Effects of Different Extraction Methods on the Molecular Composition and Biological Activities of Polysaccharides from Pleione yunnanensis
Abstract
1. Introduction
2. Results and Discussion
2.1. Protein, Total Polysaccharide, and Uronic Acid Contents
2.2. Molecular Weight and Monosaccharide Composition
2.3. Infrared Spectroscopy Analysis
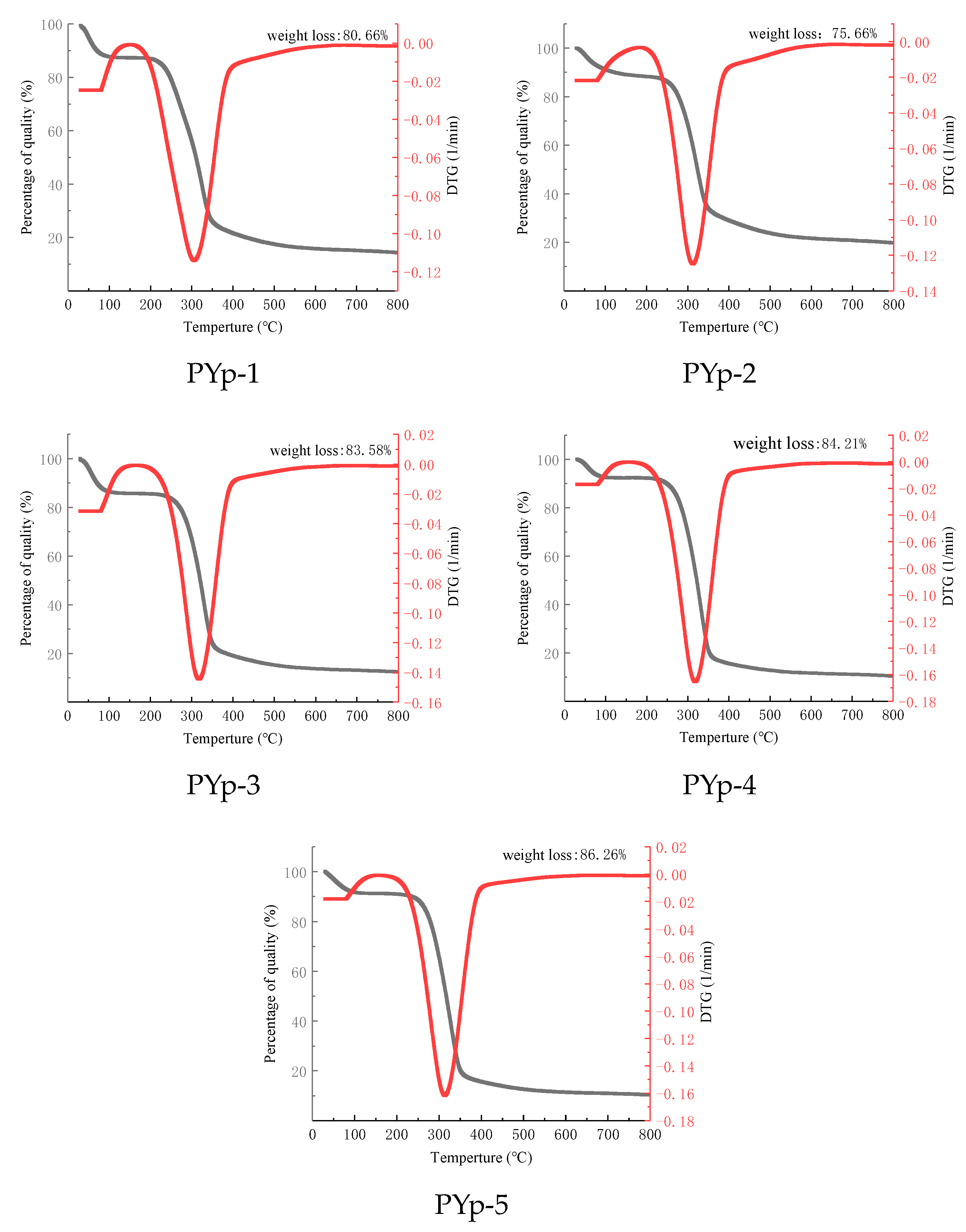
2.4. Thermogravimetric Analysis Results
2.5. Nuclear Magnetic Resonance Hydrogen Spectrum
2.6. Scanning Electron Microscope Analysis
2.7. In Vitro Antioxidant Activity Analysis
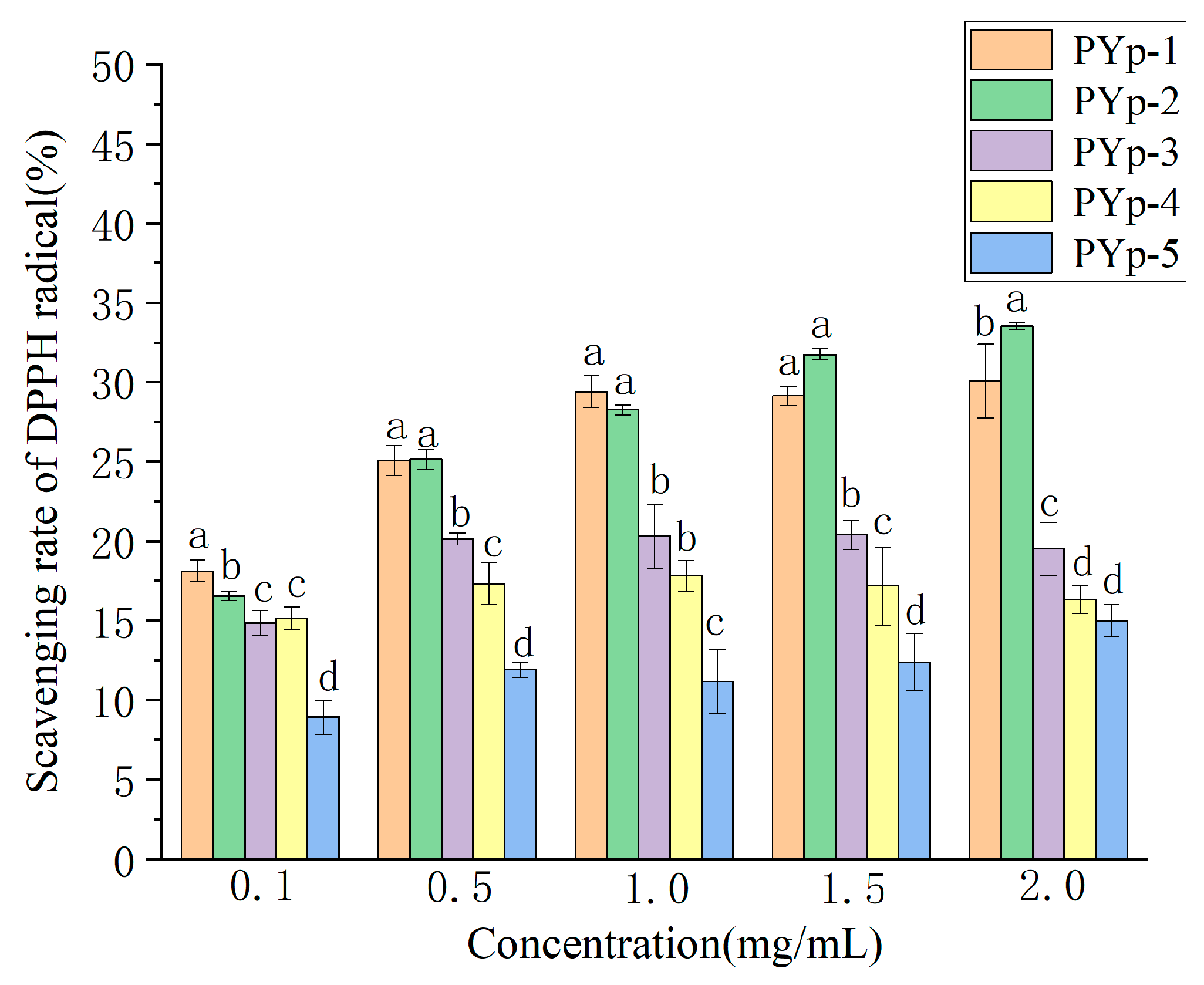
2.7.1. DPPH Radical Scavenging Activity
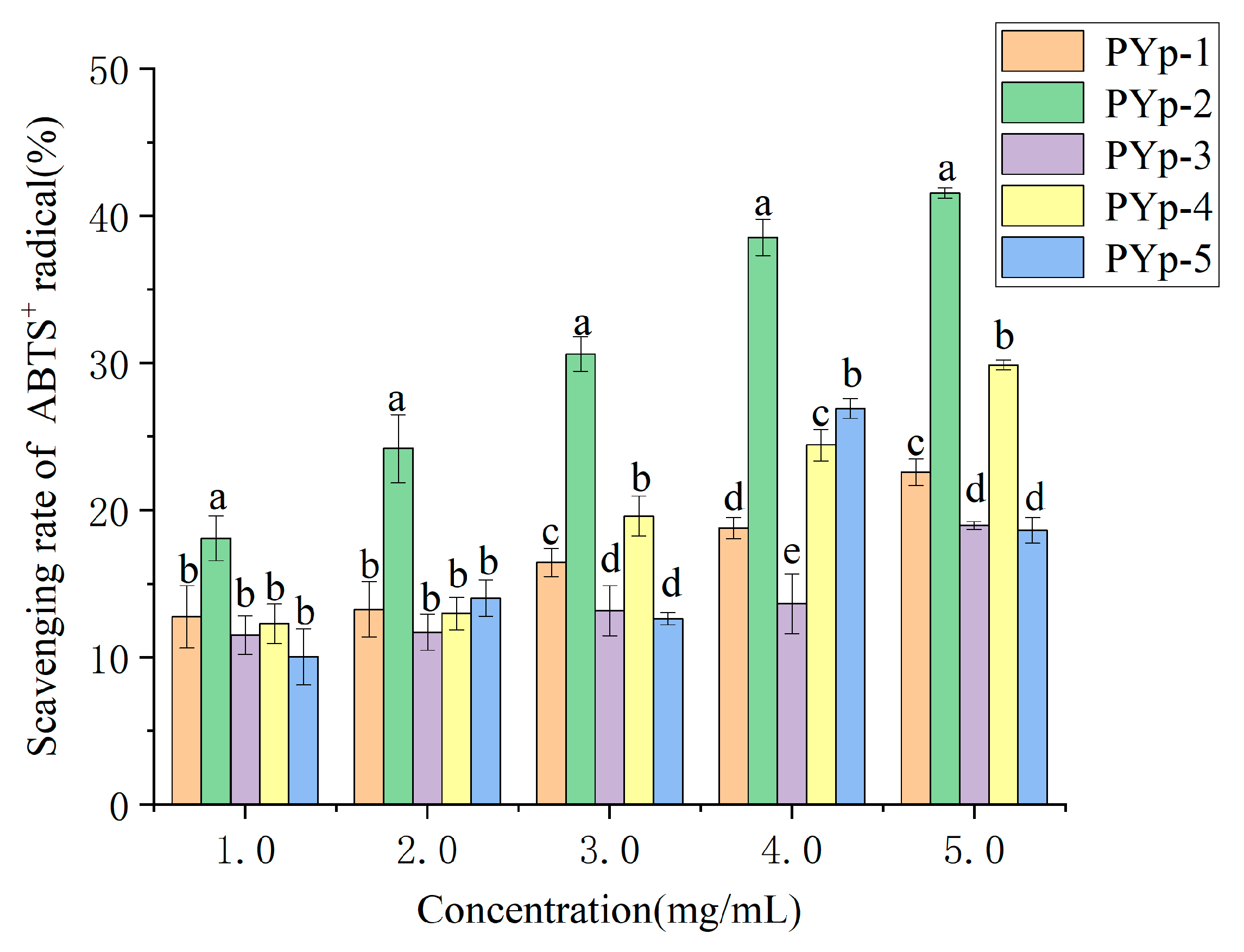
2.7.2. ABTS+ Radical Scavenging Activity
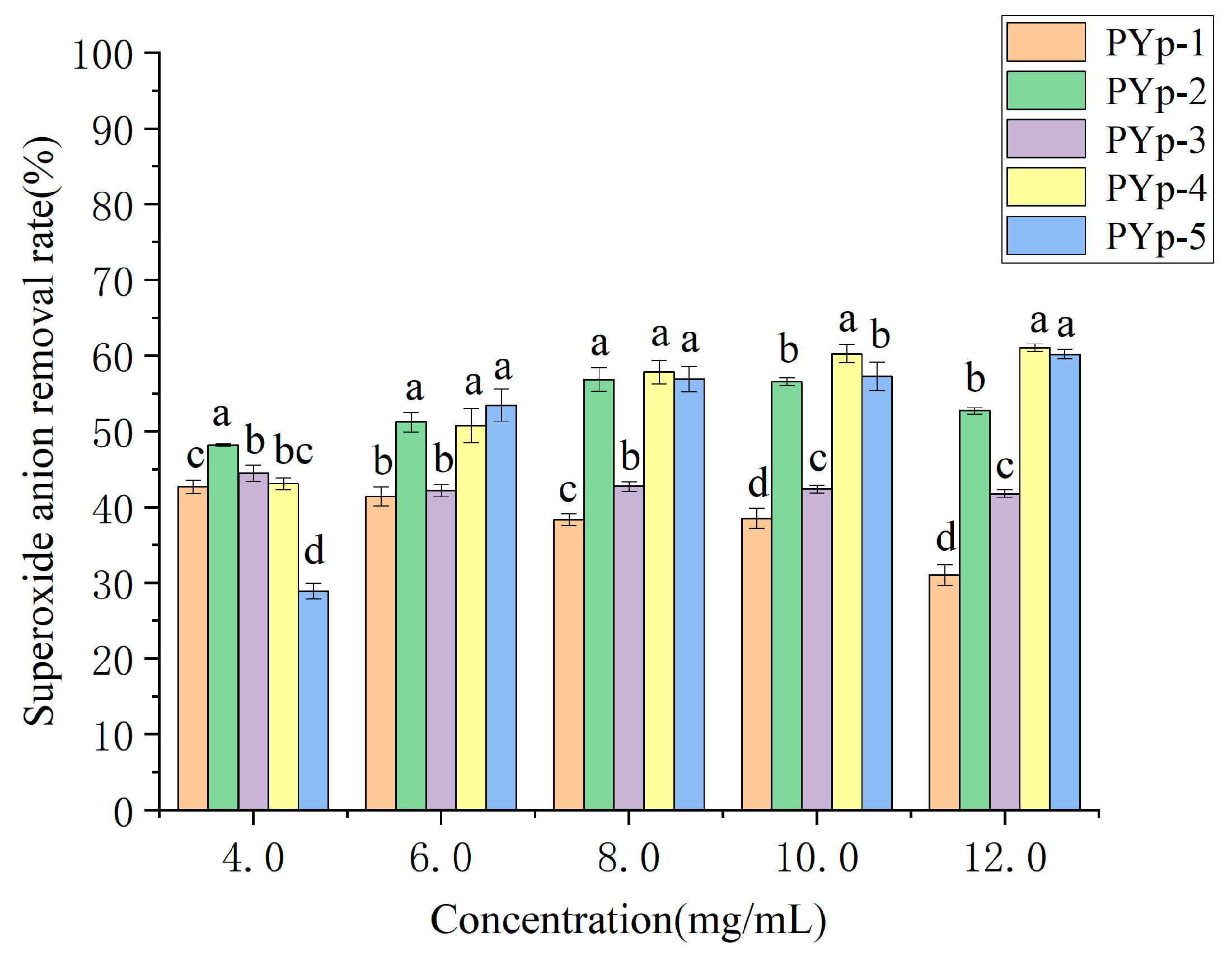
2.7.3. Superoxide Anion Radical Scavenging Ability
2.8. In Vitro Hypolipidemic Activity Results
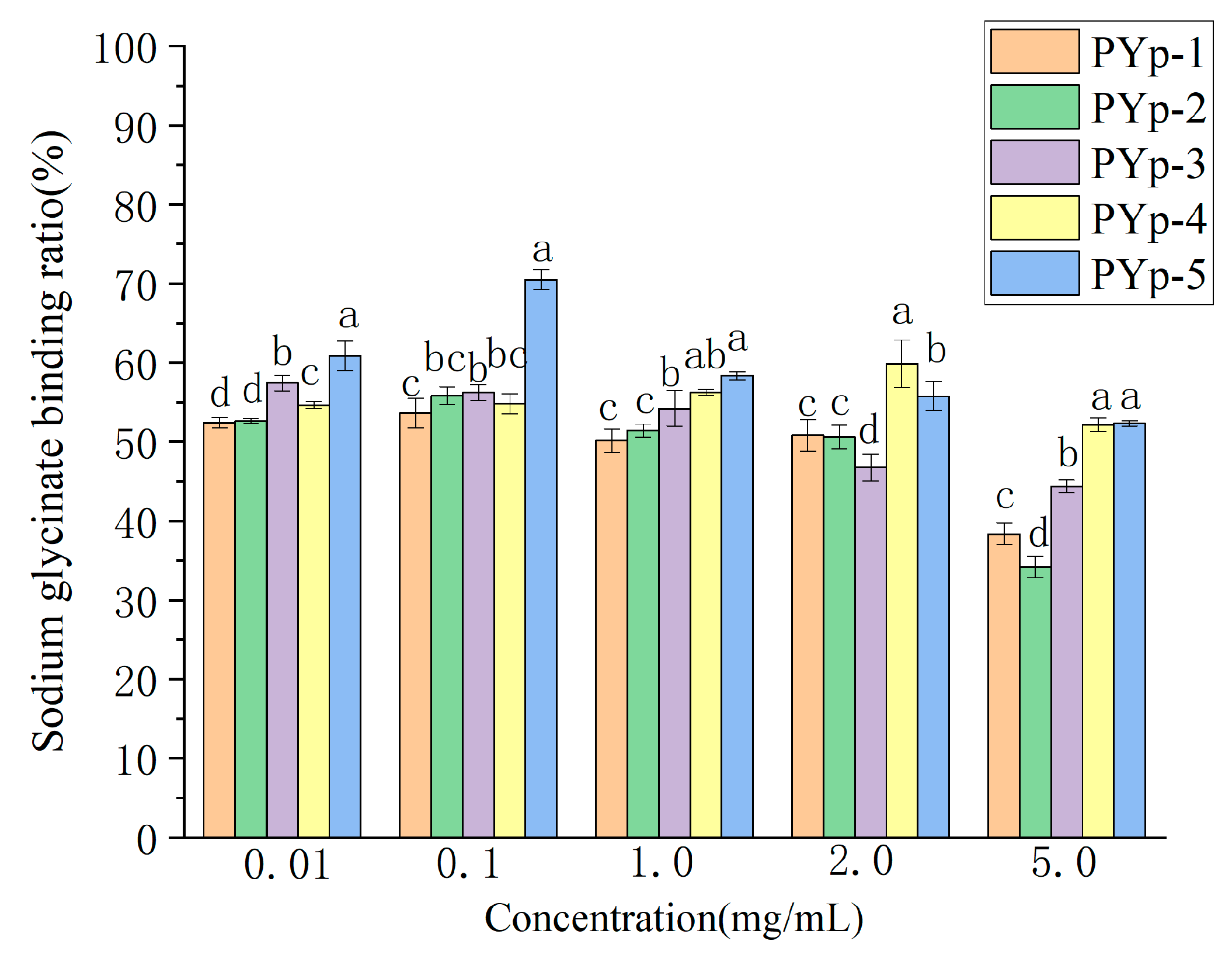
2.8.1. Sodium Glycocholate Binding Capacity Assay
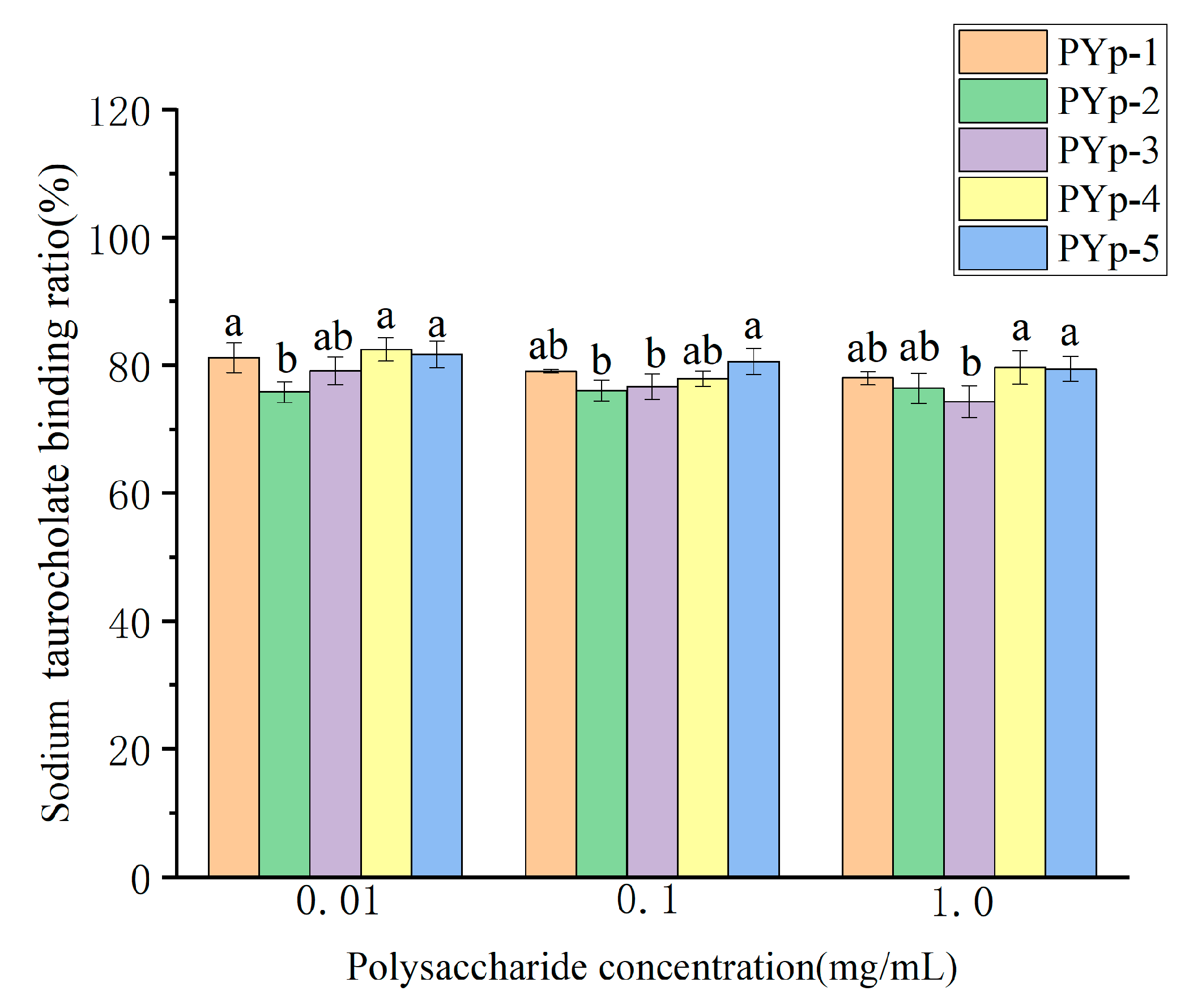
2.8.2. Sodium Taurocholate Binding Capacity Assay
3. Materials and Methods
3.1. Chemical Reagents
3.2. Extraction of P. yunnanensis Rolfe Polysaccharides
3.2.1. Preparation of Sample
3.2.2. Extraction Protocols
Microwave-Assisted Extraction
3.3. Physicochemical Characterization
3.3.1. Total Polysaccharide Content Determination
3.3.2. Uronic Acid Content Determination
3.3.3. Protein Content Determination
3.3.4. Monosaccharide Composition Analysis
3.3.5. Molecular Weight Determination
3.3.6. Fourier Transform Infrared (FTIR) Analysis
3.3.7. Nuclear Magnetic Resonance Hydrogen Spectrum Analysis
3.3.8. Scanning Electron Microscope (SEM) Analysis
3.3.9. Thermogravimetric Analysis
3.4. Assay for Antioxidant Activity
3.4.1. DPPH Radical Scavenging Ability Determination
3.4.2. ABTS+ Radical Scavenging Ability Determination
3.4.3. Superoxide Anion Radical Scavenging Ability Determination
3.5. In Vitro Hypolipidemic Ability Determination
3.5.1. Determination of Sodium Glycocholate Binding Ability
3.5.2. Determination of Sodium Taurocholate Binding Ability
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flora of China Editorial Committee. Flora of China; Science Press: Beijing, China, 1999. [Google Scholar]
- Wang, X.; Cui, B.; Wang, C.; Li, S. Chemical constituents from Pleione yunnanensis. China J. Chin. Mater. Medica 2014, 39, 851–856. [Google Scholar]
- Han, S.; Wang, X.; Cui, B.; Sun, H.; Chen, H.; Ferreira, D.; Li, S.; Hamann, M.T. Hepatoprotective Glucosyloxybenzyl 2-Hydroxy-2-isobutylsuccinates from Pleione yunnanensis. J. Nat. Prod. 2021, 84, 738–749. [Google Scholar] [CrossRef]
- Dong, H.; Wang, C.; Guo, S.; Yang, J. New Bibenzyl Derivatives from the Tubers of Pleione yunnanensis. Chem. Pharm. Bull. 2009, 57, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Huo, Y.; Zhang, Y. Latest Advancements Research on Pharmacological Activity of Polysaccharides in Chinese Medicine. Chem. Reag. 2024, 47, 1–8. [Google Scholar]
- Sun, Y.; Ren, Z.; Su, J.; Wang, C.; Dong, S. Advances in Extraction and Biological Functions of Plant Polysaccharides. China Fruit Veg. 2025, 45, 31–37. [Google Scholar]
- Wang, J. Pharmacological Studies of Pleione yunnanensis. Master’s Thesis, Yunnan College of Traditional Chinese Medicine, Kunming, China, 2012. [Google Scholar]
- Xu, X.; Zhou, Z.; Mao, Y.; Fang, L.; He, J. Study the Effect of Pleione yunnanensis Rolfe Polysaccharides on IL-2 and p53 Protein Expression. Food Res. Dev. 2016, 37, 6–10. [Google Scholar]
- Jiang, S.; Xu, J.; Su, X.; Liu, Z.; Song, W. Effects of Pseudobulbus cremastrae seu pleiones Polysaccharide on the Regulation of Immune Function and the Inhibition of Tumor Growth in Sarcoma S180 Tumor-Bearing Mice. Food Sci. 2018, 39, 216–221. [Google Scholar]
- Huang, Y.; Xie, W.; Tang, T.; Chen, H.; Zhou, X. Structural characteristics, antioxidant and hypoglycemic activities of polysaccharides from Mori Fructus based on different extraction methods. Front. Nutr. 2023, 10, 1125831. [Google Scholar] [CrossRef]
- Han, X.; Liu, H.; Zhang, Y.; Song, Z.; Yang, L.; Liu, X.; Yang, L.; Wu, M.; Tan, L. The effects of different extraction methods on the structure and antioxidant properties of Bletilla striata polysaccharide. Prep. Biochem. Biotechnol. 2025, 55, 392–402. [Google Scholar] [CrossRef]
- Zou, X.; Xiao, J.; Chi, J.; Zhang, M.; Zhang, R.; Jia, X.; Mei, D.; Dong, L.; Yi, Y.; Huang, F. Physicochemical properties and prebiotic activities of polysaccharides from Zizyphus jujube based on different extraction techniques. Int. J. Biol. Macromol. 2022, 223 Pt A, 663–672. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, W.; Wu, K.; Meng, P.; Cai, J.; Lin, C. Effects of the Physicochemical Properties and Biological Activities of Polygonatum sibiricum Polysaccharides Extracted by Different Extraction Methods. Mod. Food Sci. Technol. 2024, 40, 142–152. [Google Scholar]
- Wang, M.; Zhang, C.; Xu, Y.; Ma, M.; Yao, T.; Sui, Z. Impact of Six Extraction Methods on Molecular Composition and Antioxidant Activity of Polysaccharides from Young Hulless Barley Leaves. Foods 2023, 12, 3381. [Google Scholar] [CrossRef]
- Zhang, M.; He, C.; Wang, H.; Yu, Z.; Si, C.; Zhao, C.; Duan, J. Advances in Active Polysaccharides in Medicinal Plants of Orchidaceae. J. Trop. Subtrop. Bot. 2019, 27, 611–622. [Google Scholar]
- He, L.; Yan, X.; Liang, J.; Li, S.; He, H.; Xiong, Q.; Lai, X.; Hou, S.; Huang, S. Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohydr. Polym. 2018, 198, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Tan, X. Study on Fractional Purification with Low Concentration Alcohol, Molecular Modification of Mori Fructus Polysaccharides and Its Anti-Acute Alcoholic Liver Injury Activity. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2019. [Google Scholar]
- Zai, Q.; Chen, H.; Xie, W.; Zhou, X. Effects of different extraction methods on chemical compositions and biological activities of polysaccharides from Lycium barbarum. China J. Chin. Mater. Medica 2023, 48, 60–70. [Google Scholar]
- Gao, T.; Tang, H.; Luo, Z.; Luo, H.; Li, M. Primary Structure Analysis and Antioxidant Activity in Vitro of Crude Polysaccharide from Chuanminshen violaceum. J. Chin. Inst. Food Sci. Technol. 2021, 21, 275–282. [Google Scholar]
- Zhu, Y. Extraction, Purification, Physicochemical Properties, and Hypoglycemic Activity Evaluation of Polysaccharides from Gynura divaricata (L.) DC. Ph.D. Thesis, Zhejiang Gongshang University, Hangzhou, China, 2023. [Google Scholar]
- Liu, R.; Wang, L.; Luan, S.; Cheng, P.; Wang, Z. In vitro binding of bile salts by water—soluble AAP. Sci. Technol. Food Ind. 2015, 36, 358–361. [Google Scholar]
- Zheng, C.; Dong, Q.; Chen, H.; Cong, Q.; Ding, K. Structural characterization of a polysaccharide from Chrysanthemum morifolium flowers and its antioxidant activity. Carbohydr. Polym. 2015, 130, 113–121. [Google Scholar] [CrossRef]
- Wang, Q.; Shu, Z.; Xu, B.; Xing, N.; Jiao, W.; Yang, B.; Kuang, H. Structural characterization and antioxidant activities of polysaccharides from Citrus aurantium L. International Journal of Biological. Int. J. Biol. Macromol. 2014, 67, 112–123. [Google Scholar] [CrossRef]
- Zhao, X.; MOU, H.; Zhang, J.; Zhang, W. Optimization of the Extraction Process and in Vitro Antioxidant and HypolipidemicActivities of Polysaccharides from Sophora flavescens. Sci. Technol. Food Ind. 2024, 45, 212–220. [Google Scholar]
- Wang, L.; Zhang, B.; Xiao, J.; Qiang, H.; Chao, L.; Xiong, F. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.; Suganuma, T.; Kitahara, K.; Tanoue, H.; Ichiki, M. Monosaccharide composition of sweetpotato fiber and cell wall polysaccharides from sweetpotato, cassava, and potato analyzed by the high-performance anion exchange chromatography with pulsed amperometric detection method. J. Agric. Food Chem. 2000, 48, 3448–3454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Huang, R.; Wen, P.; Song, Y.; He, B.; Tan, J.; Hao, H.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohydr. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Lin, L.; Zhao, M. Lotus leaf polysaccharides prepared by alkaline water, deep eutectic solvent and high pressure homogenization-assisted dual enzyme extraction: A comparative study of structural features, prebiotic activities and functionalities. Food Hydrocoll. 2023, 143, 108870. [Google Scholar]
- Lin, W. Studies on the Physicochemical Properties, Biological Activity and Molecular Mechanism of Biosynthesis of Polysaccharide from Cyclocarya paliurus Leaves at Different Developmental Stages. Master’s Thesis, Zhejiang SCI-Tech University, Hangzhou, China, 2020. [Google Scholar]
- Zhang, H.; Li, C.; Liu, H.; Ma, X.; Zhang, X.; Wang, B.; Liu, Y. Extraction and Purification of Cinnamomum cassia Polysaccharides and Its Antioxidant and Hypoglycemic Activities in Vitro. Sci. Technol. Food Ind. 2024, 45, 15–24. [Google Scholar]





| Standard | Proportion/% | ||||
|---|---|---|---|---|---|
| Product | PYp-1 | PYp-2 | PYp-3 | PYp-4 | PYp-5 |
| Fuc | - | - | - | - | - |
| Ara | - | - | - | - | - |
| Rha | - | - | - | - | - |
| Gal | - | - | - | - | - |
| Glc | 45.81 ± 0.025 | 50.37 ± 0.040 | 44.89 ± 0.080 | 44.61 ± 0.012 | 43.85 ± 0.015 |
| Xyl | - | - | - | - | - |
| Man | 54.19 ± 0.010 | 49.63 ± 0.006 | 55.11 ± 0.015 | 55.39 ± 0.025 | 56.15 ± 0.010 |
| Fru | - | - | - | - | - |
| Rib | - | - | - | - | - |
| Gal-UA | - | - | - | - | - |
| Gul-UA | - | - | - | - | - |
| Glc-UA | - | - | - | - | - |
| Man-UA | - | - | - | - | - |
| Time (min) | Phase A (H2O) % | B-Phase (0.1 M NaOH) % | (C Phase 0.1 M NaOH, 0.2 M NaAc) % |
|---|---|---|---|
| 0 | 95 | 5 | 0 |
| 26 | 85 | 5 | 10 |
| 42 | 85 | 5 | 10 |
| 42.1 | 60 | 0 | 40 |
| 52 | 60 | 40 | 0 |
| 52.1 | 95 | 5 | 0 |
| 60 | 95 | 5 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Zheng, Z.; Han, X.; Chen, Y.; Liu, H.; Yang, L.; Wu, M. Effects of Different Extraction Methods on the Molecular Composition and Biological Activities of Polysaccharides from Pleione yunnanensis. Molecules 2025, 30, 1942. https://doi.org/10.3390/molecules30091942
Song Z, Zheng Z, Han X, Chen Y, Liu H, Yang L, Wu M. Effects of Different Extraction Methods on the Molecular Composition and Biological Activities of Polysaccharides from Pleione yunnanensis. Molecules. 2025; 30(9):1942. https://doi.org/10.3390/molecules30091942
Chicago/Turabian StyleSong, Zhiqin, Zhihong Zheng, Xue Han, Yaya Chen, Hai Liu, Lin Yang, and Mingkai Wu. 2025. "Effects of Different Extraction Methods on the Molecular Composition and Biological Activities of Polysaccharides from Pleione yunnanensis" Molecules 30, no. 9: 1942. https://doi.org/10.3390/molecules30091942
APA StyleSong, Z., Zheng, Z., Han, X., Chen, Y., Liu, H., Yang, L., & Wu, M. (2025). Effects of Different Extraction Methods on the Molecular Composition and Biological Activities of Polysaccharides from Pleione yunnanensis. Molecules, 30(9), 1942. https://doi.org/10.3390/molecules30091942





