Hepatoprotective Effect of Kaempferol—A Review
Abstract
1. Introduction
2. General Overview of Liver Disease Pathophysiology
3. Sources and Biological Functions of Kaempferol
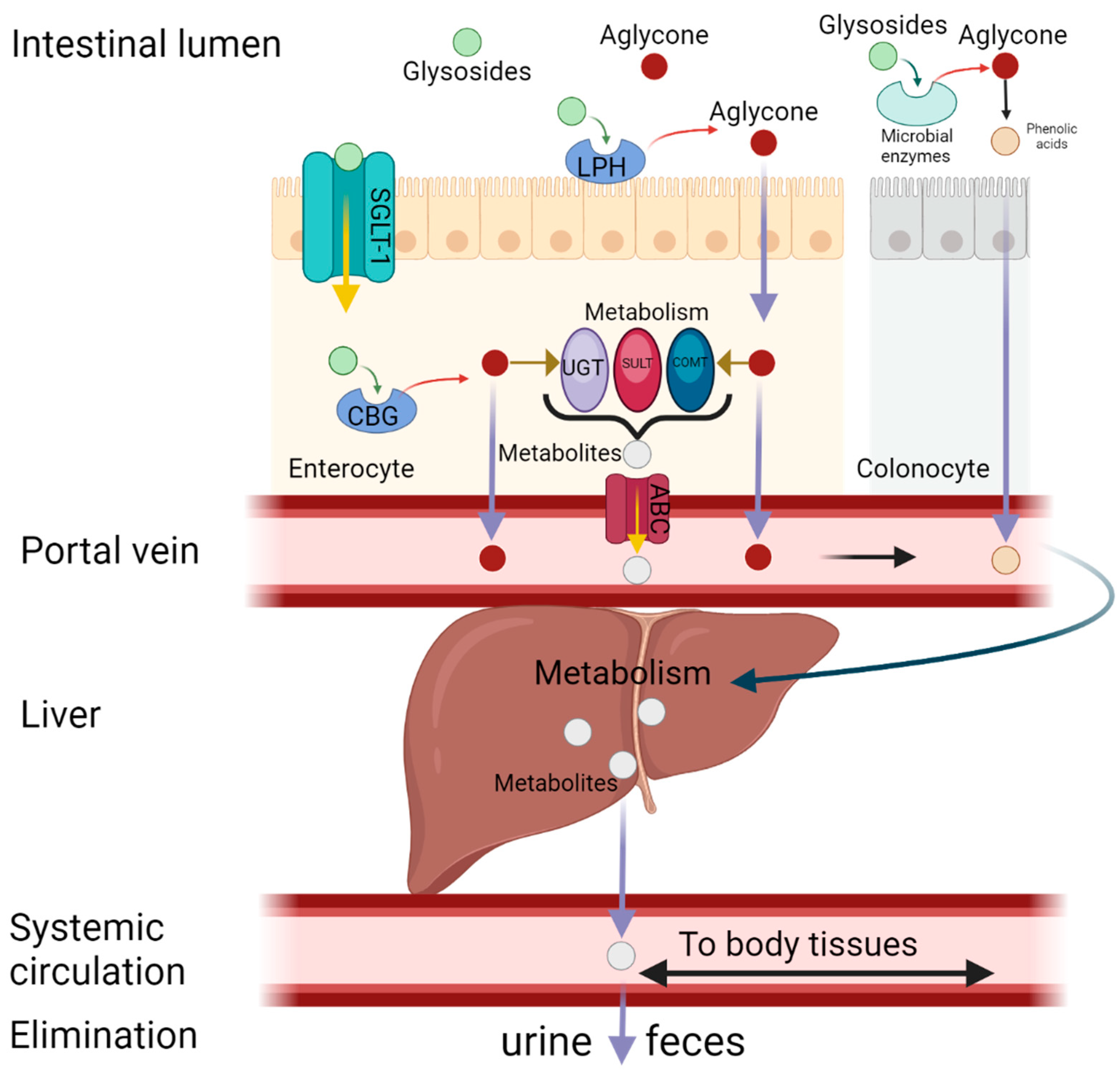
4. Metabolism and Bioavailability of Kaempferol
5. Effect of Kaempferol on Liver Condition
5.1. Reduction of Hepatic Lipid Accumulation
5.2. Inhibition of Hepatic Inflammation
5.3. Inhibition of Hepatic Oxidative Stress
5.4. Down-Regulation of Liver Fibrosis
5.5. Modulation of Gut Microbiota
6. Safety of Kaempferol and Its Possible Interactions with Conventional Medicines
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| ACC | acetyl-CoA carboxylase |
| ADH | alcohol dehydrogenase |
| ADME | absorption, distribution, metabolism, excretion |
| AhR | Aryl Hydrocarbon Receptor |
| ALK5 | activin receptor-like kinase 5 |
| ALT | alanine transferase |
| AMPK | SIRT1/AMP-activated protein kinase |
| AST | aspartate aminotransferase |
| Bax | bcl-2-like protein 4 |
| Bcl-2 | B-cell CLL/lymphoma 2 |
| CBGCGA | cytosolic enzyme β-glucosidasechlorogenic acid |
| COMT | catechol-O-methyltransferase |
| COX | cyclooxygenase |
| CPT1 | carnitine palmitoyltransferase 1 |
| CYP2E1 | cytochrome P450 2E1 |
| ECM | extracellular matrix |
| ERS | endoplasmic reticulum stress |
| FAO | fatty acid oxidation |
| FASN | fatty acid synthase |
| FOXO | forkhead box protein |
| GCG | glucagon |
| GIP | gastric inhibitory polypeptide |
| GLP-1 | glucagon-like peptide 1 |
| GSH | glutathione |
| HCC | hepatocellular carcinoma |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| HFD | high-fat diet |
| HO-1 | heme oxygenase 1 |
| HSC | hematopoietic stem cells |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| LPACT3 | lysophosphatidylcholine acyltransferase 3 |
| LPH | lactase-phlorizin hydrolase |
| LPS | lipopolysaccharide |
| LXR | liver X receptor |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| MDA | malondialdehyde |
| MFBs | myofibroblasts |
| MnSOD | manganese-dependent superoxide dismutase |
| MPO | myeloperoxidase |
| NAD+ | oxidized nicotinamide adenine dinucleotide |
| NAFLD | non-alcoholic fatty liver disease |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | nitric oxide |
| P-gp | P-glycoprotein |
| p53 | tumour protein p53 |
| PDGF | platelet-derived growth factor |
| PGC-1 | peroxisome proliferator-activated receptor gamma coactivator 1 |
| PGE | Prostaglandin E |
| PLP | pyridoxal phosphate |
| PXR | Pregnane X Receptor |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SGLT-1 | sodium-dependent glucose transporter-1 |
| SIRT1 | sirtuin 1 |
| SOD | superoxide dismutase |
| SREBPs | sterol regulatory element-binding proteins |
| STAT3 | signal transducer and activator of transcription 3. |
| SULT | sulfotransferase |
| TLR4 | toll-like receptor 4 |
| TNF-α | tumour necrosis factor-alpha |
| UGT | uridine-5ʹ-diphosphate-glucuronosyltransferase |
| α-SMA | alpha-smooth muscle actin |
References
- Trefts, E.; Gannon, M.; Wasserman, D.H. The Liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of Liver Diseases in the World. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global Burden of Liver Disease: 2023 Update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global Incidence and Prevalence of Nonalcoholic Fatty Liver Disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef]
- Heinemann, F.; Gross, P.; Zeveleva, S.; Qian, H.S.; Hill, J.; Höfer, A.; Jonigk, D.; Diehl, A.M.; Abdelmalek, M.; Lenter, M.C.; et al. Deep Learning-Based Quantification of NAFLD/NASH Progression in Human Liver Biopsies. Sci. Rep. 2022, 12, 19236. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease (accessed on 30 March 2025).
- Zhao, B.; Liu, K.; Liu, X.; Li, Q.; Li, Z.; Xi, J.; Xie, F.; Li, X. Plant-Derived Flavonoids Are a Potential Source of Drugs for the Treatment of Liver Fibrosis. Phytother. Res. 2024, 38, 3122–3145. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Markowska, J.; Kasprzak-Drozd, K.; Niziński, P.; Dragan, M.; Kondracka, A.; Gondek, E.; Oniszczuk, T.; Oniszczuk, A. Quercetin: A Promising Candidate for the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Molecules 2024, 29, 5245. [Google Scholar] [CrossRef]
- Ziółkiewicz, A.; Niziński, P.; Soja, J.; Oniszczuk, T.; Combrzyński, M.; Kondracka, A.; Oniszczuk, A. Potential of Chlorogenic Acid in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Animal Studies and Clinical Trials—A Narrative Review. Metabolites 2024, 14, 346. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Niziński, P.; Kasprzak, P.; Kondracka, A.; Oniszczuk, T.; Rusinek, A.; Oniszczuk, A. Does Resveratrol Improve Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)? Int. J. Mol. Sci. 2024, 25, 3746. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Tavakol, S.; Ahmadi, Z.; Roomiani, S.; Mohammadinejad, R.; Samarghandian, S. Therapeutic Effects of Kaempferol Affecting Autophagy and Endoplasmic Reticulum Stress. Phytother. Res. 2020, 34, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Silva dos Santos, J.; Gonçalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, L.; Yang, D.; Luo, K.; Li, X. Small-Molecule Natural Plants for Reversing Liver Fibrosis Based on Modulation of Hepatic Stellate Cells Activation: An Update. Phytomedicine 2023, 113, 154721. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.D.; Behary, J.; Zekry, A. Non-Alcoholic Fatty Liver Disease: A Review of Epidemiology, Risk Factors, Diagnosis and Management. Intern. Med. J. 2020, 50, 1038–1047. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic Fatty Liver Disease: A Precursor of the Metabolic Syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef]
- Rizzo, M.; Colletti, A.; Penson, P.E.; Katsiki, N.; Mikhailidis, D.P.; Toth, P.P.; Gouni-Berthold, I.; Mancini, J.; Marais, D.; Moriarty, P.; et al. Nutraceutical Approaches to Non-Alcoholic Fatty Liver Disease (NAFLD): A Position Paper from the International Lipid Expert Panel (ILEP). Pharmacol. Res. 2023, 189, 106679. [Google Scholar] [CrossRef]
- Katsiki, N.; Stoian, A.P.; Steiropoulos, P.; Papanas, N.; Suceveanu, A.-I.; Mikhailidis, D.P. Metabolic Syndrome and Abnormal Peri-Organ or Intra-Organ Fat (APIFat) Deposition in Chronic Obstructive Pulmonary Disease: An Overview. Metabolites 2020, 10, 465. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, S.; Ji, X.; Shen, X.; You, J.; Liang, X.; Yin, H.; Zhao, L. Current Innovations in Nutraceuticals and Functional Foods for Intervention of Non-Alcoholic Fatty Liver Disease. Pharmacol. Res. 2021, 166, 105517. [Google Scholar] [CrossRef]
- Farrell, G.C.; Larter, C.Z. Nonalcoholic Fatty Liver Disease: From Steatosis to Cirrhosis. Hepatology 2006, 43, S99–S112. [Google Scholar] [CrossRef]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ishigami, M.; Toyoda, H.; Wai-Sun Wong, V.; Peleg, N.; et al. Association Between Fibrosis Stage and Outcomes of Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Roelstraete, B.; Hagström, H.; Loomba, R.; Ludvigsson, J.F. Progression of Non-Alcoholic Fatty Liver Disease and Long-Term Outcomes: A Nationwide Paired Liver Biopsy Cohort Study. J. Hepatol. 2023, 79, 1366–1373. [Google Scholar] [CrossRef]
- Tanaka, M.; Miyajima, A. Liver Regeneration and Fibrosis after Inflammation. Inflamm. Regen. 2016, 36, 19. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of Liver Fibrosis and Its Role in Liver Cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef]
- Bai, Q.; Yan, H.; Sheng, Y.; Jin, Y.; Shi, L.; Ji, L.; Wang, Z. Long-Term Acetaminophen Treatment Induced Liver Fibrosis in Mice and the Involvement of Egr-1. Toxicology 2017, 382, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Berumen, J.; Baglieri, J.; Kisseleva, T.; Mekeel, K. Liver Fibrosis: Pathophysiology and Clinical Implications. WIREs Mech. Dis. 2021, 13, e1499. [Google Scholar] [CrossRef]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. 2022, 26, 671–683. [Google Scholar] [CrossRef]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Sultana, M.; Islam, M.A.; Khairnar, R.; Kumar, S. A Guide to Pathophysiology, Signaling Pathways, and Preclinical Models of Liver Fibrosis. Mol. Cell. Endocrinol. 2025, 598, 112448. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Liu, S.; Yang, M. Treatment of Liver Fibrosis: Past, Current, and Future. World J. Hepatol. 2023, 15, 755–774. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Uddin, M.B.; Ahmed, S.S.U.; Yu, Z.-L.; Cho, J.Y. Kaempferol: Review on Natural Sources and Bioavailability. In Kaempferol: Biosynthesis, Food Sources and Therapeutic Uses; Nova Science Publishers: New York, NY, USA, 2016; pp. 101–150. [Google Scholar]
- Periferakis, A. On the Dissemination of Acupuncture to Europe. JournalNX 2020, 6, 201–209. [Google Scholar]
- Jin, S.; Zhang, L.; Wang, L. Kaempferol, a Potential Neuroprotective Agent in Neurodegenerative Diseases: From Chemistry to Medicine. Biomed. Pharmacother. 2023, 165, 115215. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, S.; Zhang, L.; Ka, Y.; Zhou, M.; Wang, Y.; Tang, Z.; Zhang, J.; Wang, W.; Liu, W. Research Progress on Pharmacokinetics, Anti-Inflammatory and Immunomodulatory Effects of Kaempferol. Int. Immunopharmacol. 2025, 152, 114387. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. [Google Scholar] [CrossRef] [PubMed]
- Matute, A.; Tabart, J.; Cheramy-Bien, J.-P.; Pirotte, B.; Kevers, C.; Auger, C.; Schini-Kerth, V.; Dommes, J.; Defraigne, J.-O.; Pincemail, J. Compared Phenolic Compound Contents of 22 Commercial Fruit and Vegetable Juices: Relationship to Ex-Vivo Vascular Reactivity and Potential In Vivo Projection. Antioxidants 2020, 9, 92. [Google Scholar] [CrossRef]
- Phenol Explorer. Available online: http://phenol-explorer.eu/contents/polyphenol/290#chromatography-after-hydrolysis (accessed on 19 March 2025).
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yu, Y.; Dai, S.; Zhang, C.; Xue, X.; Zhou, M.; Yao, C.; Li, Y. Kaempferol Efficacy in Metabolic Diseases: Molecular Mechanisms of Action in Diabetes Mellitus, Obesity, Non-Alcoholic Fatty Liver Disease, Steatohepatitis, and Atherosclerosis. Biomed. Pharmacother. 2024, 175, 116694. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Shah, Z.A.; Saeed, F.; Imran, A.; Arshad, M.U.; Ahmad, B.; Bawazeer, S.; Atif, M.; Peters, D.G.; et al. Chemo-Preventive and Therapeutic Effect of the Dietary Flavonoid Kaempferol: A Comprehensive Review. Phytother. Res. 2019, 33, 263–275. [Google Scholar] [CrossRef]
- Hosseini, A.; Alipour, A.; Baradaran Rahimi, V.; Askari, V.R. A Comprehensive and Mechanistic Review on Protective Effects of Kaempferol against Natural and Chemical Toxins: Role of NF-κB Inhibition and Nrf2 Activation. BioFactors 2023, 49, 322–350. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Periferakis, K.; Scheau, A.-E.; Savulescu-Fiedler, I.; Caruntu, A.; Badarau, I.A.; Caruntu, C.; Scheau, C. Kaempferol: A Review of Current Evidence of Its Antiviral Potential. Int. J. Mol. Sci. 2023, 24, 16299. [Google Scholar] [CrossRef]
- Zhou, P.; Ma, Y.; Peng, J.; Hua, F. Kaempferol-3-O-Rutinoside: A Natural Flavonoid Glycosides with Multifaceted Therapeutic Potential. Neurochem. J. 2023, 17, 247–252. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, K.; Qin, S.; Jing, Y.; Liu, S.; Li, D.; Peng, C. Astragalin: A Food-Origin Flavonoid with Therapeutic Effect for Multiple Diseases. Front. Pharmacol. 2023, 14, 1265960. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Teraminami, A.; Lee, J.-Y.; Ohyama, K.; Funakoshi, K.; Kim, Y.-I.; Hirai, S.; Uemura, T.; Yu, R.; Takahashi, N.; et al. Tiliroside, a Glycosidic Flavonoid, Ameliorates Obesity-Induced Metabolic Disorders via Activation of Adiponectin Signaling Followed by Enhancement of Fatty Acid Oxidation in Liver and Skeletal Muscle in Obese-Diabetic Mice. J. Nutr. Biochem. 2012, 23, 768–776. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Hollman, P.C.; Bijsman, M.N.; van Gameren, Y.; Cnossen, E.P.; de Vries, J.H.; Katan, M.B. The Sugar Moiety Is a Major Determinant of the Absorption of Dietary Flavonoid Glycosides in Man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid Metabolism: The Interaction of Metabolites and Gut Microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. Biomed. Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.-M. The Role of Metabolism (and the Microbiome) in Defining the Clinical Efficacy of Dietary Flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, C.L.-L.; Kong, A.-N. Metabolism, Oral Bioavailability and Pharmacokinetics of Chemopreventive Kaempferol in Rats. Biopharm. Drug Dispos. 2009, 30, 356–365. [Google Scholar] [CrossRef]
- Herrera, T.E.S.; Tello, I.P.S.; Mustafa, M.A.; Jamil, N.Y.; Alaraj, M.; Atiyah Altameem, K.K.; Alasheqi, M.Q.; Hamoody, A.-H.M.; Alkhafaji, A.T.; Shakir, M.N.; et al. Kaempferol: Unveiling Its Anti-Inflammatory Properties for Therapeutic Innovation. Cytokine 2025, 186, 156846. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, I.; Al-Abbasi, F.A.; Afzal, M.; Altayb, H.N.; Nadeem, M.S.; Gupta, G. Formulation and Evaluation of Kaempferol Loaded Nanoparticles against Experimentally Induced Hepatocellular Carcinoma: In Vitro and In Vivo Studies. Pharmaceutics 2021, 13, 2086. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Khanam, N.; Nath, D. Solid Lipid Nanoparticle: A Potent Vehicle of the Kaempferol for Brain Delivery through the Blood-Brain Barrier in the Focal Cerebral Ischemic Rat. Chem.-Biol. Interact. 2024, 397, 111084. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, Antioxidant and Anti-Inflammatory Activities of Kaempferol and Its Corresponding Glycosides and the Enzymatic Preparation of Kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol—A Dietary Anticancer Molecule with Multiple Mechanisms of Action: Recent Trends and Advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Zhang, D.; Yu, C.; Jin, C.; Igarashi, K. Antioxidant and Hepatoprotective Activity of Kaempferol 3-O-β-d- (2,6-Di-O-α-l-Rhamnopyranosyl)Galactopyronoside against Carbon Tetrachloride-Induced Liver Injury in Mice. Food Sci. Biotechnol. 2017, 26, 1071–1076. [Google Scholar] [CrossRef]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent Progress Regarding Kaempferol for the Treatment of Various Diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
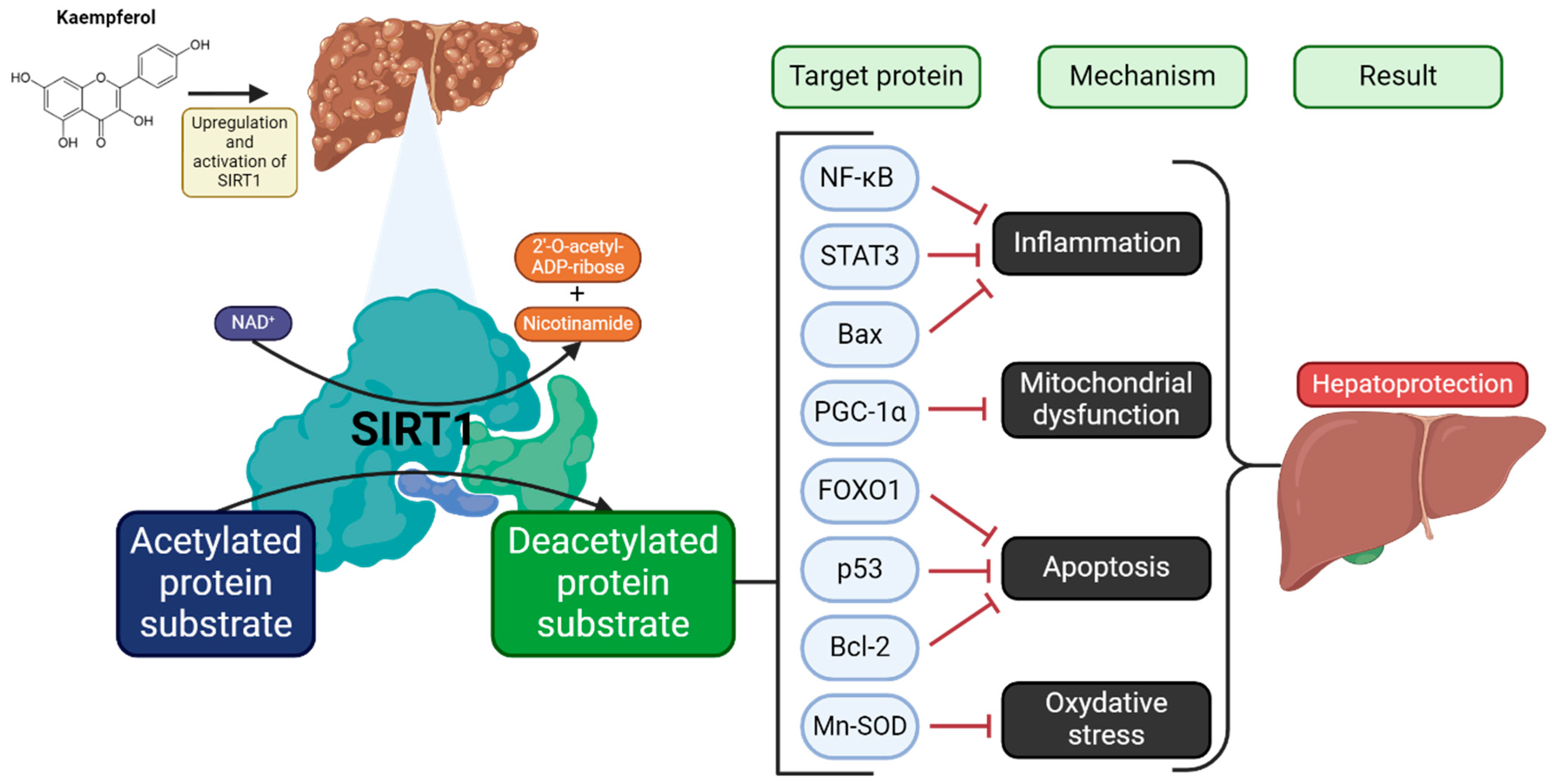
- BinMowyna, M.N.; AlFaris, N.A. Kaempferol Suppresses Acetaminophen-Induced Liver Damage by Upregulation/Activation of SIRT1. Pharm. Biol. 2021, 59, 144–154. [Google Scholar] [CrossRef]
- Alkandahri, M.Y.; Pamungkas, B.T.; Oktoba, Z.; Shafirany, M.Z.; Sulastri, L.; Arfania, M.; Anggraeny, E.N.; Pratiwi, A.; Astuti, F.D.; Indriyani; et al. Hepatoprotective Effect of Kaempferol: A Review of the Dietary Sources, Bioavailability, Mechanisms of Action, and Safety. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 1–16. [Google Scholar] [CrossRef]
- Pingili, R.B.; Challa, S.R.; Pawar, A.K.; Toleti, V.; Kodali, T.; Koppula, S. A Systematic Review on Hepatoprotective Activity of Quercetin against Various Drugs and Toxic Agents: Evidence from Preclinical Studies. Phytother. Res. 2020, 34, 5–32. [Google Scholar] [CrossRef]
- Domitrović, R.; Potočnjak, I. A Comprehensive Overview of Hepatoprotective Natural Compounds: Mechanism of Action and Clinical Perspectives. Arch. Toxicol. 2016, 90, 39–79. [Google Scholar] [CrossRef]
- Li, N.; Yin, L.; Shang, J.; Liang, M.; Liu, Z.; Yang, H.; Qiang, G.; Du, G.; Yang, X. Kaempferol Attenuates Nonalcoholic Fatty Liver Disease in Type 2 Diabetic Mice via the Sirt1/AMPK Signaling Pathway. Biomed. Pharmacother. 2023, 165, 115113. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Julia Xu, X.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A Long-Standing Partnership? Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, G.-F.; Ma, Y.-S.; Zhang, H.-W.; Zhou, Y.; Liu, G.-H.; Chen, D.-Y.; Ping, J.; Liu, Y.-H.; Mou, X.; et al. Hepatic Proteomic Changes and Sirt1/AMPK Signaling Activation by Oxymatrine Treatment in Rats With Non-Alcoholic Steatosis. Front. Pharmacol. 2020, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Dzamko, N.; van Denderen, B.J.W.; Hevener, A.L.; Jørgensen, S.B.; Honeyman, J.; Galic, S.; Chen, Z.-P.; Watt, M.J.; Campbell, D.J.; Steinberg, G.R.; et al. AMPK Β1 Deletion Reduces Appetite, Preventing Obesity and Hepatic Insulin Resistance*. J. Biol. Chem. 2010, 285, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Abbasalipourkabir, R.; Ziamajidi, N. Fish Oil and Chicoric Acid Combination Protects Better against Palmitate-Induced Lipid Accumulation via Regulating AMPK-Mediated SREBP-1/FAS and PPARα/UCP2 Pathways. Arch. Physiol. Biochem. 2020, 129, 1–9. [Google Scholar] [CrossRef]
- Shokri Afra, H.; Zangooei, M.; Meshkani, R.; Ghahremani, M.H.; Ilbeigi, D.; Khedri, A.; Shahmohamadnejad, S.; Khaghani, S.; Nourbakhsh, M. Hesperetin Is a Potent Bioactivator That Activates SIRT1-AMPK Signaling Pathway in HepG2 Cells. J. Physiol. Biochem. 2019, 75, 125–133. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, P.; Chen, Z.-L.; Zhang, S.-J.; Wang, Y.-Q.; Cai, X.; Luo, L.; Zhou, X.; Zhao, L. Emodin Attenuates Lipopolysaccharide-Induced Acute Liver Injury via Inhibiting the TLR4 Signaling Pathway In Vitro and In Vivo. Front. Pharmacol. 2018, 9, 962. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, T.; Li, W.; Muhammad, I.; Wang, H.; Sun, X.; Yang, Y.; Li, J.; Xiao, T.; Zhang, X. Baicalin Alleviates Lipopolysaccharide-Induced Liver Inflammation in Chicken by Suppressing TLR4-Mediated NF-κB Pathway. Front. Pharmacol. 2017, 8, 547. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Yan, X.-T.; Wang, K.; Tian, R.-M.; Lu, Z.-Y.; Wu, L.-L.; Xu, H.-T.; Wu, Y.-S.; Liu, X.-S.; Mao, W.; et al. Triptriolide Alleviates Lipopolysaccharide-Induced Liver Injury by Nrf2 and NF-κB Signaling Pathways. Front. Pharmacol. 2018, 9, 999. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Iwata, A.; Suzuki, K.; Suto, A.; Kawashima, S.; Saito, Y.; Owada, T.; Kobayashi, M.; Watanabe, N.; Nakajima, H. B and T Lymphocyte Attenuator Inhibits LPS-Induced Endotoxic Shock by Suppressing Toll-like Receptor 4 Signaling in Innate Immune Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 5121–5126. [Google Scholar] [CrossRef]
- Islam, M.S.; Yu, H.; Miao, L.; Liu, Z.; He, Y.; Sun, H. Hepatoprotective Effect of the Ethanol Extract of Illicium Henryi against Acute Liver Injury in Mice Induced by Lipopolysaccharide. Antioxidants 2019, 8, 446. [Google Scholar] [CrossRef]
- Liu, P.; Wu, P.; Yang, B.; Wang, T.; Li, J.; Song, X.; Sun, W. Kaempferol Prevents the Progression from Simple Steatosis to Non-Alcoholic Steatohepatitis by Inhibiting the NF-κB Pathway in Oleic Acid-Induced HepG2 Cells and High-Fat Diet-Induced Rats. J. Funct. Foods 2021, 85, 104655. [Google Scholar] [CrossRef]
- Xiang, H.; Shao, M.; Lu, Y.; Wang, J.; Wu, T.; Ji, G. Kaempferol Alleviates Steatosis and Inflammation During Early Non-Alcoholic Steatohepatitis Associated With Liver X Receptor α-Lysophosphatidylcholine Acyltransferase 3 Signaling Pathway. Front. Pharmacol. 2021, 12, 690736. [Google Scholar] [CrossRef] [PubMed]
- Alrumaihi, F.; Almatroodi, S.A.; Alharbi, H.O.A.; Alwanian, W.M.; Alharbi, F.A.; Almatroudi, A.; Rahmani, A.H. Pharmacological Potential of Kaempferol, a Flavonoid in the Management of Pathogenesis via Modulation of Inflammation and Other Biological Activities. Molecules 2024, 29, 2007. [Google Scholar] [CrossRef] [PubMed]
- Al Olayan, E.M.; Aloufi, A.S.; AlAmri, O.D.; El-Habit, O.H.; Abdel Moneim, A.E. Protocatechuic Acid Mitigates Cadmium-Induced Neurotoxicity in Rats: Role of Oxidative Stress, Inflammation and Apoptosis. Sci. Total Environ. 2020, 723, 137969. [Google Scholar] [CrossRef] [PubMed]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative Effect of Kaempferol, a Flavonoid, on Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef]
- Yang, Q.-S.; He, L.-P.; Zhou, X.-L.; Zhao, Y.; Shen, J.; Xu, P.; Ni, S.-Z. Kaempferol Pretreatment Modulates Systemic Inflammation and Oxidative Stress Following Hemorrhagic Shock in Mice. Chin. Med. 2015, 10, 6. [Google Scholar] [CrossRef]
- Cioarca-Nedelcu, R.; Atanasiu, V.; Stoian, I. Alcoholic Liver Disease-from Steatosis to Cirrhosis—A Biochemistry Approach. J. Med. Life 2021, 14, 594–599. [Google Scholar] [CrossRef]
- Renu, K.; Saravanan, A.; Elangovan, A.; Ramesh, S.; Annamalai, S.; Namachivayam, A.; Abel, P.; Madhyastha, H.; Madhyastha, R.; Maruyama, M.; et al. An Appraisal on Molecular and Biochemical Signalling Cascades during Arsenic-Induced Hepatotoxicity. Life Sci. 2020, 260, 118438. [Google Scholar] [CrossRef]
- Shih, T.-Y.; Young, T.-H.; Lee, H.-S.; Hsieh, C.-B.; Hu, O.Y.-P. Protective Effects of Kaempferol on Isoniazid- and Rifampicin-Induced Hepatotoxicity. AAPS J. 2013, 15, 753–762. [Google Scholar] [CrossRef]
- Dong, L.; Yin, L.; Quan, H.; Chu, Y.; Lu, J. Hepatoprotective Effects of Kaempferol-3-O-α-l-Arabinopyranosyl-7-O-α-l-Rhamnopyranoside on d-Galactosamine and Lipopolysaccharide Caused Hepatic Failure in Mice. Molecules 2017, 22, 1755. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, L.; Duan, J.; Li, L. Strategies to Improve the Efficiency of Mesenchymal Stem Cell Transplantation for Reversal of Liver Fibrosis. J. Cell. Mol. Med. 2019, 23, 1657–1670. [Google Scholar] [CrossRef]
- Yoshiji, H.; Nagoshi, S.; Akahane, T.; Asaoka, Y.; Ueno, Y.; Ogawa, K.; Kawaguchi, T.; Kurosaki, M.; Sakaida, I.; Shimizu, M.; et al. Evidence-Based Clinical Practice Guidelines for Liver Cirrhosis 2020. J. Gastroenterol. 2021, 56, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, A.; Gentilini, A.; Pastore, M.; Gitto, S.; Marra, F. Cellular and Molecular Mechanisms Underlying Liver Fibrosis Regression. Cells 2021, 10, 2759. [Google Scholar] [CrossRef]
- Targeting TGF-β Signal Transduction for Fibrosis and Cancer Therapy|Molecular Cancer|Full Text. Available online: https://molecular-cancer.biomedcentral.com/articles/10.1186/s12943-022-01569-x (accessed on 30 January 2025).
- Zhang, J.; Li, Y.; Liu, Q.; Huang, Y.; Li, R.; Wu, T.; Zhang, Z.; Zhou, J.; Huang, H.; Tang, Q.; et al. Sirt6 Alleviated Liver Fibrosis by Deacetylating Conserved Lysine 54 on Smad2 in Hepatic Stellate Cells. Hepatology 2021, 73, 1140–1157. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Huang, S.; Huang, Q.; Ming, Z.; Wang, M.; Li, R.; Zhao, Y. Kaempferol Attenuates Liver Fibrosis by Inhibiting Activin Receptor-like Kinase 5. J. Cell. Mol. Med. 2019, 23, 6403–6410. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, H.; Huang, Z.; Chen, X.; You, J.; Zou, T. A Critical Review of Kaempferol in Intestinal Health and Diseases. Antioxidants 2023, 12, 1642. [Google Scholar] [CrossRef]
- Duarte, L.; Gasaly, N.; Poblete-Aro, C.; Uribe, D.; Echeverria, F.; Gotteland, M.; Garcia-Diaz, D.F. Polyphenols and Their Anti-Obesity Role Mediated by the Gut Microbiota: A Comprehensive Review. Rev. Endocr. Metab. Disord. 2021, 22, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Osborn, L.J.; Schultz, K.; Massey, W.; DeLucia, B.; Choucair, I.; Varadharajan, V.; Banerjee, R.; Fung, K.; Horak, A.J.; Orabi, D.; et al. A Gut Microbial Metabolite of Dietary Polyphenols Reverses Obesity-Driven Hepatic Steatosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2202934119. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Q.; Zhao, T. Preventive Effects of Kaempferol on High-Fat Diet-Induced Obesity Complications in C57BL/6 Mice. BioMed Res. Int. 2020, 2020, 4532482. [Google Scholar] [CrossRef]
- Bian, Y.; Lei, J.; Zhong, J.; Wang, B.; Wan, Y.; Li, J.; Liao, C.; He, Y.; Liu, Z.; Ito, K.; et al. Kaempferol Reduces Obesity, Prevents Intestinal Inflammation, and Modulates Gut Microbiota in High-Fat Diet Mice. J. Nutr. Biochem. 2022, 99, 108840. [Google Scholar] [CrossRef]
- Lu, Y.; Shao, M.; Zhang, C.; Xiang, H.; Wang, J.; Wu, T.; Ji, G. Kaempferol Attenuates Nonalcoholic Steatohepatitis by Regulating Serum and Liver Bile Acid Metabolism. Front. Pharmacol. 2022, 13, 946360. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Xiong, F.; Wang, S.; Wang, K.; Zhang, Y.; Zhang, Q. Flavonoid Ingredients of Ginkgo Biloba Leaf Extract Regulate Lipid Metabolism through Sp1-Mediated Carnitine Palmitoyltranferase 1A up-Regulation. J. Biomed. Sci. 2014, 21, 87. [Google Scholar] [CrossRef]
- Fan, X.; Bai, J.; Hu, M.; Xu, Y.; Zhao, S.; Sun, Y.; Wang, B.; Hu, J.; Li, Y. Drug Interaction Study of Flavonoids toward OATP1B1 and Their 3D Structure Activity Relationship Analysis for Predicting Hepatoprotective Effects. Toxicology 2020, 437, 152445. [Google Scholar] [CrossRef] [PubMed]
- Singab, A.N.B.; Youssef, D.T.A.; Noaman, E.; Kotb, S. Hepatoprotective Effect of Flavonol Glycosides Rich Fraction from Egyptian Vicia Calcarata Desf. against CCl4-Induced Liver Damage in Rats. Arch. Pharm. Res. 2005, 28, 791–798. [Google Scholar] [CrossRef]
- Cho, S.S.; Yang, J.H.; Seo, K.H.; Shin, S.M.; Park, E.Y.; Cho, S.S.; Jo, G.U.; Eo, J.H.; Park, J.S.; Oh, D.S.; et al. Cudrania Tricuspidata Extract and Its Major Constituents Inhibit Oxidative Stress-Induced Liver Injury. J. Med. Food 2019, 22, 602–613. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Jiang, Z.; Xie, W.; Zhang, X. Hepatoprotective Effect of Kaempferol against Alcoholic Liver Injury in Mice. Am. J. Chin. Med. 2015, 43, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.-F.; Bian, Y.-Q.; Wu, R.; Sun, Y.; Chen, X.-L.; Yang, M.-D.; Zhang, Q.-R.; Hu, Y.; Sun, M.-Y.; Su, S.-B. Yinchenhao Decoction Suppresses Rat Liver Fibrosis Involved in an Apoptosis Regulation Mechanism Based on Network Pharmacology and Transcriptomic Analysis. Biomed. Pharmacother. 2019, 114, 108863. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, G.; Qiu, H.; Zhu, L.; Ren, Z.; Zhao, W.; Zhang, T.; Liu, L. The P53-Inducible Gene 3 Involved in Flavonoid-Induced Cytotoxicity through the Reactive Oxygen Species-Mediated Mitochondrial Apoptotic Pathway in Human Hepatoma Cells. Food Funct. 2015, 6, 1518–1525. [Google Scholar] [CrossRef]
- Du, Y.-C.; Lai, L.; Zhang, H.; Zhong, F.-R.; Cheng, H.-L.; Qian, B.-L.; Tan, P.; Xia, X.-M.; Fu, W.-G. Kaempferol from Penthorum Chinense Pursh Suppresses HMGB1/TLR4/NF-κB Signaling and NLRP3 Inflammasome Activation in Acetaminophen-Induced Hepatotoxicity. Food Funct. 2020, 11, 7925–7934. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Choi, H.-S.; Seo, M.-J.; Jeon, H.-J.; Kim, K.-J.; Lee, B.-Y. Kaempferol Suppresses Lipid Accumulation by Inhibiting Early Adipogenesis in 3T3-L1 Cells and Zebrafish. Food Funct. 2015, 6, 2824–2833. [Google Scholar] [CrossRef]
- Torres-Villarreal, D.; Camacho, A.; Castro, H.; Ortiz-Lopez, R.; de la Garza, A.L. Anti-Obesity Effects of Kaempferol by Inhibiting Adipogenesis and Increasing Lipolysis in 3T3-L1 Cells. J. Physiol. Biochem. 2019, 75, 83–88. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Lasa, A.; Abendaño, N.; Fernández-Quintela, A.; Mosqueda-Solís, A.; Garcia-Sobreviela, M.P.; Arbonés-Mainar, J.M.; Portillo, M.P. Phenolic Compounds Apigenin, Hesperidin and Kaempferol Reduce in Vitro Lipid Accumulation in Human Adipocytes. J. Transl. Med. 2017, 15, 237. [Google Scholar] [CrossRef]
- Lee, B.; Kwon, M.; Choi, J.S.; Jeong, H.O.; Chung, H.Y.; Kim, H.-R. Kaempferol Isolated from Nelumbo Nucifera Inhibits Lipid Accumulation and Increases Fatty Acid Oxidation Signaling in Adipocytes. J. Med. Food 2015, 18, 1363–1370. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, X.; Xu, C.; Cheng, S.; Ni, X.; Shi, Y.; Yao, Y.; Chen, L.; Hu, M.G.; Xia, D. Kaempferol Regulates the Thermogenic Function of Adipocytes in High-Fat-Diet-Induced Obesity via the CDK6/RUNX1/UCP1 Signaling Pathway. Food Funct. 2023, 14, 8201–8216. [Google Scholar] [CrossRef] [PubMed]
- Romero-Juárez, P.A.; Visco, D.B.; Manhães-de-Castro, R.; Urquiza-Martínez, M.V.; Saavedra, L.M.; González-Vargas, M.C.; Mercado-Camargo, R.; Aquino, J.d.S.; Toscano, A.E.; Torner, L.; et al. Dietary Flavonoid Kaempferol Reduces Obesity-Associated Hypothalamic Microglia Activation and Promotes Body Weight Loss in Mice with Obesity. Nutr. Neurosci. 2023, 26, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.A.F. The Mutagenic Activity of Quercetin and Kaempferol in Drosophila Melanogaster. Mutat. Res. Lett. 1982, 103, 145–147. [Google Scholar] [CrossRef]
- Silva, I.D.; Rodrigues, A.; Gaspar, J.; Mala, R.; Laires, A.; Rueff, J. Mutagenicity of Kaempferol in V79 Cells: The Role of Cytochromes P450. Teratog. Carcinog. Mutagen. 1996, 16, 229–241. [Google Scholar] [CrossRef]
- Lemos, C.; Peters, G.J.; Jansen, G.; Martel, F.; Calhau, C. Modulation of Folate Uptake in Cultured Human Colon Adenocarcinoma Caco-2 Cells by Dietary Compounds. Eur. J. Nutr. 2007, 46, 329–336. [Google Scholar] [CrossRef]
- Tu, L.-Y.; Bai, H.-H.; Cai, J.-Y.; Deng, S.-P. The Mechanism of Kaempferol Induced Apoptosis and Inhibited Proliferation in Human Cervical Cancer SiHa Cell: From Macro to Nano. Scanning 2016, 38, 644–653. [Google Scholar] [CrossRef]
- Goey, A.K.L.; Mooiman, K.D.; Beijnen, J.H.; Schellens, J.H.M.; Meijerman, I. Relevance of in Vitro and Clinical Data for Predicting CYP3A4-Mediated Herb-Drug Interactions in Cancer Patients. Cancer Treat. Rev. 2013, 39, 773–783. [Google Scholar] [CrossRef]
- Rendic, S.; Guengerich, F.P. Human Cytochrome P450 Enzymes 5-51 as Targets of Drugs and Natural and Environmental Compounds: Mechanisms, Induction, and Inhibition—Toxic Effects and Benefits. Drug Metab. Rev. 2018, 50, 256–342. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-T.-L.; Duong, V.-A.; Maeng, H.-J. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.-H.; Park, H.; Li, X.; Davidson, L.A.; Allred, C.; Patil, B.; Jayaprakasha, G.; Orr, A.A.; Mao, L.; Chapkin, R.S.; et al. Structure-Dependent Modulation of Aryl Hydrocarbon Receptor-Mediated Activities by Flavonoids. Toxicol. Sci. 2018, 164, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Lin, W.; Wu, J.; Chen, T. Flavonoids Activate Pregnane x Receptor-Mediated CYP3A4 Gene Expression by Inhibiting Cyclin-Dependent Kinases in HepG2 Liver Carcinoma Cells. BMC Biochem. 2010, 11, 23. [Google Scholar] [CrossRef]
- Kitakaze, T.; Makiyama, A.; Nakai, R.; Kimura, Y.; Ashida, H. Kaempferol Modulates TCDD- and t-BHQ-Induced Drug-Metabolizing Enzymes and Luteolin Enhances This Effect. Food Funct. 2020, 11, 3668–3680. [Google Scholar] [CrossRef]
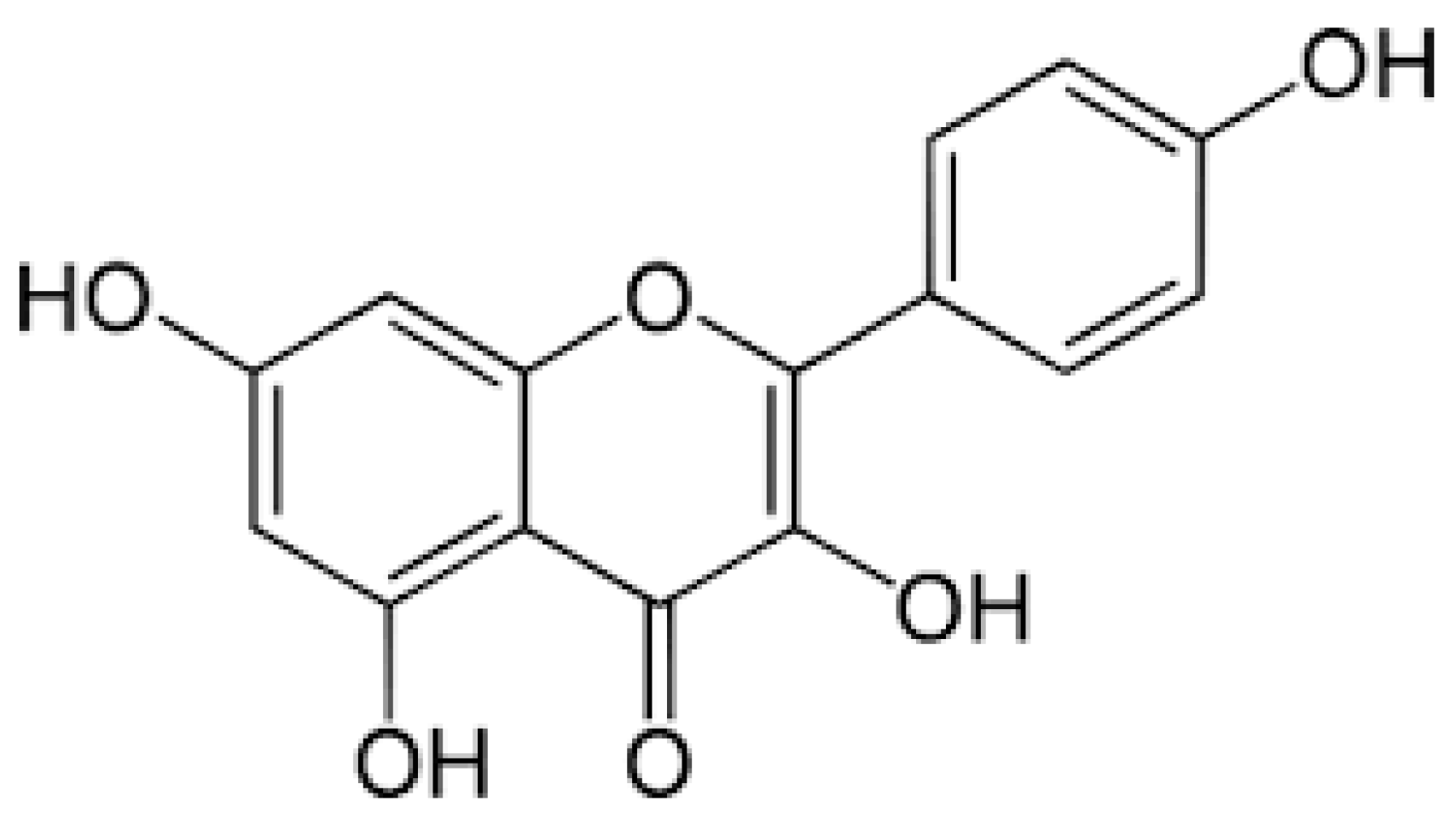

| Plant | Scientific Name | Amount | References | |
|---|---|---|---|---|
| Green chili | Capsicum annum | 39 | [40] | |
| Plant extracts [mg/kg dry mass] | Onion leaves | Allium fistulosum | 832 | |
| Papaya shoots | Carica papaya | 453 | ||
| Brinjal | Solanum melongena | 80 | ||
| Pumpkin | Cucurbita maxima | 371 | ||
| Sengkuang | Pachyrrhizus erosus | 37 | ||
| Carrot | Daucus carota | 140 | ||
| White radish | Raphanus sativus | 38 | ||
| Daun turi | Sesbania grandifolia | 21 | ||
| Lemon grass | Cymbopogon citratus | 178 | ||
| Cekur manis | Sauropus androgynus | 323 | ||
| Pegaga | Hydrocotyle asiatica | 20 | ||
| Bunga kantan | Phaeomeria speciosa | 286 | ||
| Black tea | Camellia sinensis | 118 | ||
| Beans | Phaseolus vulgaris | 14 | [41] | |
| Broccoli | Brassica oleracea var. italica | 72 | ||
| Cauliflower | Brassica oleracea var. botrytis | 270 | ||
| Plant-derived beverages [μg/mL] | Lemon juice | Citrus limon | 1.9 | [42] |
| Grapefruit juice | Citrus × paradisi | 1.1 | ||
| Pineapple juice | Ananas comosus | 1.2 | ||
| Apple juice | Malus domestica | 1.0 | ||
| Black tea | Camellia sinensis | 11.4 | [43] | |
| Plants [mg/100 g fresh weight] | Spinach | Spinacia oleracea | 7.86 | [43] |
| Garden cress | Lepidium sativum | 13.00 | ||
| Broccoli | Brassica oleracea var. italica | 5.65 | ||
| Kale | Brassica oleracea var. sabellica | 5.65 | ||
| Onion | Allium cepa | 26.74 | ||
| Rabbiteye blueberries | Vaccinium virgatum | 2.36 |
| Diseases | Model | Doses | Mechanism of Action/Effect | Ref. |
|---|---|---|---|---|
| MASLD | Male ddY mice | 4.9 mg/kg | ↓ TBARS and TNF-α caused by CCl4 free radicals | [66] |
| C57BLKS/J mice fed HFD | 87.5 µmol/kg | Regulation of hepatic lipid accumulation (activation of the SIRT1/AMPK pathway) | [74] | |
| HFD-induced SD rats | 350 µmol/kg | Prevention of advancement of simple fatty liver disease to non-alcoholic steatohepatitis (blocking the NF-κB pathway) | [85] | |
| HepG2 cells | 1 or 10 µM | |||
| C57BL/6 J mice fed HFD | 0.5 mL/100 g | Regulation of BA metabolism in the serum and liver (enhancing CYP27A1 and NTCP expression) | [106] | |
| MASLD | HepG2 cells | 50 mg/kg | ↓ fat buildup in the liver, ↑ NF-κB signalling pathway, ↑ mitochondrial beta-oxidation, ↑ expression of CPT1A | [107] |
| Liver injury | Bosentan-induced rat liver injury model and HEK-293 cells | 25 mg/kg and 1–150 µM | ↓ OATP1B1 transporter (keeping AST and ALT levels stable) | [108] |
| Male swiss albino rats | 25 mg/kg | ↓ lipid peroxidation caused by CCl4 free radicals | [109] | |
| Mice and HepG2 cells | 250 and 500 mg/kg and 100, 200, and 400 µM | ↓ AA + Fe-induced ROS, ↓ glutathione depletion | [110] | |
| ALI mice model | 10 and 20 mg/kg | ↓ antioxidant defence activity, ↑ lipid peroxidation and oxidative stress | [111] | |
| Liver fibrosis | L02, LX2, and rats | 20 µM | ↓ caspase-3 protein levels, ↑ p-ERK1/2, PI3K and Bcl-xL protein expression in L02 cells; ↑proliferation of LX2 cells, ↑ Bax and cleaved caspase-8. | [112] |
| HSCs/CCl4-induced mouse model | 2–10 µmol/L | ↓ hyaluronan, ALT, AST and Smad2/3, ↓ collagen synthesis and HSC activation; ↑activin receptor-like kinase 5 | [100] | |
| Liver cancer | HepG2 cells | 10, 20, 40, and 80 µM | ↑ ROS production, ↑ cytochrome c ↑ PIG3 mRNA and protein, ↓ mitochondrial membrane potential, ↓ f Bax/Bcl-2 and caspase-9 and -3 | [113] |
| Hepatotoxicity | Male C57BL/6 mice | 30 and 60 mg/kg | ↓ ALT and AST, ↓ liver cell damage, ↑ antioxidant enzymes and apoptosis; ↓ NLRP3 and pro-inflammatory molecules | [114] |
| Obesity | Wild-type zebrafish | 7.5, 15, and 30 µM | ↑ adipogenesis | [115] |
| The 3T3-L1 preadipocytes | 60 µM | ↑ lipolysis, ↓ adipogenesis | [116] | |
| Human mesenchymal fat cells | 1, 10 or 25 µM | ↑ lipolysis, ↓ adipogenesis | [117] | |
| The 3T3-L1 preadipocytes | 2.5, 5, 10, 20 and 40 µM | ↓ adipogenic transcription factors, ↑ PPARα-mediated signalling of FAO | [118] | |
| C57BL/6 J male mice fed HFD | 43.75, 87.5, and 175 µmol/kg | Regulation of adipocyte thermogenesis via the CDK6/RUNX1/UCP1 pathway | [119] | |
| C57BL/6 J male mice fed HFD | a high-fat diet with 0.1% kaempferol | ↑ intestinal barrier integrity, ↓ intestinal inflammation by inhibition of TLR4/NF-κB pathway | [105] | |
| C57BL/6 mice fed HFD | 350 µmol/kg | ↑ gut microbiota and ↓ the progression of insulin resistance. | [104] | |
| C57BL/6 mice fed HFD | 0.875 µmol/kg | Regulation of physiological processes concerning energy balance and inflammation | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niziński, P.; Krajewska, A.; Oniszczuk, T.; Polak, B.; Oniszczuk, A. Hepatoprotective Effect of Kaempferol—A Review. Molecules 2025, 30, 1913. https://doi.org/10.3390/molecules30091913
Niziński P, Krajewska A, Oniszczuk T, Polak B, Oniszczuk A. Hepatoprotective Effect of Kaempferol—A Review. Molecules. 2025; 30(9):1913. https://doi.org/10.3390/molecules30091913
Chicago/Turabian StyleNiziński, Przemysław, Anna Krajewska, Tomasz Oniszczuk, Beata Polak, and Anna Oniszczuk. 2025. "Hepatoprotective Effect of Kaempferol—A Review" Molecules 30, no. 9: 1913. https://doi.org/10.3390/molecules30091913
APA StyleNiziński, P., Krajewska, A., Oniszczuk, T., Polak, B., & Oniszczuk, A. (2025). Hepatoprotective Effect of Kaempferol—A Review. Molecules, 30(9), 1913. https://doi.org/10.3390/molecules30091913










