The Use of Plants That Seal Blood Vessels in Preparations Applied Topically to the Skin: A Review
Abstract
1. Introduction
2. Natural Substances of Plant Origin with a Sealing Effect on Blood Vessels
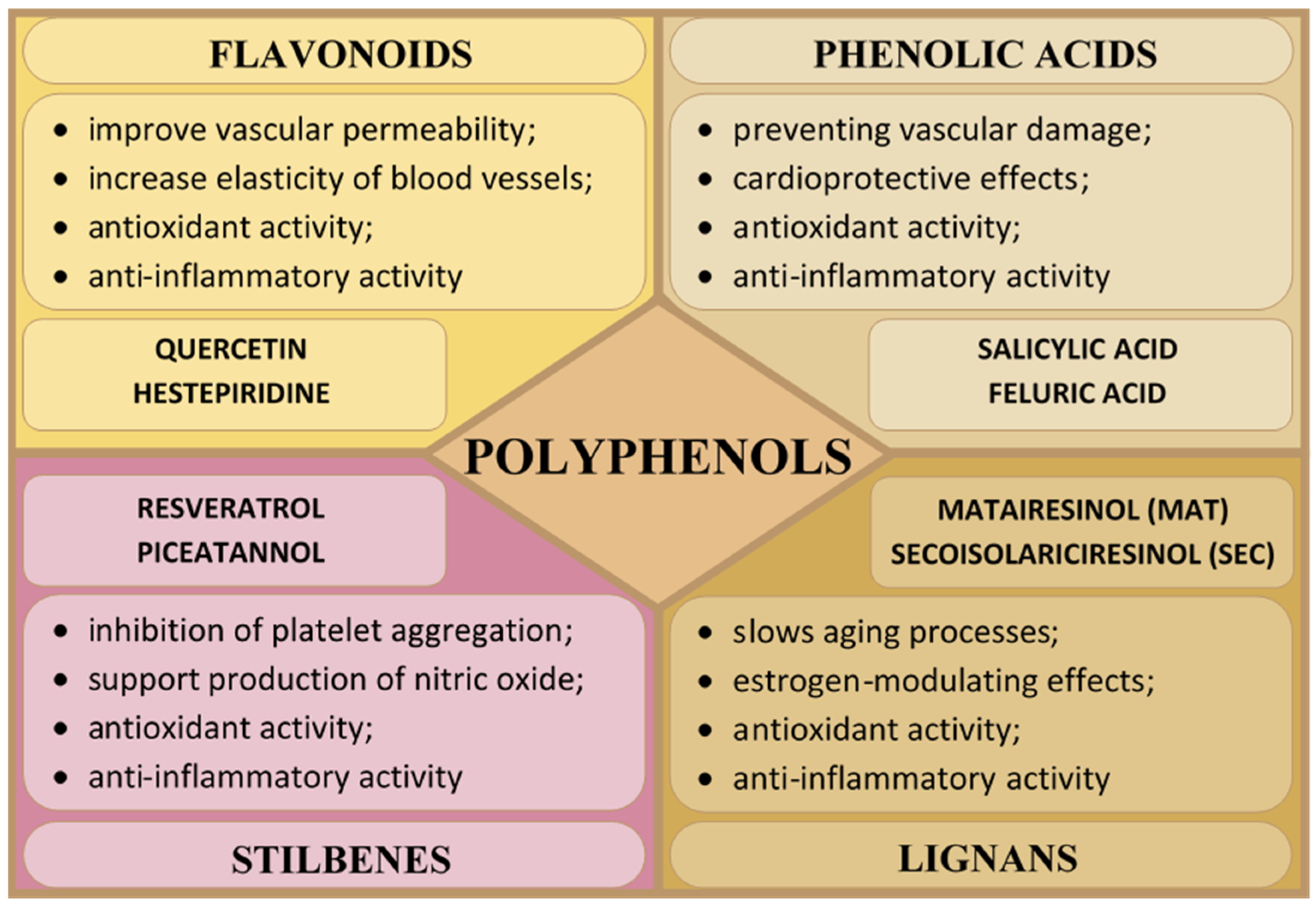
2.1. Polyphenols
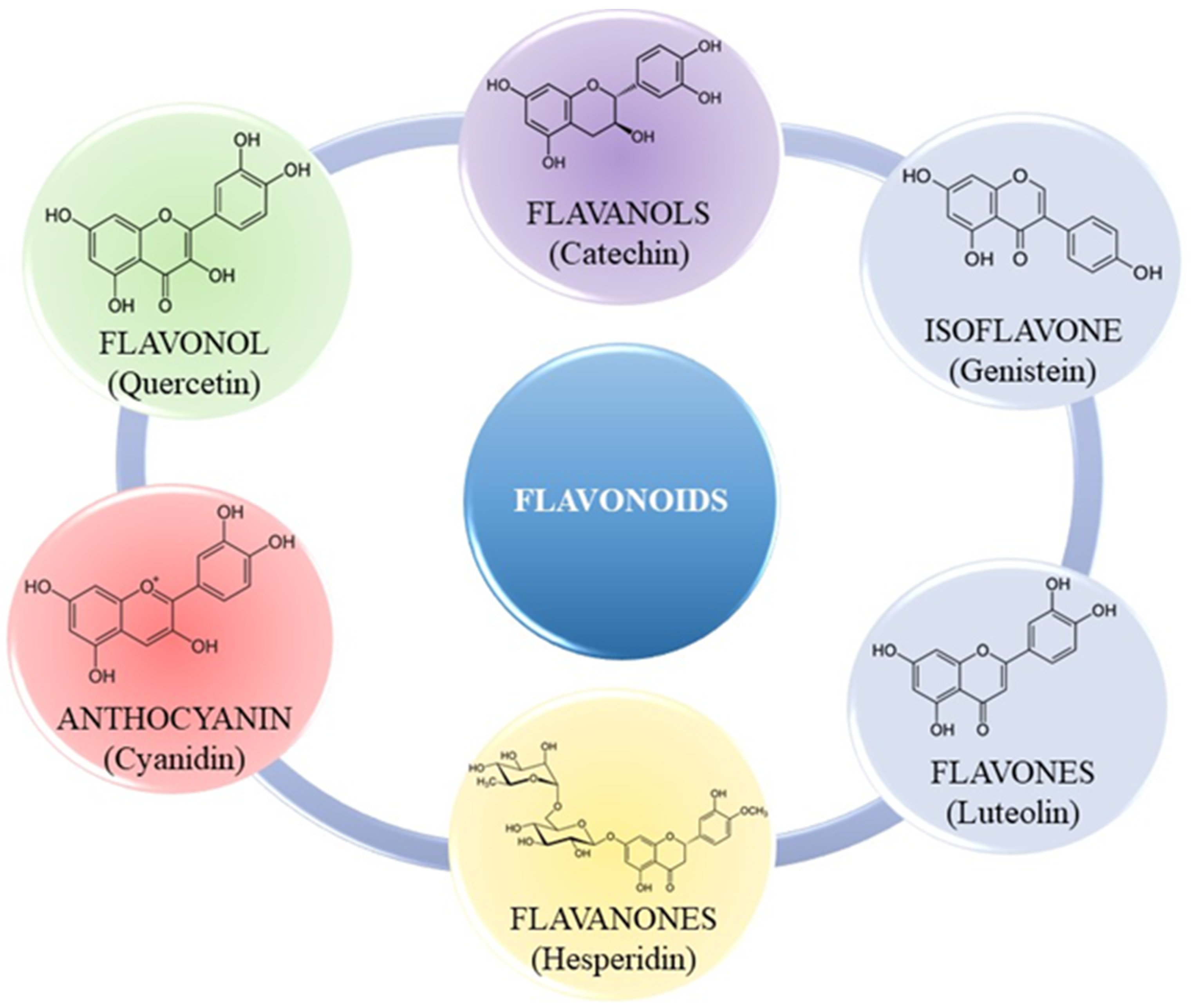
2.1.1. Flavonoids
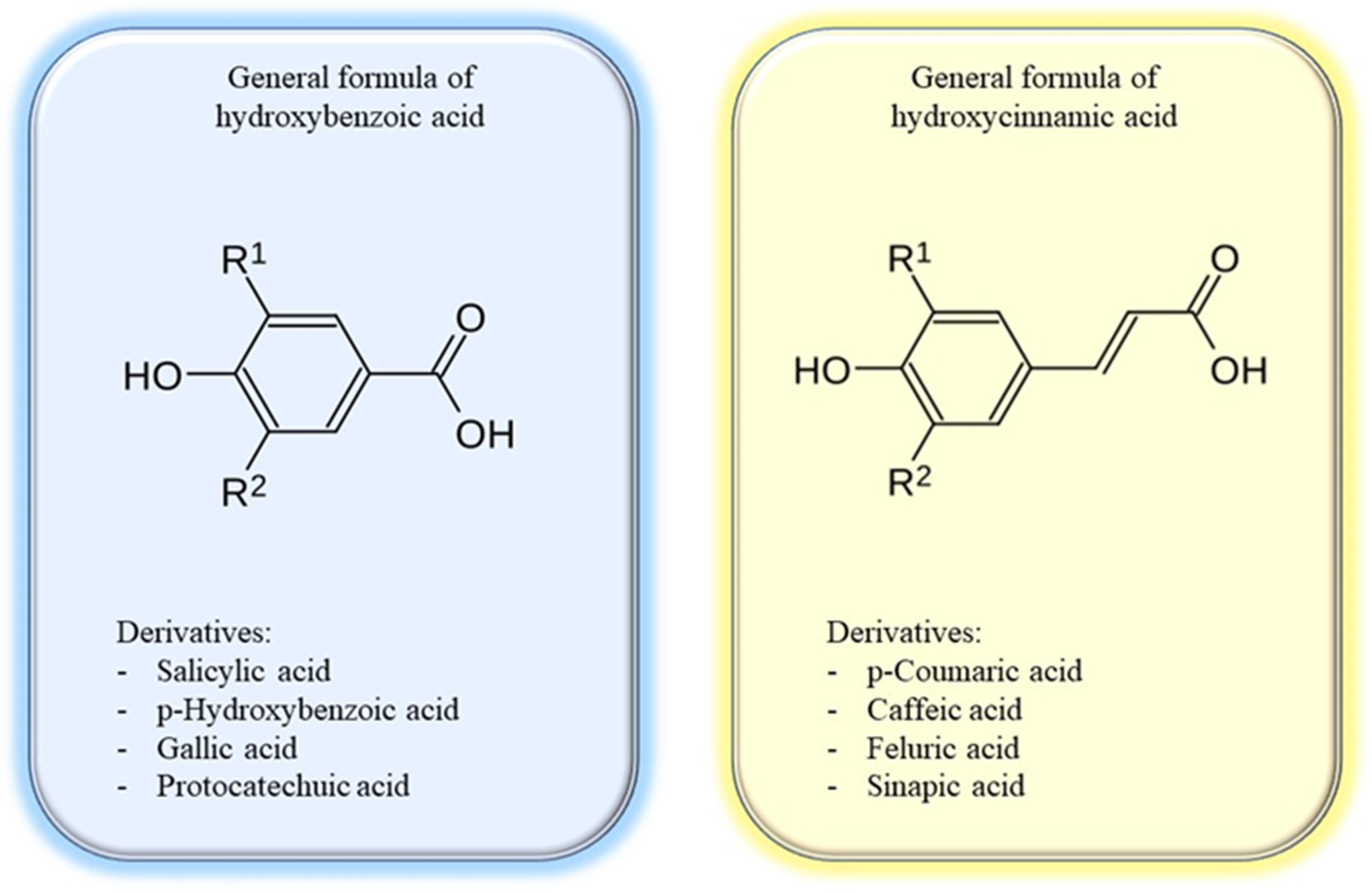
2.1.2. Phenolic Acids
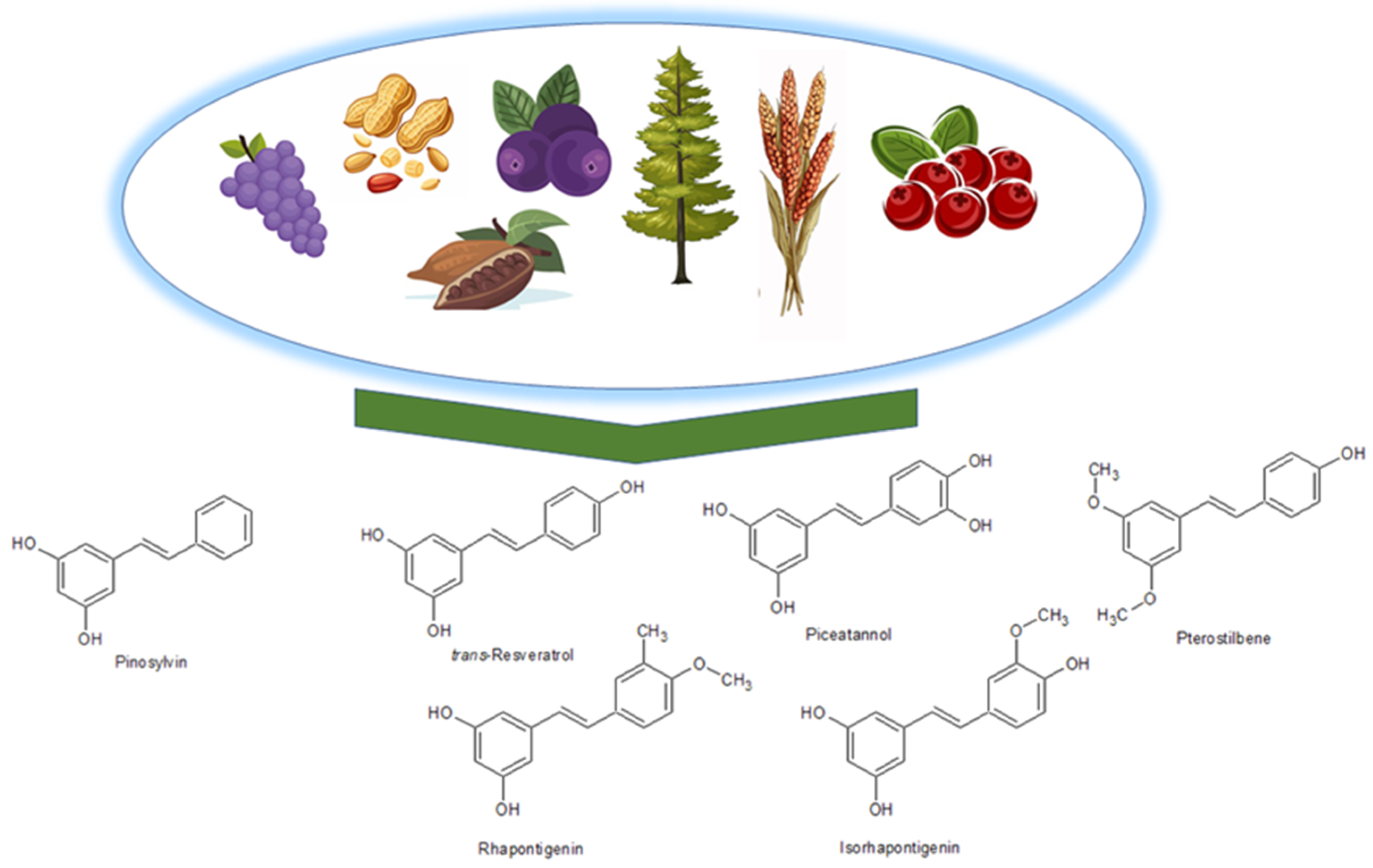
2.1.3. Stilbenes
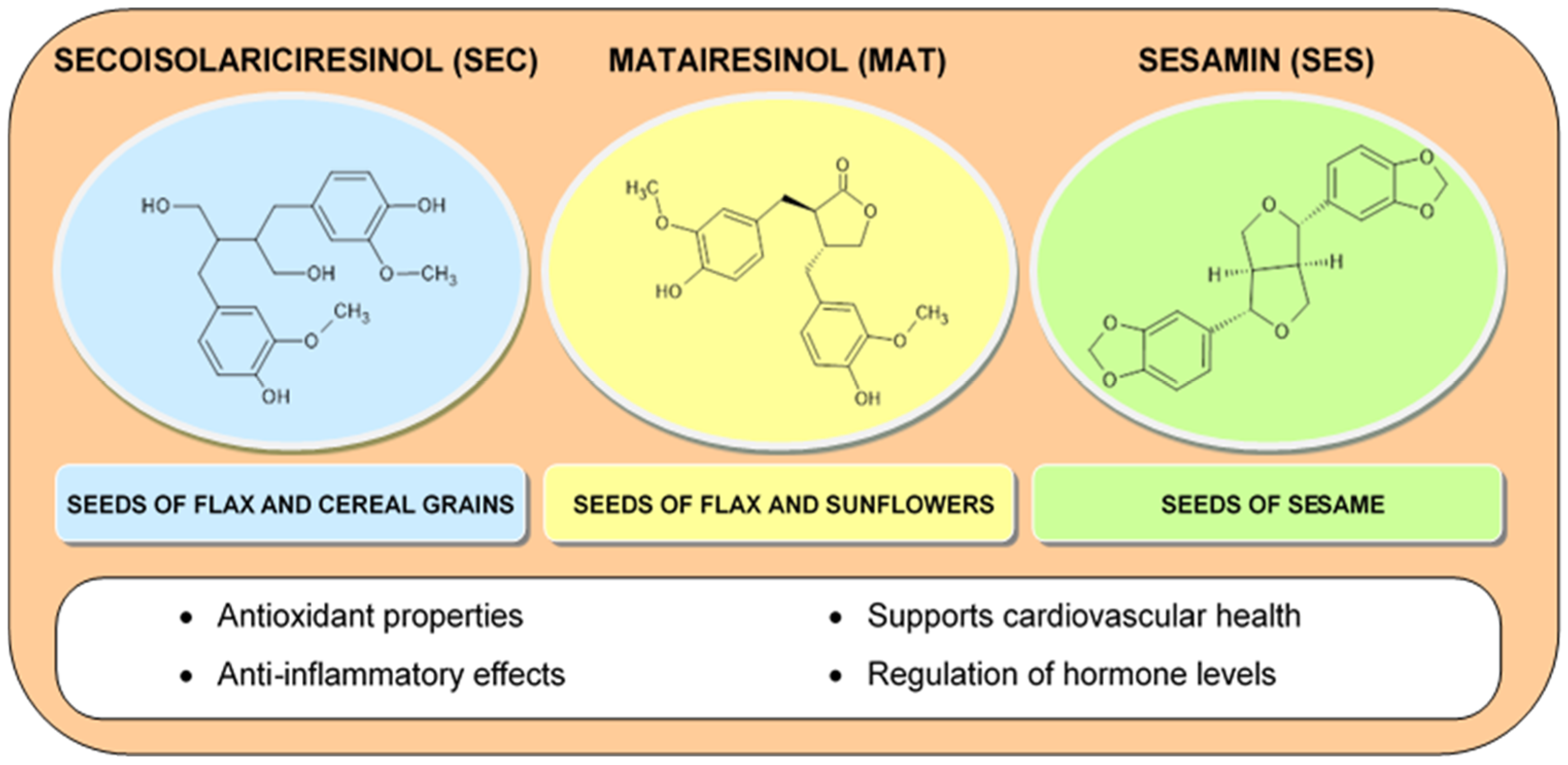
2.1.4. Lignans
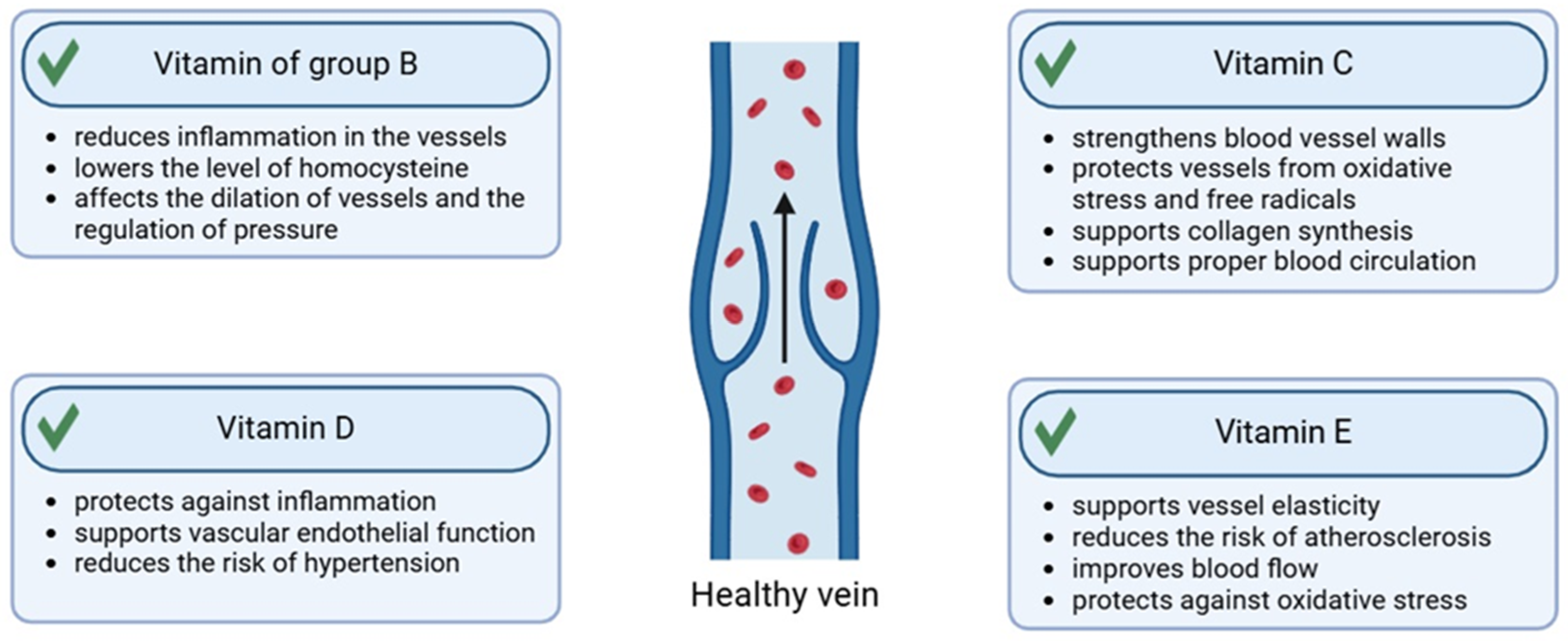
2.2. Vitamins
2.2.1. Vitamin C
2.2.2. Vitamin E
2.2.3. Vitamin D
2.2.4. Vitamins from the B Group
2.3. Saponins
2.4. Carotenoids
3. Plants in the Treatment of Vascular Skin
3.1. Camellia Sinensis
3.2. Chrysanthellum Indicum
3.3. Helichrysum Italicum
3.4. Glycyrrhiza Glabra
3.5. Artemisia Annua
3.6. Aesculus Hippocastanum
3.7. Potentilla Erecta
3.8. Achillea Millefolium
3.9. Quassia Amara
3.10. Ginkgo Biloba
3.11. Artemisia Lavandulaefolia
3.12. Calendula Officinalis
3.13. Arnica Montana
3.14. Ruscus Aculeatus
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal Plants: Past History and Future Perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Yi, E.-Y.; Han, K.-S.; Kim, Y.-J. Extract of Artemisia Lavandulaefolia Inhibits In Vitro Angiogenesis in Human Umbilical Vein Endothelial Cells. J. Cancer Prev. 2014, 19, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Eid, S.Y.; Alshammari, E.; Ashour, M.L.; Wink, M.; El-Readi, M.Z. Chrysanthemum Indicum and Chrysanthemum Morifolium: Chemical Composition of Their Essential Oils and Their Potential Use as Natural Preservatives with Antimicrobial and Antioxidant Activities. Foods 2020, 9, 1460. [Google Scholar] [CrossRef]
- Nilofar; Sinan, K.I.; Eyupoglu, O.E.; Ferrante, C.; Ahmed, S.; Etienne, O.K.; Zengin, G. Multiple Online-HPLC Methodologies and Biological Properties of Leaves and Stem Barks Extracts of Chrysanthellum Indicum. Microchem. J. 2024, 197, 109847. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of Photosynthetic Processes and the Accumulation of Secondary Metabolites in Plants in Response to Monochromatic Light Environments: A Review. Biochim. Biophys. Acta BBA-Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant Tissue Culture as a Perpetual Source for Production of Industrially Important Bioactive Compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Ng, M.-S.; Cheng, S.-S.; Lo, A.W.-Y.; Xiao, Z.; Shin, T.-S.; Chung, G.; Lam, H.-M. Understanding the Composition, Biosynthesis, Accumulation and Transport of Flavonoids in Crops for the Promotion of Crops as Healthy Sources of Flavonoids for Human Consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef]
- Chopade, V.V.; Phatak, A.A.; Upaganlawar, A.B.; Tankar, A. Green Tea (Camellia sinensis): Chemistry, Traditional, Medicinal Uses and Its Pharmacological Activities—A Review. Pharmacogn. Rev. 2008, 2, 157–162. [Google Scholar]
- Wang, X.; Lei, D.; Zhu, M.; Zhang, H.; Liao, J.; Zhang, J.; Liu, Y. Phylogeny, Genetics and Ecological Adaptation of the Chrysanthemum indicum Complex. Med. Plant Biol. 2023, 2, 17. [Google Scholar] [CrossRef]
- Kramberger, K.; Kenig, S.; Jenko Pražnikar, Z.; Kočevar Glavač, N.; Barlič-Maganja, D. A Review and Evaluation of the Data Supporting Internal Use of Helichrysum Italicum. Plants 2021, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua—Importance in Traditional Medicine and Current State of Knowledge on the Chemistry, Biological Activity and Possible Applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.; Mishra, A.; Khushtar, M. Phytochemical, Ethanomedicinal and Pharmacological Applications of Escin from Aesculus hippocastanum L. towards Future Medicine. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190115. [Google Scholar] [CrossRef]
- Wölfle, U.; Hoffmann, J.; Haarhaus, B.; Rao Mittapalli, V.; Schempp, C.M. Anti-Inflammatory and Vasoconstrictive Properties of Potentilla Erecta—A Traditional Medicinal Plant from the Northern Hemisphere. J. Ethnopharmacol. 2017, 204, 86–94. [Google Scholar] [CrossRef]
- Bagheri, A. Safety and Hemostatic Effect of Achillea millefolium L. in Localized Bleeding. Hepatol. Forum 2024, 5, 25–27. [Google Scholar] [CrossRef]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Kukula-Koch, W. Achillea Species as Sources of Active Phytochemicals for Dermatological and Cosmetic Applications. Oxid. Med. Cell. Longev. 2021, 2021, 6643827. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Health Benefits of Quassin from Quassia amara: A Comprehensive Review of Their Ethnopharmacological Importance, Pharmacology, Phytochemistry and Analytical Aspects. Curr. Nutr. Food Sci. 2020, 16, 35–44. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, H.; Zhang, Y.; Che, F.; Shen, N.; Cui, Y. Leaves, Seeds and Exocarp of Ginkgo biloba L. (Ginkgoaceae): A Comprehensive Review of Traditional Uses, Phytochemistry, Pharmacology, Resource Utilization and Toxicity. J. Ethnopharmacol. 2022, 298, 115645. [Google Scholar] [CrossRef]
- Sapkota, B.; Kunwar, P. A Review on Traditional Uses, Phytochemistry and Pharmacological Activities of Calendula officinalis Linn. Nat. Prod. Commun. 2024, 19, 1934578X241259021. [Google Scholar] [CrossRef]
- Gyawali, N.; Rayamajhi, A.; Karki, D.; Pokhrel, T.; Adhikari, A. Arnica montana L.: Traditional Uses, Bioactive Chemical Constituents, and Pharmacological Activities. In Medicinal Plants of the Asteraceae Family; Devkota, H.P., Aftab, T., Eds.; Springer Nature: Singapore, 2022; pp. 61–75. ISBN 978-981-19-6079-6. [Google Scholar]
- Rodrigues, J.P.B.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Pires, T.C.S.P.; Calhelha, R.C.; Carvalho, A.M.; Ferreira, I.C.F.R.; Barros, L. Phenolic Compounds and Bioactive Properties of Ruscus aculeatus L. (Asparagaceae): The Pharmacological Potential of an Underexploited Subshrub. Molecules 2021, 26, 1882. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hong, Y.J.; Kim, M. Angiogenesis in Chronic Inflammatory Skin Disorders. Int. J. Mol. Sci. 2021, 22, 12035. [Google Scholar] [CrossRef] [PubMed]
- Saurat, J.-H.; Halioua, B.; Baissac, C.; Cullell, N.P.; Ben Hayoun, Y.; Aroman, M.S.; Taieb, C.; Skayem, C. Epidemiology of Acne and Rosacea: A Worldwide Global Study. J. Am. Acad. Dermatol. 2024, 90, 1016–1018. [Google Scholar] [CrossRef]
- Hegdekar, N.Y.; Priya, S.; Shetty, S.S.; Jyothi, D. Formulation and Evaluation of Niosomal Gel Loaded with Asparagus Racemosus Extract for Anti-Inflammatory Activity. Indian J. Pharm. Educ. Res. 2023, 57, s63–s74. [Google Scholar] [CrossRef]
- Hoffmann, J.; Casetti, F.; Bullerkotte, U.; Haarhaus, B.; Vagedes, J.; Schempp, C.; Wölfle, U. Anti-Inflammatory Effects of Agrimoniin-Enriched Fractions of Potentilla Erecta. Molecules 2016, 21, 792. [Google Scholar] [CrossRef]
- Lv, N.; Wang, L.; Zeng, M.; Wang, Y.; Yu, B.; Zeng, W.; Jiang, X.; Suo, Y. Saponins as Therapeutic Candidates for Atherosclerosis. Phytother. Res. 2024, 38, 1651–1680. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Wang, Y.; Qiu, C. Research Progress and Prospect Analysis of the Application of Flax Lignans. J. Nat. Fibers 2024, 21, 2309909. [Google Scholar] [CrossRef]
- Jarzębski, M.; Smułek, W.; Siejak, P.; Rezler, R.; Pawlicz, J.; Trzeciak, T.; Jarzębska, M.; Majchrzak, O.; Kaczorek, E.; Kazemian, P.; et al. Aesculus hippocastanum L. as a Stabilizer in Hemp Seed Oil Nanoemulsions for Potential Biomedical and Food Applications. Int. J. Mol. Sci. 2021, 22, 887. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A Comprehensive Review on the Biological, Agricultural and Pharmaceutical Properties of Secondary Metabolites Based-Plant Origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant Carotenoids: Recent Advances and Future Perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.S.Q.; Bourkas, A.N.; Sibbald, R.G.; Sibbald, C. Biomarkers in Rosacea: A Systematic Review. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Dridi, A.; Reis, F.S.; Pires, T.C.S.P.; Calhelha, R.C.; Pereira, C.; Zaghdoudi, K.; Ferreira, I.C.F.R.; Barros, L.; Barreira, J.C.M. Aesculus hippocastanum L.: A Simple Ornamental Plant or a Source of Compelling Molecules for Industry? Separations 2023, 10, 160. [Google Scholar] [CrossRef]
- Raudone, L.; Vilkickyte, G.; Marksa, M.; Radusiene, J. Comparative Phytoprofiling of Achillea millefolium Morphotypes: Assessing Antioxidant Activity, Phenolic and Triterpenic Compounds Variation across Different Plant Parts. Plants 2024, 13, 1043. [Google Scholar] [CrossRef]
- Manana, G.; Nino, G.; Bochoidze, I. The Content of Phenolic Compounds and Antioxidant Activity of the Butcher’s Broom Plant (Ruscus aculeatus L). World Sci. 2023, 1, 95–100. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Mohammed, H.A.; Al-Khalaf, A.M.; Khan, O.; Mostafa, M.A.H.; Al Haidari, R.A.; Taha, H.H.; Khan, R.A. Ginkgo biloba Leaves Extract’s Cosmeceutical Evaluation: A Preliminary Assessments on Human Volunteers towards Achieving Improved Skin Condition and Rejuvenation. Drug Dev. Ind. Pharm. 2023, 49, 281–292. [Google Scholar] [CrossRef]
- Zacarias, C.A.; de Mendonça Florenziano, R.F.; de Andrade, T.A.M.; de Aro, A.A.; do Amaral, M.E.C.; dos Santos, G.M.T.; Esquisatto, M.A.M. Arnica montana L. Associated with Microcurrent Accelerates the Dermis Reorganisation of Skin Lesions. Int. J. Exp. Pathol. 2023, 104, 81–95. [Google Scholar] [CrossRef]
- Shafeie, N.; Tabatabai Naini, A.; Jahromi, H. Comparison of Different Concentrations of Calendula officinalis Gel on Cutaneous Wound Healing. Biomed. Pharmacol. J. 2015, 8, 979–992. [Google Scholar] [CrossRef]
- Guo, J.; Li, K.; Lin, Y.; Liu, Y. Protective Effects and Molecular Mechanisms of Tea Polyphenols on Cardiovascular Diseases. Front. Nutr. 2023, 10, 1202378. [Google Scholar] [CrossRef]
- Hettwer, S.; Gyenge, E.B.; Suter, B.; Schöffel, L.; Peyer, S.; Obermayer, B. Skin and Vascular Fitness. SOFW J. 2023, 149, 8–12. [Google Scholar]
- Zheng, X.-Q.; Zhang, X.-H.; Gao, H.-Q.; Huang, L.-Y.; Ye, J.-J.; Ye, J.-H.; Lu, J.-L.; Ma, S.-C.; Liang, Y.-R. Green Tea Catechins and Skin Health. Antioxidants 2024, 13, 1506. [Google Scholar] [CrossRef] [PubMed]
- Klayman, D.L. Qinghaosu (Artemisinin): An Antimalarial Drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- MKhater; Greco, F.; Osborn, H.M.I. Antiangiogenic Activity of Flavonoids: A Systematic Review and Meta-Analysis. Molecules 2020, 25, 4712. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Odai, T.; Terauchi, M.; Kato, K.; Hirose, A.; Miyasaka, N. Effects of Grape Seed Proanthocyanidin Extract on Vascular Endothelial Function in Participants with Prehypertension: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2844. [Google Scholar] [CrossRef]
- Obrzut, W. Natural Active Ingredients in Cosmetics for Mature Skin. Aesthetic Cosmetol. Med. 2024, 13, 161–166. [Google Scholar] [CrossRef]
- Bencsik, T.; Balázs, V.L.; Farkas, Á.; Csikós, E.; Horváth, A.; Ács, K.; Kocsis, M.; Doseděl, M.; Fialová, S.B.; Czigle, S.; et al. Herbal Drugs in Chronic Venous Disease Treatment: An Update. Fitoterapia 2024, 179, 106256. [Google Scholar] [CrossRef]
- Tian, R.; Miao, L.; Cheang, W.-S. Effects of Pterostilbene on Cardiovascular Health and Disease. Curr. Issues Mol. Biol. 2024, 46, 9576–9587. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Ma, W.; Zhang, H.; Meng, X.; Zhang, H.; Guo, M.; Ling, X.; Li, L. Anti-Inflammatory Activity of Panax Notoginseng Flower Saponins Quantified Using LC/MS/MS. Molecules 2023, 28, 2416. [Google Scholar] [CrossRef]
- Karimi, A.; Majlesi, M.; Rafieian-Kopaei, M. Herbal versus Synthetic Drugs; Beliefs and Facts. J. Nephropharmacol. 2015, 4, 27–30. [Google Scholar]
- Salem, H.A.; Wadie, W. Effect of Niacin on Inflammation and Angiogenesis in a Murine Model of Ulcerative Colitis. Sci. Rep. 2017, 7, 7139. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose–Response Meta-Analysis. Mol. Nutr. Food Res. 2021, 65, 2001019. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Chaudhuri, P.K. Structural Characteristics, Bioavailability and Cardioprotective Potential of Saponins. Integr. Med. Res. 2018, 7, 33–43. [Google Scholar] [CrossRef]
- Zerres, S.; Stahl, W. Carotenoids in Human Skin. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2020, 1865, 158588. [Google Scholar] [CrossRef]
- Lademann, J.; Meinke, M.C.; Sterry, W.; Darvin, M.E. Carotenoids in Human Skin: Carotenoids in Human Skin. Exp. Dermatol. 2011, 20, 377–382. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Ma, E.Z.; Khachemoune, A. Flavonoids and Their Therapeutic Applications in Skin Diseases. Arch. Dermatol. Res. 2022, 315, 321–331. [Google Scholar] [CrossRef]
- Vacca, R.A.; Valenti, D.; Caccamese, S.; Daglia, M.; Braidy, N.; Nabavi, S.M. Plant Polyphenols as Natural Drugs for the Management of Down Syndrome and Related Disorders. Neurosci. Biobehav. Rev. 2016, 71, 865–877. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Lall, R.; Syed, D.; Adhami, V.; Khan, M.; Mukhtar, H. Dietary Polyphenols in Prevention and Treatment of Prostate Cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, G.; Silva, C.C.; Cabral, S. Leishmanicidal activity of flavonoids natural and synthetic: A minireview. Mintage J. Pharm. Med. Sci. 2018, 7, 25–34. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Ekalu, A.; Habila, J.D. Flavonoids: Isolation, Characterization, and Health Benefits. Beni-Suef Univ. J. Basic. Appl. Sci. 2020, 9, 45. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic Potential of Flavonoids in Cancer: ROS-Mediated Mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Okuno, Y.; Marumoto, S.; Miyazawa, M. Antimutagenic Activity of Flavonoids from Sozuku. Nat. Prod. Res. 2019, 33, 862–865. [Google Scholar] [CrossRef]
- Alcaraz, M.; Olivares, A.; Achel, D.G.; García-Gamuz, J.A.; Castillo, J.; Alcaraz-Saura, M. Genoprotective Effect of Some Flavonoids against Genotoxic Damage Induced by X-Rays In Vivo: Relationship between Structure and Activity. Antioxidants 2021, 11, 94. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Ciumarnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, S.C.; Rachisan, A.L.; Negrean, V.; Perne, M.G.; Doncan, V.I.; Alexescu, T.G.; Para, I.; et al. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of Food Matrix on the Content and Bioavailability of Flavonoids. Trends Food Sci. Technol. 2021, 117, 15–33. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Wójciak, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Hoian, U.; Klimczak, K.; Szczepanek, D.; Sowa, I. Evaluation of the Antioxidant, Cytoprotective and Antityrosinase Effects of Schisandra Chinensis Extracts and Their Applicability in Skin Care Product. Molecules 2022, 27, 8877. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, Metabolism and Bioavailability of Flavonoids: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Mariutti, L.R.B.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Cánovas, G.V.; Orlien, V. Bioaccessibility of Bioactive Compounds from Fruits and Vegetables after Thermal and Nonthermal Processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Grundy, M.M.-L.; Edwards, C.H.; Mackie, A.R.; Gidley, M.J.; Butterworth, P.J.; Ellis, P.R. Re-Evaluation of the Mechanisms of Dietary Fibre and Implications for Macronutrient Bioaccessibility, Digestion and Postprandial Metabolism. Br. J. Nutr. 2016, 116, 816–833. [Google Scholar] [CrossRef]
- Grootaert, C.; Kamiloglu, S.; Capanoglu, E.; Van Camp, J. Cell Systems to Investigate the Impact of Polyphenols on Cardiovascular Health. Nutrients 2015, 7, 9229–9255. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Billowria, K.; Ali, R.; Rangra, N.K.; Kumar, R.; Chawla, P.A. Bioactive Flavonoids: A Comprehensive Review on Pharmacokinetics and Analytical Aspects. Crit. Rev. Anal. Chem. 2022, 54, 1002–1016. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J.; et al. Biosynthesis and Metabolic Actions of Simple Phenolic Acids in Plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-Antioxidant Activity Relationship of Methoxy, Phenolic Hydroxyl, and Carboxylic Acid Groups of Phenolic Acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Contardi, M.; Lenzuni, M.; Fiorentini, F.; Summa, M.; Bertorelli, R.; Suarato, G.; Athanassiou, A. Hydroxycinnamic Acids and Derivatives Formulations for Skin Damages and Disorders: A Review. Pharmaceutics 2021, 13, 999. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An Overview on the Role of Dietary Phenolics for the Treatment of Cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef]
- Tsao, R.; Deng, Z. Separation Procedures for Naturally Occurring Antioxidant Phytochemicals. J. Chromatogr. B 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Amoah, S.; Sandjo, L.; Kratz, J.; Biavatti, M. Rosmarinic Acid—Pharmaceutical and Clinical Aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef]
- da Silva, A.P.G.; Sganzerla, W.G.; John, O.D.; Marchiosi, R. A Comprehensive Review of the Classification, Sources, Biosynthesis, and Biological Properties of Hydroxybenzoic and Hydroxycinnamic Acids. Phytochem. Rev. 2023. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of Stilbene Biosynthesis in Plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef]
- Banik, K.; Ranaware, A.M.; Harsha, C.; Nitesh, T.; Girisa, S.; Deshpande, V.; Fan, L.; Nalawade, S.P.; Sethi, G.; Kunnumakkara, A.B. Piceatannol: A Natural Stilbene for the Prevention and Treatment of Cancer. Pharmacol. Res. 2020, 153, 104635. [Google Scholar] [CrossRef]
- De Filippis, B.; Ammazzalorso, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R. Anticancer Activity of Stilbene-Based Derivatives. ChemMedChem 2017, 12, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Loh, Y.C.; Tew, W.Y.; Yam, M.F. Vasorelaxant Effect of 3,5,4′-Trihydroxy-Trans-Stilbene (Resveratrol) and Its Underlying Mechanism. Inflammopharmacology 2020, 28, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Kubyshkin, A.; Shevandova, A.; Petrenko, V.; Fomochkina, I.; Sorokina, L.; Kucherenko, A.; Gordienko, A.; Khimich, N.; Zyablitskaya, E.; Makalish, T.; et al. Anti-Inflammatory and Antidiabetic Effects of Grape-Derived Stilbene Concentrate in the Experimental Metabolic Syndrome. J. Diabetes Metab. Disord. 2020, 2, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Sui, M.; Malinen, K.; Pesonen, T.; Isohanni, T.; Vuorinen, T. Spruce Bark Stilbenes as a Nature-Inspired Sun Blocker for Sunscreens. Green Chem. 2022, 24, 2962–2974. [Google Scholar] [CrossRef]
- Lin, M.-H.; Hung, C.-F.; Sung, H.-C.; Yang, S.-C.; Yu, H.-P.; Fang, J.-Y. The Bioactivities of Resveratrol and Its Naturally Occurring Derivatives on Skin. J. Food Drug Anal. 2021, 29, 15–38. [Google Scholar] [CrossRef]
- Hu, W.-H.; Dai, D.K.; Zheng, B.Z.-Y.; Duan, R.; Dong, T.T.-X.; Qin, Q.-W.; Tsim, K.W.-K. Piceatannol, a Natural Analog of Resveratrol, Exerts Anti-Angiogenic Efficiencies by Blockage of Vascular Endothelial Growth Factor Binding to Its Receptor. Molecules 2020, 25, 3769. [Google Scholar] [CrossRef]
- Bai, D.; Chen, H.; Liu, L.; Xiang, N.; Zhang, J.; Wu, C.; Zhang, J.; Wang, F. Ionic Liquid Microcapsules for the Topical Delivery of Pterostilbene: Enhanced Transdermal Delivery and Anti-Wrinkle and Skin Brightening Effects. J. Mol. Liq. 2024, 395, 123842. [Google Scholar] [CrossRef]
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More Than Resveratrol: New Insights into Stilbene-Based Compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef]
- Nantarat, N.; Nakagawa, K.; Miyamoto, R.; Chansakaow, S.; Sirithunyalug, J.; Leelapornpisid, P. Free Radical Scavenging Capability of Various Defatted Sesame Seed Cakes and Hulls Using EPR Compared with In Vitro Testing and HPLC Analysis. J. Oleo Sci. 2019, 68, 1279–1285. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Influence of Flaxseed (Linum Usitatissimum) on Female Reproduction. Planta Med. 2023, 89, 608–615. [Google Scholar] [CrossRef]
- Szewczyk, A.A.; Zgórka, G. Plant Polyphenols in Cosmetics—A Review. Eur. J. Med. Technol. 2019, 3, 1–10. [Google Scholar]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current Knowledge of Schisandra Chinensis (Turcz.) Baill. (Chinese Magnolia Vine) as a Medicinal Plant Species: A Review on the Bioactive Components, Pharmacological Properties, Analytical and Biotechnological Studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Barnaś, M.; Ekiert, H. Phytochemical Studies and Biological Activity of Three Chinese Schisandra Species (Schisandra Sphenanthera, Schisandra Henryi and Schisandra Rubriflora): Current Findings and Future Applications. Phytochem. Rev. 2019, 18, 109–128. [Google Scholar] [CrossRef]
- Sangiorgio, P.; Errico, S.; Verardi, A.; Moliterni, S.; Tamasi, G.; Rossi, C.; Balducchi, R. Bioactive Lignans from Flaxseed: Biological Properties and Patented Recovery Technologies. Nutraceuticals 2023, 3, 58–74. [Google Scholar] [CrossRef]
- Naik, N.J.; Madhusudhan, B. In Vitro Free Radical Scavenging, Anti-Hyaluronidase and Anti-Elastase Potential of Flaxseed Lignans Concentrate. Int. J. Pure Appl. Biosci. 2018, 6, 172–179. [Google Scholar] [CrossRef]
- Andargie, M.; Vinas, M.; Rathgeb, A.; Möller, E.; Karlovsky, P. Lignans of Sesame (Sesamum indicum L.): A Comprehensive Review. Molecules 2021, 26, 883. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Mansour, Z.R. Sesamol, a Major Lignan in Sesame Seeds (Sesamum indicum): Anti-Cancer Properties and Mechanisms of Action. Eur. J. Pharmacol. 2019, 855, 75–89. [Google Scholar] [CrossRef]
- Woo, S.-Y.; Hoshino, S.; Wong, C.P.; Win, N.N.; Awouafack, M.D.; Prema; Ngwe, H.; Zhang, H.; Hayashi, F.; Abe, I.; et al. Lignans with Melanogenesis Effects from Premna serratifolia Wood. Fitoterapia 2019, 133, 35–42. [Google Scholar] [CrossRef]
- Woo, S.-Y.; Wong, C.P.; Win, N.N.; Hoshino, S.; Prema; Ngwe, H.; Abe, I.; Morita, H. A New Tetrahydrofuran Lignan from Premna serratifolia Wood. Nat. Prod. Commun. 2019, 14, 1934578X1901400130. [Google Scholar] [CrossRef]
- Win, N.N.; Woo, S.-Y.; Ngwe, H.; Prema; Wong, C.P.; Ito, T.; Okamoto, Y.; Tanaka, M.; Imagawa, H.; Asakawa, Y.; et al. Tetrahydrofuran Lignans: Melanogenesis Inhibitors from Premna Integrifolia Wood Collected in Myanmar. Fitoterapia 2018, 127, 308–313. [Google Scholar] [CrossRef]
- Patel, Y.S.; Patel, B.M. Patel Secoisolariciresinol Diglucoside Lignan Concentrate of Flaxseeds Exhibits Chemoprotective Role in Non-Melanoma Skin Cancer through Inhibition of CDK4 and Upregulation of P53. Indian J. Exp. Biol. 2022, 59, 688–696. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C—Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Gref, R.; Deloménie, C.; Maksimenko, A.; Gouadon, E.; Percoco, G.; Lati, E.; Desmaële, D.; Zouhiri, F.; Couvreur, P. Vitamin C–Squalene Bioconjugate Promotes Epidermal Thickening and Collagen Production in Human Skin. Sci. Rep. 2020, 10, 16883. [Google Scholar] [CrossRef]
- Pullar, J.; Carr, A.; Vissers, M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of Vitamin C in Skin Diseases. Front. Physiol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Vissers, M.C.M.; Das, A.B. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Front. Physiol. 2018, 9, 809. [Google Scholar] [CrossRef]
- Al-Niaimi, F.; Chiang, N.Y.Z. Topical Vitamin C and the Skin: Mechanisms of Action and Clinical Applications. J. Clin. Aesthet. Dermatol. 2017, 10, 14–17. [Google Scholar]
- Duarah, S.; Durai, R.D.; Narayanan, V.B. Nanoparticle-in-Gel System for Delivery of Vitamin C for Topical Application. Drug Deliv. Transl. Res. 2017, 7, 750–760. [Google Scholar] [CrossRef]
- Basrowi, R.W.; Dilantika, C. Optimizing Iron Adequacy and Absorption to Prevent Iron Deficiency Anemia: The Role of Combination of Fortified Iron and Vitamin C. World Nutr. J. 2021, 5, 33–39. [Google Scholar] [CrossRef]
- Bhoot, H.R.; Zamwar, U.M.; Chakole, S.; Anjankar, A. Dietary Sources, Bioavailability, and Functions of Ascorbic Acid (Vitamin C) and Its Role in the Common Cold, Tissue Healing, and Iron Metabolism. Cureus 2023, 15, e49308. [Google Scholar] [CrossRef] [PubMed]
- Manosso, L.M.; Camargo, A.; Dafre, A.L.; Rodrigues, A.L.S. Vitamin E for the Management of Major Depressive Disorder: Possible Role of the Anti-Inflammatory and Antioxidant Systems. Nutr. Neurosci. 2022, 25, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Cameli, N. Vitamin E Supplementation in Inflammatory Skin Diseases. Dermatol. Ther. 2021, 34, e15160. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Muñoz, P.; Munné-Bosch, S. Vitamin E in Plants: Biosynthesis, Transport, and Function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef]
- Praça, F.G.; Viegas, J.S.R.; Peh, H.Y.; Garbin, T.N.; Medina, W.S.G.; Bentley, M.V.L.B. Microemulsion Co-Delivering Vitamin A and Vitamin E as a New Platform for Topical Treatment of Acute Skin Inflammation. Mater. Sci. Eng. C 2020, 110, 110639. [Google Scholar] [CrossRef]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The Role of Oxidative Stress, Antioxidants and Vascular Inflammation in Cardiovascular Disease (a Review). Vascul. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- Keen, M.; Hassan, I. Vitamin E in Dermatology. Indian Dermatol. Online J. 2016, 7, 311. [Google Scholar] [CrossRef]
- Pegoraro, N.S.; Barbieri, A.V.; Camponogara, C.; Mattiazzi, J.; Brum, E.S.; Marchiori, M.C.L.; Oliveira, S.M.; Cruz, L. Nanoencapsulation of Coenzyme Q10 and Vitamin E Acetate Protects against UVB Radiation-Induced Skin Injury in Mice. Colloids Surf. B Biointerfaces 2017, 150, 32–40. [Google Scholar] [CrossRef]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-aging and Brightening Effects of a Topical Treatment Containing Vitamin C, Vitamin E, and Raspberry Leaf Cell Culture Extract: A Split-face, Randomized Controlled Trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Qaragholi, Z.M.; Raghif, A.R.A.-; Kadhim, E.J. Antiaging Effects of a Poly Herbal Extract in Comparison with Vitamin E on Aging Induced Mice. Pak. J. Med. Health Sci. 2022, 16, 445–448. [Google Scholar] [CrossRef]
- Molavi Vasei, F.; Zamanian, M.Y.; Golmohammadi, M.; Mahmoodi, M.; Khademalhosseini, M.; Tavakoli, T.; Esmaeili, O.S.; Zarei, S.; Reza Mirzaei, M.; Hajizadeh, M.R. The Impact of Vitamin E Supplementation on Oxidative Stress, Cognitive Functions, and Aging-Related Gene Expression in Aged Mice. Food Sci. Nutr. 2024, 12, 9834–9845. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Kattoor, A.J.; Saldeen, T.; Mehta, J.L. Vitamin E and Its Anticancer Effects. Crit. Rev. Food Sci. Nutr. 2019, 59, 2831–2838. [Google Scholar] [CrossRef]
- Pereira, G.G.; Detoni, C.B.; Balducci, A.G.; Rondelli, V.; Colombo, P.; Guterres, S.S.; Sonvico, F. Hyaluronate Nanoparticles Included in Polymer Films for the Prolonged Release of Vitamin E for the Management of Skin Wounds. Eur. J. Pharm. Sci. 2016, 83, 203–211. [Google Scholar] [CrossRef]
- Mostafa, W.Z.; Hegazy, R.A. Vitamin D and the Skin: Focus on a Complex Relationship: A Review. J. Adv. Res. 2015, 6, 793–804. [Google Scholar] [CrossRef]
- Papandreou, D.; Hamid, Z.-T.-N. The Role of Vitamin D in Diabetes and Cardiovascular Disease: An Updated Review of the Literature. Dis. Markers 2015, 2015, 580474. [Google Scholar] [CrossRef]
- Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; März, W.; Pilz, S. Vitamin D and Cardiovascular Disease: An Updated Narrative Review. Int. J. Mol. Sci. 2021, 22, 2896. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asatourian, A.; Ershadifar, S.; Moghadam, M.M.; Sheibani, N. Vitamins and Regulation of Angiogenesis: [A, B1, B2, B3, B6, B9, B12, C, D, E, K]. J. Funct. Foods 2017, 38, 180–196. [Google Scholar] [CrossRef]
- Rembe, J.-D.; Fromm-Dornieden, C.; Stuermer, E.K. Effects of Vitamin B Complex and Vitamin C on Human Skin Cells: Is the Perceived Effect Measurable? Adv. Skin Wound Care 2018, 31, 225–233. [Google Scholar] [CrossRef]
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Kujovská Krčmová, L.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; Remião, F.; et al. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Apostolopoulos, V. Vitamin B1, B2, B3, B5, and B6 and the Immune System. In Nutrition and Immunity; Mahmoudi, M., Rezaei, N., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 115–125. ISBN 978-3-030-16072-2. [Google Scholar]
- Zhao, Y.; Wang, H.; Li, Y.; Yang, X.; Li, Y.; Wang, T. The Action of Topical Application of Vitamin B12 Ointment on Radiodermatitis in a Porcine Model. Int. Wound J. 2023, 20, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yu, G.; Chen, X.; Li, X. Nicotinic Acid Inhibits Angiogenesis Likely through Cytoskeleton Remodeling. Organogenesis 2017, 13, 183–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jolly, A.; Kim, H.; Moon, J.-Y.; Mohan, A.; Lee, Y.-C. Exploring the Imminent Trends of Saponins in Personal Care Product Development: A Review. Ind. Crops Prod. 2023, 205, 117489. [Google Scholar] [CrossRef]
- Góral, I.; Wojciechowski, K. Surface Activity and Foaming Properties of Saponin-Rich Plants Extracts. Adv. Colloid Interface Sci. 2020, 279, 102145. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Mersni, D.; Lourith, N. Plant-Derived Saponins and Their Prospective for Cosmetic and Personal Care Products. Bot. Stud. 2024, 65, 32. [Google Scholar] [CrossRef]
- de Costa, F.; Yendo, A.C.A.; Fleck, J.D.; Gosmann, G.; Fett-Neto, A.G. Immunoadjuvant and Anti-Inflammatory Plant Saponins: Characteristics and Biotechnological Approaches Towards Sustainable Production. Mini-Rev. Med. Chem. 2011, 11, 857–880. [Google Scholar] [CrossRef]
- Passos, F.R.S.; Araújo-Filho, H.G.; Monteiro, B.S.; Shanmugam, S.; Araújo, A.A.d.S.; Almeida, J.R.G.d.S.; Thangaraj, P.; Júnior, L.J.Q.; Quintans, J.d.S.S. Anti-Inflammatory and Modulatory Effects of Steroidal Saponins and Sapogenins on Cytokines: A Review of Pre-Clinical Research. Phytomedicine 2022, 96, 153842. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, Pharmacology and Treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Benítez-González, A.; Stinco, C.M. A Comprehensive Review on the Colorless Carotenoids Phytoene and Phytofluene. Arch. Biochem. Biophys. 2015, 572, 188–200. [Google Scholar] [CrossRef]
- Young, A.; Lowe, G. Carotenoids—Antioxidant Properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Huang, C.-S.; Hu, M.-L.; Chuang, C.-H. Multi-Carotenoids at Physiological Levels Inhibit VEGF-Induced Tube Formation of Endothelial Cells and the Possible Mechanisms of Action Both In Vitro and Ex Vivo. Nutr. Cancer 2018, 70, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine Algal Carotenoids Inhibit Angiogenesis by Down-Regulating FGF-2-Mediated Intracellular Signals in Vascular Endothelial Cells. Mol. Cell. Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef]
- Gebregziabher, B.S.; Gebremeskel, H.; Debesa, B.; Ayalneh, D.; Mitiku, T.; Wendwessen, T.; Habtemariam, E.; Nur, S.; Getachew, T. Carotenoids: Dietary Sources, Health Functions, Biofortification, Marketing Trend and Affecting Factors—A Review. J. Agric. Food Res. 2023, 14, 100834. [Google Scholar] [CrossRef]
- Domingo, D.S.; Camouse, M.M.; Hsia, A.H.; Matsui, M.; Maes, D.; Ward, N.L.; Cooper, K.D.; Baron, E.D. Anti-Angiogenic Effects of Epigallocatechin-3-Gallate in Human Skin. Int. J. Clin. Exp. Pathol. 2010, 3, 705–709. [Google Scholar]
- Chiu, A.E.; Chan, J.L.; Kern, D.G.; Kohler, S.; Rehmus, W.E.; Kimball, A.B. Double-Blinded, Placebo-Controlled Trial of Green Tea Extracts in the Clinical and Histologic Appearance of Photoaging Skin. Dermatol. Surg. 2005, 31, 855–860. [Google Scholar] [CrossRef]
- Rigopoulos, D.; Kalogeromitros, D.; Gregoriou, S.; Pacouret, J.; Koch, C.; Fisher, N.; Bachmann, K.; Brown, M.; Schwarz, E.; Camel, E.; et al. Randomized Placebo-controlled Trial of a Flavonoid-rich Plant Extract-based Cream in the Treatment of Rosacea. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 564–568. [Google Scholar] [CrossRef]
- Weber, T.M.; Ceilley, R.I.; Buerger, A.; Kolbe, L.; Trookman, N.S.; Rizer, R.L.; Schoelermann, A. Skin Tolerance, Efficacy, and Quality of Life of Patients with Red Facial Skin Using a Skin Care Regimen Containing Licochalcone A. J. Cosmet. Dermatol. 2006, 5, 227–232. [Google Scholar] [CrossRef]
- Li, T.; Zeng, Q.; CHEN, X.; Hu, Y.; Zhang, H.; Yu, A.; Wang, H. Effects of Artesunate on Rosacea-like Inflammation in Mouse Models. Chin. J. Dermatol. 2017, 12, 650–653. [Google Scholar]
- Wang, G.-J.; Gao, X.-Y.; Wu, Y.; He, H.-Q.; Yu, Y.; Qin, H.-H.; Shen, W.-T. Evaluation of the Efficacy and Tolerance of Artemether Emulsion for the Treatment of Papulopustular Rosacea: A Randomized Pilot Study. J. Dermatol. Treat. 2019, 30, 809–812. [Google Scholar] [CrossRef]
- Dumitriu, B.; Olariu, L.; Nita, R.; Zglimbea, L.; Rosoiu, N. Vascular Anti-Inflammatory Effects of Natural Compounds from Aesculus hippocastanum and Hedera Helix. Rom. Biotechnol. Lett. 2013, 18, 7963–7974. [Google Scholar]
- Ferrari, A.; Diehl, C. Evaluation of the Efficacy and Tolerance of a Topical Gel With 4% Quassia Extract in the Treatment of Rosacea. J. Clin. Pharmacol. 2012, 52, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-H.; Wang, H.-Y.; Xia, Y.-T.; Dai, D.K.; Xiong, Q.-P.; Dong, T.T.-X.; Duan, R.; Chan, G.K.-L.; Qin, Q.-W.; Tsim, K.W.-K. Kaempferol, a Major Flavonoid in Ginkgo Folium, Potentiates Angiogenic Functions in Cultured Endothelial Cells by Binding to Vascular Endothelial Growth Factor. Front. Pharmacol. 2020, 11, 526. [Google Scholar] [CrossRef]
- Roh, K.-B.; Jang, Y.; Cho, E.; Park, D.; Kweon, D.-H.; Jung, E. Chlorogenic Acid Isomers Isolated from Artemisia Lavandulaefolia Exhibit Anti-Rosacea Effects In Vitro. Biomedicines 2022, 10, 463. [Google Scholar] [CrossRef]
- van Exsel, D.C.E.; Pool, S.M.W.; van Uchelen, J.H.; Edens, M.A.; van der Lei, B.; Melenhorst, W.B.W.H. Arnica Ointment 10% Does Not Improve Upper Blepharoplasty Outcome: A Randomized, Placebo-Controlled Trial. Plast. Reconstr. Surg. 2016, 138, 66–73. [Google Scholar] [CrossRef]
- Kang, J.Y.; Tran, K.D.; Seiff, S.R.; Mack, W.P.; Lee, W.W. Assessing the Effectiveness of Arnica montana and Rhododendron Tomentosum (Ledum Palustre) in the Reduction of Ecchymosis and Edema After Oculofacial Surgery: Preliminary Results. Ophthal. Plast. Reconstr. Surg. 2017, 33, 47–52. [Google Scholar] [CrossRef]
- Berg, D. Venous Constriction by Local Administration of Ruscus Extract. Fortschr. Med. 1990, 108, 473–476. [Google Scholar]
- Yousefian, F.; Van Alfen, B.; Yadlapati, S.; Goldberg, D.J.; Gold, M. Cosmeceuticals and Other Techniques to Reduce Bruising From Surgical and Cosmetic Procedures. Dermatol. Rev. 2025, 6, e70013. [Google Scholar] [CrossRef]
- Cosman, T.L.; Arthur, H.M.; Natarajan, M.K. Prevalence of Bruising at the Vascular Access Site One Week after Elective Cardiac Catheterisation or Percutaneous Coronary Intervention. J. Clin. Nurs. 2011, 20, 1349–1356. [Google Scholar] [CrossRef]
- Bouaziz, J.D.; Duong, T.A.; Jachiet, M.; Velter, C.; Lestang, P.; Cassius, C.; Arsouze, A.; Domergue Than Trong, E.; Bagot, M.; Begon, E.; et al. Vascular Skin Symptoms in COVID-19: A French Observational Study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e451. [Google Scholar] [CrossRef]
- Sharangi, A.B. Medicinal and Therapeutic Potentialities of Tea (Camellia sinensis L.)—A Review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Shii, T.; Tanaka, T.; Watarumi, S.; Matsuo, Y.; Miyata, Y.; Tamaya, K.; Tamaru, S.; Tanaka, K.; Matsui, T.; Kouno, I. Polyphenol Composition of a Functional Fermented Tea Obtained by Tea-Rolling Processing of Green Tea and Loquat Leaves. J. Agric. Food Chem. 2011, 59, 7253–7260. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial Effects of Green Tea—A Review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Douma, S.; Zamboulis, C.; Ferro, A. Nitric Oxide Dysfunction in Vascular Endothelium and Platelets: Role in Essential Hypertension. J. Hypertens. 2009, 27, 2310–2320. [Google Scholar] [CrossRef]
- Deana, R.; Turetta, L.; Donella-Deana, A.; Donà, M.; Brunati, A.; Michiel, L.; Garbisa, S. Green Tea Epigallocatechin-3-Gallate Inhibits Platelet Signalling Pathways Triggered by Both Proteolytic and Non-Proteolytic Agonists. Thromb. Haemost. 2003, 89, 866–874. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kim, Y.-J.; Kim, H.-H.; Cho, H.-J.; Ryu, J.-H.; Rhee, M.H.; Park, H.-J. Inhibitory Effects of Epigallocatechin-3-Gallate on Microsomal Cyclooxygenase-1 Activity in Platelets. Biomol. Ther. 2013, 21, 54–59. [Google Scholar] [CrossRef]
- Chen, M.; Wang, K.; Zhang, Y.; Zhang, M.; Ma, Y.; Sun, H.; Jin, Z.; Zheng, H.; Jiang, H.; Yu, P.; et al. New Insights into the Biological Activities of Chrysanthemum Morifolium: Natural Flavonoids Alleviate Diabetes by Targeting α-Glucosidase and the PTP-1B Signaling Pathway. Eur. J. Med. Chem. 2019, 178, 108–115. [Google Scholar] [CrossRef]
- Galbany-Casals, M.; Blanco-Moreno, J.M.; Garcia-Jacas, N.; Breitwieser, I.; Smissen, R.D. Genetic Variation in Mediterranean Helichrysum italicum (Asteraceae; Gnaphalieae): Do Disjunct Populations of Subsp. Microphyllum Have a Common Origin? Plant Biol. 2011, 13, 678–687. [Google Scholar] [CrossRef]
- Bianchini, A.; Tomi, P.; Costa, J.; Bernardini, A.F. Composition ofHelichrysum Italicum (Roth) G. Don Fil. Subsp.Italicum Essential Oils from Corsica (France). Flavour Fragr. J. 2001, 16, 30–34. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza glabra): A Phytochemical and Pharmacological Review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Kushiev, H.; Noble, A.D.; Abdullaev, I.; Toshbekov, U. Remediation of Abandoned Saline Soils Using Glycyrrhiza glabra: A Study from the Hungry Steppes of Central Asia. Int. J. Agric. Sustain. 2005, 3, 102–113. [Google Scholar] [CrossRef]
- Asl, M.N.; Hosseinzadeh, H. Review of Pharmacological Effects of Glycyrrhiza Sp. and Its Bioactive Compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.d.S.; Bonafé, G.A.; Carvalho Santos, J.; Martinez, C.A.R.; Ortega, M.M.; Ribeiro, M.L. The Anti-Inflammatory Properties of Licorice (Glycyrrhiza glabra)-Derived Compounds in Intestinal Disorders. Int. J. Mol. Sci. 2022, 23, 4121. [Google Scholar] [CrossRef] [PubMed]
- Semenescu, I.; Similie, D.; Diaconeasa, Z.; Danciu, C. Recent Advances in the Management of Rosacea through Natural Compounds. Pharmaceuticals 2024, 17, 212. [Google Scholar] [CrossRef]
- Nurlybekova, A.; Kudaibergen, A.; Kazymbetova, A.; Amangeldi, M.; Baiseitova, A.; Ospanov, M.; Aisa, H.A.; Ye, Y.; Ibrahim, M.A.; Jenis, J. Traditional Use, Phytochemical Profiles and Pharmacological Properties of Artemisia Genus from Central Asia. Molecules 2022, 27, 5128. [Google Scholar] [CrossRef]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Huang, H.; Tao, K.; Qin, Z.; Guo, L.; Fitzgerald, C.; Fernández, J.R.; Pérez, E. Novel Anti-Inflammatory Artemisia Naphta Oil Extract Efficacious in in Vivo Mouse Models of Atopic Dermatitis and Psoriasis. Arch. Microbiol. Immunol. 2024, 8, 182–192. [Google Scholar] [CrossRef]
- Owczarek, A.; Kolodziejczyk-Czepas, J.; Woźniak-Serwata, J.; Magiera, A.; Kobiela, N.; Wąsowicz, K.; Olszewska, M.A. Potential Activity Mechanisms of Aesculus hippocastanum Bark: Antioxidant Effects in Chemical and Biological In Vitro Models. Antioxidants 2021, 10, 995. [Google Scholar] [CrossRef]
- Dahash, S.L.; Abass, O.K.; Abdul-Razaq, M.M.; Al-Kuraishy, H.M.; Al-Gareeb, A.I. Aesculus hippocastanum-Derived Extract β-Aescin and In Vitro Antibacterial Activity. J. Microsc. Ultrastruct. 2021, 9, 26–30. [Google Scholar] [CrossRef]
- Wilkinsos, J.; Brown, A. Horse Chestnut—Aesculus hippocastanum: Potential Applications in Cosmetic Skin-Care Products. Int. J. Cosmet. Sci. 1999, 21, 437–447. [Google Scholar] [CrossRef]
- Özkan, E.E.; Mesut, B.; Polat, A.; Tan, N. In Vitro Skin Permeation of Escin in the New Gel Formulation of Aesculus hippocastanum (Horse Chestnut). J. Fac. Pharm. Istanb. 2016, 46, 79–88. [Google Scholar]
- Dudek-Makuch, M.; Studzińska-Sroka, E.; Korybalska, K.; Czepulis, N.; Łuczak, J.; Rutkowski, R.; Marczak, Ł.; Długaszewska, J.; Grabowska, K.; Stobiecki, M.; et al. Biological Activity of Aesculus hippocastanum Flower Extracts on Vascular Endothelial Cells Cultured In Vitro. Phytochem. Lett. 2019, 30, 367–375. [Google Scholar] [CrossRef]
- Tomczyk, M.; Latté, K.P. Potentilla—A Review of Its Phytochemical and Pharmacological Profile. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Augustynowicz, D.; Latté, K.P.; Tomczyk, M. Recent Phytochemical and Pharmacological Advances in the Genus potentilla L. Sensu Lato—An Update Covering the Period from 2009 to 2020. J. Ethnopharmacol. 2021, 266, 113412. [Google Scholar] [CrossRef]
- Hoffmann, J.; Gendrisch, F.; Schempp, C.M.; Wölfle, U. New Herbal Biomedicines for the Topical Treatment of Dermatological Disorders. Biomedicines 2020, 8, 27. [Google Scholar] [CrossRef]
- Shadrack, R.S.; Kotra, K.K.; Livu, L.; Tari, D.; Tabi, H.; Trenkner, L.; Bausini, M.F.E. Evaluating the Efficacy of a New Lice Treatment Shampoo Containing Quassia amara Using Physical and Tolerance Approaches. Res. Sq. 2024, preprints. [Google Scholar] [CrossRef]
- Hoffmann, J.; Wölfle, U.; Schempp, C.M.; Casetti, F. Tannins from Potentilla officinalis Display Antiinflammatory Effects in the UV Erythema Test and on Atopic Skin. JDDG J. Dtsch. Dermatol. Ges. 2016, 14, 917–922. [Google Scholar] [CrossRef]
- Konarska, A.; Weryszko-Chmielewska, E.; Sulborska-Różycka, A.; Kiełtyka-Dadasiewicz, A.; Dmitruk, M.; Gorzel, M. Herb and Flowers of Achillea millefolium Subsp. millefolium L.: Structure and Histochemistry of Secretory Tissues and Phytochemistry of Essential Oils. Molecules 2023, 28, 7791. [Google Scholar] [CrossRef]
- Radušienė, J.; Karpavičienė, B.; Raudone, L.; Vilkickyte, G.; Çırak, C.; Seyis, F.; Yayla, F.; Marksa, M.; Rimkienė, L.; Ivanauskas, L. Trends in Phenolic Profiles of Achillea millefolium from Different Geographical Gradients. Plants 2023, 12, 746. [Google Scholar] [CrossRef]
- Verma, R.; Rahman, L.; Chanotiya, C.; Verma, R.; Chauhan, A.; Yadav, A.; Singh, A.; Yadav, A. Essential Oil Composition of Lavandula Angustifolia Mill. Cultivated in the Mid Hills of Uttarakhand, India. J. Serbian Chem. Soc. 2010, 75, 343–348. [Google Scholar] [CrossRef]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, Phytochemistry and Pharmacological Properties of Achillea millefolium L.: A Review. Phytother. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L.; Gou, G.; Xin, X.; Li, J.; Aisa, H.A. Guaianolides from Achillea millefolium L. and Their Anti-Inflammatory Activity. Phytochemistry 2023, 210, 113647. [Google Scholar] [CrossRef] [PubMed]
- Odonne, G.; Tareau, M.-A.; van Andel, T. Geopolitics of Bitterness: Deciphering the History and Cultural Biogeography of Quassia amara L. J. Ethnopharmacol. 2021, 267, 113546. [Google Scholar] [CrossRef]
- Ajaiyeoba, E.O.; Abalogu, U.I.; Krebs, H.C.; Oduola, A.M.J. In Vivo Antimalarial Activities of Quassia amara and Quassia undulata Plant Extracts in Mice. J. Ethnopharmacol. 1999, 67, 321–325. [Google Scholar] [CrossRef]
- More, M.P.; Motule, A.S.; Dongare, P.N.; Patinge, P.A.; Jawarkar, R.D.; Bakal, R.L.; Manwar, J.V. Manwar Pharmacognosy, Phytochemistry, Pharmacology and Clinical Application of Ginkgo biloba. GSC Biol. Pharm. Sci. 2021, 16, 229–240. [Google Scholar] [CrossRef]
- Lin, H.; Li, W.; Lin, C.; Wu, H.; Zhao, Y. International Biological Flora: Ginkgo biloba. J. Ecol. 2022, 110, 951–982. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic Potential of Natural Compounds in Inflammation and Chronic Venous Insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Dahija, S.; Hassan, S.T.S. Biflavonoids: Important Contributions to the Health Benefits of Ginkgo (Ginkgo biloba L.). Plants 2022, 11, 1381. [Google Scholar] [CrossRef]
- Draelos, Z.D. Cosmeceuticals for Rosacea. Clin. Dermatol. 2017, 35, 213–217. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, L.; Wang, F.; Lü, J. Phytochemical Profile and Anti-Inflammatory Activity of the Fraction from Artemisia lavandulaefolia. Chem. Biodivers. 2021, 18, e2000989. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Seago, J.L. Anatomical and Histochemical Traits of Roots and Stems of Artemisia Lavandulaefolia and A. Selengensis (Asteraceae) in the Jianghan Floodplain, China. Flora 2018, 239, 87–97. [Google Scholar] [CrossRef]
- Cha, J.-D.; Kim, Y.-H.; Kim, J.-Y. Essential Oil and 1,8-Cineole from Artemisia Lavandulaefolia Induces Apoptosis in KB Cells via Mitochondrial Stress and Caspase Activation. Food Sci. Biotechnol. 2010, 19, 185–191. [Google Scholar] [CrossRef]
- Lü, J.L.; Li, Z.; Guo, L.M.; Zhang, L.B. Sesquiterpene Lactones with COX -2 Inhibition Activity from Artemisia lavandulaefolia. Chem. Biodivers. 2018, 15, e1700548. [Google Scholar] [CrossRef]
- Qi, H.; Shi, Y.; Wu, H.; Niu, C.; Sun, X.; Wang, K. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm. Sin. B 2022, 12, 723–734. [Google Scholar] [CrossRef]
- Silva, D.; Ferreira, M.S.; Sousa-Lobo, J.M.; Cruz, M.T.; Almeida, I.F. Anti-Inflammatory Activity of Calendula officinalis L. Flower Extract. Cosmetics 2021, 8, 31. [Google Scholar] [CrossRef]
- Haładyn, K.; Wojdyło, A.; Nowicka, P. Isolation of Bioactive Compounds (Carotenoids, Tocopherols, and Tocotrienols) from Calendula officinalis L., and Their Interaction with Proteins and Oils in Nanoemulsion Formulation. Molecules 2024, 29, 4184. [Google Scholar] [CrossRef]
- Dhingra, G.; Dhakad, P.; Tanwar, S. Review on Phytochemical Constituents and Pharmacological Activities of Plant Calendula officinalis Linn. Biol. Sci. 2022, 2, 216–228. [Google Scholar] [CrossRef]
- Ashwlayan, V.D.; Kumar, A.; Verma, M.; Garg, V.K.; Gupta, S. Therapeutic Potential of Calendula officinalis. Pharm. Pharmacol. Int. J. 2018, 6, 149–155. [Google Scholar] [CrossRef]
- Deka, B.; Bhattacharjee, B.; Shakya, A.; Ikbal, A.M.A.; Goswami, C.; Sarma, S. Mechanism of Action of Wound Healing Activity of Calendula officinalis: A Comprehensive Review. Pharm. Biosci. J. 2021, 9, 28–44. [Google Scholar] [CrossRef]
- Re, T.A.; Mooney, D.; Antignac, E.; Dufour, E.; Bark, I.; Srinivasan, V.; Nohynek, G. Application of the Threshold of Toxicological Concern Approach for the Safety Evaluation of Calendula Flower (Calendula officinalis) Petals and Extracts Used in Cosmetic and Personal Care Products. Food Chem. Toxicol. 2009, 47, 1246–1254. [Google Scholar] [CrossRef]
- Tamboli, F.A.; Kolekar, Y.S.; More, H.N.; Mulani, S.A.; Mali, N.P. Medicinal Plants Used in Cosmetics for Skin and Hair Care. Int. J. Pharm. Chem. Anal. 2021, 8, 36–40. [Google Scholar] [CrossRef]
- Nawrot, J.; Gornowicz-Porowska, J.; Kroma, A.; Nowak, G. Arnika Górska Jako Roślina Lecznicza. Postępy Fitoter. 2021, 22, 32–35. [Google Scholar] [CrossRef]
- Schmidt, T.J. Arnica montana L.: Doesn’t Origin Matter? Plants 2023, 12, 3532. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, F.M.; Robledo, S.M.; Schmidt, T.J. Evaluation of Pharmacokinetic and Toxicological Parameters of Arnica Tincture after Dermal Application In Vivo. Pharmaceutics 2022, 14, 2379. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Kimel, K. Arnica montana and Its Medicinal Value in the Light of Scientific Research. Farm. Pol. 2022, 78, 491–502. [Google Scholar] [CrossRef]
- Iannitti, T.; Morales-Medina, J.C.; Bellavite, P.; Rottigni, V.; Palmieri, B. Effectiveness and Safety of Arnica montana in Post-Surgical Setting, Pain and Inflammation. Am. J. Ther. 2016, 23, e184–e197. [Google Scholar] [CrossRef]
- Leu, S.; Havey, J.; White, L.E.; Martin, N.; Yoo, S.S.; Rademaker, A.W.; Alam, M. Accelerated Resolution of Laser-Induced Bruising with Topical 20% Arnica: A Rater-Blinded Randomized Controlled Trial: Topical Treatments for Bruising. Br. J. Dermatol. 2010, 163, 557–563. [Google Scholar] [CrossRef]
- Stevinson, C.; Devaraj, V.S.; Fountain-Barber, A.; Hawkins, S.; Ernst, E. Homeopathic Arnica for Prevention of Pain and Bruising: Randomized Placebo-Controlled Trial in Hand Surgery. J. R. Soc. Med. 2003, 96, 60–65. [Google Scholar] [CrossRef]
- Thomas, P.A.; Mukassabi, T.A. Biological Flora of the British Isles: Ruscus aculeatus. J. Ecol. 2014, 102, 1083–1100. [Google Scholar] [CrossRef]
- Masullo, M.; Pizza, C.; Piacente, S. Ruscus Genus: A Rich Source of Bioactive Steroidal Saponins. Planta Med. 2016, 82, 1513–1524. [Google Scholar] [CrossRef]
- Jäger, K.; Eichlisberger, R.; Jeanneret, C.; Lobs, K. Pharmacodynamic Effects of Ruscus Extract (Cyclo 3 Fort®) On Superficial and Deep Veins in Patients with Primary Varicose Veins: Assessment by Duplexsonography. Clin. Drug Investig. 1999, 17, 265–273. [Google Scholar] [CrossRef]
- Leanpolchareanchai, J.; Teeranachaideekul, V. Topical Microemulsions: Skin Irritation Potential and Anti-Inflammatory Effects of Herbal Substances. Pharmaceuticals 2023, 16, 999. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Torbati, M.; Mahmoudi, J.; Valizadeh, H. Medicinal Plants as Potential Hemostatic Agents. J. Pharm. Pharm. Sci. 2020, 23, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Büyükyıldırım, T.; Şenol Deniz, F.S. Natural Treatment Approaches for Varicose Veins: A Brief Review of the Literature. Trak. Univ. J. Nat. Sci. 2024, 25, 121–132. [Google Scholar] [CrossRef]



| Plant | Family | Traditional Uses | Ref. |
|---|---|---|---|
| Camellia sinensis | Theaceae | Promoting the excretion of urine; controlling bleeding; helping heal wounds; improving heart health; regulating flatulence, body temperature, and blood sugar; promoting digestion; improving mental processes | [9] |
| Chrysanthellum indicum | Asteraceae | The treatment of common cold; fever; migraine; conjunctivitis; eye irritation; hypertension; inflammation; ulcerative colitis; vertigo; ophthalmia with swelling as well as skin infections | [10] |
| Helichrysum italicum | Asteraceae | Health purposes in the respiratory and digestive tracts as well as the skin; wound healing; antimicrobial uses; gall and bladder disorders; analgesic; common types of preparations are infusions, decoctions for both oral and external use, followed by vapors, juices, and powders | [11] |
| Glycyrrhiza glabra | Fabaceae | Respiratory disorders; hyperdipsia; epilepsy; fever; sexual debility; paralysis; stomach ulcers; rheumatism; skin diseases; hemorrhagic diseases; jaundice | [12] |
| Artemisia annua | Asteraceae | The treatment of jaundice; antibacterial; antipyretic agent in malaria; tuberculosis; wounds; hemorrhoids; viral, bacterial, and autoimmune diseases | [13] |
| Aesculus hippocastanum | Sapindaceae Juss | For flatulence; anorexia; antiseptic; antioxidant; antipyretic; analgesic and antiaging agent; in treatment of infections of ear, nose, and throat regions; source of non-edible starch and timber | [14] |
| Potentilla erecta | Rosaceae | Astringent agent to treat bleeding and inflammation of the skin as well as mucosa and diarrhea | [15] |
| Achillea millefolium | Asteraceae | An antihemorrhagic; wound-healing agent; remedy for ulcers, skin diseases (wounds); snakebites and varicose veins; skin inflammation | [16,17] |
| Quassia amara | Simaroubaceae | Antimalarial; stomachic; antianemic; antibiotic; cytotoxic; antiamoebic activity; reproductive function; insecticidal, larvicidal, and vermifuge properties | [18] |
| Ginkgo biloba | Ginkgoaceae | Relieve cough; reduce phlegm; clear poison and relieve diarrhea | [19] |
| Artemisia lavandulaefolia | Asteraceae | Various diseases; digestive; anthelmintic; effective odor remover; gastrointestinal diseases; constipation; pain; belly pain; asthma; gynecological problems | [2] |
| Calendula officinalis | Asteraceae | Gynecological issues; digestive disorders; inflammation of the oral and pharyngeal mucosa; eye conditions; skin injuries; burns; vision problems; menstrual irregularities; diaphoretic; blood purifier; helps lower blood sugar levels; tinctures, ointments, and balms; antitumor; astringent; diuretic; antipyretic; anti-inflammatory properties; wounds; acne; scars; herpes; frostbite; chickenpox; mumps; gangrene; ulcers; insect bites; boils; skin inflammation; toothaches; oral sores; gargles; varicose veins, soothing agents for diaper rash; hemorrhoids; conjunctivitis; mouth inflammation; homeopathy | [20] |
| Arnica montana | Asteraceae | Bruises; sprains; back pain; rheumatoid arthritis; phlebitis | [21] |
| Ruscus aculeatus | Asparagaceae | Diuretic effects; urinary tract disorders; laxative | [22] |
| Flavonoid Class | Flavonoid Example | Source |
|---|---|---|
| Flavonol | Quercetin | Apples |
| Flavanol | Catechins | Teas |
| Isoflavone | Genistein | Soybeans |
| Flavone | Luteolin | Ginko biloba |
| Flavanone | Hestepridin | Oranges |
| Anthocyanin | Cyanidin | Blackberries |
| Main Phenolic Acids | Derivative Phenolic Acid Examples | Source |
|---|---|---|
| Hydroxybenzoic acids | Gallic acid | A. montana |
| Syringic acid | Açaí (Euterpe oleraceae Mart.) | |
| p-Hydroxybenzoic acid | Soybeans, A. montana | |
| Protocatechuic acid | Ginkgo biloba | |
| Hydroxycinnamic acids | Ferulic acid | Corn |
| Caffeic acid | Potato | |
| p-Coumaric acid | Tomato | |
| Sinapic acid | Celery |
| Carotenoid Class | Carotenoid Example | Source |
|---|---|---|
| Carotenes | β-carotene | Carrots |
| Lycopene | Tomatoes | |
| Xanthophylls | Lutein | Egg yolk |
| Zeaxanthin | Watermelon | |
| Capsanthine | Peppers | |
| Astaxanthin | Crustaceans |
| Group of Active Compounds | Examples of Compounds | Effects of Different Plant Compounds | Ref. |
|---|---|---|---|
| Vitamins | Vitamin C | Collagen synthesis, strengthening vessels, improving circulation, antioxidant protection | [45,115] |
| Vitamin E | Strong antioxidant, protection of blood vessels from damage, anticancer, photoprotective, and antiaging effect, skin regeneration | [123,124,125,126] [130,131,132,133,134,135] [136] | |
| Vitamin D | Reduction of atherogenesis | [137,138,139] | |
| Vitamin B2 | Antioxidant, maintaining healthy blood vessel walls | [140,141,142,143] | |
| Vitamin B3 | Improvement of endothelial function, lowering the level of bad cholesterol (LDL), increasing the level of good cholesterol (HDL) | [52,140] | |
| Vitamin B5 | Improvement of endothelial function, lowering the level of bad cholesterol (LDL), increasing the level of good cholesterol (HDL) | [52,140] | |
| Vitamin B6 | Lowering homocysteine levels | [140,141,143] | |
| Vitamin B7 | Participation in maintaining balance | [140,141,143] | |
| Vitamin B9 | Lowering homocysteine levels, influence on the regeneration of blood vessels | [140,141,143] | |
| Vitamin B12 | Anti-inflammatory, antifibrotic, antiradiation effects, lowering homocysteine levels | [144,145] | |
| Carotenoids | β-carotene | Participation in the photosynthesis process, protection against UV radiation and oxidative stress, influence on the hormonal balance of plants | [32,153] |
| Lutein | |||
| Zeaxanthin | |||
| Lycopene | |||
| Polyphenols | Flavonoids | Antioxidant, anticancer, anti-inflammatory, and antimutagenic effects improving the elasticity of blood vessels | [64,65,66,67,68] [44,70,71,72] |
| Phenolic acids | Anti-inflammatory, antiallergic, antimicrobial, antioxidant, antithrombotic, cardioprotective, anticancer, hepatoprotective, and antidiabetic effects | [80,81,82,83,84,85,86] | |
| Stilbenes | Anti-inflammatory, anticancer, and antioxidant effects, prevention of excessive platelet aggregation, UVB skin protection, collagen synthesis, inhibition of melanogenesis | [49,90,91,92,93,94,95,96,97,98] | |
| Lignans | Antioxidant, anti-inflammatory, and antiaging effects, estrogen-modulating effect | [28,72,103,104,105,106,107,108] | |
| Saponins | Diuretic, emetic, expectorant, and antiseptic effects | [15,25,27,54,149] |
| Plant | Study Model | Dose/Treatment Time | Results/Effect | Ref. |
|---|---|---|---|---|
| Camellia sinensis | In vivo randomized, double-blind, split-face trial four volunteers, age 40–59 years with significant erythema and telangiectasia In vivo double blind, placebo-controlled trial, 40 women with moderate photoaging | A cream containing 2.5% w/w of epigallocatechin gallate applied on the skin face/6 weeks Topical 10% green tea and oral 300 mg green tea supplementation for clinical and histological purposes/twice daily as well as placebo for 8 weeks | Angiogenic growth, vascular endothelial growth factor (VEGF), and hypoxia-inducible factor-1 (HIF-1) Reduction in visible telangiectasia (no significant differences) | [157] [158] |
| Chrysanthellum indicum | In vivo multicenter randomized, double-blind, parallel group, placebo-controlled study, 246 people aged 18–80 with clinically moderate rosacea | A cream containing 1% extract of Ch. indicum and placebo/12 weeks, applied twice a day | Reduction in erythema score (41.3%), overall rosacea severity compared to baseline and placebo | [159] |
| Helichrysum italicum | In vivo double-blind, placebo-controlled in vivo study, 43 women aged 30–65 | H. italicum extract on the legs (5%), the eyes (3%), and the face (1%)/applied for 28 days twice daily | Reduction of red spots and visible capillaries | [41] |
| Glycyrrhiza glabra | In vivo study, 62 women with mild to moderate red facial skin, mean age of 48 years | The products contained extract from the licorice root of Glycyrrhiza inflata/8 weeks | Significant improvements in average erythema scores were observed at 4 and 8 weeks | [160] |
| Artemisia annua | In vivo study, mouse models with rosacea-like inflammation, 25 male mice aged 7 weeks In vivo randomized, investigator-blinded, parallel-group study, 130 patients with papulopustular rosacea | Treatment groups gavaged with 25, 50, or 100 mg/kg artesunate solution/7 weeks Emulsion with artemisinin derivative (1%) or metronidazole emulsion (3%)/4 weeks with observation up to 8 weeks | Reduction of erythema, number of inflammatory cells, and myeloperoxidase activity (MPO) Reduction of erythema, papules, and pustules and good tolerance of the cream with artemisinin derivative by patients | [161] [162] |
| Aeskulus hippocastanum | In vitro study, flow cytometric studies of adhesion and molecule expression, determination of proinflammatory cytokines IL-6 and IL-8 in the extracellular environment | Isolated compounds from A. hypocastanum | Inhibition of IL-6 and IL-8 secretion and blockage of the VEGF proangiogenic factor | [163] |
| Potentilla erecta | In vitro study, human keratinocyte cell line HaCaT in occlusive patch test and a collagen contraction assay In vivo randomized, prospective, placebo-controlled, double-blind study, 40 healthy non-smoking individuals of both genders, age 18–54 years, skin phototypes II and III | P. erecta extract enriched argrimoniin P. erecta cream (2%) compared to 1% hydrocortisone acetate/twice daily for two weeks | Reduced IL-6 and PGE2 or NF-κB activation in cells, narrowing of blood vessels Reduction of erythema with atopic skin. In the UV erythema test, PO cream significantly reduced the erythema index compared to the vehicle. The anti-inflammatory effects of PO cream were comparable to those of 1% hydrocortisone acetate cream | [15] [26] |
| Achillea millefolium | In vivo study, liver incision and histopathological analysis, 12 female Wistar rats | A. millefolium methanolic extract (150 mg/kg)/analysis after 4, 6, and 8 weeks | Significantly reduced bleeding time, histopathological analysis showed no signs of toxicity or hepatic damage after 4, 6, and 8 weeks in the female rats | [16] |
| Quassia amara | In vivo study, local application of the preparation, 30 persons with rosea | Q. amara gel (4%)/twice a day for 6 weeks | Reduced flushing, erythema, telangiectasia | [164] |
| Ginkgo biloba | In vitro study with human umbilical vein endothelial cells (HUVECs) of the signaling triggered by VEGF | Kaempferol identified in G. biloba | Positive effects of kaempferol on macrophage migration and tissue regeneration via modulation of macrophages via the VEGFR signaling pathway | [165] |
| Artemisia lavandulaefolia | In vitro study, human epidermal keratinocytes In vitro study, human umbilical vein endothelial cells (HUVECs) | The effect of chlorogenic acid isomers isolated from A. lavandulaefolia on the expression of KLK5 in HEKn and extracellular enzymatic activity The effect of A. lavandulaefolia extract on the formation of the HUVEC blood vessel network | Inhibition of KLK5 protease activity Inhibited HUVEC tubule formation, inhibited HUVEC migration and invasion, inhibited angiogenesis | [166] [2] |
| Calendula officinalis | In vivo study histopathological and biochemical tests, 75 white male rats | Gel containing C. officinalis extract (5%, 7%, and 10%)/14 and 21 days | On day 14, the wound size decreased in all groups and on day 21 after injury the wound size in the treated lesions was smaller than in the control | [39] |
| Arnica montana | Randomized, placebo-controlled trial, 36 patients In vivo after undergoing common oculofacial procedures, including blepharoplasty, browpexy, and rhinoplasty, 27 patients | Arnica ointment 10%/6 weeks Local hydrogel pads containing A. montana 50%/up to 6 days after surgery | There were no significant differences in the overall periorbital appearance regarding edema, petechiae, or erythema Reduction of postoperative petechiae and edema after ophthalmic and facial procedures | [167] [168] |
| Ruscus aculeatus | In vivo randomized, double-blind study, 18 healthy volunteers | Application of a preparation containing 64 to 96 mg of R. aculeatus extract | Reduction in the diameter of the femoral vein | [169] |
| Criterion | Topical Use | Oral Use | Ref. |
|---|---|---|---|
| Action | Topical effect, application of the preparation on the skin in the disease area | Systemic effect | [158,159,162,164,193,234,235,236] |
| Effectiveness | Positive effect on skin lesion, e.g., rosacea, erythema | Wide application in various diseases and disorders of body functions—systemic effect | |
| Dosage | Low risk of overdose because the absorption of active ingredients is reduced | Risk of exceeding the recommended dose and occurrence of side effects | |
| Side effects | Irritation | Increased risk of side effects by exceeding the recommended dose (e.g., hepatotoxicity) | |
| Effects on the digestive system | No effect on the digestive system | Gastrointestinal disorders | |
| Examples of herbs used | Chrysanthellum indicum, Quassia amara, Aeskulus hippocastanum | Camellia sinensis, Artemisia annua, Aeskulus hippocastanum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, B.H.; Muzykiewicz-Szymańska, A.; Florkowska, K.; Tkacz, M.; Wilk, B.; Kucharski, Ł.; Madalińska, A.; Nowak, A. The Use of Plants That Seal Blood Vessels in Preparations Applied Topically to the Skin: A Review. Molecules 2025, 30, 1973. https://doi.org/10.3390/molecules30091973
Roman BH, Muzykiewicz-Szymańska A, Florkowska K, Tkacz M, Wilk B, Kucharski Ł, Madalińska A, Nowak A. The Use of Plants That Seal Blood Vessels in Preparations Applied Topically to the Skin: A Review. Molecules. 2025; 30(9):1973. https://doi.org/10.3390/molecules30091973
Chicago/Turabian StyleRoman, Barbara Hanna, Anna Muzykiewicz-Szymańska, Katarzyna Florkowska, Magdalena Tkacz, Bartłomiej Wilk, Łukasz Kucharski, Agata Madalińska, and Anna Nowak. 2025. "The Use of Plants That Seal Blood Vessels in Preparations Applied Topically to the Skin: A Review" Molecules 30, no. 9: 1973. https://doi.org/10.3390/molecules30091973
APA StyleRoman, B. H., Muzykiewicz-Szymańska, A., Florkowska, K., Tkacz, M., Wilk, B., Kucharski, Ł., Madalińska, A., & Nowak, A. (2025). The Use of Plants That Seal Blood Vessels in Preparations Applied Topically to the Skin: A Review. Molecules, 30(9), 1973. https://doi.org/10.3390/molecules30091973









