Advances in α-Lipoic Acid for Disease Prevention: Mechanisms and Therapeutic Insights
Abstract
1. Introduction
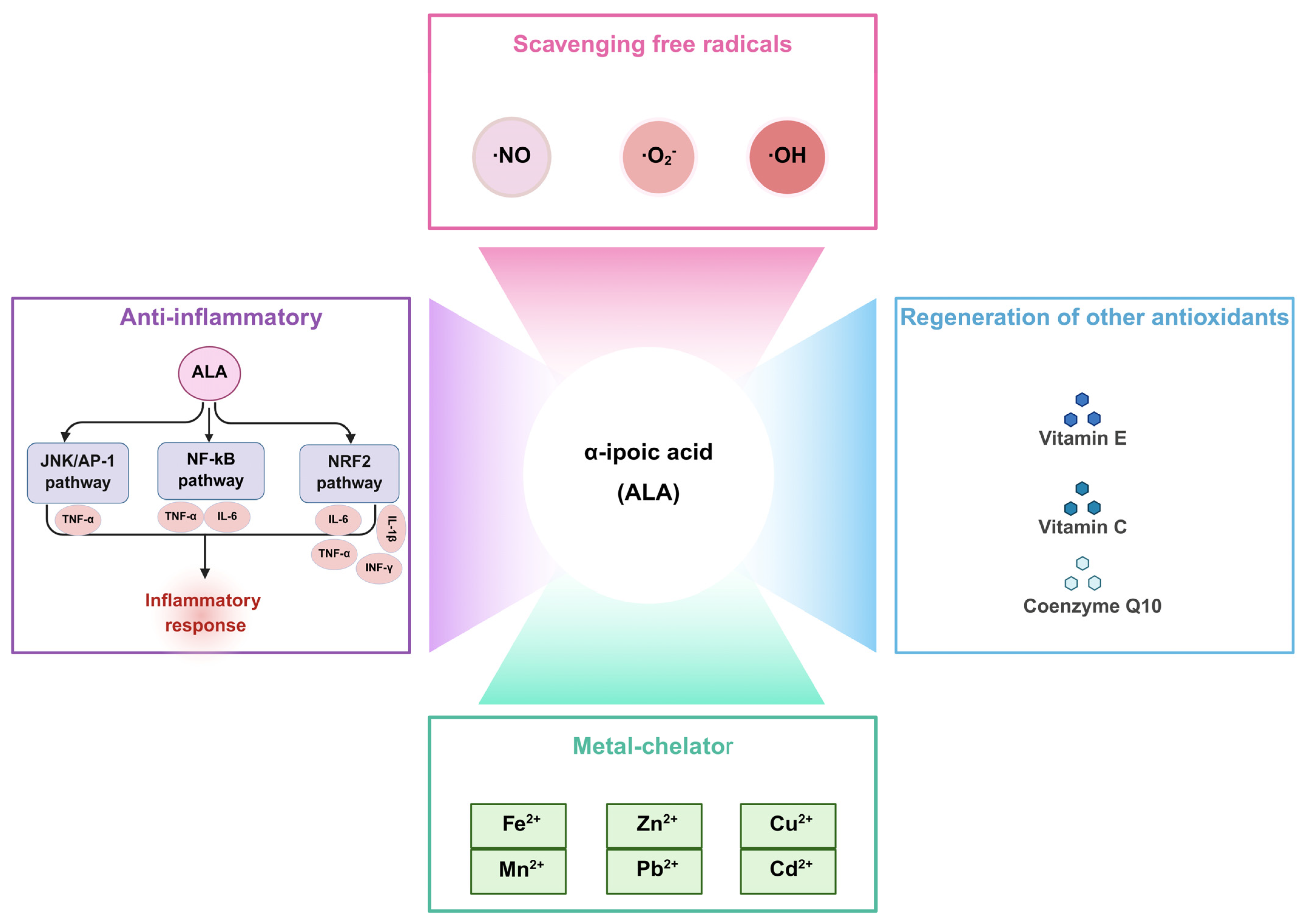
2. Biological Functions of ALA
2.1. Scavenging Free Radicals
2.2. Regeneration of Other Antioxidants
2.3. Metal Chelator
2.4. Anti-Inflammatory
3. Pharmacological Application of ALA
3.1. Neurodegeneration
3.1.1. Alzheimer’s Disease
3.1.2. Parkinson’s Disease
3.2. Pulmonary Diseases
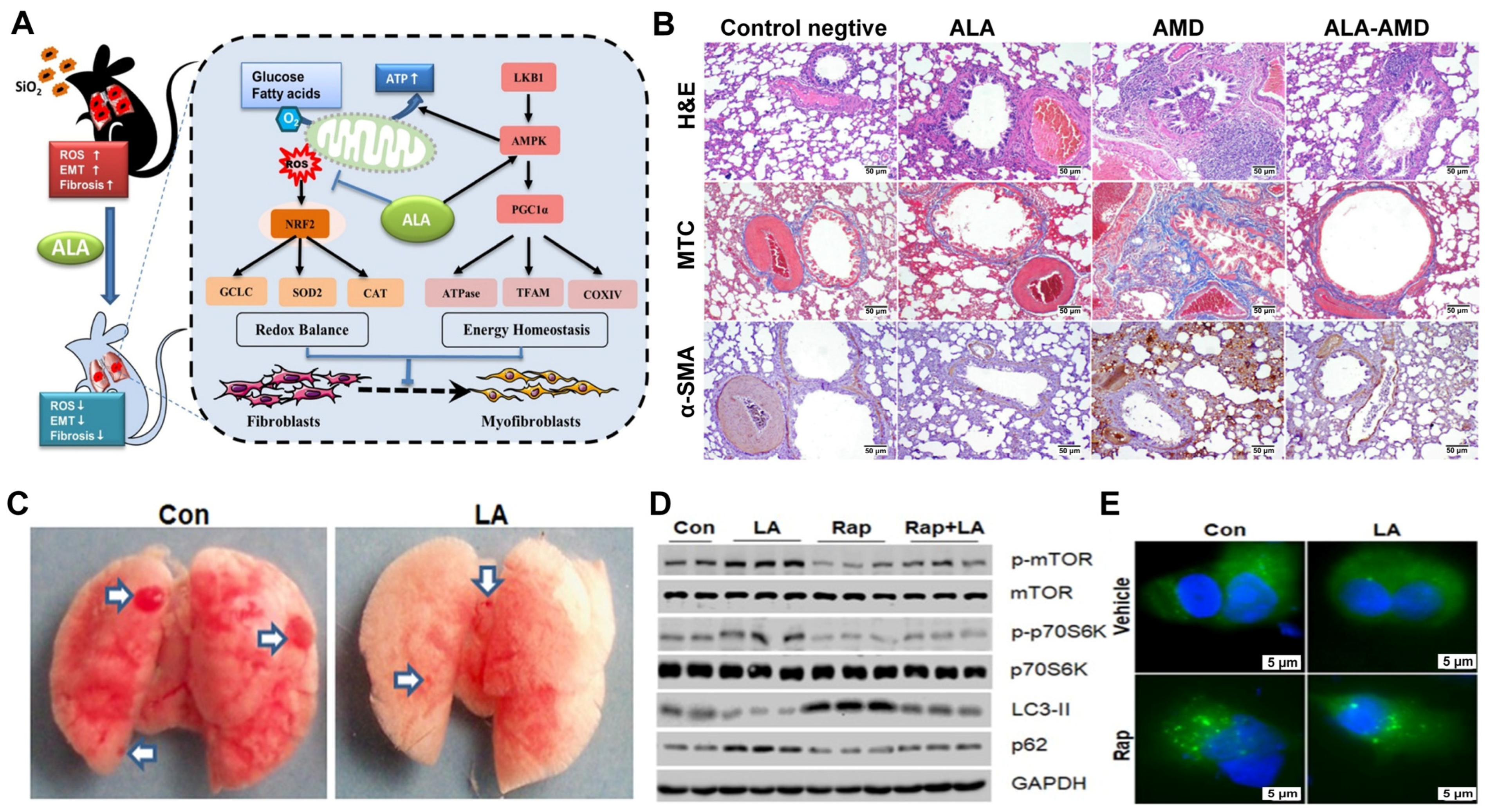
3.2.1. Pulmonary Fibrosis
3.2.2. Lung Cancer
3.3. Cardiovascular Diseases
3.3.1. Myocardial Infarction
3.3.2. Myocardial Ischemia–Reperfusion
3.3.3. Heart Failure
3.4. Diabetes
| Research Object | ALA Treatment | Outcomes | Reference |
|---|---|---|---|
| 460 patients with mild/moderate DSPN | 600 mg/kg/day, orally, 4 years | ↑NIS, NIS-LL, NSC | [65] |
| 54 T2DM patients | 600 mg/kg/day, orally, 6 months | ↑NO; ↓AGEs, ADMA, TNF-α, CAS, DN4, CPT | [66] |
| 200 patients with DPN | 600 mg/kg, twice-daily, orally, 6 months | ↑NSS, NDS, VAS, VPT | [67] |
| 1242 patients with DSPN | 600/800/1200 mg/kg/day, orally, 24 months | ↑TSS, NDS, NIS, and the global satisfaction score | [68] |
| 54 T2DM patients with DSPN | 600 mg/kg/day, orally, 6 months | ↑NO; ↓serum kallistatin, TNF-α, ADMA, NTSS-6, DN4 | [69] |
| 28 male BALB/C mice | 10 mg/kg, intraperitoneally weekly, 5 weeks | ↓grade 2 cataract | [71] |
| Female Brown Norway rats (7 weeks) | 30 mg/kg/day, orally, 10 months | ↓lens opacities, blood glucose levels | [72] |
| 30 diabetic dogs | 2 mg/kg/day, orally 200 days | ↑time to cataract formation; ↓lens opacities | [74] |
4. Novel Therapeutic Strategies of ALA
4.1. Derivatives of ALA
4.1.1. N2L
4.1.2. L-F001
4.1.3. CPI-613
4.1.4. Others
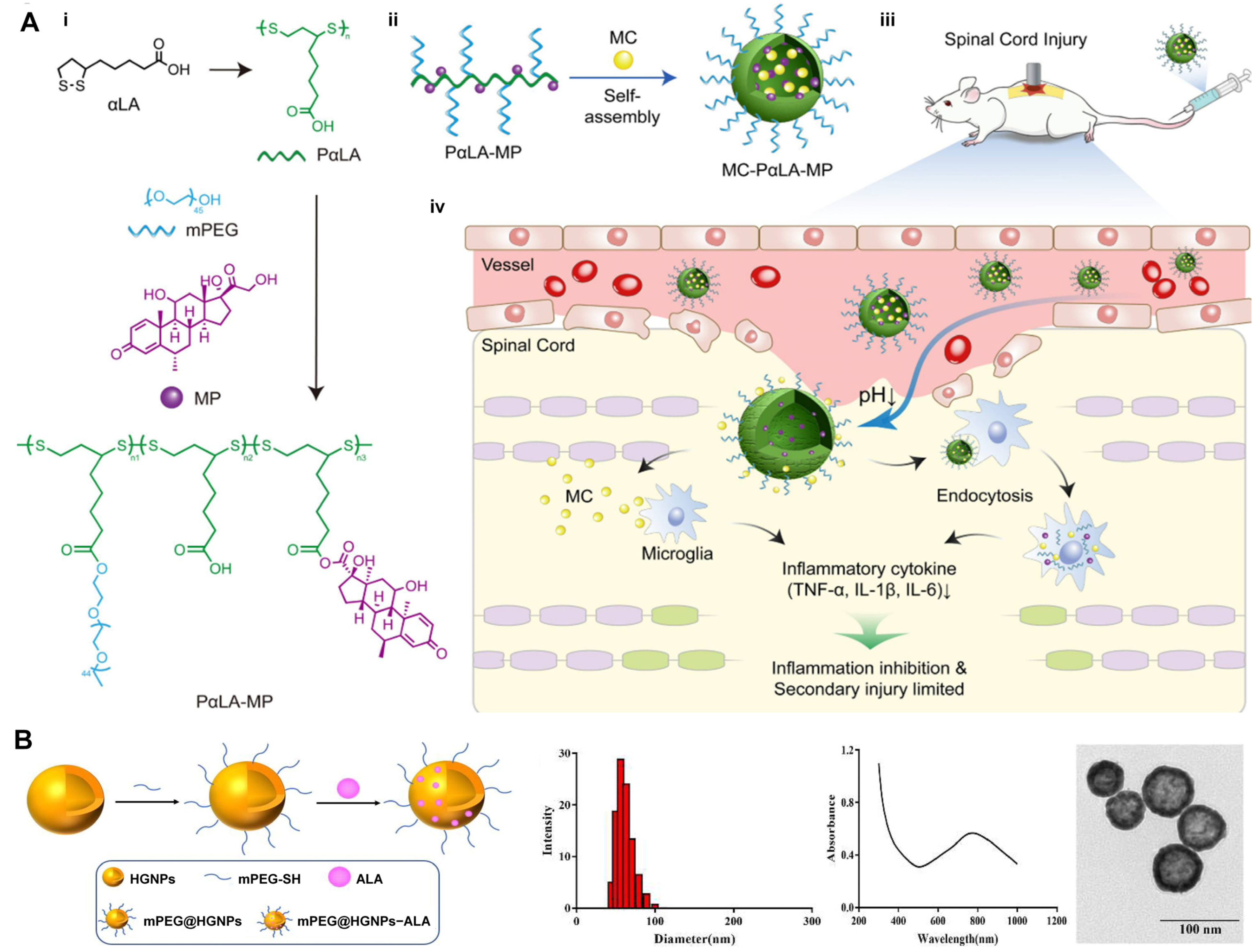
4.2. Delivery Systems of ALA
4.2.1. Nanoparticles
4.2.2. Hydrogels
4.2.3. Electrospinning
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| ABI | Ankle Brachial Index |
| ADMA | Asymmetric dimethylarginine |
| ADAScog | Alzheimer’s disease assessment scale—cognitive subscale |
| ALAM10 | Amyloid precursor protein-like protein 10 |
| ALDH1A1 | Aldehyde dehydrogenase 1 family member A1 |
| ALDH2 | Aldehyde dehydrogenase 2 |
| ALT | Alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| AMI | Acute myocardial infarction |
| APP | Amyloid precursor protein |
| ATP | Adenosine triphosphate |
| ATG13 | Autophagy-related 13 |
| ACSL4 | Acyl-coA synthetase long chain family member 4 |
| AR | Aldose reductase |
| AGEs | Advanced glycation end products |
| BAX | BCL-2-associated x protein |
| BCL-2 | B-cell lymphoma 2 |
| BNIP3L | BCL2/adenovirus E1B 19 kDa interacting protein 3-like |
| Caspase-3 | Cysteine-aspartic protease 3 |
| CAS | Composite autonomic score |
| CAT | Catalase |
| CHO | C/EBP homologous protein |
| CD133 | Cluster of differentiation 133 |
| CD44 | Cluster of differentiation 44 |
| CK-MB | Creatine kinase-mb |
| COX | Cyclooxygenase |
| COL1-α1 | Collagen type I alpha 1 chain |
| COL3-α1 | Collagen type III alpha 1 chain |
| COS | Chitosan oligosaccharide |
| CPT | Current perception threshold |
| Cyt-c | Cytochrome c |
| DESP | Deep eutectic supramolecular polymer |
| DN4 | Douleur neuropathique en 4 questions |
| DRP1 | Dynamin-related protein 1 |
| DPN | Diabetic peripheral neuropathy |
| DSPN | Diabetic sensorimotor polyneuropathy |
| ERK | Extracellular signal-regulated kinase |
| ERI | Erythropoietin resistance index |
| FIS1 | Mitochondrial fission 1 protein |
| FBG | Fasting blood-glucose |
| FMD | Flow-mediated dilation |
| FOXO1 | Forkhead box O1 |
| FRAP | Ferric reducing antioxidant power |
| Frizzled | Frizzled class receptor 2 |
| FUNDC1 | Fun14 domain containing 1 |
| GPR109A | G protein-coupled receptor 109A |
| FTH1 | Ferritin heavy chain 1 |
| GSK3β | Glycogen synthase kinase 3 beta |
| GSH | Glutathione |
| GPX | Glutathione peroxidase |
| GRB2 | Growth factor receptor bound protein 2 |
| HMGB1 | High mobility group box 1 |
| HbA1 | Glycosylated hemoglobin |
| HGNPs | Hollow gold nanoparticles |
| IADL | Instrumental activities of daily living |
| LAMC | Alpha-lipoic acid-modified chitosan |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IS | Infarct size |
| IKK-β | Inhibitor of κB kinase beta |
| IRP2 | Iron regulatory protein 2 |
| IκB-α | Inhibitor of kappa B alpha |
| JNK | c-Jun N-terminal kinase |
| LC3 | Microtubule-associated protein 1 light chain 3 |
| LDH | Lactate dehydrogenase |
| LVEF | Left ventricular ejection fraction |
| MC | Minocycline |
| MDA | Malondialdehyde |
| MFN | Mitofusin |
| MMP | Matrix metalloproteinase |
| MMSE | Mini-Mental State Examination |
| MNPs | Melanin nanoparticles |
| mPEG | methoxy poly(ethylene glycol) |
| MPO | Myeloperoxidase |
| M-β-CD | Methyl-β-cyclodextrin (M-β-CD) |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NIS-LL | Neuropathy impairment score of the lower limbs |
| NSC | Neuropathy symptoms and change |
| NPs | Nanoparticles |
| NO | Nitric oxide radical |
| NOX-4 | NADPH oxidase 4 |
| NDS | Neurological deficit score |
| NSS | Neurological symptom score |
| NTSS-6 | Neurological total symptom score-6 |
| NF-κB | Nuclear factor kappa B |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| p70S6K | Ribosomal protein S6 kinase (S6K1) |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PDK1 | Pyruvate dehydrogenase kinase 1 |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator-1alpha |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| p-AKT | Phosphorylated protein kinase B |
| p-PI3k | Phosphorylated phosphoinositide 3-kinase |
| PFC | Pulmonary function capacity |
| ROS | Reactive oxygen species |
| 6-OHDA | 6-Hydroxydopamine |
| SIRT1 | Sirtuin 1 |
| SLC7A11 | Solute carrier family 7 member 11 |
| SOD | Superoxide dismutase |
| SMAD3 | SMAD family member 3 |
| TAC | Total antioxidant capacity |
| TGF-β1 | Transforming growth factor beta 1 |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| TOM20 | Translocase of outer mitochondrial membrane 20 |
| TSCI | Traumatic spinal cord injury |
| VAS | Visual analog scale |
| VPT | Vibration perception threshold |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
References
- Reed, L.J.; DeBUSK, B.G.; Gunsalus, I.C.; Hornberger, C.S. Crystalline Alpha-Lipoic Acid; a Catalytic Agent Associated with Pyruvate Dehydrogenase. Science 1951, 114, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Q.; Ling, X.; Wang, H.-J.; Chen, F.-E. A-Lipoic Acid Chemistry: The Past 70 Years. RSC Adv. 2023, 13, 36346–36363. [Google Scholar] [CrossRef]
- Petca, A.; Bot, M.; Maru, N.; Calo, I.G.; Borislavschi, A.; Dumitrascu, M.C.; Petca, R.-C.; Sandru, F.; Zvanca, M.E. Benefits of α-Lipoic Acid in High-Risk Pregnancies (Review). Exp. Ther. Med. 2021, 22, 1232. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Singh, T.G.; Dahiya, R.S.; Abdel-Daim, M.M. A-Lipoic Acid, an Organosulfur Biomolecule a Novel Therapeutic Agent for Neurodegenerative Disorders: An Mechanistic Perspective. Neurochem. Res. 2022, 47, 1853–1864. [Google Scholar] [CrossRef]
- Gorąca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic Acid—Biological Activity and Therapeutic Potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K.; Bishen, S.M.; Mishra, H.; Khurana, A. Molecular and Therapeutic Insights of Alpha-Lipoic Acid as a Potential Molecule for Disease Prevention. Rev. Bras. Farmacogn. 2023, 33, 272–287. [Google Scholar] [CrossRef]
- Biewenga, G.P.; Haenen, G.R.; Bast, A. The Pharmacology of the Antioxidant Lipoic Acid. Gen. Pharmacol. 1997, 29, 315–331. [Google Scholar] [CrossRef]
- Taherian, S.-S.; Khayamabed, R.; Tavalaee, M.; Nasr-Esfahani, M.H. Alpha-Lipoic Acid Minimises Reactive Oxygen Species-Induced Damages during Sperm Processing. Andrologia 2019, 51, e13314. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, H.; Liao, Y.; Zhou, S.; Yang, Y.; Zhang, M.; Guo, Y.; Xie, T.; Chen, S.; Ouyang, W.; et al. Chronic Sleep Deprivation Impairs Visual Functions via Oxidative Damage in Mice. Am. J. Pathol. 2024, 194, 307–320. [Google Scholar] [CrossRef]
- Maczurek, A.; Hager, K.; Kenklies, M.; Sharman, M.; Martins, R.; Engel, J.; Carlson, D.A.; Münch, G. Lipoic Acid as an Anti-Inflammatory and Neuroprotective Treatment for Alzheimer’s Disease. Adv. Drug Deliv. Rev. 2008, 60, 1463–1470. [Google Scholar] [CrossRef]
- Seifar, F.; Khalili, M.; Khaledyan, H.; Amiri Moghadam, S.; Izadi, A.; Azimi, A.; Shakouri, S.K. A-Lipoic Acid, Functional Fatty Acid, as a Novel Therapeutic Alternative for Central Nervous System Diseases: A Review. Nutr. Neurosci. 2019, 22, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-Lipoic Acid as a Dietary Supplement: Molecular Mechanisms and Therapeutic Potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef]
- Bast, A.; Haenen, G.R.M.M. Lipoic Acid: A Multifunctional Antioxidant. BioFactors 2003, 17, 207–213. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Muresan, A.; Vergely, C. Alpha-Lipoic Acid: Molecular Mechanisms and Therapeutic Potential in Diabetes. Can. J. Physiol. Pharmacol. 2015, 93, 1021–1027. [Google Scholar] [CrossRef]
- Camiolo, G.; Tibullo, D.; Giallongo, C.; Romano, A.; Parrinello, N.L.; Musumeci, G.; Di Rosa, M.; Vicario, N.; Brundo, M.V.; Amenta, F.; et al. A-Lipoic Acid Reduces Iron-Induced Toxicity and Oxidative Stress in a Model of Iron Overload. Int. J. Mol. Sci. 2019, 20, 609. [Google Scholar] [CrossRef] [PubMed]
- Patwa, J.; Thakur, A.; Flora, S.J.S. Alpha Lipoic Acid and Monoisoamyl-DMSA Combined Treatment Ameliorates Copper-Induced Neurobehavioral Deficits, Oxidative Stress, and Inflammation. Toxics 2022, 10, 718. [Google Scholar] [CrossRef]
- Soták, M.; Clark, M.; Suur, B.E.; Börgeson, E. Inflammation and Resolution in Obesity. Nat. Rev. Endocrinol. 2025, 21, 45–61. [Google Scholar] [CrossRef]
- Alshammari, G.M.; Abdelhalim, M.A.; Al-Ayed, M.S.; Al-Harbi, L.N.; Yahya, M.A. The Protective Effect of α-Lipoic Acid against Gold Nanoparticles (AuNPs)-Mediated Liver Damage Is Associated with Upregulating Nrf2 and Suppressing NF-κB. Nutrients 2022, 14, 3327. [Google Scholar] [CrossRef] [PubMed]
- Zwierz, M.; Chabowski, A.; Sztolsztener, K. α-Lipoic Acid—A Promising Agent for Attenuating Inflammation and Preventing Steatohepatitis in Rats Fed a High-Fat Diet. Arch. Biochem. Biophys. 2023, 750, 109811. [Google Scholar] [CrossRef]
- Wang, H.-H.; Lin, C.-A.J.; Tseng, Y.-M.; Lee, H.-I.; Lee, Y.-N.; Yeh, H.-I.; Yang, P.-S.; Peng, H.-Y.; Wu, Y.-J. Dihydrolipoic Acid-Coated Gold Nanocluster Bioactivity against Senescence and Inflammation through the Mitochondria-Mediated JNK/AP-1 Pathway. Nanomed. Nanotechnol. Biol. Med. 2021, 36, 102427. [Google Scholar] [CrossRef]
- Costa, D.V.S.; Costa, D.V.S.; Sousa, C.N.S.; Silva, A.M.H.P.; Medeiros, I.S.; Martins, D.S.; Martins, C.S.; Pequeno, A.L.V.; Lima-Júnior, R.C.P.; Soares, P.M.G.; et al. The Alpha-Lipoic Acid Improves Survival and Prevents Irinotecan-Induced Inflammation and Intestinal Dysmotility in Mice. Pharmaceuticals 2020, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Vauzour, D. Natural Products and Neuroprotection 3.0. Int. J. Mol. Sci. 2023, 24, 3885. [Google Scholar] [CrossRef]
- González-Cofrade, L.; de Las Heras, B.; Apaza Ticona, L.; Palomino, O.M. Molecular Targets Involved in the Neuroprotection Mediated by Terpenoids. Planta Med. 2019, 85, 1304–1315. [Google Scholar] [CrossRef]
- Dieter, F.; Esselun, C.; Eckert, G.P. Redox Active α-Lipoic Acid Differentially Improves Mitochondrial Dysfunction in a Cellular Model of Alzheimer and Its Control Cells. Int. J. Mol. Sci. 2022, 23, 9186. [Google Scholar] [CrossRef] [PubMed]
- Metsla, K.; Kirss, S.; Laks, K.; Sildnik, G.; Palgi, M.; Palumaa, T.; Tõugu, V.; Palumaa, P. A-Lipoic Acid Has the Potential to Normalize Copper Metabolism, Which Is Dysregulated in Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 85, 715–728. [Google Scholar] [CrossRef]
- Staykov, H.; Lazarova, M.; Hassanova, Y.; Stefanova, M.; Tancheva, L.; Nikolov, R. Neuromodulatory Mechanisms of a Memory Loss-Preventive Effect of Alpha-Lipoic Acid in an Experimental Rat Model of Dementia. J. Mol. Neurosci. 2022, 72, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Hu, F.; Hu, Z.; Luo, F.; Li, X.; Xing, S.; Sun, L.; Long, D. Neuroprotective Effect of α-Lipoic Acid against Aβ25-35-Induced Damage in BV2 Cells. Molecules 2023, 28, 1168. [Google Scholar] [CrossRef]
- Pei, X.; Hu, F.; Luo, F.; Huang, X.; Li, X.; Xing, S.; Long, D. The Neuroprotective Effects of Alpha-Lipoic Acid on an Experimental Model of Alzheimer’s Disease in PC12 Cells. J. Appl. Toxicol. 2022, 42, 285–294. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Dong, X.; Meng, Z.; Ji, L.; Kang, Y.; Liu, M.; Zhou, W.; Song, W. Alpha-Lipoic Acid Alleviates Cognitive Deficits in Transgenic App23/Ps45 Mice Through a Mitophagy-Mediated Increase in Adam10 A-Secretase Cleavage of App. Alzheimer’s Res. Ther. 2024, 16, 160. [Google Scholar] [CrossRef]
- Zheng, Q.; Ma, P.; Yang, P.; Zhai, S.; He, M.; Zhang, X.; Tu, Q.; Jiao, L.; Ye, L.; Feng, Z.; et al. Alpha Lipoic Acid Ameliorates Motor Deficits by Inhibiting Ferroptosis in Parkinson’s Disease. Neurosci. Lett. 2023, 810, 137346. [Google Scholar] [CrossRef]
- Tai, S.; Zheng, Q.; Zhai, S.; Cai, T.; Xu, L.; Yang, L.; Jiao, L.; Zhang, C. Alpha-Lipoic Acid Mediates Clearance of Iron Accumulation by Regulating Iron Metabolism in a Parkinson’s Disease Model Induced by 6-OHDA. Front. Neurosci. 2020, 14, 612. [Google Scholar] [CrossRef]
- Das, N.R.; Vaidya, B.; Khare, P.; Bishnoi, M.; Sharma, S.S. Combination of Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) Agonist and PPAR Gamma Co-Activator 1α (PGC-1α) Activator Ameliorates Cognitive Deficits, Oxidative Stress, and Inflammation in Rodent Model of Parkinson’s Disease. Curr. Neurovasc. Res. 2021, 18, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, Y.; Zhang, L.; Zhang, C.; Zhao, Y.; Zhang, Y.; Li, S.; Chang, C.; Zhang, X.; Yang, G. Alpha-Lipoic Acid Attenuates MPTP/MPP+-Induced Neurotoxicity: Roles of SIRT1-Dependent PGC-1α Signaling Pathways. Neurotox. Res. 2022, 40, 410–419. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Zhao, Y.; Zhang, Y.; Gao, Y.; Zhang, X.; Yang, G. Alpha-Lipoic Acid Improved Motor Function in MPTP-Induced Parkinsonian Mice by Reducing Neuroinflammation in the Nigral and Spinal Cord. Neurosci. Lett. 2022, 781, 136669. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Wang, H. A-Lipoic Acid Alleviates Ferroptosis in the MPP+-Induced PC12 Cells via Activating the PI3K/Akt/Nrf2 Pathway. Cell Biol. Int. 2021, 45, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Hager, K.; Kenklies, M.; McAfoose, J.; Engel, J.; Münch, G. Alpha-Lipoic Acid as a New Treatment Option for Alzheimer’s Disease--a 48 Months Follow-up Analysis. J. Neural Transm. Suppl. 2007, 72, 189–193. [Google Scholar]
- Shinto, L.; Quinn, J.; Montine, T.; Dodge, H.H.; Woodward, W.; Baldauf-Wagner, S.; Waichunas, D.; Bumgarner, L.; Bourdette, D.; Silbert, L.; et al. A Randomized Placebo-Controlled Pilot Trial of Omega-3 Fatty Acids and Alpha Lipoic Acid in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 38, 111–120. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z.; Huang, T.; Zhou, H. Fibrosis to Carcinogenesis: Unveiling the Causal Dynamics between Pulmonary Fibrosis and Lung Cancer. Front. Oncol. 2024, 14, 1452559. [Google Scholar] [CrossRef]
- Gu, J.; Xu, J.; Jiao, A.; Cai, N.; Gu, T.; Wu, P.; Cheng, X.; Chen, B.; Chen, Y.; Liu, X. Comprehensive Analysis of Single-Cell Transcriptomics and Genetic Factors Reveals the Mechanisms and Preventive Strategies for the Progression from Pulmonary Fibrosis to Lung Cancer. Int. Immunopharmacol. 2024, 140, 112803. [Google Scholar] [CrossRef]
- Chang, M.; Xu, G.; Xiong, C.; Yang, X.; Yan, S.; Tao, Y.; Li, H.; Li, Y.; Yao, S.; Zhao, Y. Alpha-Lipoic Acid Attenuates Silica-Induced Pulmonary Fibrosis by Improving Mitochondrial Function via AMPK/PGC1α Pathway Activation in C57BL/6J Mice. Toxicol. Lett. 2021, 350, 121–132. [Google Scholar] [CrossRef]
- Yan, S.; Zhao, Y.; Yan, J.; Guan, Y.; Lyu, M.; Xu, G.; Yang, X.; Bai, Y.; Yao, S. Low Expression of Lipoic Acid Synthase Aggravates Silica-Induced Pulmonary Fibrosis by Inhibiting the Differentiation of Tregs in Mice. Antioxid. Redox Signal. 2024, 41, 216–232. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; R Mousa, M. The Protective Potential of Alpha Lipoic Acid on Amiodarone-Induced Pulmonary Fibrosis and Hepatic Injury in Rats. Mol. Cell. Biochem. 2021, 476, 3433–3448. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, G.; Li, H.; Chang, M.; Guan, Y.; Li, Y.; Wu, W.; Yao, S. Overexpression of Endogenous Lipoic Acid Synthase Attenuates Pulmonary Fibrosis Induced by Crystalline Silica in Mice. Toxicol. Lett. 2020, 323, 57–66. [Google Scholar] [CrossRef]
- Elhadidy, M.G.; Elmasry, A.; Elsayed, H.R.H.; El-Nablaway, M.; Hamed, S.; Elalfy, M.M.; Rabei, M.R. Modulation of COX-2 and NADPH Oxidase-4 by Alpha-Lipoic Acid Ameliorates Busulfan-Induced Pulmonary Injury in Rats. Heliyon 2021, 7, e08171. [Google Scholar] [CrossRef] [PubMed]
- Azmoonfar, R.; Amini, P.; Yahyapour, R.; Rezaeyan, A.; Tavassoli, A.; Motevaseli, E.; Khodamoradi, E.; Shabeeb, D.; Musa, A.E.; Najafi, M. Mitigation of Radiation-Induced Pneumonitis and Lung Fibrosis Using Alpha-Lipoic Acid and Resveratrol. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Zhang, X.; Qi, T.; Cheng, H.; Kong, Q.; Liu, L.; Cao, X.; Ding, Z. Alpha-Lipoic Acid Inhibits Lung Cancer Growth via mTOR-Mediated Autophagy Inhibition. FEBS Open Bio 2020, 10, 607–618. [Google Scholar] [CrossRef]
- Phiboonchaiyanan, P.P.; Chanvorachote, P. Suppression of a Cancer Stem-like Phenotype Mediated by Alpha-Lipoic Acid in Human Lung Cancer Cells through down-Regulation of β-Catenin and Oct-4. Cell. Oncol. 2017, 40, 497–510. [Google Scholar] [CrossRef]
- Yang, L.; Wen, Y.; Lv, G.; Lin, Y.; Tang, J.; Lu, J.; Zhang, M.; Liu, W.; Sun, X. A-Lipoic Acid Inhibits Human Lung Cancer Cell Proliferation through Grb2-Mediated EGFR Downregulation. Biochem. Biophys. Res. Commun. 2017, 494, 325–331. [Google Scholar] [CrossRef]
- Yue, L.; Ren, Y.; Yue, Q.; Ding, Z.; Wang, K.; Zheng, T.; Chen, G.; Chen, X.; Li, M.; Fan, L. A-Lipoic Acid Targeting Pdk1/Nrf2 Axis Contributes to the Apoptosis Effect of Lung Cancer Cells. Oxidative Med. Cell. Longev. 2021, 2021, 6633419. [Google Scholar] [CrossRef]
- Puchsaka, P.; Chaotham, C.; Chanvorachote, P. A-Lipoic Acid Sensitizes Lung Cancer Cells to Chemotherapeutic Agents and Anoikis via Integrin Β1/Β3 Downregulation. Int. J. Oncol. 2016, 49, 1445–1456. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar]
- Yang, Z.; Tian, Y.; Berr, S.S.; French, B.A. Therapeutic Efficacy of Alpha-Lipoic Acid against Acute Myocardial Infarction and Chronic Left Ventricular Remodeling in Mice. Cardiol. Res. Pract. 2020, 2020, 6759808. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Qi, B.; Liu, Y.; Cheng, X.; Feng, J.; Gao, W.; Li, T. A-Lipoic Acid Alleviates Myocardial Injury and Induces M2b Macrophage Polarization after Myocardial Infarction via HMGB1/NF-kB Signaling Pathway. Int. Immunopharmacol. 2023, 121, 110435. [Google Scholar] [CrossRef] [PubMed]
- Nemati, S.; Zavvari-Oskuye, Z.; Bafadam, S.; Mokhtari, B.; Badalzadeh, R.; Vakili, A. Impact of Combined Alpha-Lipoic Acid and Mitoquinone Supplementation on Myocardial Infarction in Aged Rats: Heart Performance and Molecular Mechanisms. Exp. Gerontol. 2024, 189, 112402. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hady, E.; Mohamed, F.; Ahmed, M.; Abdel-Salam, M.; Ayobe, M. Supplementation of Lipoic Acid, Zinc and Clopidogrel Reduces Mortality Rate and Incidence of Ventricular Arrhythmia in Experimental Myocardial Infarction. Front. Physiol. 2021, 12, 582223. [Google Scholar] [CrossRef]
- Oskuye, Z.Z.; Mehri, K.; Mokhtari, B.; Bafadam, S.; Nemati, S.; Badalzadeh, R. Cardioprotective Effect of Antioxidant Combination Therapy: A Highlight on MitoQ plus Alpha-Lipoic Acid Beneficial Impact on Myocardial Ischemia-Reperfusion Injury in Aged Rats. Heliyon 2024, 10, e28158. [Google Scholar] [CrossRef] [PubMed]
- Gholami, S.; Mokhtari, B.; Badalzadeh, R. Alpha-Lipoic Acid Potentiates the Anti-Arrhythmic Effects of Ischemic Postconditioning in the Setting of Cardiac Ischemia/Reperfusion Injury in Diabetic Rats. J. Diabetes Metab. Disord. 2022, 21, 707–716. [Google Scholar] [CrossRef]
- Mokhtari, B.; Abdoli-Shadbad, M.; Alihemmati, A.; Javadi, A.; Badalzadeh, R. Alpha-Lipoic Acid Preconditioning plus Ischemic Postconditioning Provides Additional Protection against Myocardial Reperfusion Injury of Diabetic Rats: Modulation of Autophagy and Mitochondrial Function. Mol. Biol. Rep. 2022, 49, 1773–1782. [Google Scholar] [CrossRef]
- Gholami, S.; Badalzadeh, R.; Alihemmati, A. Alpha-Lipoic Acid Enhances Ischemic Postconditioning-Mediated Improvement of Myocardial Infarction and Apoptosis in Diabetic Rats with Ischemia/Reperfusion Injury. Can. J. Physiol. Pharmacol. 2023, 101, 682–691. [Google Scholar] [CrossRef]
- Qi, B.; Zheng, Y.; Gao, W.; Qi, Z.; Gong, Y.; Liu, Y.; Wang, Y.; Cheng, X.; Ning, M.; Lang, Y.; et al. Alpha-Lipoic Acid Impedes Myocardial Ischemia-Reperfusion Injury, Myocardial Apoptosis, and Oxidative Stress by Regulating HMGB1 Expression. Eur. J. Pharmacol. 2022, 933, 175295. [Google Scholar] [CrossRef]
- Pop, C.; Ștefan, M.-G.; Muntean, D.-M.; Stoicescu, L.; Gal, A.F.; Kiss, B.; Morgovan, C.; Loghin, F.; Rochette, L.; Lauzier, B.; et al. Protective Effects of a Discontinuous Treatment with Alpha-Lipoic Acid in Obesity-Related Heart Failure with Preserved Ejection Fraction, in Rats. Antioxidants 2020, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yin, L.; Sun, X.; Wu, J.; Dong, Z.; Hu, K.; Sun, A.; Ge, J. Alpha-Lipoic Acid Protects against Pressure Overload-Induced Heart Failure via ALDH2-Dependent Nrf1-FUNDC1 Signaling. Cell Death Dis. 2020, 11, 599. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Price, R.S.; Chen, K.S.; Feldman, E.L. The Importance of Rare Subtypes in Diagnosis and Treatment of Peripheral Neuropathy: A Review. JAMA Neurol. 2015, 72, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Tesfaye, S.; Spallone, V.; Gurieva, I.; Al Kaabi, J.; Mankovsky, B.; Martinka, E.; Radulian, G.; Nguyen, K.T.; Stirban, A.O.; et al. Screening, Diagnosis and Management of Diabetic Sensorimotor Polyneuropathy in Clinical Practice: International Expert Consensus Recommendations. Diabetes Res. Clin. Pract. 2022, 186, 109063. [Google Scholar] [CrossRef] [PubMed]
- Papanas, N.; Maltezos, E. α-Lipoic Acid, Diabetic Neuropathy, and Nathan’s Prophecy. Angiology 2012, 63, 81–83. [Google Scholar] [CrossRef]
- Csiha, S.; Hernyák, M.; Molnár, Á.; Lőrincz, H.; Katkó, M.; Paragh, G.; Bodor, M.; Harangi, M.; Sztanek, F.; Berta, E. Alpha-Lipoic Acid Treatment Reduces the Levels of Advanced End Glycation Products in Type 2 Diabetes Patients with Neuropathy. Biomedicines 2025, 13, 438. [Google Scholar] [CrossRef]
- El-Nahas, M.R.; Elkannishy, G.; Abdelhafez, H.; Elkhamisy, E.T.; El-Sehrawy, A.A. Oral Alpha Lipoic Acid Treatment for Symptomatic Diabetic Peripheral Neuropathy: A Randomized Double-Blinded Placebo-Controlled Study. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1531–1534. [Google Scholar] [CrossRef]
- Hsieh, R.-Y.; Huang, I.-C.; Chen, C.; Sung, J.-Y. Effects of Oral Alpha-Lipoic Acid Treatment on Diabetic Polyneuropathy: A Meta-Analysis and Systematic Review. Nutrients 2023, 15, 3634. [Google Scholar] [CrossRef]
- Hernyák, M.; Tóth, L.I.; Csiha, S.; Molnár, Á.; Lőrincz, H.; Paragh, G.; Harangi, M.; Sztanek, F. Kallistatin as a Potential Marker of Therapeutic Response during Alpha-Lipoic Acid Treatment in Diabetic Patients with Sensorimotor Polyneuropathy. Int. J. Mol. Sci. 2024, 25, 13276. [Google Scholar] [CrossRef]
- Bierbrauer, K.L.; Comini, L.R.; Leonhard, V.; Escobar Manzanelli, M.A.; Castelli, G.; Farfán, S.; Alasino, R.V.; Beltramo, D.M. Eudragit Films as Carriers of Lipoic Acid for Transcorneal Permeability. Polymers 2023, 15, 1793. [Google Scholar] [CrossRef]
- Kan, E.; Kiliçkan, E.; Ayar, A.; Çolak, R. Effects of Two Antioxidants; α-Lipoic Acid and Fisetin against Diabetic Cataract in Mice. Int. Ophthalmol. 2015, 35, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Sun, L.; Hata, I.; Sakamoto, Y.; Sasaki, H.; Sasaki, K. Efficacy of Alpha-Lipoic Acid against Diabetic Cataract in Rat. Jpn. J. Ophthalmol. 2007, 51, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Williams, D. An Oral Antioxidant Formulation Delaying and Potentially Reversing Canine Diabetic Cataract: A Placebo-Controlled Double-Masked Pilot Study. Int. J. Diabetes Clin. Res. 2015, 2, 1–5. [Google Scholar] [CrossRef]
- Williams, D.L. Effect of Oral Alpha Lipoic Acid in Preventing the Genesis of Canine Diabetic Cataract: A Preliminary Study. Vet. Sci. 2017, 4, 18. [Google Scholar] [CrossRef]
- Chen, X.; Gao, J.; Jiang, Y.; Huang, P.; Xie, Y.; Pi, R.; Zhu, S.; Yao, M. Determination of Newly Synthesized Lipoic Acid-Niacin Dimer in Rat Plasma by UPLC/Electrospray Ionization Tandem Mass Spectrometry: Assay Development, Validation and Application to a Pharmacokinetic Study. Biomed. Chromatogr. 2014, 28, 213–217. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Liao, R.; Li, Q.; Pi, R.; Yang, X. N2L, a Novel Lipoic Acid-Niacin Dimer Protects HT22 Cells against β-Amyloid Peptide-Induced Damage through Attenuating Apoptosis. Metab. Brain Dis. 2019, 34, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.-L.; Yu, Y.-Z.; Yu, H.-H.; Wang, G.-F.; Pi, R.-B.; Xu, Z.; Zhang, C.; Zhou, W.-J.; Li, D.-D.; Chen, X.-G.; et al. Protective Effects of Lipoic Acid-Niacin Dimers against Blue Light-Induced Oxidative Damage to Retinal Pigment Epithelium Cells. Int. J. Ophthalmol. 2019, 12, 1262–1271. [Google Scholar] [CrossRef]
- Jiang, Y.; Jin, M.; Chen, J.; Yan, J.; Liu, P.; Yao, M.; Cai, W.; Pi, R. Discovery of a Novel Niacin-Lipoic Acid Dimer N2L Attenuating Atherosclerosis and Dyslipidemia with Non-Flushing Effects. Eur. J. Pharmacol. 2020, 868, 172871. [Google Scholar] [CrossRef]
- Peng, W.; Zhu, Z.; Yang, Y.; Hou, J.; Lu, J.; Chen, C.; Liu, F.; Pi, R. N2L, a Novel Lipoic Acid-Niacin Dimer, Attenuates Ferroptosis and Decreases Lipid Peroxidation in HT22 Cells. Brain Res. Bull. 2021, 174, 250–259. [Google Scholar] [CrossRef]
- Shen, W.; Wang, L.; Pi, R.; Li, Z.; Wang, R. L-F001, a Multifunctional ROCK Inhibitor Prevents Paraquat-Induced Cell Death through Attenuating ER Stress and Mitochondrial Dysfunction in PC12 Cells. Biochem. Biophys. Res. Commun. 2015, 464, 794–799. [Google Scholar] [CrossRef]
- Luo, L.; Chen, J.; Su, D.; Chen, M.; Luo, B.; Pi, R.; Wang, L.; Shen, W.; Wang, R. L-F001, a Multifunction ROCK Inhibitor Prevents 6-OHDA Induced Cell Death through Activating Akt/GSK-3beta and Nrf2/HO-1 Signaling Pathway in PC12 Cells and Attenuates MPTP-Induced Dopamine Neuron Toxicity in Mice. Neurochem. Res. 2017, 42, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Ouyang, Y.; Wang, S.; Hou, J.; Zhu, Z.; Yang, Y.; Zhou, R.; Pi, R. L-F001, a Multifunctional Fasudil-Lipoic Acid Dimer Prevents RSL3-Induced Ferroptosis via Maintaining Iron Homeostasis and Inhibiting JNK in HT22 Cells. Front. Cell. Neurosci. 2022, 16, 774297. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, L.; Jin, N.; Sha, S.; Ouyang, Y. L-F001, a Multifunctional Fasudil-Lipoic Acid Dimer, Antagonizes Hypoxic-Ischemic Brain Damage by Inhibiting the TLR4/MyD88 Signaling Pathway. Brain Behav. 2023, 13, e3280. [Google Scholar] [CrossRef]
- Neitzel, C.; Demuth, P.; Wittmann, S.; Fahrer, J. Targeting Altered Energy Metabolism in Colorectal Cancer: Oncogenic Reprogramming, the Central Role of the TCA Cycle and Therapeutic Opportunities. Cancers 2020, 12, 1731. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, Z.; Huang, Z.; Tang, Y.; Yang, D.; Huang, J.; He, L.; Liu, M.; Chen, Z.; Teng, Y. CPI-613 Rewires Lipid Metabolism to Enhance Pancreatic Cancer Apoptosis via the AMPK-ACC Signaling. J. Exp. Clin. Cancer Res. 2020, 39, 73. [Google Scholar] [CrossRef]
- Arnold, C.; Demuth, P.; Seiwert, N.; Wittmann, S.; Boengler, K.; Rasenberger, B.; Christmann, M.; Huber, M.; Brunner, T.; Linnebacher, M.; et al. The Mitochondrial Disruptor Devimistat (CPI-613) Synergizes with Genotoxic Anticancer Drugs in Colorectal Cancer Therapy in a Bim-Dependent Manner. Mol. Cancer Ther. 2022, 21, 100–112. [Google Scholar] [CrossRef]
- Udumula, M.P.; Rashid, F.; Singh, H.; Pardee, T.; Luther, S.; Bhardwaj, T.; Anjaly, K.; Piloni, S.; Hijaz, M.; Gogoi, R.; et al. Targeting Mitochondrial Metabolism with CPI-613 in Chemoresistant Ovarian Tumors. J. Ovarian Res. 2024, 17, 226. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Jiang, Y.; Xiang, R.; Gong, H.; Gong, Y.; Xu, H.; Ma, Z.; Xie, Y.; Zhu, Y.; et al. The Function and Mechanism of Clinical Trial Agent CPI-613 in Multiple Myeloma. Biochem. Pharmacol. 2024, 232, 116717. [Google Scholar] [CrossRef]
- Baguisi, A.; Casale, R.A.; Kates, S.A.; Lader, A.S.; Stewart, K.; Beeuwkes, R. CMX-2043 Efficacy in a Rat Model of Cardiac Ischemia-Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 563–569. [Google Scholar] [CrossRef]
- Tcheng, J.E.; Gibson, M.; Krucoff, M.W.; Patel, M.R.; Ajit, M.; Hiremath, J.; Ponde, C.; Ramsaran, E.; Clark, G.; Lader, A.S.; et al. SUPPORT-1 (Subjects Undergoing PCI and Perioperative Reperfusion Treatment): A Prospective, Randomized Trial of CMX-2043 in Patients Undergoing Elective Percutaneous Coronary Intervention. J. Cardiovasc. Pharmacol. 2020, 76, 189–196. [Google Scholar] [CrossRef]
- Lv, S.-Y.; He, S.; Ling, X.-L.; Wang, Y.-Q.; Huang, C.; Long, J.-R.; Wang, J.-Q.; Qin, Y.; Wei, H.; Yu, C.-Y. Review of Lipoic Acid: From a Clinical Therapeutic Agent to Various Emerging Biomaterials. Int. J. Pharm. 2022, 627, 122201. [Google Scholar] [CrossRef] [PubMed]
- Sagawa, N.; Ohno, S.; Hiratsuka, T.; Kondo, N.; Iwata, H.; Bando, H.; Ohyama, T.; Ishida, M.; Kono, Y.; Nakajima, K.; et al. The Utility of DHL-HisZnNa, a Novel Antioxidant, against Anticancer Agent-Induced Alopecia in Breast Cancer Patients: A Multicenter Phase II Clinical Trial. Breast Cancer Res. Treat. 2019, 176, 625–630. [Google Scholar] [CrossRef]
- Kono, Y.; Inomata, M.; Hagiwara, S.; Hiratsuka, T.; Suzuki, K.; Koga, H.; Shiraishi, N.; Noguchi, T.; Kitano, S. Antiproliferative Effects of a New α-Lipoic Acid Derivative, DHL-HisZnNa, in HT29 Human Colon Cancer Cells in Vitro. Expert Opin. Ther. Targets 2012, 16 (Suppl. S1), S103–S109. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, T.; Inomata, M.; Kono, Y.; Yokoyama, S.; Shiraishi, N.; Kitano, S. DHL-TauZnNa, a Newly Synthesized α-Lipoic Acid Derivative, Induces Autophagy in Human Colorectal Cancer Cells. Oncol. Rep. 2013, 29, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Bellini, C.; Mancin, F.; Papini, E.; Tavano, R. Nanotechnological Approaches to Enhance the Potential of α-Lipoic Acid for Application in the Clinic. Antioxidants 2024, 13, 706. [Google Scholar] [CrossRef]
- Halder, S.; Mibe, Y.; Rikimura, S.; Kuromi, K.; Sato, H.; Onoue, S. Strategic Application of Liposomal System to R-α-Lipoic Acid for the Improvement of Nutraceutical Properties. Drug Dev. Ind. Pharm. 2022, 48, 239–246. [Google Scholar] [CrossRef]
- Metwaly, H.H.; Fathy, S.A.; Abdel Moneim, M.M.; Emam, M.A.; Soliman, A.F.; El-Naggar, M.E.; Omara, E.A.; El-Bana, M.A. Chitosan and Solid Lipid Nanoparticles Enhance the Efficiency of Alpha-Lipoic Acid against Experimental Neurotoxicity. Toxicol. Mech. Methods 2022, 32, 268–279. [Google Scholar] [CrossRef]
- Lin, F.; Liu, Y.; Luo, W.; Liu, S.; Wang, Y.; Gu, R.; Liu, W.; Xiao, C. Minocycline-Loaded Poly(α-Lipoic Acid)-Methylprednisolone Prodrug Nanoparticles for the Combined Anti-Inflammatory Treatment of Spinal Cord Injury. Int. J. Nanomed. 2022, 17, 91–104. [Google Scholar] [CrossRef]
- Xi, Y.; Pan, W.; Liu, Y.; Liu, J.; Xu, G.; Su, Y.; Chen, D.; Ye, X. A-Lipoic Acid Loaded Hollow Gold Nanoparticles Designed for Osteoporosis Treatment: Preparation, Characterization and in Vitro Evaluation. Artif. Cells Nanomed. Biotechnol. 2023, 51, 131–138. [Google Scholar] [CrossRef]
- Cui, C.; Sun, Y.; Nie, X.; Yang, X.; Wang, F.; Liu, W. A Coenzyme-Based Deep Eutectic Supramolecular Polymer Bioadhesive. Adv. Funct. Mater. 2023, 33, 2307543. [Google Scholar] [CrossRef]
- Li, Q.; Liu, K.; Jiang, T.; Ren, S.; Kang, Y.; Li, W.; Yao, H.; Yang, X.; Dai, H.; Chen, Z. Injectable and Self-Healing Chitosan-Based Hydrogel with MOF-Loaded α-Lipoic Acid Promotes Diabetic Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112519. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Li, Q.; Liu, K.; Wu, X.; Xu, H.; Chen, Z.; Dai, H. Efficacy of Melanin-Loaded Lipoic Acid-Modified Chitosan Hydrogel in Diabetic Wound Healing. Carbohydr. Polym. 2024, 340, 122215. [Google Scholar] [CrossRef]
- Zeng, J.; Fang, H.; Pan, H.; Gu, H.; Zhang, K.; Song, Y. Rapidly Gelled Lipoic Acid-Based Supramolecular Hydrogel for 3D Printing of Adhesive Bandage. ACS Appl. Mater. Interfaces 2024, 16, 53515–53531. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, F.; Li, J.; Yang, Y.; Guo, D.; Zhang, Y.; Yang, A.; He, X.; Cheng, Y. Green Polymer Hydrogels from a Natural Monomer with Inherent Antioxidative Capability for Efficient Wound Healing and Spinal Cord Injury Treatment. Biomater. Sci. 2023, 11, 3683–3694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xia, Y.; Li, X.; Zhang, Q.; Wu, Y.; Cui, C.; Liu, J.; Liu, W. Arginine-Solubilized Lipoic Acid-Induced β-Sheets of Silk Fibroin-Strengthened Hydrogel for Postoperative Rehabilitation of Breast Cancer. Bioact. Mater. 2024, 40, 667–682. [Google Scholar] [CrossRef]
- Jia, M.; Lu, R.; Liu, C.; Zhou, X.; Li, P.; Zhang, S. In Situ Implantation of Chitosan Oligosaccharide-Doped Lipoic Acid Hydrogel Breaks the “Vicious Cycle” of Inflammation and Residual Tumor Cell for Postoperative Skin Cancer Therapy. ACS Appl. Mater. Interfaces 2023, 15, 32824–32838. [Google Scholar] [CrossRef]
- Li, W.; Yin, Y.; Zhou, H.; Fan, Y.; Yang, Y.; Gao, Q.; Li, P.; Gao, G.; Li, J. Recent Advances in Electrospinning Techniques for Precise Medicine. Cyborg Bionic Syst. 2024, 5, 0101. [Google Scholar] [CrossRef]
- Lian, S.; Lamprou, D.; Zhao, M. Electrospinning Technologies for the Delivery of Biopharmaceuticals: Current Status and Future Trends. Int. J. Pharm. 2024, 651, 123641. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Liu, Y.; Cai, L.; Zhou, L.; Jiang, H.; Chen, J. Encapsulating MoS2-Nanoflowers Conjugated with Chitosan Oligosaccharide into Electrospun Nanofibrous Scaffolds for Photothermal Inactivation of Bacteria. J. Nanostruct. Chem. 2024, 14, 137–151. [Google Scholar] [CrossRef]
- Ruchika; Bhardwaj, N.; Saneja, A. Orally Fast Dissolving α-Lipoic Acid Electrospun Nanofibers Mitigates Lipopolysaccharide Induced Inflammation in RAW 264.7 Macrophages. Int. J. Biol. Macromol. 2024, 264, 130623. [Google Scholar] [CrossRef]
- Xie, D.-M.; Zhong, Q.; Xu, X.; Li, Y.; Chen, S.; Li, M.; Peng, C. Alpha Lipoic Acid–Loaded Electrospun Fibrous Patch Films Protect Heart in Acute Myocardial Infarction Mice by Inhibiting Oxidative Stress. Int. J. Pharm. 2023, 632, 122581. [Google Scholar] [CrossRef] [PubMed]






| Cells Line/Animal | ALA Treatment | Mechanism | Reference |
|---|---|---|---|
| Alzheimer’s disease | |||
| SH-SY5Y cells | 100 µM, 1 mM, 24 h | ↑ATP, MMP; ↓ROS | [24] |
| SH-SY5Y cells; Fruit fly | 0–50 µM, 24 h; 2 mM, ALA-food, 14 days | ↑Copper metabolism, Locomotor activity; ↓Eye photoreceptor cells | [25] |
| Male Wistar rats (160–180 g) | 30 mg/kg/day, injected intraperitoneally, 11 days | ↑STL, DA, NA; ↓AChE activity | [26] |
| BV2 cells | 100 µM, 24 h | ↑BCL-2, IκB-α, SOD, GPX, CAT, p-GSK3β, β-catenin; ↓BAX, Caspase-3, NF-κB, p65, GSK3β, p-β-catenin | [27] |
| PC12 cells | 1, 10, 100 µM, 48 h | ↑BCL-2, IκB-α, Frizzled 2, p-GSK3β, β-catenin; ↓BAX, Caspase-3, NF-κB, p65, GSK3β, p-β-catenin | [28] |
| 20E2 cells; APP23/PS45 transgenic mice (2 months) | 400 µM, 24 h; 5 mg/kg/day, injected intraperitoneally, 4 months | ↑ALAM10, C83, LC3, BNIP3L; ↓APP, C89, C99, P62 | [29] |
| Parkinson’s disease | |||
| PC12 cells; Mice | 10 µM, 24 h; 50 mg/kg/day, injected intraperitoneally, 14 days | ↑FTH1, GPX4, x-CT, SIRT1, NRF2; ↓DMT1, ROS | [30] |
| Male Sprague–Dawley (SD) rats (250–300 g) | 100 mg/kg/day, injected intraperitoneally, 14 days | ↑SOD, GSH; ↓ROS, Iron, TH, IRP2, DMT1 | [31] |
| Male Sprague–Dawley (SD) rats (250–300 g) | 30 mg/kg/day, injected intraperitoneally, 7 days | ↑GSH, PGC1α, TFAM, NRF2; ↓MDA, IL-6 | [32] |
| SH-SY5Y cells; Male C57BL/6 mice (22–27 g) | 200 µM, 24 h; 50 mg/kg/day, injected intraperitoneally, 14 days | ↑SIRT1, PGC1α, TH, SOD; ↓MDA, DCF | [33] |
| Male C57BL/6 mice (23–28 g) | 50 mg/kg/day, injected intraperitoneally, 14 days | ↓NF-κB, TNF-α, iNOS | [34] |
| PC12 cells | 0, 0.1, 1, 10 and 20 mM, 24 h | ↑SLC7A11, GPX4, p-P13k, p-AKT, NRF2 | [35] |
| 43 AD patients | 600 mg/kg/day, orally, 48 months | ↑MMSE, ADAScog | [36] |
| 39 AD patients | 600 mg/kg/day, orally, 12 months | ↑MMSE, IADL | [37] |
| Cells Line/Animal | ALA Treatment | Mechanism | Reference |
|---|---|---|---|
| Pulmonary fibrosis | |||
| Male C57BL/6 J mice (8 weeks) | 3.0 mg/mL, gavage, 28 days | ↑TFAM, COX-4, ATPase, p-AMPKα, PGC1α, NRF2; ↓TGF-β1, α-SMA, PAI-1, COL1-α1, COL3-α1, KEAP-1 | [40] |
| C57BL/6J mice | 100 mg/kg/day, orally, 28 days | ↑E-cadherin, TAC, CAT, GSH, ATP, PPARα, TFAM; ↓Collagen, α-SMA, Vimentin, MDA, ROS | [41] |
| Male Wistar rats (125–130 g) | 100 mg/kg/day, orally, 21 days | ↑GSH, TAC; ↓TGF-β1, IFN-γ, ALT, AST, MTC, α-SMA | [42] |
| C57BLKS/J mice (8 weeks) | Overexpressed LIAS gene | ↑NRF2, P65, IKK-β, I-κB; ↓TNF-α, TGF-β1, Vimentin, α-SMA, PAI-1, COL1-α1, COL3-α1 | [43] |
| Male albino rats (200–240 g) | 200 mg/kg/day, injected intraperitoneally, 6 weeks | ↑COX-2, SOD, GPX; ↓NOX4, TNF-α, IL-6, IL-1β, IL-10, α-SMA, Caspase 3, NOX-4 | [44] |
| Mice | 200 mg/kg/day, gavage, 2 weeks | ↓Inflammation, Pulmonary edema, Collagen deposition. | [45] |
| Non-small cell lung cancer | |||
| A549 cells; Nude mice (6 weeks) | 5 mM, 24 h; 50 mg/kg/day, orally, 18 days | ↑LDH, BAX/BCL-2, p62, p-p70S6K/p70S6K, p-mTOR/mTOR; ↓LC3-II, VPS34, Beclin-1, ATG13 | [46] |
| H460, H292, H23 cells | 0–5 μM, 48 h | ↓CD133, ALDH1A1, CD44, Oct-4, β-catenin, p-Akt, EMT (E-cadherin, Vimentin, Snail, Slug) | [47] |
| NCI-H1975, A549 cells | 2.0 mM, 24 h | ↓Grb2, p-EGFR, p-ERK, CDK2/4/6, Cyclin D3/E1 | [48] |
| A549, PC9 cells | 1.5 mM, 24 h or 48 h | ↑ROS, Caspase-9; ↓BCL-2, PDK1, NRF2 | [49] |
| H460 cells | 0–10 μM, 48 h | ↓Integrin β1 and β3, p-AKT, BCL-2 | [50] |
| Cells Line/Animal | ALA Treatment | Mechanism | Reference |
|---|---|---|---|
| Myocardial infarction | |||
| C57Bl/6 mice | 75 mg/kg/day, orally, 7 days | ↑LVEF; ↓LVR, MDA, NOX, MPO | [52] |
| H9C2, RAW264.7 cells; Male C57BL/6J mice (20–25 g) | 100 μM,12 h; 30 mg/kg/day, injected intraperitoneally, 7 days | ↑IL-10, TGF-β, BCL-2, p62, CD206; ↓IL-1β, IL-6, HMGB1, NF-κB. BAX, LC3 II/LC3 I | [53] |
| Male Wistar rats (400–450 g) | 100 mg/kg/day, gavage, 14 days | ↓TNF-α, IL-1β, IL-6, Caspase-3, BAX, Cyt-c | [54] |
| Wistar rats of both sexes (150–170 g) | 50 mg/kg/day gavage, 10 days | ↑SOD, CAT; ↓Incidence of mortality, CK-MB, MDA | [55] |
| Myocardial ischemia–reperfusion | |||
| Male Wistar rats (22–24 months) | 10 mg/kg/day, gavage, 14 days | ↑MFN1, MFN2, FOXO1; ↓LDH, IS, DRP1, FIS1 | [56] |
| Male Wistar rats (200–250 g) | 100 mg/kg/day, orally, 5 weeks | ↑NO, Connexin-43; ↓PFC, VF | [57] |
| Male Wistar rats (200–250 g) | 100 mg/kg/day, orally, 5 weeks | ↓Mitochondrial ROS, LC3, p62 | [58] |
| Male Wistar rats (200–250 g) | 100 mg/kg/day, orally, 5 weeks | ↑BCL-2; ↓BAX, cleaved Caspase-3 | [59] |
| H9C2 cells; Male Sprague–Dawley (SD) rats (250 g ± 10 g) | 100 Μm, 12 h; 30 mg/kg/day, injected intraperitoneally | ↑SOD, BCL-2; ↓IL-6, TNF-α, IL-1β, BAX, HMGB1, TLR4, NF-κB | [60] |
| Heart failure | |||
| Sprague–Dawley rats | 50 mg/kg/day, injected intraperitoneally, 2 weeks/month for 9 months | ↑GSH/GSSG, TAC, NOX; ↓MDA, TNF-α, IL-6 | [61] |
| NRCMs cells; Male C57BL/6 mice (20–25 g) | 10 μM,48 h; 0.2% (wt/wt), drinking, 4 weeks | ↑BCL-2, TOM20, ALDH2, FUNDC1, LC3, NRF1; ↓cleaved Caspase-3, BAX, P62 | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jiang, S.; He, Y.; Pang, P.; Shan, H. Advances in α-Lipoic Acid for Disease Prevention: Mechanisms and Therapeutic Insights. Molecules 2025, 30, 1972. https://doi.org/10.3390/molecules30091972
Wang Y, Jiang S, He Y, Pang P, Shan H. Advances in α-Lipoic Acid for Disease Prevention: Mechanisms and Therapeutic Insights. Molecules. 2025; 30(9):1972. https://doi.org/10.3390/molecules30091972
Chicago/Turabian StyleWang, Yonglian, Shuxia Jiang, Yaoxuan He, Ping Pang, and Hongli Shan. 2025. "Advances in α-Lipoic Acid for Disease Prevention: Mechanisms and Therapeutic Insights" Molecules 30, no. 9: 1972. https://doi.org/10.3390/molecules30091972
APA StyleWang, Y., Jiang, S., He, Y., Pang, P., & Shan, H. (2025). Advances in α-Lipoic Acid for Disease Prevention: Mechanisms and Therapeutic Insights. Molecules, 30(9), 1972. https://doi.org/10.3390/molecules30091972






