Batch and Continuous Lipase-Catalyzed Production of Dietetic Structured Lipids from Milk Thistle, Grapeseed, and Apricot Kernel Oils
Abstract
1. Introduction
2. Results and Discussion
2.1. Oil Characterization
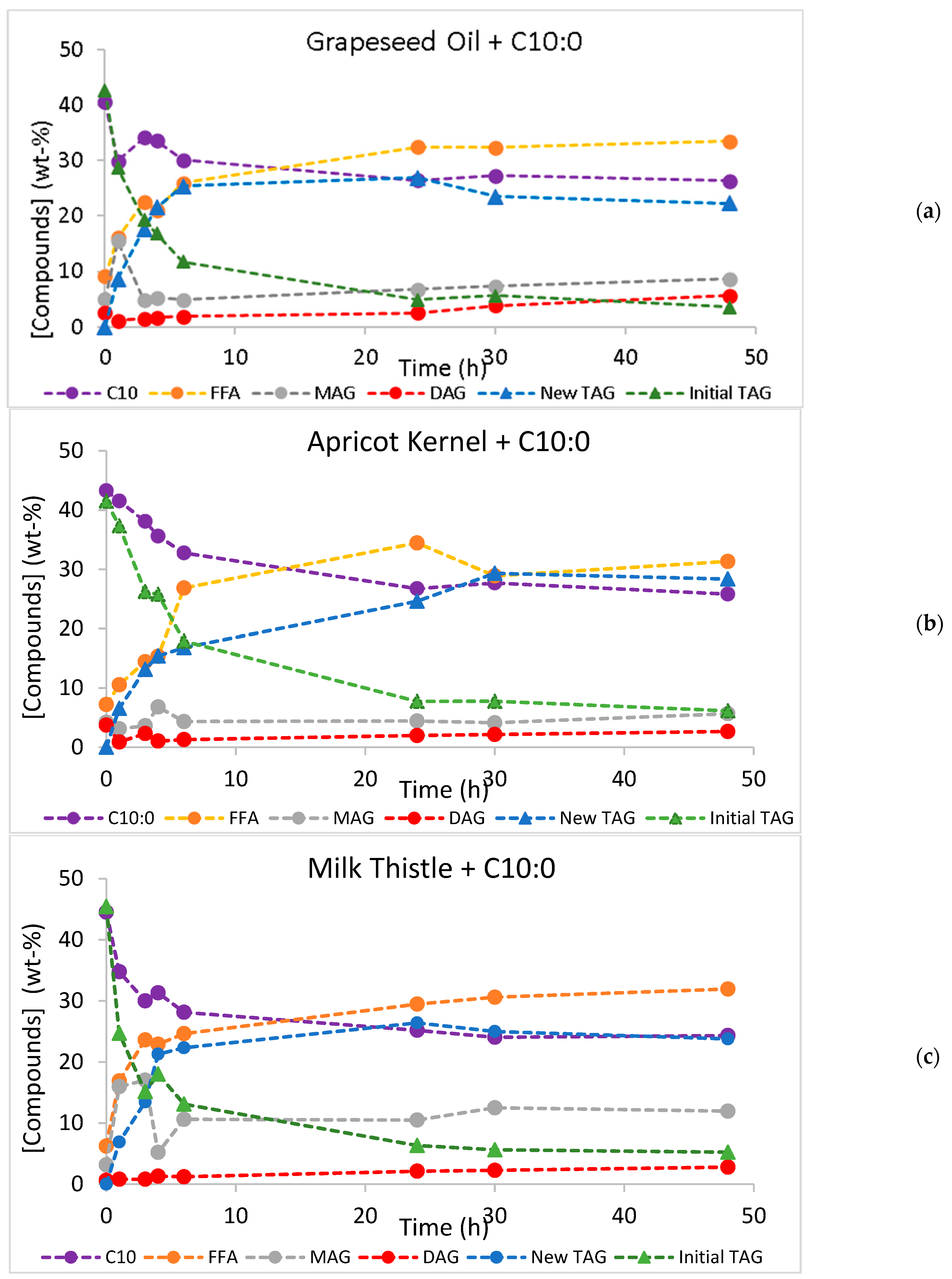
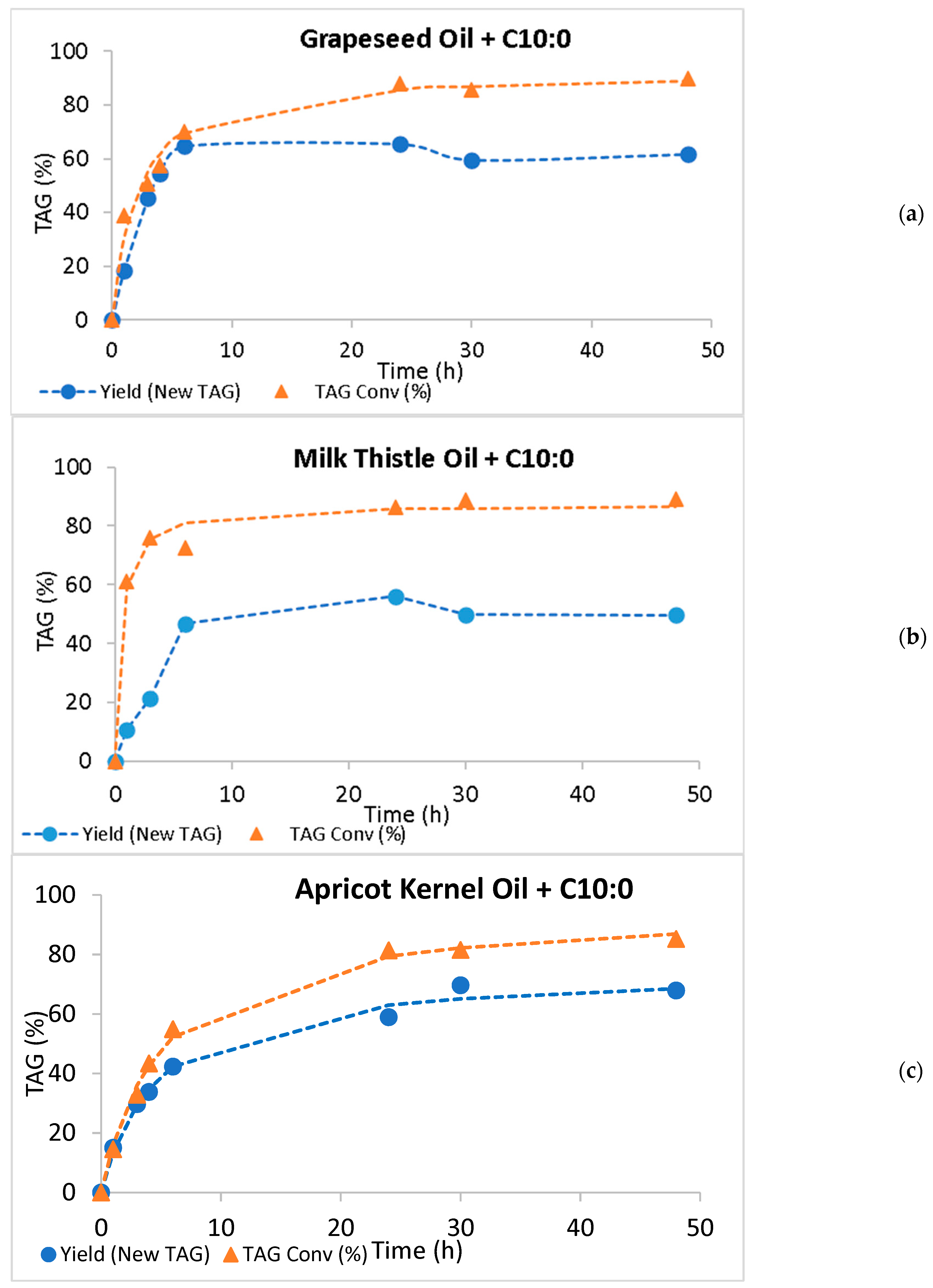
2.2. Batch Production of Low-Calorie TAGs by Acidolysis and Interesterification
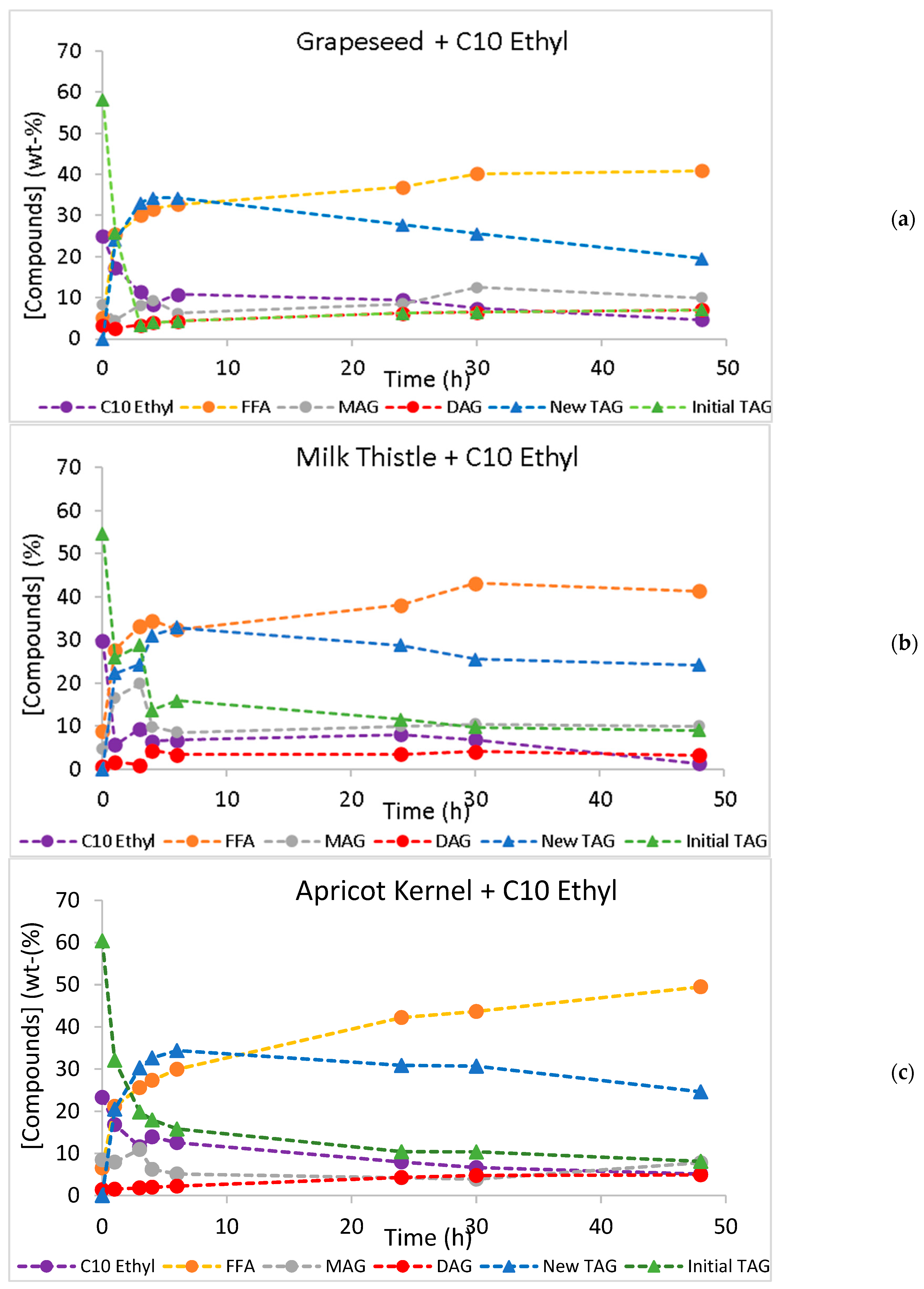
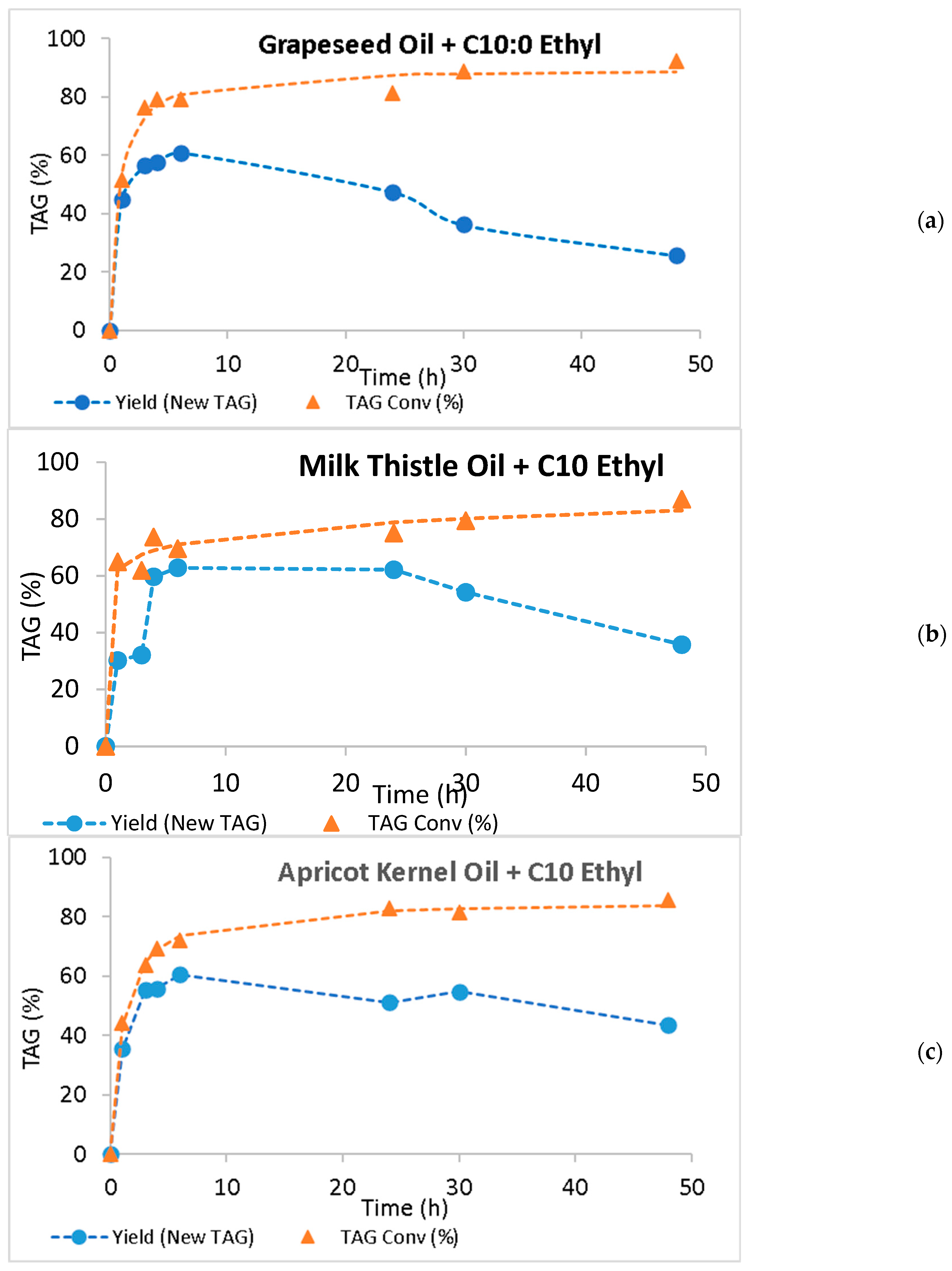
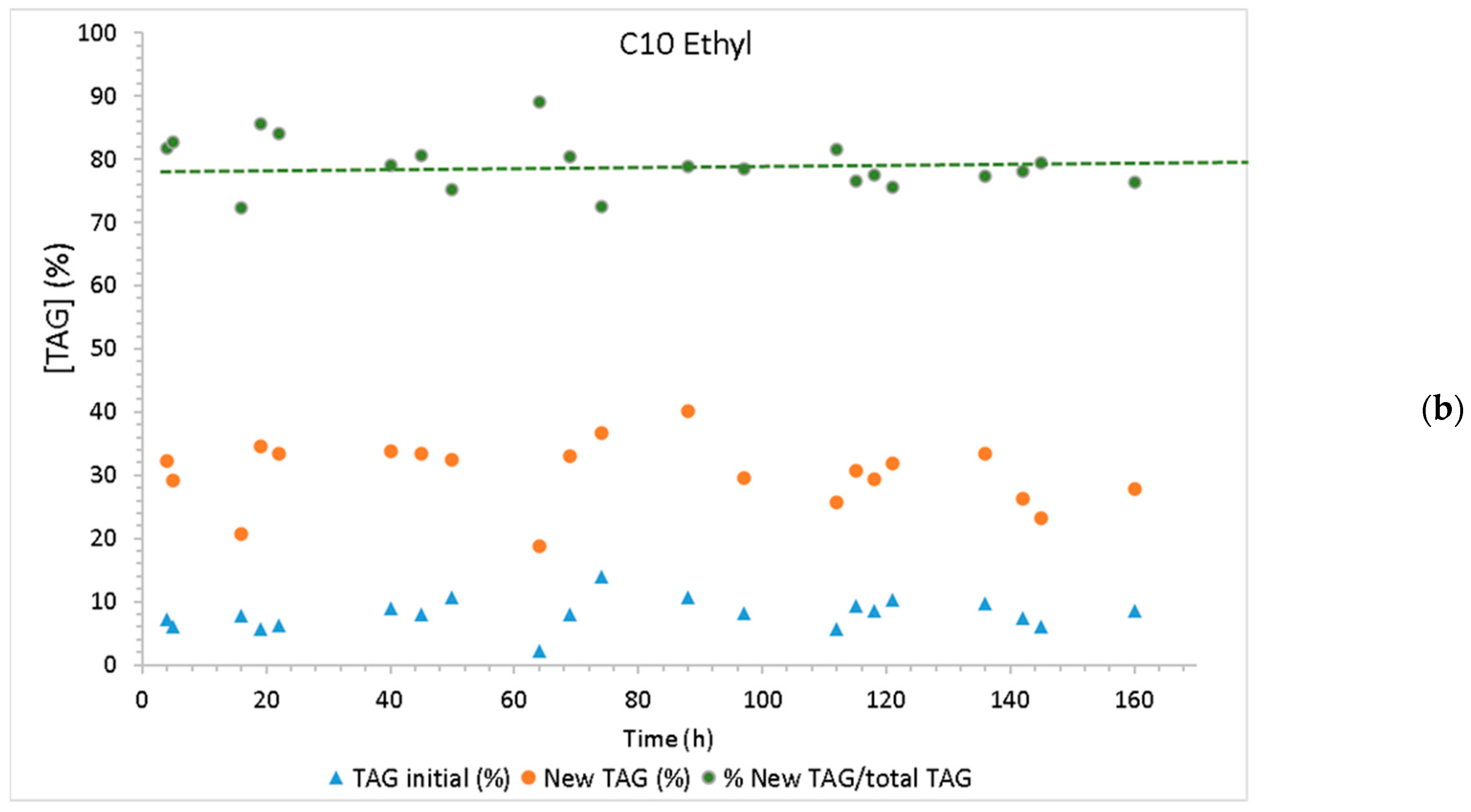
2.3. Continuous Production of Low-Calorie TAGs
3. Materials and Methods
3.1. Materials
3.2. Oil Characterization
3.3. Lipase-Catalyzed Batch Reactions
3.4. Lipase-Catalyzed Continuous Reactions
3.5. Analysis of Compounds Along Time-Course Reactions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; WHO. Standard for Named Vegetable Oils; CXS 210-1999; Codex Alimentarius Commission: Rome, Italy, 2001; Volume 8, pp. 11–25. [Google Scholar]
- FAO; WHO. Standard for Named Vegetable Oils; CXS 210-1999, Amended in 2015; Codex Alimentarius Commission: Rome, Italy, 2015; p. 13. [Google Scholar]
- FAO; WHO. Standard for Named Vegetable Oils; CXS 210-1999, Amended in 2022; Codex Alimentarius Commission: Rome, Italy, 2022; p. 16. [Google Scholar]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; Montserrat-de la Paz, S. Grape (Vitis vinifera L.) Seed Oil: A Functional Food from the Winemaking Industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef] [PubMed]
- Bokelmann, J.M. Milk Thistle (Silybum marianum): Seeds, Flower Heads. In Medicinal Herbs in Primary Care, 1st ed.; Bokelmann, J.M., Ed.; Elsevier: Philadelphia, PA, USA, 2022; pp. 495–509. ISBN 9780323846769. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wu, S.C. Health Benefits of Silybum marianum: Phytochemistry, Pharmacology, and Applications. J. Agric. Food Chem. 2020, 68, 11644–11664. [Google Scholar] [CrossRef]
- Pawar, K.R.; Nema, P.K. Apricot kernel characterization, oil extraction, and its utilization: A review. Food Sci. Biotechnol. 2023, 32, 249–263. [Google Scholar] [CrossRef]
- Vaknin, Y.; Hadas, R.; Schafferman, D.; Murkhovsky, L.; Bashan, N. The potential of milk thistle (Silybum marianum L.), an Israeli native, as a source of edible sprouts rich in antioxidants. Int. J. Food Sci. Nutr. 2008, 59, 339–346. [Google Scholar] [CrossRef]
- Ayduğan, A.; Ok, S.; Yılmaz, E. Cold-pressed milk thistle seed oil: Physico-chemical properties, Composition and Sensory Analysis. Grasas y Aceites 2022, 73, e481. [Google Scholar] [CrossRef]
- Hadolin, M.; Skerget, M.; Knez, Z.; Bauman, D. High pressure extraction of vitamin E-rich oil from Silybum marianum. Food Chem. 2001, 74, 355–364. [Google Scholar] [CrossRef]
- El-Mallah, M.H.; El-Shami, S.M.; Hassanein, M.M. Detailed studies on some lipids of Silybum marianum (L.) seed oil. Grasas y Aceites 2003, 54, 397–402. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Wang, S.; Liu, H.; Li, B.Z.; Che, L. Constituents and thermal properties of milk thistle seed oils extracted with three methods. LWT-Food Sci. Technol. 2020, 126, 109282. [Google Scholar] [CrossRef]
- Willis, M.W.; Lencki, R.W.; Marangoni, A.G. Lipid Modification Strategies in the Production of Nutritionally Functional Fats and Oils. Crit. Rev. Food Sci. Nutr. 1998, 38, 639–674. [Google Scholar] [CrossRef]
- Gunstone, F.D. Enzymes as biocatalysts in the modification of natural lipids. J. Sci. Food Agric. 1999, 79, 1535–1549. [Google Scholar] [CrossRef]
- Osborn, H.T.; Akoh, C.C. Structured Lipids—Novel fats with medical, nutraceutical, and food applications. Compr. Rev. Food Sci. Food Saf. 2002, 1, 93–120. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Pokorny, J. The development and application of novel vegetable oils tailor-made for specific human dietary needs. Eur. J. Lipid Sci. Technol. 2003, 105, 769–778. [Google Scholar] [CrossRef]
- Jala, R.; Hu, P.; Yang, T.; Jiang, Y.; Zheng, Y.; XU, X. Lipases as Biocatalysts for the Synthesis of Structured Lipids. In Lipases and Phospholipases; Sandoval, G., Ed.; Humana Press: New York, NY, USA, 2012; Volume 861, pp. 403–433. [Google Scholar] [CrossRef]
- Kim, B.H.; Akoh, C.C. Recent research trends on the enzymatic synthesis of structured lipids. J. Food Sci. 2015, 80, C1713–C1724. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Dias, S.; Osório, N.M.; Tecelão, C. Bioprocess Technologies for Production of Structured Lipids as Nutraceuticals. In Current Developments in Biotechnology and Bioengineering. Technologies for Production of Nutraceuticals and Functional Food Products; Rai, A., Singh, S.P., Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; book 21; pp. 209–237. ISBN 978-0-12-823506-5. [Google Scholar] [CrossRef]
- Akoh, C.C. Food Lipids Chemistry, Nutrition, and Biotechnology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 1047. ISBN 9781498744874. [Google Scholar] [CrossRef]
- Xu, X.; Balchen, S.; Høy, C.-E.; Alder-Nissen, J. Production of Specific-Structured Lipids by Enzymatic Interesterification in a Pilot Continuous Enzyme Bed Reactor. J. Am. Oil Chem. Soc. 1998, 75, 1573–1579. [Google Scholar] [CrossRef]
- Xu, X.; Porsgaard, T.; Zang, H.; Adler-Nissen, J.; Høy, C.-E. Production of structured lipids in a packed-bed reactor with Thermomyces lanuginosa lipase. J. Am. Oil Chem. Soc. 2002, 79, 561–565. [Google Scholar] [CrossRef]
- Nunes, P.A.; Pires-Cabral, P.; Ferreira-Dias, S. Production of olive oil enriched with medium chain fatty acids catalysed by commercial immobilised lipases. Food Chem. 2011, 127, 993–998. [Google Scholar] [CrossRef]
- Caballero, E.; Soto, C.; Olivares, A.; Altamirano, C. Potential use of avocado oil on structured lipids MLM-type production catalysed by commercial immobilised lipases. PLoS ONE 2014, 9, e107749. [Google Scholar] [CrossRef]
- Costa, C.M.; Osório, N.M.; Canet, A.; Rivera, I.; Sandoval, G.; Valero, F.; Ferreira-Dias, S. Production of MLM type structured lipids from grapeseed oil catalyzed by non-commercial lipases. Eur. J. Lipid Sci. Technol. 2018, 120, 1700320. [Google Scholar] [CrossRef]
- Abed, S.M.; Wei, W.; Ali, A.H.; Korma, S.A.; Mousa, A.H.; Hassan, H.M.; Jin, Q.; Wang, X. Synthesis of structured lipids enriched with medium-chain fatty acids via solvent-free acidolysis of microbial oil catalyzed by Rhizomucor miehei lipase. LWT—Food Sci. Technol. 2018, 93, 306–315. [Google Scholar] [CrossRef]
- Bassan, N.; Rodrigues, R.H.; Monti, R.; Tecelão, C.; Ferreira-Dias, S.; Paula, A.V. Enzymatic modification of grapeseed (Vitis vinifera L.) oil aiming to obtain dietary triacylglycerols in a batch reactor. LWT—Food Sci. Technol. 2019, 99, 600–606. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Lee, W.J.; Lai, O.M.; Tan, C.P.; Wang, Y. Production of Structured triacylglycerol via Enzymatic Interesterification of Medium-Chain Triacylglycerol and Soybean Oil Using a Pilot-Scale Solvent-Free Packed Bed Reactor. J. Am. Oil Chem. Soc. 2020, 97, 271–280. [Google Scholar] [CrossRef]
- Mota, D.A.; Rajan, D.; Heinzl, G.C.; Osório, N.M.; Gominho, J.; Krause, L.C.; Soares, C.M.F.; Nampoothiri, K.M.; Sukumaran, R.K.; Ferreira-Dias, S. Production of low-calorie structured lipids from spent coffee grounds or olive pomace crude oils catalyzed by immobilized lipase in magnetic nanoparticles. Bioresour. Technol. 2020, 307, 123–223. [Google Scholar] [CrossRef] [PubMed]
- Mota, D.A.; Santos, J.C.B.; Faria, D.; Lima, A.S.; Krause, L.C.; Soares, C.M.F.; Ferreira-Dias, S. Synthesis of dietetic structured lipids from spent coffee grounds crude oil catalyzed by commercial immobilized lipases and immobilized Rhizopus oryzae lipase on biochar and hybrid support. Processes 2020, 8, 1542. [Google Scholar] [CrossRef]
- Martínez-Galán, P.J.; Ontibón-Echeverri, C.M.; Costa, M.C.; Batista-Duharte, A.; Batista, V.G.; Mesa, V.; Monti, R.; De Paula, A.V.; Baviera, A.M. Enzymatic synthesis of capric acid-rich structured lipids and their effects on mice with high-fat diet-induced obesity. Food Res. Int. 2021, 148, 110602. [Google Scholar] [CrossRef] [PubMed]
- Simões, T.; Ferreira, J.; Lemos, M.F.L.; Augusto, A.; Félix, R.; Silva, S.F.J.; Ferreira-Dias, S.; Tecelão, C. Argan oil as a rich source of linoleic fatty acid for dietetic structured lipids production. Life 2021, 11, 1114. [Google Scholar] [CrossRef]
- Cozentino, I.D.S.C.; Rodrigues, M.D.F.; Mazziero, V.T.; Cerri, M.O.; Cavallini, D.C.U.; de Paula, A.V. Enzymatic synthesis of structured lipids from grape seed (Vitis vinifera L.) oil in associated packed bed reactors. Biotechnol. Appl. Biochem. 2022, 69, 101–109. [Google Scholar] [CrossRef]
- Heinzl, G.C.; Mota, D.A.; Martinis, V.; Martins, A.S.; Soares, C.M.F.; Osório, N.; Gominho, J.; Nampoothiri, K.M.; Sukumaran, R.K.; Pereira, H.; et al. Integrated bioprocess for structured lipids, emulsifiers and biodiesel production using crude acidic olive pomace oils. Bioresour. Technol. 2022, 346, 126646. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Gogate, P.; Annapure, U. Process intensification of acidolysis reaction catalysed by enzymes for synthesis of designer lipids using sonication. Chem. Eng. J. 2022, 428, 131374. [Google Scholar] [CrossRef]
- Kadhum, A.A.H.; Shamma, M.N. Edible lipids modification processes: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 48–58. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Feng, F.; Wei, W.; Zhang, H. Synthesis of Medium and Long-Chain Triacylglycerols by Enzymatic Acidolysis of Algal Oil and Lauric Acid. LWT—Food Sci. Technol. 2021, 136, 110309. [Google Scholar] [CrossRef]
- Pacheco, B.J.S.; Domingues, O.; Reina, M.P.; de Neto, A.B.; Andrade, G.S.S.; de Paula, A.V. Improved Synthesis of Dietary Triglycerides by Using Lipase Supported on Clay Carriers. Biotechnol. J. 2022, 17, e2100491. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.M. Reactor Engineering. In Bioprocess Engineering Principles; Doran, P.M., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; pp. 333–391. ISBN 978-0-12-220855-3. [Google Scholar] [CrossRef]
- Souza-Gonçalves, J.; Fialho, A.; Soares, C.M.F.; Osório, N.M.; Ferreira-Dias, S. Continuous Production of Dietetic Structured Lipids using Crude Acidic Olive Pomace Oils. Molecules 2023, 28, 2637. [Google Scholar] [CrossRef]
- Remonatto, D.; Santaella, N.; Lerin, L.A.; Bassan, J.C.; Cerri, M.O.; de Paula, A.V. Solvent-Free Enzymatic Synthesis of Dietary Triacylglycerols from Cottonseed Oil in a Fluidized Bed Reactor. Molecules 2023, 28, 5384. [Google Scholar] [CrossRef] [PubMed]
- Fomuso, B.L.; Akoh, C.C. Lipase catalyzed acidolysis of olive oil and caprylic acid in a bench-scale packed bed bioreactor. Food Res. Int. 2002, 35, 15–21. [Google Scholar] [CrossRef]
- Kim, B.H.; Akoh, C.C. Modelling of lipase catalyzed acidolysis of sesame oil and caprylic acid by response surface methodology: Optimization of reaction conditions by considering both acyl incorporation and migration. J. Agric. Food Chem. 2005, 53, 8033–8037. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Akoh, C.C. Solvent-free enzymatic synthesis of structured lipids from peanut oil and caprylic acid in a stirred tank batch reactor. J. Am. Oil Chem. Soc. 1998, 75, 1533–1537. [Google Scholar] [CrossRef]
- Jennings, B.H.; Akoh, C.C. Lipase catalyzed modification of rice bran oil to incorporate capric acid. J. Agric. Food Chem. 2000, 48, 4439–4443. [Google Scholar] [CrossRef]
- Sousa, V.; Campos, V.; Nunes, P.; Pires-Cabral, P. Incorporation of capric acid in pumpkin seed oil by sn-1,3 regioselective lipase-catalyzed acidolysis. OCL 2018, 25, A302. [Google Scholar] [CrossRef]
- Atsakou, A.E.; Remonatto, D.; Júnior, R.H.M.; Paz-Cedeno, F.R.; Masarin, F.; Andrade, G.S.S.; Gattas, E.A.L.; de Paula, A.V. Synthesis of dietary lipids from pumpkin (Cucurbita pepo. L) oil obtained by enzymatic extraction: A sustainable approach. 3 Biotech 2023, 13, 358. [Google Scholar] [CrossRef]
- Van Nguyen, L.; Shahidi, F. Structured lipids from virgin coconut oil and omega-3 fatty acids: Process optimization. J. Am. Oil Chem. Soc. 2025. [Google Scholar] [CrossRef]
- Liu, X.; Akoh, C.C. Enzymatic Synthesis and Characterization of MLM-type Structured Lipid Using Grapeseed Oil and Capric Acid. J. Oleo Sci. 2024, 73, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Caicedo-Paz, A.V.; Mussagy, C.U.; Mesa, V.; Junior, R.H.M.; Valenzuela, R.; de Paula, A.V.; Martinez-Galan, J.P. Enzymatic synthesis of structured lipids from sacha inchi (Plukenetia volubilis) oil with capric acid via acidolysis reaction in stirred tank and packed bed mini reactors. Food Biosci. 2024, 58, 103769. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Guo, Z.; Chen, B. Synthesis of novel medium-long-medium type structured lipids from microalgae oil via two-step enzymatic reactions. Process Biochem. 2018, 68, 108–116. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Cui, R.; Zhang, C.; Xu, M.; Liu, W.; Wang, M.; Liu, R.; Xu, L.; Song, L. Effect of screw pressing temperature on apricot (Prunus armeniaca L.) kernels oil quality properties and apricot kernels protein isolate functional properties. LWT—Food Sci. Technol. 2024, 213, 117002. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, P.C.; Tilakratne, B.M.K.S.; Verma, A.K. Studies on physico-chemical characteristics and fatty acid composition of wild apricot (Prunus armeniaca Linn.) kernel oil. Indian. J. Nat. Prod. Resour. 2012, 3, 366–370. Available online: https://www.researchgate.net/publication/289239944_Studies_on_physico-chemical_characteristics_and_fatty_acid_composition_of_wild_apricot_Prunus_armeniaca_Linn_kernel_oil (accessed on 23 April 2025).
- De Marchi, F.; Seraglia, R.; Molin, L.; Traldi, P.; De Rosso, M.; Panighel, A.; Vedova, A.D.; Gardiman, M.; Giust, M.; Flamini, R. Seed oil triglyceride profiling of thirty-two hybrid grape varieties. J. Mass Spectrom. 2012, 47, 1113–1119. [Google Scholar] [CrossRef]
- Xu, X. Engineering of enzymatic reactions and reactors for lipid modification and synthesis. Eur. J. Lipid Sci. Technol. 2003, 105, 289–304. [Google Scholar] [CrossRef]
- Heisler, A.; Rabiller, C.; Hublin, L. Lipase catalysed isomerisation of 1,2-(2,3)-diglyceride into 1,3-diglyceride. The crucial role of water. Biotechnol. Lett. 1991, 13, 327–332. [Google Scholar] [CrossRef]
- Method COI/T.20/Doc. No 34/Rev.1; Determination of Free Fatty Acids, Cold Method. Internacional Olive Council: Madrid, Spain, 2017; p. 4.
- Method COI/T.20/Doc. No 35/Rev.1; Determination of Peroxide Value. Internacional Olive Council: Madrid, Spain, 2017; p. 5.
- Method COI/T.20/Doc. No 33/Rev.1; Determination of Fatty Acid Methyl Esters by Gas Chromatography. Internacional Olive Council: Madrid, Spain, 2017; p. 18.
- Method COI/T.20/Doc. No 25/Rev. 2; Evaluation of the Coherence of TAG Composition with the Fatty Acid Composition. Internacional Olive Council: Madrid, Spain, 2018; p. 15.
- EN 14105; Fat and Oil Derivatives–Fatty Acid Methyl Esters (FAME)–Determination on Free and Total Glycerol and Mono-, di-, Triglyceride Contents. European Committee for Standardization: Brussels, Belgium, 2011.


| Oil | Acid Value (mg KOH/g) | Peroxide Value (meqO2/kg) |
|---|---|---|
| Grapeseed | 0.18 ± 0.00 | 14.05 ± 0.07 |
| Milk Thistle | 1.85 ± 0.00 | 2.04 ± 0.06 |
| Apricot Kernel | 0.979 ± 0.00 | 1.25 ± 0.01 |
| Fatty Acid | Short Name | Grapeseed Oil | Milk Thistle Oil | Apricot Kernel Oil |
|---|---|---|---|---|
| Myristic acid | C14:0 | 0.05 ± 0.002 | 0.09 ± 0.001 | 0.02 ± 0.006 |
| Pentadecanoic acid | C15:0 | 1.0 ± 0.001 | 0.02 ± 0.000 | 0.00 ± 0.00 |
| Palmitic acid | C16:0 | 7.01 ± 0.221 | 7.71 ± 0.118 | 5.16 ± 0.059 |
| Palmitoleic acid | C16:1 | 0.21 ± 0.008 | 0.10 ± 0.004 | 0.66 ± 0.006 |
| Heptadecanoic acid | C17:0 | 0.06 ± 0.002 | 0.06 ± 0.003 | 0.04 ± 0.000 |
| Heptadecenoic acid | C17:1 | 0.03 ± 0.003 | 0.03 ± 0.002 | 0.12 ± 0.001 |
| Stearic acid | C18:0 | 4.15 ± 0.005 | 5.52 ± 0.037 | 1.16 ± 0.001 |
| trans-Oleic acid | T18:1 | 0.05 ± 0.006 | 0.01 ± 0.004 | 0.02 ± 0.003 |
| Oleic acid | C18:1 | 19.96 ± 0.066 | 28.02 ± 0.157 | 68.61 ± 0.331 |
| trans-Linoleic acid | T18:2 | 0.79 ± 0.103 | 0.04 ± 0.001 | 0.03 ± 0.004 |
| Linoleic acid | C18:2 | 66.62 ± 0.093 | 51.55 ± 0.061 | 23.89 ± 0.227 |
| trans-Linolenic acid | T18:3 | 0.07 ± 0.003 | 0.00 ± 0.000 | 0.00 ± 0.00 |
| Linolenic acid | C18:3 | 0.29 ± 0.000 | 0.17 ± 0.006 | 0.09 ± 0.006 |
| Arachidic acid | C20:0 | 0.19 ± 0.000 | 3.05 ± 0.150 | 0.1 ± 0.005 |
| Eicosenoic acid | C20:1 | 0.17 ± 0.005 | 0.94 ± 0.038 | 0.1 ± 0.003 |
| Heneicosanoic acid | C21:0 | 0.12 ± 0.002 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Eicosadienoic acid | C20:2 | 0.002 ± 0.021 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Behenic acid | C22:0 | 0.17 ± 0.000 | 1.88 ± 0.251 | 0.00 ± 0.00 |
| Erucic acid | C22:1 | 0.003 ± 0.000 | 0.03 ± 0.004 | 0.00 ± 0.00 |
| Lignoceric acid | C24:0 | 0.06 ± 0.000 | 0.52 ± 0.113 | 0.00 ± 0.00 |
| Nervonic acid | C24:1 | 0.00 ± 0.000 | 0.10 ± 0.068 | 0.00 ± 0.00 |
| Σ SFAs | 11.82 | 18.86 | 6.48 | |
| Σ MUFAs | 20.42 | 29.23 | 69.50 | |
| Σ PUFAs | 67.76 | 51.76 | 24.01 |
| ECN | TAGs | Grapeseed Oil | Milk Thistle Oil | Apricot Kernel Oil |
|---|---|---|---|---|
| 42 | LLL + (OLLn + PoLL) | 34.99 ± 0.023 | 14.93 ± 0.665 | 2.50 ± 0.011 |
| 44 | OLL + (OOLn + PoOL) | 23.98 ± 0.102 | 20.98 ± 0.099 | 13.49 ± 0.029 |
| 44 | PLL + PoPoO | 11.94 ± 0.314 | 9.12 ± 0.120 | 1.47 ± 0.035 |
| 46 | OOL + LnPP | 7.34 ± 0.245 | 12.07 ± 0.099 | 27.78 ± 0.031 |
| 46 | PoOO | 7.38 ± 0.028 | 6.81 ± 0.141 | 1.26 ± 0.012 |
| 46 | SLL + PLO | 5.188 ± 0.083 | 6.24 ± 0.042 | 6.32 ± 0.050 |
| 48 | PLP | 0.38 ± 0.014 | 7.77 ± 0.106 | 0.34 ± 0.001 |
| 48 | OOO + PoPP | 1.99 ± 0.030 | 4.91 ± 0.071 | 35.24 ± 0.038 |
| 48 | SOL | 2.35 ± 0.072 | 0.69 ± 0.019 | 0.98 ± 0.005 |
| 48 | POO | 1.23 ± 0.048 | 2.17 ± 0.056 | 6.43 ± 0.021 |
| 48 | POP | 0.54 ± 0.001 | 2.02 ± 0.020 | 0.41 ± 0.002 |
| Σ ECN42 | 35.13 | 15.19 | 2.79 | |
| Σ ECN44 | 36.67 | 31.52 | 15.81 | |
| Σ ECN46 | 19.95 | 26.82 | 35.61 | |
| Σ ECN48 | 7.01 | 19.15 | 43.47 | |
| Σ ECN50 | 1.00 | 4.03 | 1.97 | |
| Σ ECN52 | 0.24 | 3.25 | 0.30 |
| System | New TAG Yield Maximum Value (%) | Time (h) | TAG Conversion Maximum Value (%) | Time (h) | Initial Rate (% New TAGs/h) |
|---|---|---|---|---|---|
| GO + C10:0 | 64.7 ± 1.37 | 6 | 90.0 ± 0.060 | 48 | 13.6 |
| GO + C10 Ethyl | 61.0 ± 0.147 | 6 | 92.2 ± 0.219 | 48 | 44.8 |
| TO + C10:0 | 56.1 ± 0.117 | 24 | 89.1 ± 0.056 | 48 | 7.14 |
| TO + C10 Ethyl | 62.8 ± 0.176 | 6 | 87.0 ± 0.010 | 48 | 30.2 |
| AO + C10:0 | 69.7 ± 0.383 | 30 | 85.2 ± 0.084 | 48 | 9.9 |
| AO + C10 Ethyl | 60.6 ± 0.166 | 6 | 85.7 ± 0.035 | 48 | 35.5 |
| Substrates | Biocatalyst | Best Reaction Conditions | Best Results | Ref. |
|---|---|---|---|---|
| Acidolysis | ||||
| Olive oil + C10:0 | Lipozyme RM IM Lipozyme TL IM Novozym 435 | Enzyme load: 5% (w/w) MR = 1:2 Time: 24 h T: 45 °C | Incorporation: RM IM: 27.1 mol%; TL IM: 28.8 mol% Novozym 435: 30.4 mol% | [23] |
| Grapeseed oil + C10:0 | rROL in Amberlite IRA 96 Self-immobilized C. papaya lipase | Enzyme load: 5% (w/w) MR = 1:2 Time: 48 h T: 40 °C | New TAG Yield: rROL: 54.3% C. papaya lipase: 38.2% | [25] |
| Grapeseed oil + C10:0 | Lipozyme RM IM Lipozyme TL IM | Enzyme load: 5% (w/w) MR = 1:2 Time: 24 h T: 45 °C | Incorporation: RM IM: 34.5 mol % TL IM: 27.0 mol % | [27] |
| Crude OP oil + C10:0 Crude SCG oil + C10:0 | Lipozyme TL IM ROL-MNP | Enzyme load: 5% (w/w) MR = 1:2 T: 50 °C | New TAG Yield: Lipozyme TL IM (48 h): Crude OP oil: ~40% Crude SCG oil: ~48% ROL-MNP (3–5 h): Crude OP oil: ~50% Crude SCG oil: ~50% | [29] |
| Crude SCG oil + C10:0 | Lipozyme RM IM Lipozyme TL IM | Enzyme load: 5% (w/w) MR = 1:2 T: 50 °C | New TAG Yield: RM IM: ~70% (7 h) TL IM ~50% (48 h) | [30] |
| Pumpkin oil + C10:0 | Lipozyme RM IM | Enzyme load: 5% (w/w) MR = 1:9 Time: 24 h T: 60 °C | Incorporation: 48.4 mol% | [47] |
| Grapeseed oil + C10:0 | Lipozyme RM IM Lipozyme 435 | Enzyme load: 5% (w/w) MR = 1:3 Time: 12 h T: 65 °C | Incorporation: RM IM: 60.08 mol% Lipozyme 435: 50.78 mol%; | [49] |
| Sacha inchi oil + C10:0 | Rhizopus oryzae lipase immobilized in corn cob powder | Bach PBR with recirculation: MR = 1:3 Time: 120 h T: 45 °C | Incorporation: 36 mol% | [50] |
| Interesterification | ||||
| Crude OP oil + C10 Ethyl Crude SCG oil + C10 Ethyl | Lipozyme TL IM ROL-MNP | Enzyme load: 5% (w/w) MR = 1:2 T: 50 °C | New TAG Yield: Lipozyme TL IM (7 h): Crude OP oil: ~52% Crude SCG oil: ~65% ROL-MNP (3–5 h): Crude OP oil: 46% Crude SCG oil: 26% | [29] |
| Crude SCG oil + C10 Ethyl | Lipozyme RM IM | Enzyme load: 5% (w/w) MR = 1:2 Time: 7 h T: 50 °C | New TAG Yield: ~70% | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbaş, Ş.; Osório, N.M.; Ferreira-Dias, S. Batch and Continuous Lipase-Catalyzed Production of Dietetic Structured Lipids from Milk Thistle, Grapeseed, and Apricot Kernel Oils. Molecules 2025, 30, 1943. https://doi.org/10.3390/molecules30091943
Akbaş Ş, Osório NM, Ferreira-Dias S. Batch and Continuous Lipase-Catalyzed Production of Dietetic Structured Lipids from Milk Thistle, Grapeseed, and Apricot Kernel Oils. Molecules. 2025; 30(9):1943. https://doi.org/10.3390/molecules30091943
Chicago/Turabian StyleAkbaş, Şuheda, Natália M. Osório, and Suzana Ferreira-Dias. 2025. "Batch and Continuous Lipase-Catalyzed Production of Dietetic Structured Lipids from Milk Thistle, Grapeseed, and Apricot Kernel Oils" Molecules 30, no. 9: 1943. https://doi.org/10.3390/molecules30091943
APA StyleAkbaş, Ş., Osório, N. M., & Ferreira-Dias, S. (2025). Batch and Continuous Lipase-Catalyzed Production of Dietetic Structured Lipids from Milk Thistle, Grapeseed, and Apricot Kernel Oils. Molecules, 30(9), 1943. https://doi.org/10.3390/molecules30091943








