A New Protein–Ligand Trapping System to Rapidly Screen and Discover Small-Molecule Inhibitors of PD-L1 from Natural Products
Abstract
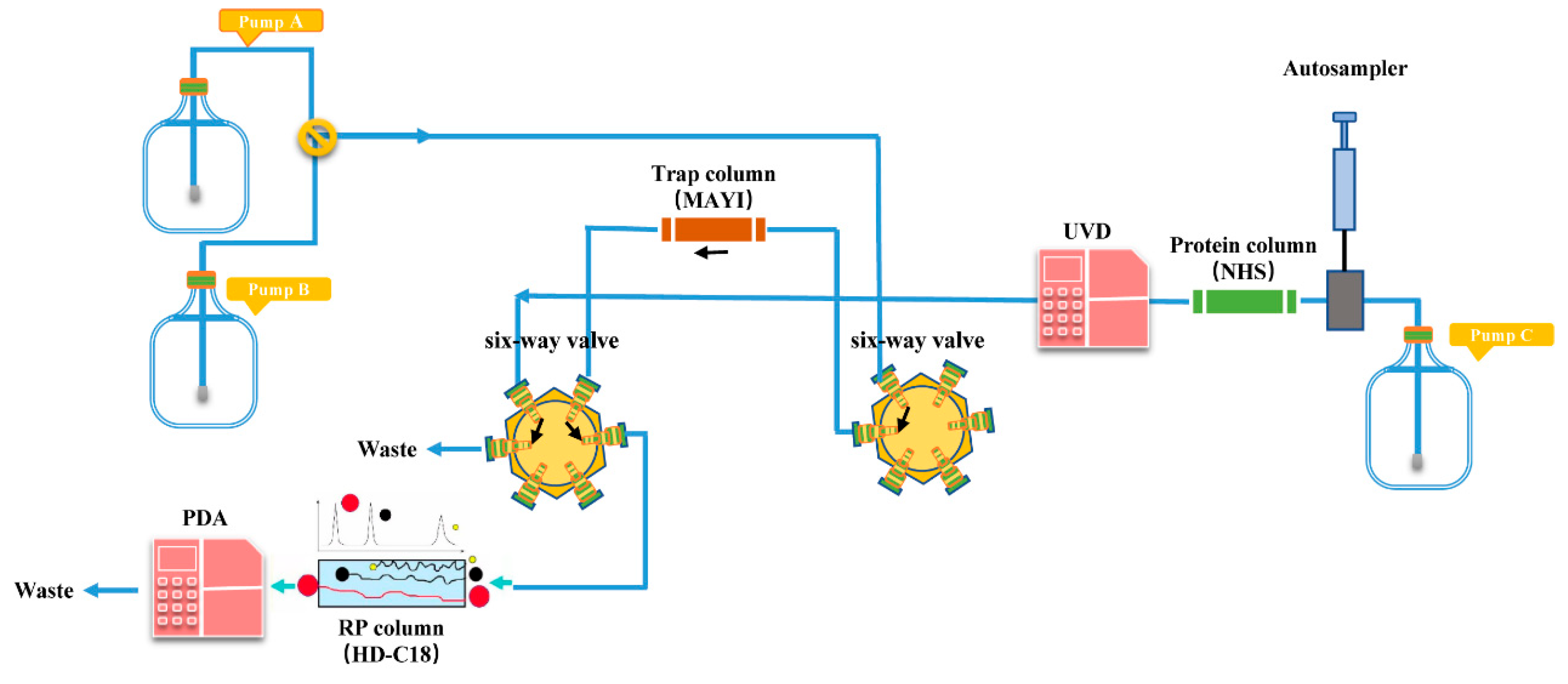
1. Introduction
2. Results and Discussion
2.1. The Effectiveness of the New PLT System Validated by Positive Medicine
2.2. PD-L1 Binding Active Compounds in TA Screened by New PLT System
2.3. SPR Validation Results: PD-L1 Binding Affinity at the Molecular Level
2.4. Cytopharmacology Validation Results: PD-1/PD-L1 Inhibiting Activity at the Cellular Level
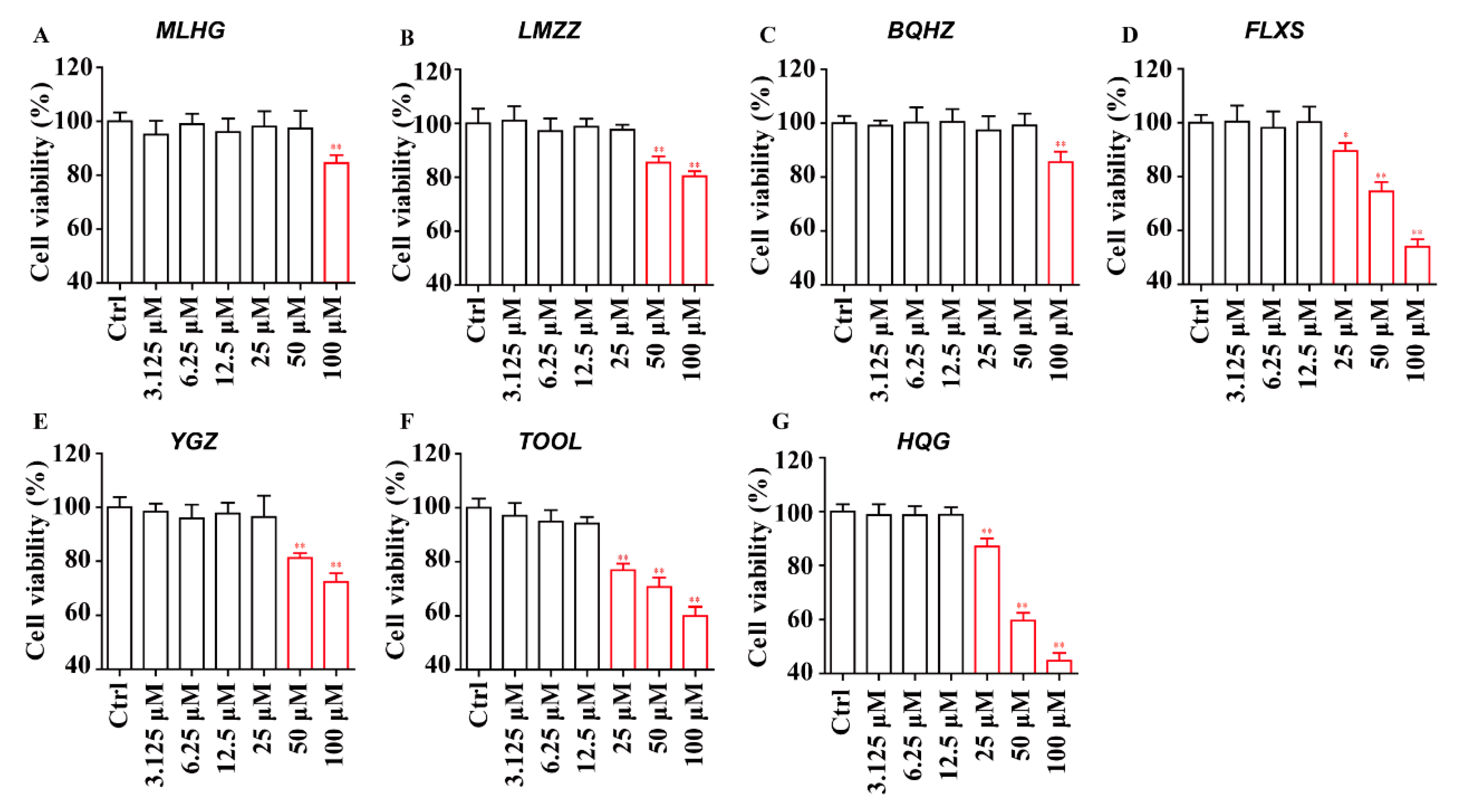
2.4.1. Cytotoxicity and Concentration Design of the In Vitro Study
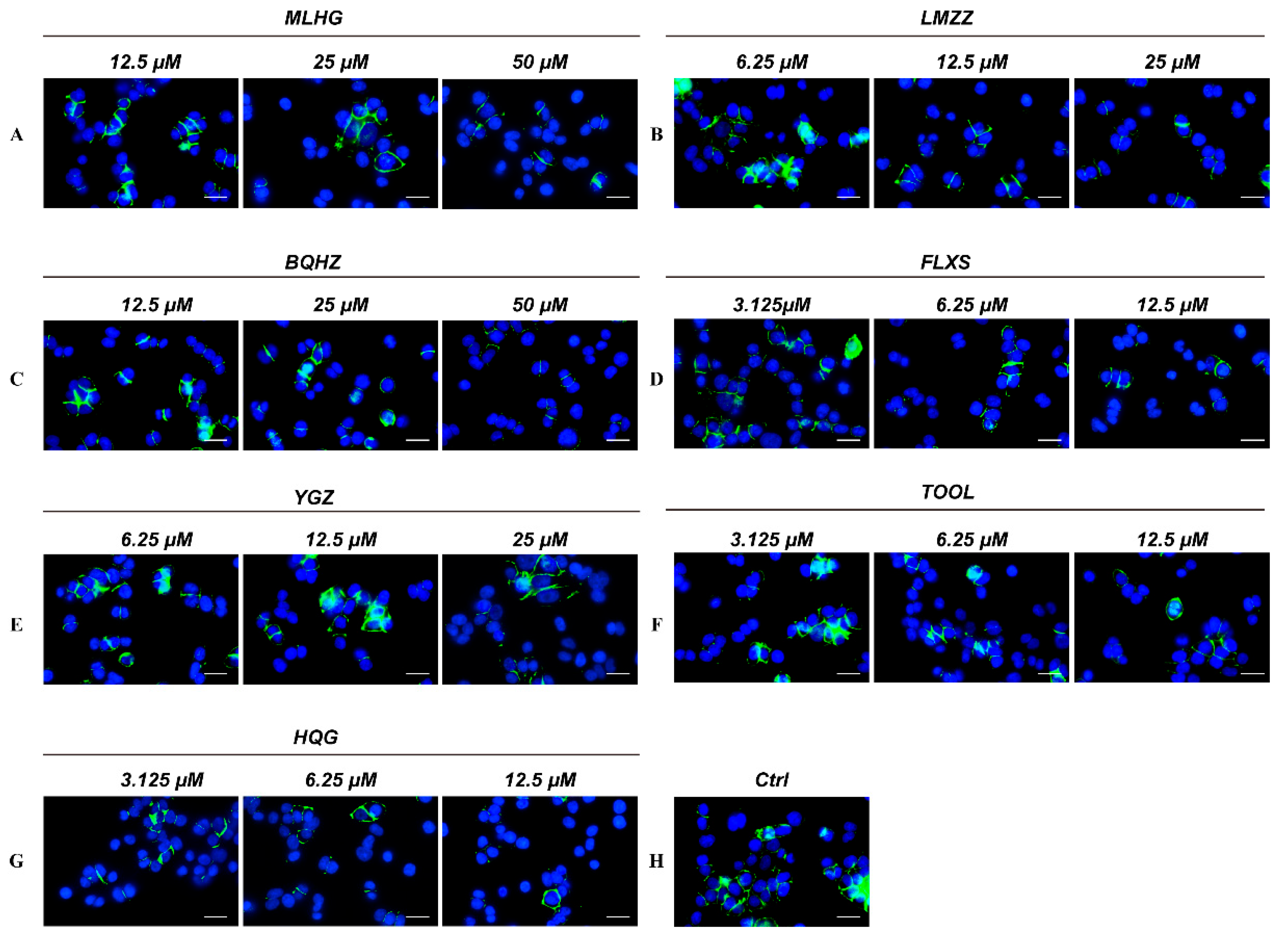
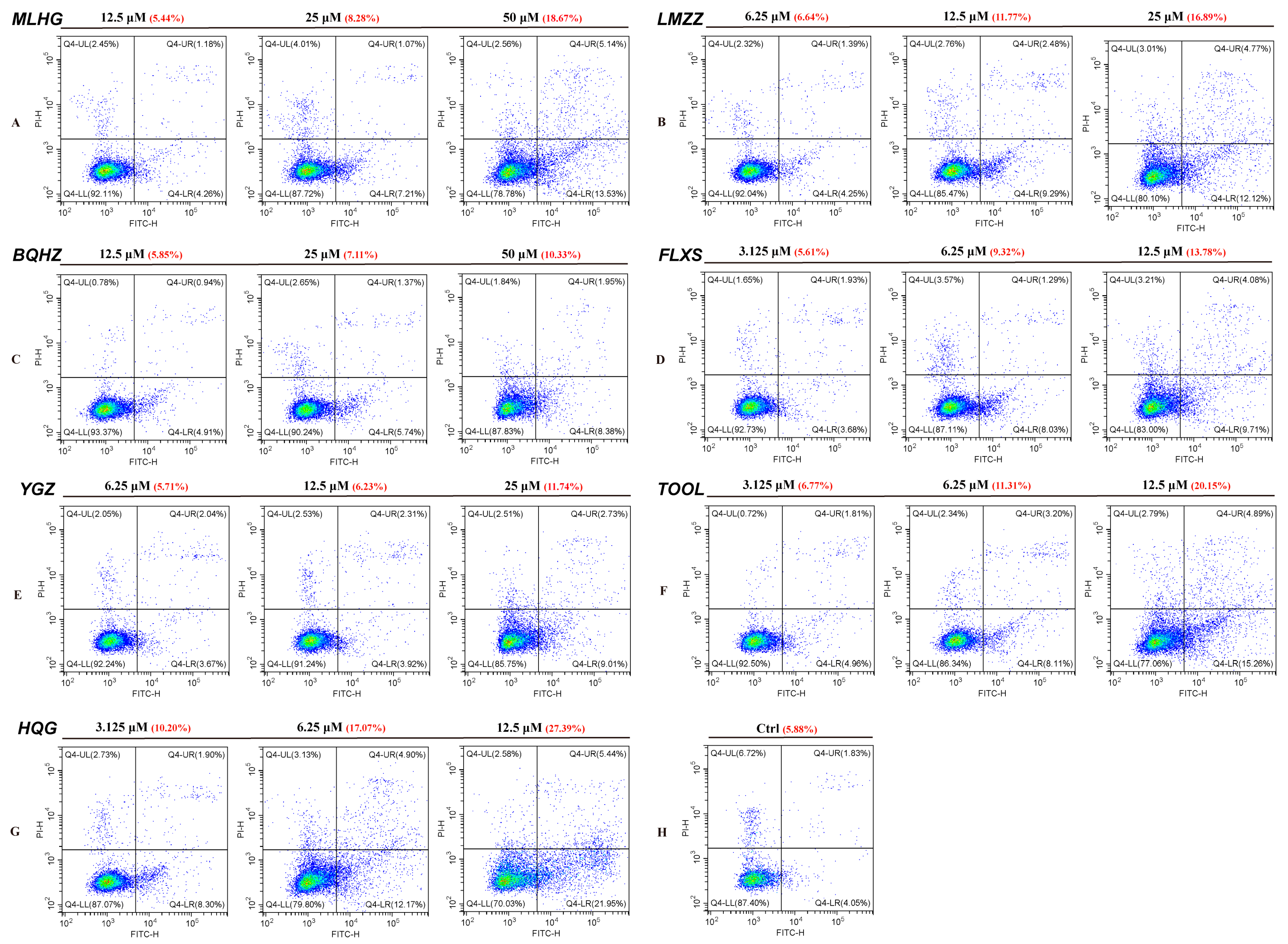
2.4.2. Immunofluorescence Validation Results: PD-1/PD-L1 Inhibiting Activity
2.4.3. Inhibitors May Promote A549 Apoptosis When Cocultured with PBMCs Induced by PD-1/PD-L1
3. Experimental Section
3.1. Materials
3.1.1. Reagents and Chemicals
3.1.2. Escherichia coli and Cell Culture
3.1.3. TA Sample Solution Preparation and Positive Medicine Selection
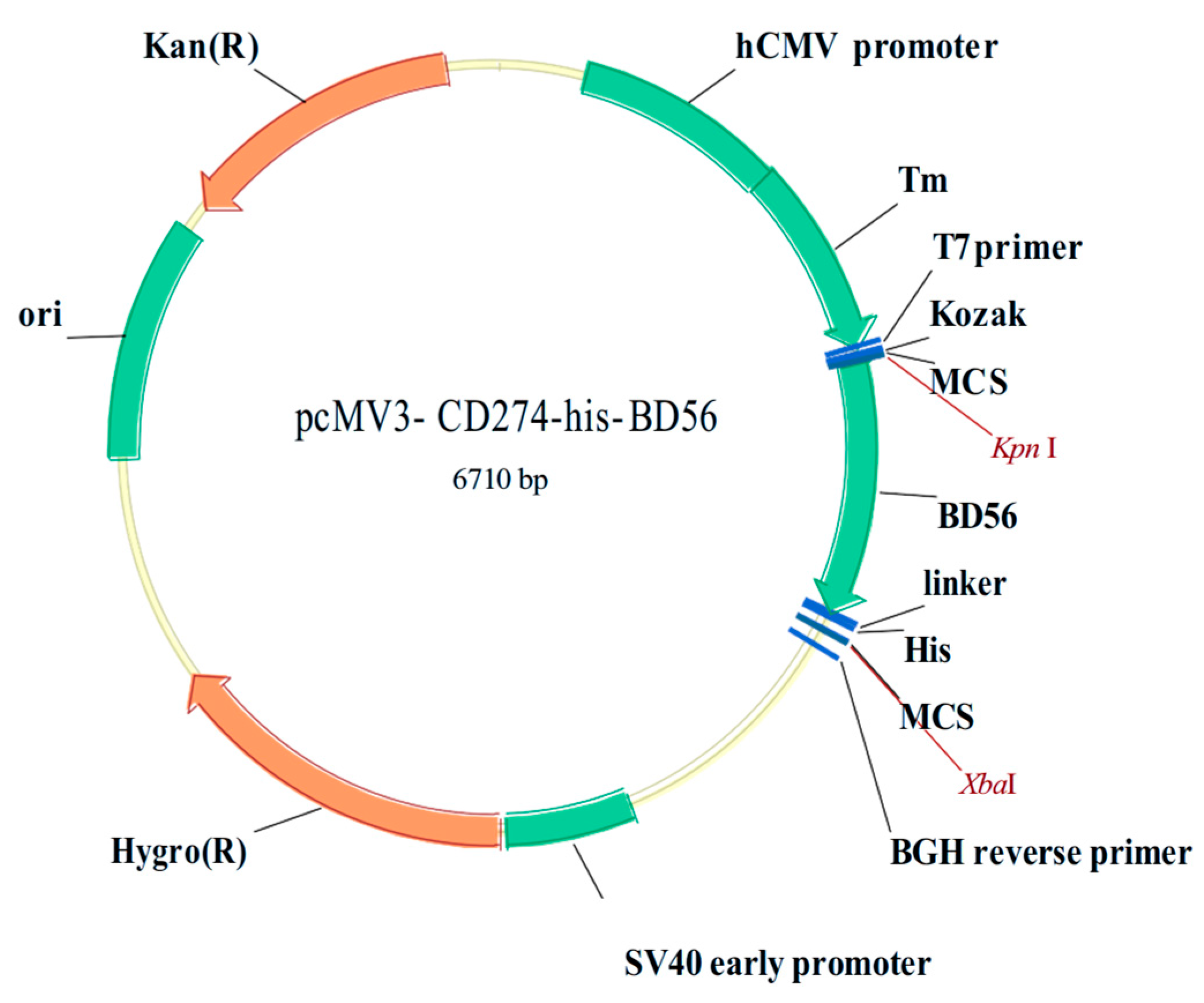
3.1.4. PD-L1 DNA Plasmid (Extracellular Fragment) Preparation and hPD-L1 Recombinant Protein Expression in Mammalian Cells
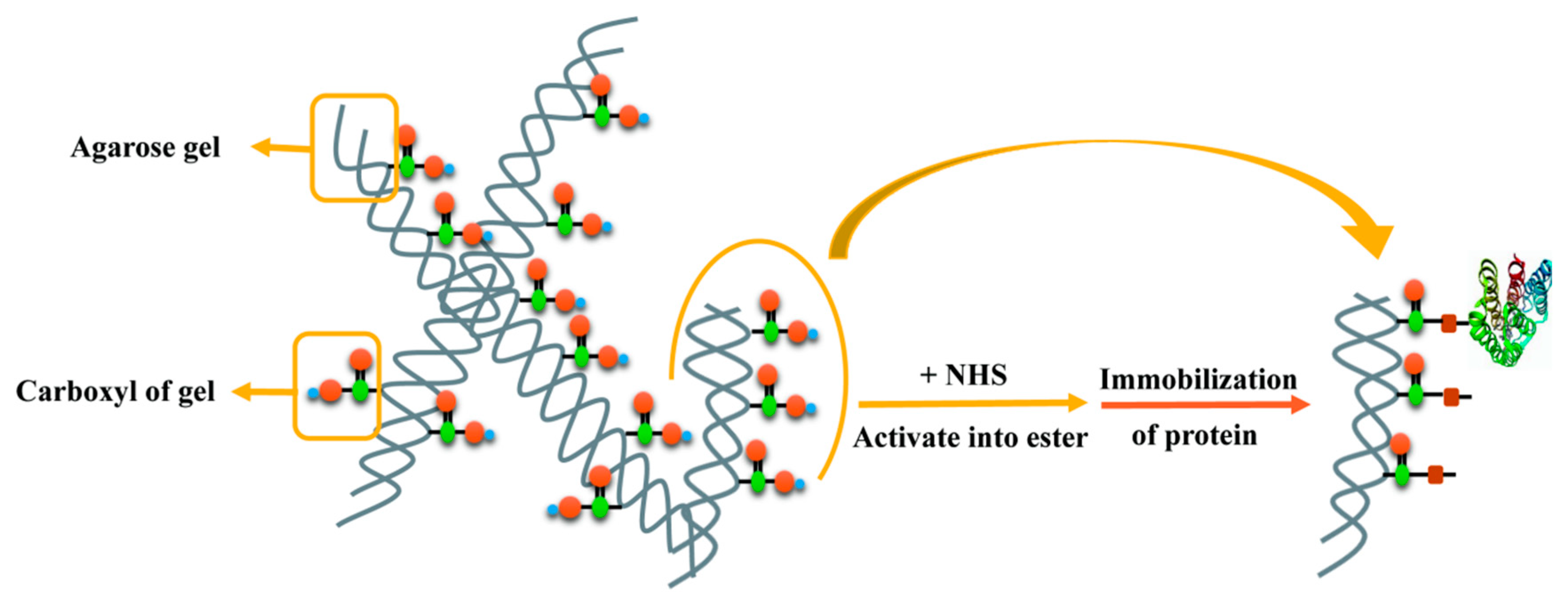
3.1.5. PD-L1 Protein Immobilized Affinity Column (PD-L1 Protein Column) Synthesis
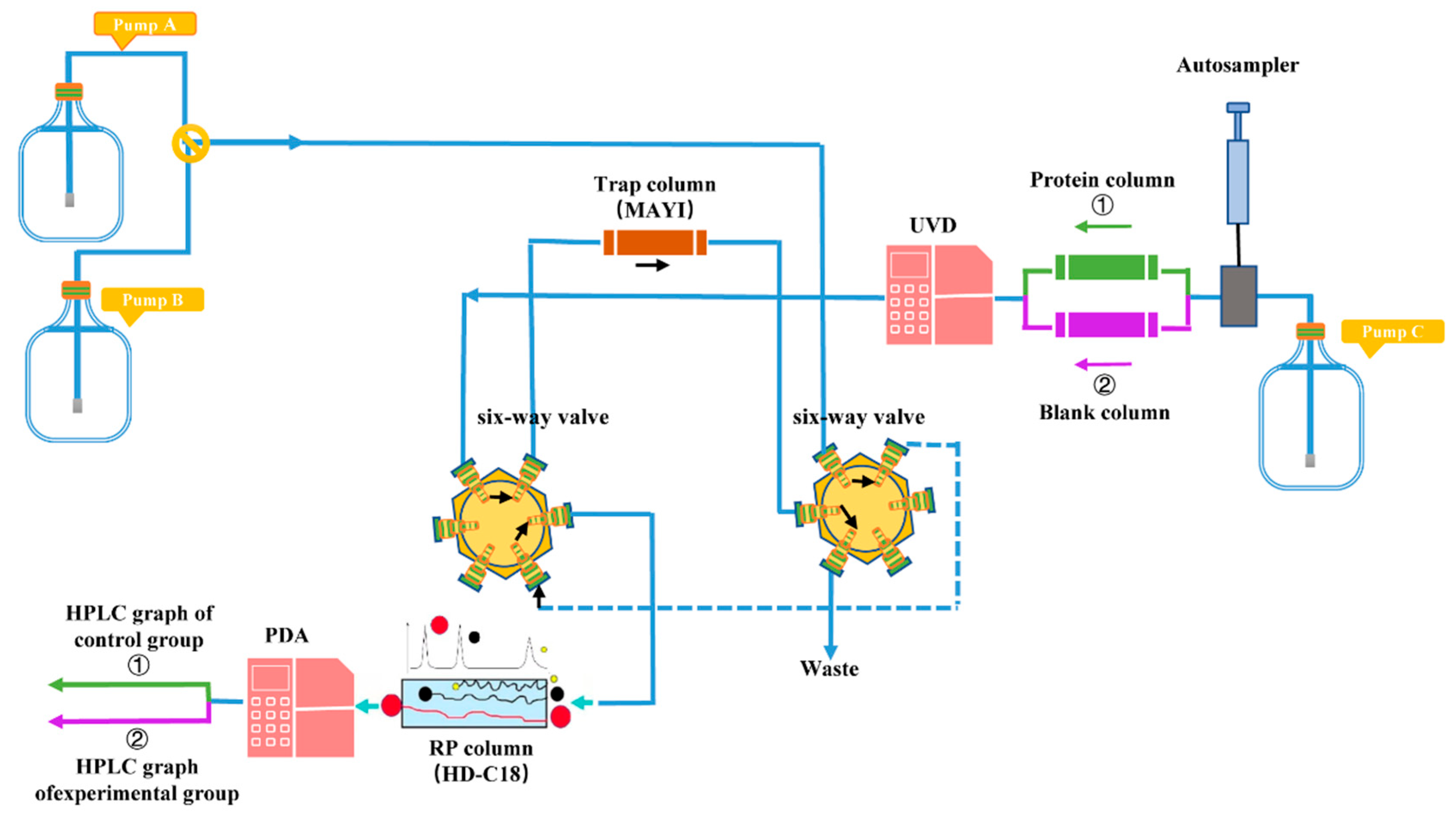
3.2. Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013, 342, 1432–1433. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. Raising the bar: The curative potential of human cancer immunotherapy. Sci. Transl. Med. 2012, 4, 127ps8. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.H.; Westwood, J.A.; Slaney, C.Y.; Darcy, P.K. Clinical application of genetically modified T cells in cancer therapy. Clin. Transl. Immunol. 2014, 3, e16. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chan, T.A. Cancer: Antitumour immunity gets a boost. Nature 2014, 515, 496–498. [Google Scholar] [CrossRef]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Terme, M.; Ullrich, E.; Aymeric, L.; Meinhardt, K.; Desbois, M.; Delahaye, N.; Viaud, S.; Ryffel, B.; Yagita, H.; Kaplanski, G.; et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011, 71, 5393–5399. [Google Scholar] [CrossRef]
- Agata, Y.; Kawasaki, A.; Nishimura, H.; Ishida, Y.; Tsubata, T.; Yagita, H.; Honjo, T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996, 8, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004, 4, 336–347. [Google Scholar] [CrossRef]
- Yamazaki, T.; Akiba, H.; Iwai, H.; Matsuda, H.; Aoki, M.; Tanno, Y.; Shin, T.; Tsuchiya, H.; Pardoll, D.M.; Okumura, K.; et al. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002, 169, 5538–5545. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Zak, K.M.; Grudnik, P.; Magiera, K.; Dömling, A.; Dubin, G.; Holak, T.A. Structural biology of the immune checkpoint receptor PD-1 and its ligands PD-L1/PD-L2. Structure 2017, 25, 1163–1174. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Wilkinson, E. Nivolumab success in untreated metastatic melanoma. Lancet Oncol. 2015, 16, e9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Y.; Zhang, W.; Yang, S.; Wang, C.; Guo, Q.; Shi, W. Clinical analysis of docetaxel combined with PD-1/PD-L1 inhibitor in second-line treatment of advanced non-small cell lung cancer. Chin. J. Lung Cancer 2021, 24, 605–612. [Google Scholar]
- Flessner, M.F.; Choi, J.; Credit, K.; Deverkadra, R.; Henderson, K. Resistance of tumor interstitial pressure to the penetration of intraperitoneally delivered antibodies into metastatic ovarian tumors. Clin. Cancer Res. 2005, 11, 3117–3125. [Google Scholar] [CrossRef]
- Inman, B.A.; Longo, T.A.; Ramalingam, S.; Harrison, M.R. Atezolizumab: A PD-L1-blocking antibody for bladder cancer. Clin. Cancer Res. 2017, 23, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Agrawal, L.; Bacal, A.; Jain, S.; Singh, V.; Emanuele, N.; Emanuele, M.; Meah, F. Immune checkpoint inhibitors and endocrine side effects, a narrative review. Postgrad. Med. 2020, 132, 206–214. [Google Scholar] [CrossRef]
- Chang, L.S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr. Rev. 2019, 40, 17–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, H.T.; Shin, W.; Chae, J.; Choi, J.; Kim, S.H.; Lim, H.; Won Heo, T.; Park, K.Y.; Lee, Y.J.; et al. Structural basis of checkpoint blockade by monoclonal antibodies in cancer immunotherapy. Nat. Commun. 2016, 7, 13354. [Google Scholar] [CrossRef]
- Lin, X.; Lu, X.; Luo, G.; Xiang, H. Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur. J. Med. Chem. 2020, 186, 111876. [Google Scholar] [CrossRef]
- Carvalho, S.; Levi-Schaffer, F.; Sela, M.; Yarden, Y. Immunotherapy of cancer: From monoclonal to oligoclonal cocktails of anti-cancer antibodies: IUPHAR Review 18. Br. J. Pharmacol. 2016, 173, 1407–1424. [Google Scholar] [CrossRef]
- Andrews, A.J.A.H.; Benefits, D. Treating with checkpoint inhibitors-figure $1 million per patient. Am. Health Drug Benefits 2015, 8, 9. [Google Scholar]
- Guzik, K.; Tomala, M.; Muszak, D.; Konieczny, M.; Hec, A.; Baszkiewicz, U.; Pustua, M.; Butera, R.; Dmling, A.; Holak, T.A. Development of the inhibitors that target the PD-1/PD-L1 interaction—A brief Look at progress on small molecules, peptides and macrocycles. Molecules 2019, 24, 2071. [Google Scholar] [CrossRef]
- Li, T.T.; Jiang, J.W.; Qie, C.X.; Xuan, C.X.; Hu, X.L.; Liu, W.M.; Chen, W.T.; Liu, J. Identification of active small-molecule modulators targeting the novel immune checkpoint VISTA. BMC Immunol. 2021, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, G.; Fu, X.; Wang, P.P.; Tan, C.P.; Chen, C. Ultrasonic collaborative pulse extraction of sugarcane polyphenol with good antiaging and α-glucosidase inhibitory activity. Int. J. Biol. Macromol. 2025, 297, 139930. [Google Scholar] [CrossRef]
- Yang, N.; Yue, R.; Ma, J.; Li, W.; Zhao, Z.; Li, H.; Shen, Y.; Hu, Z.; Lv, C.; Xu, X.; et al. Nitidine chloride exerts anti-inflammatory action by targeting Topoisomerase I and enhancing IL-10 production. Pharmacol. Res. 2019, 148, 104368. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, R.; Ghelardoni, S.; Carnicelli, V.; Frascarelli, S.; Ronca, F.; Ronca-Testoni, S. Ca2+ channel remodeling in perfused heart: Effects of mechanical work and interventions affecting Ca2+ cycling on sarcolemmal and sarcoplasmic reticulum Ca2+ channels. FASEB J. 2002, 16, 1976–1978. [Google Scholar] [CrossRef]
- Xin, C.L.; Hui, W.D.; Zhou, L.; Shu, S.D.; Zhi, L.L. Essential oil composition and larvicidal activity of Toddalia asiatica roots against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2013, 112, 1197–1203. [Google Scholar]
- Tsai, I.L.; Wun, M.F.; Teng, C.M.; Ishikawa, T.; Chen, I.S. Anti-platelet aggregation constituents from Formosan Toddalia asiatica. Phytochemistry 1998, 48, 1377–1382. [Google Scholar] [CrossRef]
- Hu, J.; Shi, X.; Chen, J.; Mao, X.; Zhu, L.; Yu, L.; Shi, J. Alkaloids from Toddalia asiatica and their cytotoxic, antimicrobial and antifungal activities. Food Chem. 2014, 148, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; Liu, Y.B.; Ma, S.G.; Li, L.; Li, Y.; Jiang, J.D.; Qu, J.; Yu, S.S. Antiviral spirotriscoumarins A and B: Two pairs of oligomeric coumarin enantiomers with a spirodienone-sesquiterpene skeleton from Toddalia asiatica. Org. Lett. 2016, 18, 5146–5149. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S.W.; Zhang, X.Y.; Liu, Y.; Liu, X.H.; Zhang, S.; Wang, W.; Wang, J.; Wang, W. Separation of new coumarin glycosides from Toddalia asiatica using offline two-dimensional high-performance liquid chromatography. Plants 2020, 9, 428. [Google Scholar] [CrossRef]
- Zeng, Z.; Tian, R.; Feng, J.; Yang, N.A.; Yuan, L. A systematic review on traditional medicine Toddalia asiatica (L.) Lam.: Chemistry and medicinal potential. Saudi Pharm. J. 2021, 29, 781–798. [Google Scholar] [CrossRef]
- Hao, X.Y.; Peng, L.; Ye, L.; Huang, N.H.; Shen, Y.M. A study on anti-inflammatory and analgesic effects of alkaloids of Toddalia asiatica. J. Chin. Integr. Med. 2004, 2, 450–452. [Google Scholar] [CrossRef][Green Version]
- Stephen Irudayaraj, S.; Sunil, C.; Duraipandiyan, V.; Ignacimuthu, S. Antidiabetic and antioxidant activities of Toddalia asiatica (L.) Lam.: Leaves in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2012, 143, 515–523. [Google Scholar] [CrossRef]
- Karunai Raj, M.; Balachandran, C.; Duraipandiyan, V.; Agastian, P.; Ignacimuthu, S. Antimicrobial activity of Ulopterol isolated from Toddalia asiatica (L.) Lam.: A traditional medicinal plant. J. Ethnopharmacol. 2012, 140, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Zhang, Z.; Xu, B.; Zhao, S.; Ding, Y.; Wu, X.; Wu, R.; Lv, Y.; Dong, J. Baicalein and baicalin promote antitumor immunity by suppressing PD-L1 expression in hepatocellular carcinoma cells. Int. Immunopharmacol. 2019, 75, 105824. [Google Scholar] [CrossRef]
- Tian, W.A.; Xie, X.B.; Cao, P.C. Magnoflorine improves sensitivity to doxorubicin (DOX) of breast cancer cells via inducing apoptosis and autophagy through AKT/mTOR and p38 signaling pathways. Biomed. Pharmacother. 2020, 121, 109139. [Google Scholar]
- Sun, X.L.; Zhang, X.W.; Zhai, H.J.; Zhang, D.; Ma, S.Y. Magnoflorine inhibits human gastric cancer progression by inducing autophagy, apoptosis and cell cycle arrest by JNK activation regulated by ROS. Biomed. Pharmacother. 2020, 125, 109118. [Google Scholar] [CrossRef] [PubMed]
- Voukeng, I.C.; Lafontaine, D.L.J. The natural alkaloid nitidine chloride targets RNA polymerase I to inhibit ribosome biogenesis and repress cancer cell growth. Cell Death Discov. 2025, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Guo, Y.; Zhang, J.B.; Wei, X.H. Induction of apoptosis by chelerythrine chloride through mitochondrial pathway and Bcl-2 family proteins in human hepatoma SMMC-7721 cell. Arch. Pharm. Res. 2011, 34, 791–800. [Google Scholar] [CrossRef]
- Vázquez, R.; Riveiro, M.E.; Vermeulen, M.; Mondillo, C.; Coombes, P.H.; Crouch, N.R.; Ismail, F.; Mulholland, D.A.; Baldi, A.; Shayo, C.; et al. Toddaculin, a natural coumarin from Toddalia asiatica, induces differentiation and apoptosis in U-937 leukemic cells. Phytomedicine 2012, 15, 8–9. [Google Scholar] [CrossRef]
- Cao, Y.; Fang, J.; Shi, Y.; Wang, H.; Chen, X.; Liu, Y.; Zhu, Z.; Cao, Y.; Hong, Z.; Chai, Y. Screening potential P-glycoprotein inhibitors by combination of a detergent-free membrane protein extraction with surface plasmon resonance biosensor. Acta Pharm. Sin. B 2022, 12, 3113–3123. [Google Scholar] [CrossRef]
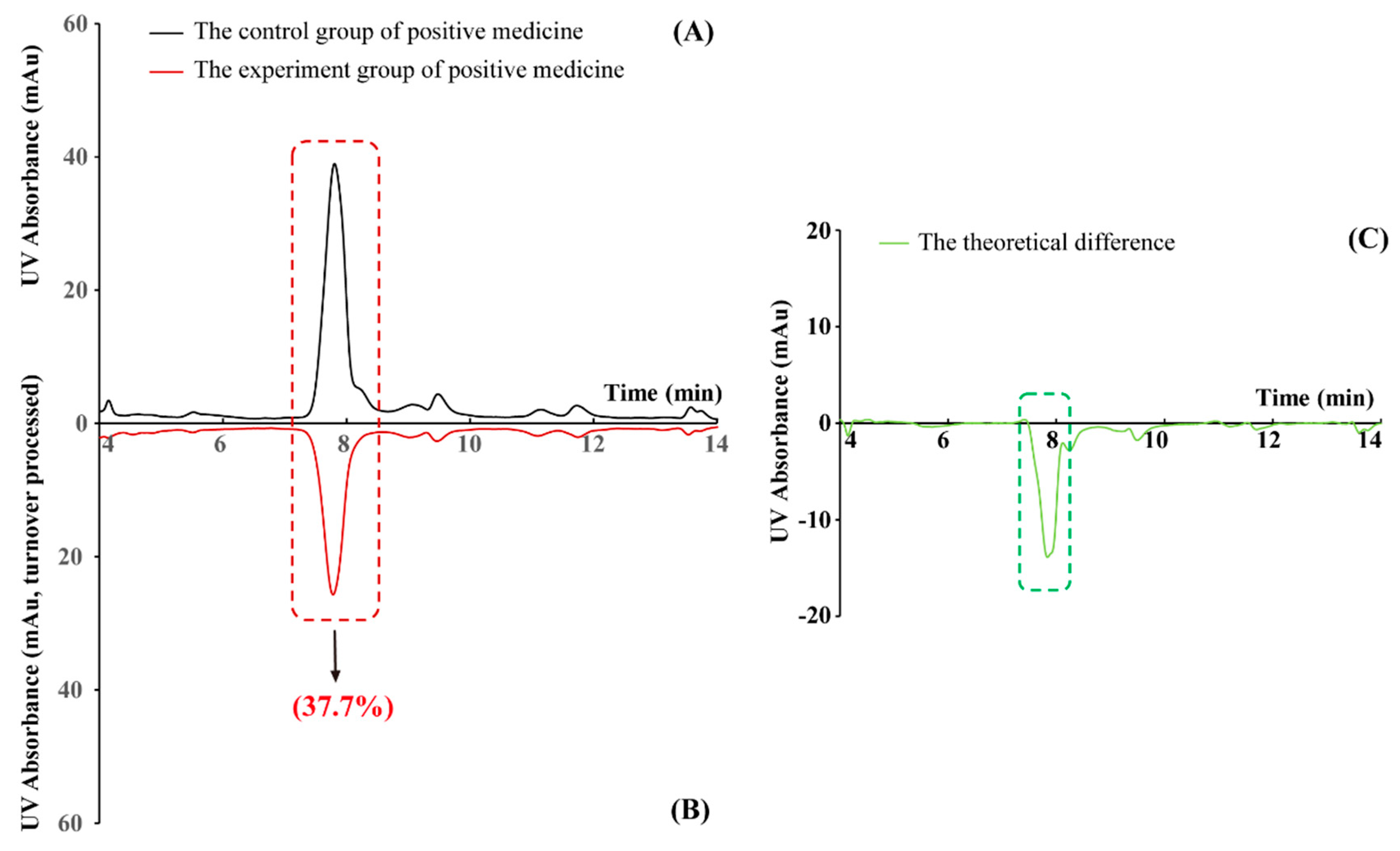
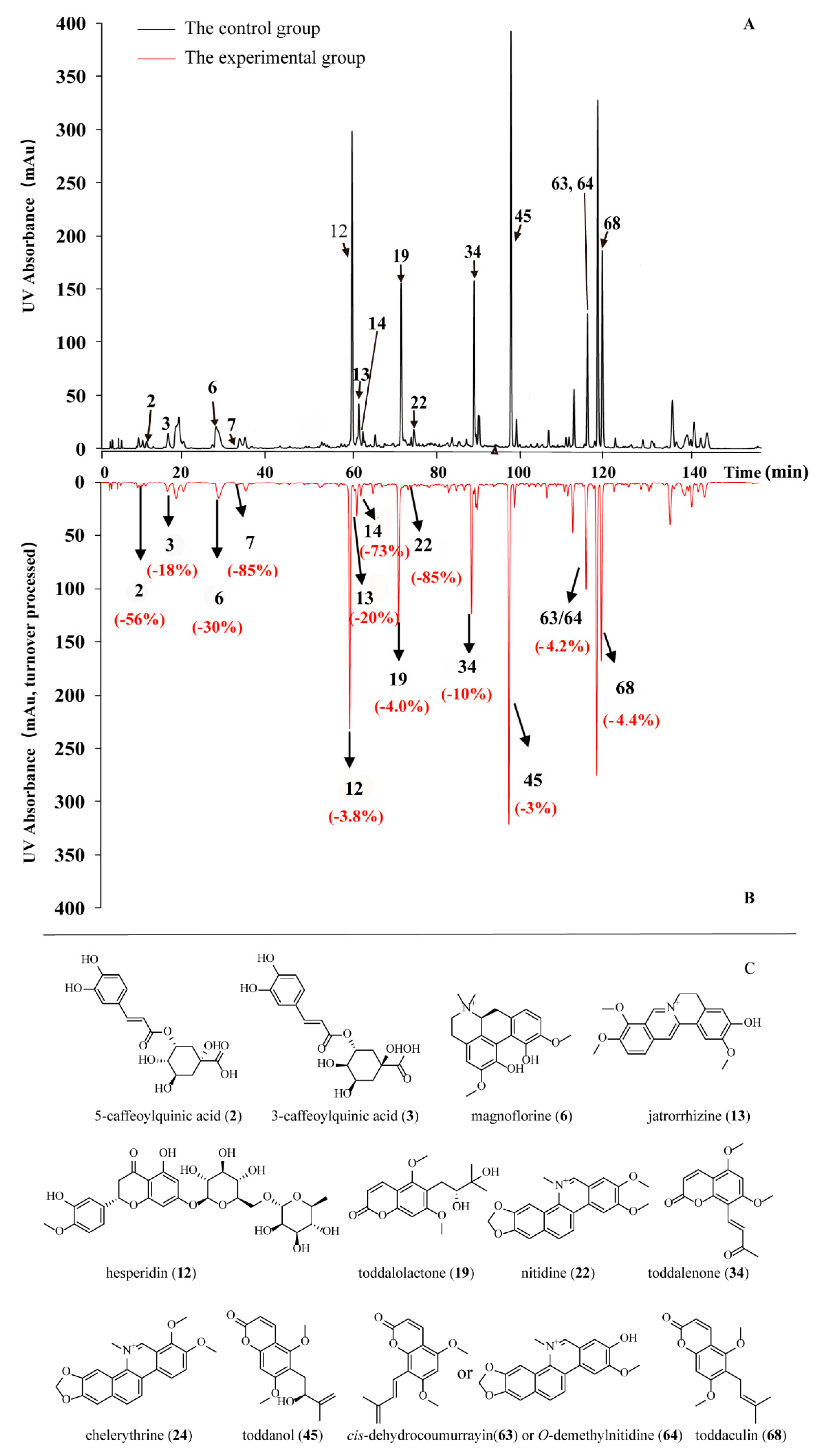

| NO. | Peak | TR (min) | An | Ap | RCP (%) | Peak Area Drop Value |
|---|---|---|---|---|---|---|
| 2 | 7 | 10.278 | 217,068 | 95,476 | −56.015626 | 121,592 |
| 3 | 8 | 15.546 | 497,316 | 405,786 | −18.404797 | 91,530 |
| 6 | 11 | 26.808 | 1,489,829 | 1,042,284 | −30.040025 | 447,545 |
| 7 | 12 | 32.328 | 395,747 | 58,265 | −85.27721 | 337,482 |
| 12 | 15 | 58.773 | 5,312,326 | 5,110,540 | −3.7984491 | 201,786 |
| 13 | 16 | 60.376 | 818,092 | 656,451 | −19.758291 | 161,641 |
| 14 | 17 | 61.762 | 108,244 | 29,147 | −73.072872 | 79,097 |
| 19 | 20 | 70.308 | 3,480,298 | 3,342,819 | −3.9502077 | 137,479 |
| 22 | 22 | 73.297 | 425,276 | 62,145 | −85.387137 | 363,131 |
| 34 | 27 | 87.459 | 2,469,279 | 2,220,585 | −10.071523 | 248,694 |
| 45 | 29 | 96.103 | 6,282,474 | 6,069,301 | −3.3931378 | 213,173 |
| 63/64 | 39 | 114.078 | 2,164,023 | 2,073,044 | −4.2041605 | 90,979 |
| 68 | 41 | 116.525 | 5,727,162 | 5,472,947 | −4.4387604 | 254,215 |
| 24 | 23 | 76.127 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Sun, S.; Yin, R.; Lin, Z.; Wang, D.; Wang, W.; Fu, X.; Wang, J.; Lei, X.; Sun, M.; et al. A New Protein–Ligand Trapping System to Rapidly Screen and Discover Small-Molecule Inhibitors of PD-L1 from Natural Products. Molecules 2025, 30, 1754. https://doi.org/10.3390/molecules30081754
Huang Y, Sun S, Yin R, Lin Z, Wang D, Wang W, Fu X, Wang J, Lei X, Sun M, et al. A New Protein–Ligand Trapping System to Rapidly Screen and Discover Small-Molecule Inhibitors of PD-L1 from Natural Products. Molecules. 2025; 30(8):1754. https://doi.org/10.3390/molecules30081754
Chicago/Turabian StyleHuang, Yazhuo, Senfeng Sun, Runxin Yin, Zongtao Lin, Daidong Wang, Wanwan Wang, Xiangyu Fu, Jing Wang, Xinyu Lei, Mimi Sun, and et al. 2025. "A New Protein–Ligand Trapping System to Rapidly Screen and Discover Small-Molecule Inhibitors of PD-L1 from Natural Products" Molecules 30, no. 8: 1754. https://doi.org/10.3390/molecules30081754
APA StyleHuang, Y., Sun, S., Yin, R., Lin, Z., Wang, D., Wang, W., Fu, X., Wang, J., Lei, X., Sun, M., Chen, S., & Wang, H. (2025). A New Protein–Ligand Trapping System to Rapidly Screen and Discover Small-Molecule Inhibitors of PD-L1 from Natural Products. Molecules, 30(8), 1754. https://doi.org/10.3390/molecules30081754






