shariati, M.; Akhtar, N.; Riaz, Z.; Altaf, S.H.; et al. Chemical constituents, experimental and clinical pharmacology of Rosa damascena: A literature review. J. Pharm. Pharmacol. 2020, 72, 161–174. [Google Scholar] [CrossRef]
- Maia, W.M.; de Andrade, F.D.; Filgueiras, L.A.; Mendes, A.N.; Assunção, A.F.; Rodrigues, N.D.; Marques, R.B.; Maia Filho, A.L.; de Sousa, D.P.; Lopes, L.D. Antidepressant activity of rose oxide essential oil: Possible involvement of serotonergic transmission. Heliyon 2021, 7, e06620. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Shafei, M.N.; Saberi, Z.; Amini, S. Pharmacological effects of Rosa damascena. Iran. J. Basic Med. Sci. 2011, 14, 295–307. [Google Scholar]
- Kumar, A.; Gautam, R.D.; Singh, S.; Chauhan, R.; Kumar, M.; Kumar, D.; Kumar, A.; Singh, S. Phenotyping floral traits and essential oil profiling revealed considerable variations in clonal selections of damask rose (Rosa damascena Mill.). Sci. Rep. 2023, 13, 8101. [Google Scholar] [CrossRef]

| Rose Samples | Essential Oil/% (v/w) |
|---|---|
| Jinbian Rose | 0.030 ± 0.006 |
| Kushui Rose | 0.028 ± 0.004 |
| Pingyin Rose | 0.032 ± 0.007 |
| Compounds | Rt | Content %-Jinbian Rose | Content %-Kushui Rose | Content %-Pingyin Rose |
|---|---|---|---|---|
| Furfural | 14.981 | 0.46 ± 0.05 | 0.44 ± 0.05 | 0.55 ± 0.08 |
| Octadecane | 15.542 | / | / | 0.49 ± 0.04 |
| Benzaldehyde | 16.750 | 0.30 ± 0.09 | 0.57 ± 0.10 | / |
| Hexadecane | 21.451 | 0.56 ± 0.05 | 0.83 ± 0.07 | / |
| Levomenthol | 21.604 | / | / | 0.54 ± 0.06 |
| Benzeneacetaldehyde | 21.613 | 1.33 ± 0.14 | / | / |
| Aromandendrene | 21.756 | / | 0.95 ± 0.04 | / |
| Copaene | 22.450 | / | 0.22 ± 0.02 | / |
| trans-calamenene | 22.905 | / | 2.44 ± 0.04 | / |
| Pentacosane | 22.945 | / | / | 6.02 ± 0.07 * |
| alpha-Terpineol | 23.440 | / | 0.39 ± 0.04 | 0.14 ± 0.02 |
| 2-Dodecanol | 24.192 | / | 1.02 ± 0.06 | / |
| Heptadecane | 24.296 | 0.50 ± 0.04 | / | / |
| Dodecane | 24.354 | / | 0.57 ± 0.05 | / |
| Citronellol | 25.105 | 0.85 ± 0.07 | 0.62 ± 0.05 | 0.65 ± 0.06 |
| Acetic acid, 2-phenylmethyl ester | 25.981 | 0.27 ± 0.03 | / | 0.44 ± 0.05 |
| 2-Tridecanone | 26.162 | 0.49 ± 0.04 | 1.66 ± 0.18 | 0.63 ± 0.06 |
| Tridecanal | 26.438 | 0.52 ± 0.05 | 0.52 ± 0.09 | / |
| Nonadecane | 26.656 | 10.37 ± 0.16 * | / | / |
| Octacosane | 27.751 | 0.35 ± 0.04 | / | 0.62 ± 0.06 |
| Geraniol | 27.018 | / | 0.56 ± 0.05 | 0.51 ± 0.06 |
| Phenylethyl Alcohol | 28.493 | 0.57 ± 0.05 | 0.70 ± 0.08 | 0.54 ± 0.05 |
| 2-Hexadecanol | 29.540 | / | 2.47 ± 0.10 * | / |
| 2-Tetradecanol | 29.616 | / | 1.68 ± 0.08 | / |
| Nonadecane | 29.740 | / | 1.41 ± 0.05 | 1.22 ± 0.08 |
| n-Pentadecanol | 29.939 | / | 0.96 ± 0.08 | 0.510 ± 0.04 |
| 9-Nonadecene | 30.101 | / | 0.46 ± 0.04 | / |
| Methyleugenol | 32.794 | 2.85 ± 0.08 * | 0.82 ± 0.06 | 8.55 ± 0.21 * |
| Mandelic acid | 33.279 | 0.31 ± 0.03 | 0.74 ± 0.05 | / |
| 2-Pentadecanone | 33.517 | / | / | / |
| Methoxyacetic acid, 2-tetradecyl | 33.746 | / | / | 1.10 ± 0.07 |
| Eicosane | 33.936 | 3.36 ± 0.15 * | 1.54 ± 0.09 | / |
| Octanoic acid | 33.420 | 1.55 ± 0.10 | / | 0.23 ± 0.03 |
| Nerolidol 2 | 34.088 | 2.59 ± 0.10 | / | / |
| Nerolidyl acetate | 34.260 | / | 0.35 ± 0.04 | / |
| Octanoic acid | 34.545 | / | 0.92 ± 0.07 | / |
| n-Tridecan-1-ol | 35.373 | 0.33 ± 0.04 | / | / |
| Cedrol | 36.496 | / | 1.36 ± 0.08 | / |
| 1-Hexadecanol, acetate | 36.591 | / | / | 1.61 ± 0.09 |
| Pent-2-ynal | 38.066 | / | 0.41 ± 0.08 | / |
| Heneicosane | 38.523 | 13.47 ± 0.12 * | 8.55 ± 0.24 * | 9.20 ± 0.18 * |
| Eugenol | 38.761 | 0.77 ± 0.05 | 0.88 ± 0.08 | 3.54 ± 0.09 * |
| Nonanoic acid | 39.236 | 1.21 ± 0.07 | / | 0.63 ± 0.04 |
| 1-Undecanol | 39.817 | / | / | 0.44 ± 0.04 |
| Isolongifolen-5-one | 40.074 | / | 2.89 ± 0.05* | / |
| 2-Benzyl-3-isopropyl-cyclopentanone | 41.140 | / | 0.83 ± 0.06 | / |
| alpha-Bisabolol | 41.416 | 0.55 ± 0.05 | 1.89 ± 0.09 | 1.03 ± 0.05 |
| Hexadecanoic acid, methyl ester | 41.891 | 0.65 ± 0.06 | / | / |
| Decanoic acid, methyl ester | 42.091 | / | 0.47 ± 0.05 | / |
| Hentriacontane | 42.405 | / | / | 1.01 ± 0.05 |
| Docosane | 42.443 | 0.55 ± 0.06 | / | / |
| Pentacosane | 42.576 | / | 5.72 ± 0.21 | / |
| n-Decanoic acid | 43.623 | 1.03 ± 0.05 | 1.42 ± 0.08 | 1.08 ± 0.09 |
| Citronellyl butyrate | 45.050 | / | 2.81 ± 0.09 | / |
| Isophytol | 45.431 | / | 0.33 ± 0.08 | / |
| Tricosyl acetate | 45.450 | / | / | 0.425 ± 0.05 |
| Tricosone | 47.011 | 6.12 ± 0.12 * | 11.79 ± 0.16 * | 12.87 ± 0.18 * |
| 1-Methoxymethyl-2-methylbenzene | 47.981 | 1.78 ± 0.06 | / | / |
| 1,4-Pentadiene | 47.991 | / | 2.79 ± 0.10 * | / |
| Benzenemethanol, 2,4-dimethyl- | 48.295 | / | / | 0.62 ± 0.05 |
| 2,3-Dimethylanisole | 48.486 | / | 1.52 ± 0.09 | / |
| Tetracosane | 50.893 | / | / | 1.07 ± 0.08 |
| Isolongifolene, 9,10-dehydro- | 52.264 | / | / | 1.42 ± 0.07 |
| Dodecanoic acid | 52.444 | 2.34 ± 0.10 | 2.83 ± 0.08 * | 1.375 ± 0.11 |
| trans-calamenene | 52.463 | / | 1.56 ± 0.10 | / |
| 4-Isopropylcinnamic acid | 52.930 | / | / | 0.56 ± 0.06 |
| Eicosane | 55.128 | / | / | 7.56 ± 0.18 * |
| Pentacosane | 55.423 | 5.12 ± 0.10 * | / | / |
| 3,4-Dimethylanisole | 56.412 | 0.88 ± 0.09 | / | |
| Tridecanoic acid | 56.812 | 4.83 ± 0.07 | / | / |
| 2-Aminoresorcinol | 57.355 | / | / | 0.83 ± 0.06 |
| Phytol | 57.964 | 1.93 ± 0.04 | 2.19 ± 0.07 | 0.63 ± 0.05 |
| Benzoic acid, 2-phenylmethyl ester | 60.076 | 0.34 ± 0.03 | / | / |
| Tetradecanoic acid | 60.885 | 2.90 ± 0.08 * | 0.55 ± 0.07 | 0.66 ± 0.05 |
| cis, cis, cis-7,10,13-Hexadecatrienal | 61.256 | / | 0.56 ± 0.05 | / |
| Heptacosane | 62.807 | 2.52 ± 0.10 | 2.374 ± 0.14 | 1.985 ± 0.13 |
| Hexatriacontane | 62.731 | / | 1.96 ± 0.10 * | |
| Octacosyl trifluoroacetate | 63.958 | 0.62 ± 0.03 | / | / |
| Pentadecanoic acid | 64.596 | 0.51 ± 0.04 | / | / |
| n-Hexadecanoic acid | 68.697 | 14.15 ± 0.14 * | 12.17 ± 0.54 * | 6.62 ± 0.23 * |
| Octadecanoic acid | 76.500 | 0.54 ± 0.09 | / | 0.62 ± 0.05 |
| Oleic Acid | 77.737 | 0.96 ± 0.10 | / | / |
| 9,12-Octadecadienoic acid (Z, Z)- | 80.84 | 3.44 ± 0.38 | 3.70 ± 0.10 * | 0.58 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Cai, J.; Wang, L.; Zhu, S.; Chen, Y.; Chen, Y.; Zhong, J.; Li, J.; Hu, P.; Ye, Q. Identification of Chemical Components in Three Types of Rose Essential Oils Based on Gas Chromatography-Mass Spectrometry (GC-MS) and Chemometric Methods. Molecules 2025, 30, 1974. https://doi.org/10.3390/molecules30091974
Xu M, Cai J, Wang L, Zhu S, Chen Y, Chen Y, Zhong J, Li J, Hu P, Ye Q. Identification of Chemical Components in Three Types of Rose Essential Oils Based on Gas Chromatography-Mass Spectrometry (GC-MS) and Chemometric Methods. Molecules. 2025; 30(9):1974. https://doi.org/10.3390/molecules30091974
Chicago/Turabian StyleXu, Min, Jia Cai, Long Wang, Shunpeng Zhu, Yangxi Chen, Yuchen Chen, Jie Zhong, Jiaxin Li, Peng Hu, and Qiang Ye. 2025. "Identification of Chemical Components in Three Types of Rose Essential Oils Based on Gas Chromatography-Mass Spectrometry (GC-MS) and Chemometric Methods" Molecules 30, no. 9: 1974. https://doi.org/10.3390/molecules30091974
APA StyleXu, M., Cai, J., Wang, L., Zhu, S., Chen, Y., Chen, Y., Zhong, J., Li, J., Hu, P., & Ye, Q. (2025). Identification of Chemical Components in Three Types of Rose Essential Oils Based on Gas Chromatography-Mass Spectrometry (GC-MS) and Chemometric Methods. Molecules, 30(9), 1974. https://doi.org/10.3390/molecules30091974






