Unlocking the Health Secrets of Onions: Investigating the Phytochemical Power and Beneficial Properties of Different Varieties and Their Parts
Abstract
1. Introduction
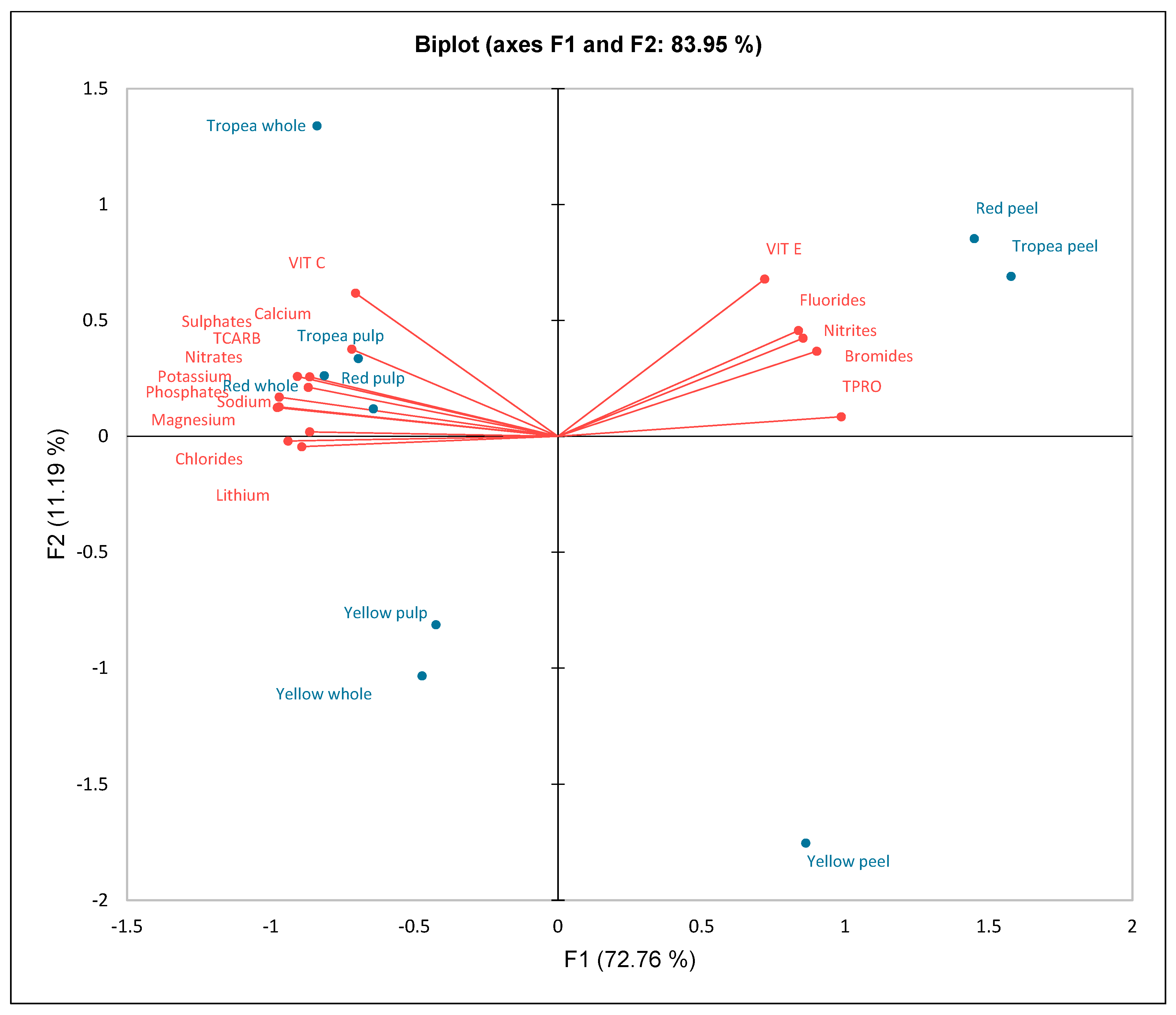
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Extract Preparation
4.2. Chemicals
4.3. Protein and Carbohydrate Detection
4.4. Determination of Total Phenolic Compounds, Total Flavonoids, and Vitamins A, C, and E
4.5. Determination of Antioxidant Activities
4.6. RP-DAD-HPLC Identification of Phenolic and Flavonoid Components
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suleria, H.A.R.; Butt, M.S.; Anjum, F.M.; Saeed, F.; Khalid, N. Onion: Nature protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2013, 55, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Taglienti, A.; Araniti, F.; Piscopo, A.; Tiberini, A. Characterization of Volatile Organic Compounds in ‘Rossa di Tropea’ Onion by Means of Headspace Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry (HS/SPME GC–MS) and Sensory Analysis. Agronomy 2021, 11, 874. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Średnicka-Tober, D.; Barański, M.; Hallmann, E.; Góralska-Walczak, R.; Kopczyńska, K.; Rembiałkowska, E.; Górski, J.; Leifert, C.; Rempelos, L. The Effect of Different Fertilization Regimes on Yield, Selected Nutrients, and Bioactive Compounds Profiles of Onion. Agronomy 2021, 11, 883. [Google Scholar] [CrossRef]
- Yang, J.; Meyers, K.J.; Van Der Heide, J.; Liu, R.H. Varietal differences in phenolic content and antioxidant and antiproliferative activities of onions. J. Agric. Food Chem. 2004, 52, 6787–6793. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, Y.-J.; Shin, Y. Comparative analysis of polyphenol content and antioxidant activity of different parts of five onion cultivars harvested in Korea. Antioxidants 2024, 13, 197. [Google Scholar] [CrossRef]
- Abbasi, A.; Sanej, K.D.; Moradi, S.; Bazzaz, S.; Esmaeili, A.; Ghafourian, K.; Sabahi, S.; Lahouty, M.; Akrami, S.; Aslani, R.; et al. Bioactive Compounds and Biological Activities of Allium sativum L. In Reference Series in Phytochemistry; Springer Nature: Cham, Switzerland, 2023; pp. 1–40. [Google Scholar] [CrossRef]
- Iwar, K.; Ochar, K.; Seo, Y.A.; Ha, B.-K.; Kim, S.-H. Alliums as potential antioxidants and anticancer agents. Int. J. Mol. Sci. 2024, 25, 8079. [Google Scholar] [CrossRef]
- Jurgiel-Malecka, G.; Gibczynska, M.; Nawrocka-Pezik, M. Comparison of chemical composition of selected cultivars of white, yellow and red onions. Bulg. J. Agric. Sci. 2015, 21, 736–741. [Google Scholar]
- Kwak, J.-H.; Seo, J.M.; Kim, N.-H.; Arasu, M.V.; Kim, S.; Yoon, M.K.; Kim, S.-J. Variation of quercetin glycoside derivatives in three onion (Allium cepa L.) varieties. Saudi J. Biol. Sci. 2016, 24, 1387–1391. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidative phenolic constituents of skins of onion varieties and their activities. J. Funct. Foods 2013, 5, 1191–1203. [Google Scholar] [CrossRef]
- Lakshmikanthan, M.; Muthu, S.; Krishnan, K.; Altemimi, A.B.; Haider, N.N.; Govindan, L.; Selvakumari, J.; Alkanan, Z.T.; Cacciola, F.; Francis, Y.M. A Comprehensive Review on Anthocyanin-rich foods: Insights into Extraction, Medicinal Potential, and Sustainable Applications. J. Agric. Food Res. 2024, 17, 101245. [Google Scholar] [CrossRef]
- Olalusi, A. Hot Air Drying and Quality of Red and White Varieties of Onion (Allium cepa). J. Agric. Chem. Environ. 2014, 3, 13–19. [Google Scholar] [CrossRef]
- Russo, M.T.; Cefaly, V.; Di Sanzo, R.; Carabetta, S.; Postorino, S.; Serra, D. Characterization of different “tropea red onion” (Allium cepa L.) ecotypes by aroma precursors, aroma profiles and polyphenolic composition. Acta Hortic. 2012, 939, 197–203. [Google Scholar] [CrossRef]
- Ren, F.; Zhou, S. Phenolic Components and Health Beneficial Properties of Onions. Agriculture 2021, 11, 872. [Google Scholar] [CrossRef]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, X.; Qu, Y.; Jiang, Y.; Liu, X.; Xiao, Y.; Lv, K.; Xu, Y.; Liu, K. Neuroprotective effects of healthful plant-based diets on retinal structure: Insights from a large cohort. J. Nutr. Health Aging 2024, 29, 100431. [Google Scholar] [CrossRef]
- Devirgiliis, C.; Guberti, E.; Mistura, L.; Raffo, A. Effect of fruit and vegetable consumption on Human Health: An update of the literature. Foods 2024, 13, 3149. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Kothari, D.; Lee, W.-D.; Kim, S.-K. Allium flavonols: Health benefits, molecular targets, and bioavailability. Antioxidants 2020, 9, 888. [Google Scholar] [CrossRef]
- Taleghani, A.; Ayati, Z.; Eghbali, S.; Emami, S.A.; Tayarani-Najaran, Z. Health benefits of Allium spp. in metabolic syndrome: A review. S. Afr. J. Bot. 2024, 167, 217–255. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; Regueiro, J.; Simal-Gándara, J.; Rodrigues, A.S.; Almeida, D.P.F. Increasing the Added-Value of Onions as a Source of Antioxidant Flavonoids: A Critical Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Samota, M.K.; Sharma, M.; Kaur, K.; Sarita, N.; Yadav, D.K.; Pandey, A.K.; Tak, Y.; Rawat, M.; Thakur, J.; Rani, H. Onion anthocyanins: Extraction, stability, bioavailability, dietary effect, and health implications. Front. Nutr. 2022, 9, 917617. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Muskała, M.; Merecz-Sadowska, A.; Sikora, J.; Picot, L.; Sitarek, P. Anti-Inflammatory and anticancer effects of anthocyanins in in vitro and in vivo studies. Antioxidants 2024, 13, 1143. [Google Scholar] [CrossRef]
- González-De-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Carrera, C.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Flavonol Composition and Antioxidant Activity of Onions (Allium cepa L.) Based on the Development of New Analytical Ultrasound-Assisted Extraction Methods. Antioxidants 2021, 10, 273. [Google Scholar] [CrossRef]
- Cheng, A.; Chen, X.; Jin, Q.; Wang, W.; Shi, J.; Liu, Y. Comparison of phenolic content and antioxidant capacity of red and yellow onions. Czech J. Food Sci. 2013, 31, 501–508. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Almeida, D.P.F.; Simal-Gándara, J.; Pérez-Gregorio, M.R. Onions: A source of flavonoids. In InTech eBooks; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Chadorshabi, S.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red Onion Skin Active Ingredients, Extraction and Biological Properties for Functional Food Applications. Food Chem. 2022, 386, 132737. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Carr, A.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Singh, D.P.; Sarma, B.K.; Upadhyay, G.; Singh, H.B. Polyphenolics from various extracts/fractions of red onion (Allium cepa) peel with potent antioxidant and antimutagenic activities. Food Chem. Toxicol. 2009, 47, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Polyphenol-Rich Dry Common Beans (Phaseolus vulgaris L.) and Their Health Benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.H. Overview of the vital roles of macro minerals in the human body. J. Trace Elem. Miner. 2023, 4, 100076. [Google Scholar] [CrossRef]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef]
- Daglia, M.; Lorenzo, A.; Nabavi, S.; Talas, Z.; Nabavi, S. Polyphenols: Well Beyond The Antioxidant Capacity: Gallic Acid and Related Compounds as Neuroprotective Agents: You are What You Eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef]
- Cikman, O.; Soylemez, O.; Ozkan, O.F.; Kiraz, H.A.; Sayar, I.; Ademoglu, S.; Taysi, S.; Karaayvaz, M. Antioxidant activity of syringic acid prevents oxidative stress in L-arginine–Induced acute pancreatitis: An experimental study on rats. Int. Surg. 2015, 100, 891–896. [Google Scholar] [CrossRef]
- Chen, C. Sinapic acid and its derivatives as medicine in oxidative Stress-Induced diseases and aging. Oxidative Med. Cell. Longev. 2015, 2016, 3571614. [Google Scholar] [CrossRef]
- Tošović, J.; Bren, U. Antioxidative action of ellagic Acid—A Kinetic DFT Study. Antioxidants 2020, 9, 587. [Google Scholar] [CrossRef]
- Mallamaci, R.; Conforti, F.; Statti, G.; Avato, P.; Barbarossa, A.; Meleleo, D. Phenolic Compounds from Tropea Red Onion as Dietary Agents for Protection against Heavy Metals Toxicity. Life 2024, 14, 495. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of flavonoids on disease prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef]
- Čižmárová, B.; Hubková, B.; Tomečková, V.; Birková, A. Flavonoids as promising natural compounds in the prevention and treatment of selected skin diseases. Int. J. Mol. Sci. 2023, 24, 6324. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Calderaro, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Panuccio, M.R. Soil salinity improves nutritional and health promoting compounds in three varieties of lentil (Lens culinaris Med.). Food Biosci. 2020, 35, 100571. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Panuccio, M.R. Sulfur bentonite-organic-based fertilizers as tool for improving bio-compounds with antioxidant activities in red onion. J. Sci. Food Agric. 2019, 100, 785–793. [Google Scholar] [CrossRef]
- Aremu, S.; Nweze, C. Determination of vitamin A content from selected Nigerian fruits using spectrophotometric method. Bangladesh J. Sci. Ind. Res. 2017, 52, 153–158. [Google Scholar] [CrossRef]
- Davies, S.H.R.; Masten, S.J. Spectrophotometric method for ascorbic acid using dichlorophenolindophenol: Elimination of the interference due to iron. Anal. Chim. Acta 1991, 248, 225–227. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]

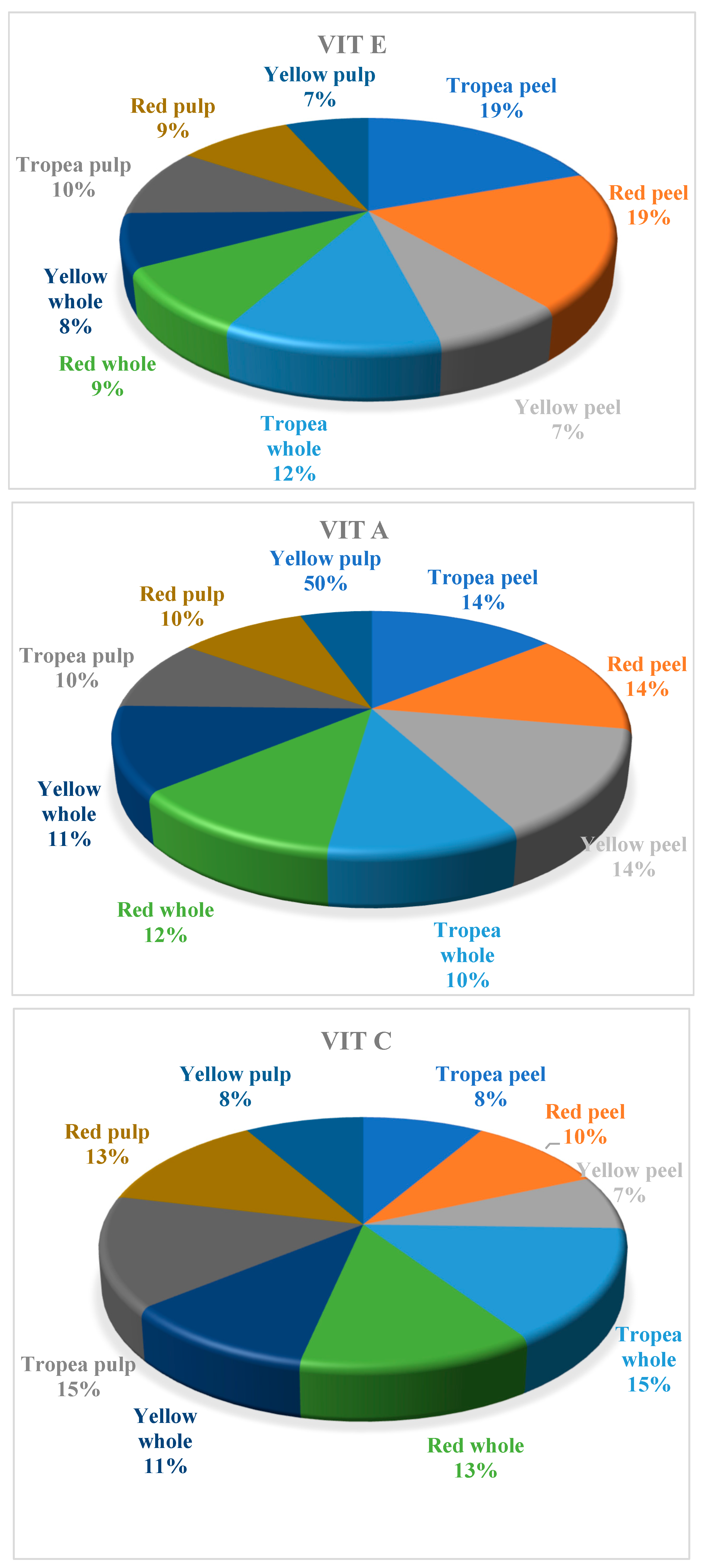
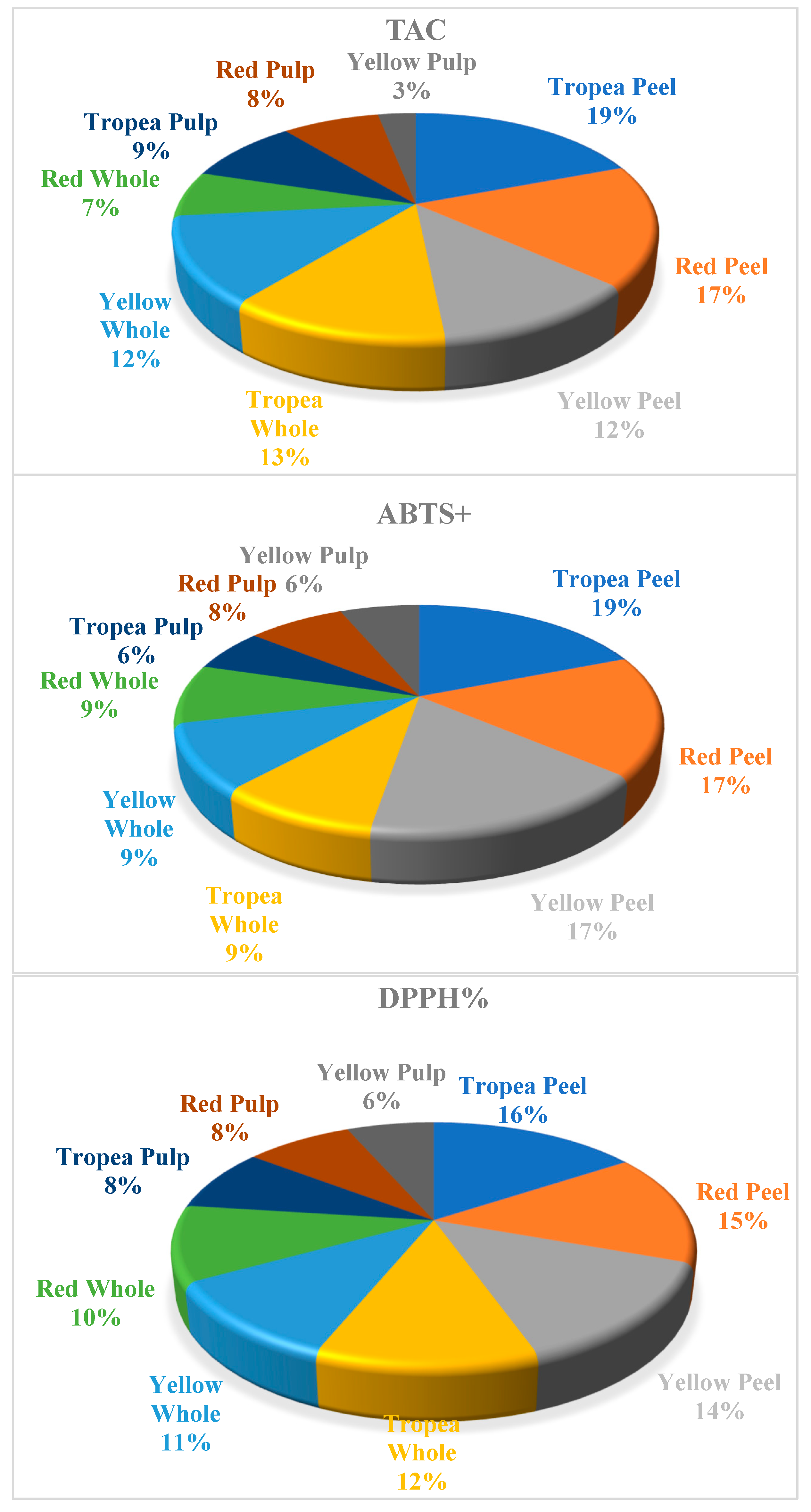
| Cations | Lithium | Sodium | Potassium | Magnesium | Calcium |
|---|---|---|---|---|---|
| Tropea peel | n.d. | 0.5 ± 0.02 | 1.1 c ± 0.04 | 0.2 c ± 0.01 | 7.7 c ± 0.02 |
| Red peel | n.d. | 0.7 d ± 0.05 | 1.1 c ± 0.01 | 0.2 c ± 0.21 | 7.6 c ± 0.12 |
| Yellow peel | n.d. | 0.5 d ± 0.01 | 1.1 c ± 0.12 | 0.2 c ± 0.08 | 7.6 c ± 0.23 |
| Tropea whole | 0.04 a ± 0.01 | 1.44 c ± 0.32 | 39.8 a ± 1.56 | 2.8 a ± 0.04 | 16.1 a ± 0.11 |
| Red whole | 0.09 a ± 0.01 | 1.80 b ± 0.01 | 35.8 b ± 2.01 | 2.4 a ± 0.01 | 8.5 c ± 0.32 |
| Yellow whole | 0.06 a ± 0.01 | 1.64 b ± 0.01 | 34.2 c ± 2.45 | 2.3 a ± 0.02 | 10.1 b ± 0.12 |
| Tropea pulp | 0.07 a ± 0.01 | 2.31 a ± 0.01 | 38.4 a ± 3.01 | 2.7 a ± 0.07 | 10.5 b ± 0.23 |
| Red pulp | 0.07 a ± 0.01 | 2.63 a ± 0.01 | 31.9 b ± 1.01 | 2.6 a ± 0.02 | 12.5 b ± 0.16 |
| Yellow pulp | 0.07 a ± 0.01 | 2.28 a ± 0.01 | 31.6 b ± 4.01 | 2.5 a ± 0.04 | 11 b ± 0.19 |
| Anions | Fluorides | Chlorides | Nitrites | Bromides | Nitrates | Phosphates | Sulfates |
|---|---|---|---|---|---|---|---|
| Tropea peel | 0.011 a ± 0.03 | 0.123 b ± 0.05 | 0.029 a ± 0.01 | 0.044 a ± 0.01 | 0.004 c ± 0.01 | n.d. | n.d. |
| Red peel | 0.010 a ± 0.06 | 0.122 b ± 0.04 | 0.032 a ± 0.03 | 0.039 a ± 0.03 | n.d. | n.d. | 0.055 c ± 0.01 |
| Yellow peel | 0.002 a ± 0.01 | 0.153 d ± 0.03 | n.d. | 0.008 b ± 0.08 | n.d. | n.d. | 0.072 c ± 0.01 |
| Tropea whole | 0.003 a ± 0.01 | 0.345 b ± 0.09 | n.d. | 0.001 c ± 0.01 | 0.032 a ± 0.01 | 0.827 a ± 0.08 | 0.193 a ± 0.02 |
| Red whole | 0.002 a ± 0.03 | 0.306 b ± 0.07 | n.d. | 0.008 b ± 0.02 | 0.026 a ± 0.01 | 0.831 a ± 0.02 | 0.139 b ± 0.05 |
| Yellow whole | 0.003 a ± 0.07 | 0.408 a ± 0.02 | 0.002 b ± 0.02 | 0.001 c ± 0.01 | 0.025 a ± 0.02 | 0.596 c ± 0.06 | 0.096 c ± 0.08 |
| Tropea pulp | 0.002 a ± 0.01 | 0.345 b ± 0.04 | n.d. | 0.001 c ± 0.02 | 0.012 b ± 0.01 | 0.859 a ± 0.01 | 0.187 a ± 0.02 |
| Red pulp | 0.002 a ± 0.01 | 0.410 a ± 0.01 | n.d. | n.d. | 0.027 a ± 0.01 | 0.953 a ± 0.23 | 0.150 b ± 0.01 |
| Yellow pulp | 0.002 a ± 0.06 | 0.295 c ± 0.12 | n.d. | n.d. | 0.016 b ± 0.05 | 0.706 b ± 0.12 | 0.083 c ± 0.03 |
| PEEL | PULP | WHOLE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Polyphenolic Acids | Tropea | Red | Yellow | Tropea | Red | Yellow | Tropea | Red | Yellow |
| mg/100 g | mg/g | mg/g | mg/g | mg/g | mg/g | mg/g | mg/g | mg/g | |
| Gallic acid | 4.6 ± 0.7 a | 0.87 ± 0.7 c | 0.4 ± 0.04 c | 0.33 ± 0.07 c | 0.2 ± 0.05 c | 1.6 ± 0.5 b | 3.4 ± 0.5 ab | 0.8 ± 0.03 c | 0.53 ± 0.03 c |
| Protocathecuic acid | 7.3 ± 2 a | 10.3 ± 1.9 a | n.d. | 1.4 ± 0.3 d | 0.8 ± 0.3 d | 0.6 ± 0.05 d | 4.8 ± 0.9 b | 1.8 ± 0.07 c | 1.93 ± 0.06 c |
| Siryngic acid | 13.2 ± 1.1 a | 4.2 ± 0.6 b | n.d. | n.d. | n.d. | 2.5 ± 0.23 c | n.d. | n.d. | n.d. |
| p-Coumaric acid | 4.8 ± 0.3 a | 4.6 ± 0.2 a | 3.3 ± 0.5 ab | 1.47 ± 0.9 c | 1.5 ± 0.8 c | 2.23 ± 0.6 b | 2.31 ± 0.6 b | 2.5 ± 0.7 b | 2.5 ± 0.6 b |
| m-Coumaric acid | 6.8 ± 1.3 a | 2.5 ± 0.5 b | 0.6 ± 0.02 c | 0.3 ± 0.02 c | 0.3 ± 0.04 c | 0.3 ± 0.02 c | 0.30 ± 0.2 c | 0.4 ± o.o3 c | 0.3 ± 0.01 c |
| o-Coumaric acid | 6.9 ± 1.3 a | 8.7 ± 1.2 a | 0.97 ± 0.6 abc | 0.36 ± 0.03 c | 1.5 ± 0.6 b | 0.4 ± 0.06 c | 1.5 ± 0.5 b | 1.5 ± 0.3 b | 1.5 ± 0.6 b |
| Trans-cinnamic acid | 3.6 ± 0.6 a | 3.3 ± 0.4 a | 1.4 ± 0.5 b | 0.6 ± 0.03 c | 0.64 ± 0.05 c | 0.65 ± 0.03 c | 0.65 ± 0.04 c | 0.62 ± 0.06 c | 0.63 ± 0.02 c |
| Vanillic acid | 1 ± 0.5 a | 1.5 ± 0.9 a | 0.4 ± 0.03 c | 0.6 ± 0.03 b | 0.1 ± 0.01 c | 0.3 ± 0.03 c | 0.23 ± 0.01 c | 0.33 ± 0.02 c | 0.33 ± 0.03 c |
| Trans-4-hydroxycinnamic acid | 0.23 ± 0.03 c | 0.23 ± 0.06 c | 0.2 ± 0.02 c | 1.33 ± 0.6 a | 0.13 ± 0.06 c | 0.22 ± 0.01 c | 0.55 ± 0.06 b | 0.133 ± 0.01 c | 0.142 ± 0.03 c |
| Sinapic acid | 1.7 ± 0.6 a | 0.4 ± 0.02 b | 0.2 ± 0.01 b | 1.3 ± 0.4 a | 0.27 ± 0.03 b | 0.35 ± 0.02 b | 0.40 ± 0.03 b | 0.33 ± 0.04 b | 0.30 ± 0.02 b |
| 3-hydroxycinnamic acid | 3 ± 0.5 a | 2.2 ± 0.1 b | 0.2 ± 0.01 c | 0.1 ± 0.02 c | 0.1 ± 0.02 c | n.d. | 0.1 ± 0.02 c | n.d. | 0.2 ± 0.02 c |
| 2,5-dihydroxybenzoic acid | 16.8 ± 2 a | 5.5 ± 1.1 bc | 3.8 ± 0.9 c | 9.1 ± 1.2 b | 0.5 ± 0.02 d | 3.3 ± 0.8 b | 9.2 ± 1.4 b | 2.2 ± 0.7 c | 2.4 ± 0.8 c |
| Caffeic acid | 4.4 ± 1.0 b | 3.8 ± 0.9 b | 0.67 ± 0.02 c | 0.25 ± 0.02 c | 0.2 ± 0.02 c | 0.1 ± 0.01 c | 9.27 ± 1.2 a | 0.13 ± 0.02 c | 0.1 ± 0.01 c |
| Ellagic acid | 6 ± 1.1 b | 1.36 ± 0.2 bc | 18.7 ± 2.2 a | 1.1 ± 0.2 bc | 0.47 ± 0.02 c | 0.1 ± 0.01 c | 0.43 ± 0.03 c | 0.57 ± 0.04 c | 2.8 ± 0.5 b |
| Chlorogenic acid | 32.8 ± 1.9 a | 18.9 ± 1.7 b | 3.6 ± 0.5 d | 4.1 ± 0.5 d | 0.83 ± 0.02 g | 2.8 ± 0.3 f | 8.5 ± 1 c | 1.7 ± 0.4 e | 0.87 ± 0.03 g |
| Ferulic acid | 51.3 ± 1.6 a | 51.1 ± 1.2 a | 50.1 ± 1.7 a | 25.6 ± 1.6 b | 25.3 ± 1.8 b | 48.1 ± 2 a | 25.3 ± 1.9 b | 25.3 ± 1.6 b | 25.4 ± 1.7 b |
| PEEL | PULP | WHOLE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Flavonoids | Tropea | Red | Yellow | Tropea | Red | Yellow | Tropea | Red | Yellow |
| Isorhametin-3-glucoside | 60 ± 2 a | 35 ± 2.1 b | 11 ± 1.2 e | 17 ± 3 d | 25 ± 3 c | 16 ± 1.2 d | 22 ± 2 c | 17 ± 2 d | 12 ± 1 e |
| Isorhamnetin | 19.6 ± 3 a | 9.5 ± 2 b | 7.4 ± 1.2 b | 1.2 ± 0.6 c | 1.4 ± 0.5 c | 1.6 ± 0.9 c | 0.2 ± 0.06 d | 0.8 ± 0.03 d | 1.8 ± 0.6 c |
| Apigenin | 16 ± 1.4 a | 12 ± 0.9 b | 6.8 ± 0.6 b | n.d. | n.d. | n.d. | 0.6 ± 0.06 c | 0.3 ± 0.02 c | 0.1 ± 0.02 c |
| Procyanidine 2 | 72 ± 5.6 a | 86 ± 4.6 b | 68 ± 3.6 c | 4.4 ± 0.6 f | 4.8 ± 0.7 f | 4.2 ± 2.6 f | 42 ± 3.7 e | 44 ± 4.1 e | 54 ± 3.7 d |
| Cyanidin-3-O-glucoside | 29.6 ± 1.7 c | 19.6 ± 1.0 d | 10.3 ± 0.7 e | 112.4 ± 6.7 a | 128.2 ± 7 a | 103.2 ± 8 a | 115.2 ± 7 a | 101 ± 8 a | 83.8 ± 9 b |
| Catechin | 361 ± 14 b | 573 ± 18 a | 120 ± 11 c | 17 ± 1 e | 17 ± 1.2 e | 14 ± 1.5 e | 39 ± 1.5 d | 43 ± 1.7 d | 50 ± 2.1 d |
| Myricetin | 8.4 ± 1.2 a | 7.2 ± 1 a | 6.4 ± 1.1 a | 0.6 ± 0.05 c | 0.4 ± 0.04 c | 0.6 ± 0.06 c | 0.4 ± 0.03 c | 1.4 ± 0.3 b | 0.8 ± 0.07 c |
| Luteolin | 404 ± 33 a | 460 ± 27 a | 53 ± 18 b | 8.8 ± 10 c | 14 ± 1.5 c | 5.6 ± 1 d | 41.4 ± 18 b | 42.2 ± 16 b | 0.6 ± 0.06 e |
| Spiraeoside | 691 ± 32 a | 786 ± 28 b | 184 ± 14 c | 133 ± 18 d | 206 ± 15 c | 121 ± 11 d | 178 ± 15 b | 152 ± 18 b | 161 ± 14 b |
| Quercetin | 419 ± 21 b | 491 ± 18 a | 251 ± 18 c | 12.8 ± 1.8 d | 4.4 ± 0.5 g | 10 ± 0.7 f | 34 ± 36 e | 29 ± 1.8 e | 12 ± 1.6 f |
| Kaempferol | 15 ± 1.9 a | 19 ± 1.9 a | 4.4 ± 1.2 b | 0.2 ± 0.05 c | 1 ± 0.07 c | n.d. | 1.4 ± 0.08 c | 1.6 ± 0.06 c | 0.4 ± 0.01 d |
| Vicenin-2 | 34.2 ± 18 a | 32.2 ± 12 a | 10.6 ± 5 b | 4.6 ± 1.2 c | 9.2 ± 2 b | 3.4 ± 1 d | 7.6 ± 1.3 b | 6.4 ± 1.2 b | 3.5 ± 0.8 d |
| Rutin | 0.4 ± 0.01 c | 3 ± 0.6 a | 0.8 ± 0.06 d | 0.6 ± 0.2 c | 1.2 ± 0.3 b | 0.4 c | 0.2 ± 0.02 c | 1.3 ± 0.2 b | 0.4 ± 0.01 c |
| Quercetin-3 beta-D-glucoside | 12.2 ± 1.a | 11.4 ± 1.1 a | 2.8 ± 0.8 c | 8.4 ± 1.6 b | 8.2 ± 1.8 b | 1.8 ± 0.8 c | 7.4 ± 1.1 b | 6.5 ± 1.3 b | 1.1 ± 0.4 c |
| Naringenin | 1.2 ± 0.1 a | 1 ± 0.2 a | 0.6 ± 0.02 b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Delphinidin | 7.8 ± 1.4 a | 4.8 ± 1 b | 1.2 ± 0.3 c | 0.6 ± 0.1 d | 1.2 ± 0.3 c | 0.2 ± 0.01 e | 2.9 ± 1 bc | 2.7 ± 1.1 bc | 0.6 ± 0.03 c |
| Naringin | 2.2 ± 0.3 a | 2.7 ± 0.4 a | 3.2 ± 0.4 a | 1 ± 0.5 b | 0.2 ± 0.02 c | 1 ± 0.3 b | 0.6 ± 0.3 b | 0.8 ± 0.1 b | 0.6 ± 0.2 b |
| Variables | Lithium | Sodium | Potassium | Magnesium | Calcium | Fluorides | Chlorides | Nitrites | Bromides | Nitrates | Phosphates | Sulfates | TCARB | TPRO | VIT A | VIT C | VIT E |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lithium | 1 | 0.904 | 0.898 | 0.902 | 0.402 | −0.712 | 0.838 | −0.701 | −0.731 | 0.750 | 0.920 | 0.659 | 0.846 | −0.911 | 0.158 | 0.570 | −0.653 |
| Sodium | 0.904 | 1 | 0.842 | 0.891 | 0.534 | −0.671 | 0.851 | −0.649 | −0.735 | 0.662 | 0.918 | 0.675 | 0.723 | −0.844 | 0.043 | 0.562 | −0.599 |
| Potassium | 0.898 | 0.842 | 1 | 0.991 | 0.711 | −0.714 | 0.921 | −0.741 | −0.803 | 0.874 | 0.965 | 0.834 | 0.927 | −0.968 | 0.218 | 0.759 | −0.594 |
| Magnesium | 0.902 | 0.891 | 0.991 | 1 | 0.742 | −0.724 | 0.929 | −0.747 | −0.817 | 0.869 | 0.982 | 0.825 | 0.915 | −0.966 | 0.163 | 0.734 | −0.607 |
| Calcium | 0.402 | 0.534 | 0.711 | 0.742 | 1 | −0.450 | 0.682 | −0.513 | −0.604 | 0.765 | 0.707 | 0.730 | 0.694 | −0.653 | −0.015 | 0.671 | −0.280 |
| Fluorides | −0.712 | −0.671 | −0.714 | −0.724 | −0.450 | 1 | −0.722 | 0.988 | 0.970 | −0.570 | −0.722 | −0.711 | −0.662 | 0.836 | −0.110 | −0.340 | 0.953 |
| Chlorides | 0.838 | 0.851 | 0.921 | 0.929 | 0.682 | −0.722 | 1 | −0.745 | −0.830 | 0.872 | 0.914 | 0.754 | 0.770 | −0.936 | 0.412 | 0.664 | −0.648 |
| Nitrites | −0.701 | −0.649 | −0.741 | −0.747 | −0.513 | 0.988 | −0.745 | 1 | 0.972 | −0.625 | −0.735 | −0.713 | −0.689 | 0.848 | −0.136 | −0.363 | 0.939 |
| Bromides | −0.731 | −0.735 | −0.803 | −0.817 | −0.604 | 0.970 | −0.830 | 0.972 | 1 | −0.669 | −0.790 | −0.756 | −0.701 | 0.900 | −0.217 | −0.423 | 0.918 |
| Nitrates | 0.750 | 0.662 | 0.874 | 0.869 | 0.765 | −0.570 | 0.872 | −0.625 | −0.669 | 1 | 0.861 | 0.698 | 0.882 | −0.864 | 0.275 | 0.696 | −0.456 |
| Phosphates | 0.920 | 0.918 | 0.965 | 0.982 | 0.707 | −0.722 | 0.914 | −0.735 | −0.790 | 0.861 | 1 | 0.847 | 0.921 | −0.941 | 0.055 | 0.767 | −0.582 |
| Sulfates | 0.659 | 0.675 | 0.834 | 0.825 | 0.730 | −0.711 | 0.754 | −0.713 | −0.756 | 0.698 | 0.847 | 1 | 0.828 | −0.799 | −0.076 | 0.880 | −0.481 |
| TCARB | 0.846 | 0.723 | 0.927 | 0.915 | 0.694 | −0.662 | 0.770 | −0.689 | −0.701 | 0.882 | 0.921 | 0.828 | 1 | −0.893 | −0.031 | 0.771 | −0.494 |
| TPRO | −0.911 | −0.844 | −0.968 | −0.966 | −0.653 | 0.836 | −0.936 | 0.848 | 0.900 | −0.864 | −0.941 | −0.799 | −0.893 | 1 | −0.278 | −0.633 | 0.754 |
| VIT A | 0.158 | 0.043 | 0.218 | 0.163 | −0.015 | −0.110 | 0.412 | −0.136 | −0.217 | 0.275 | 0.055 | −0.076 | −0.031 | −0.278 | 1 | −0.048 | −0.261 |
| VIT C | 0.570 | 0.562 | 0.759 | 0.734 | 0.671 | −0.340 | 0.664 | −0.363 | −0.423 | 0.696 | 0.767 | 0.880 | 0.771 | −0.633 | −0.048 | 1 | −0.084 |
| VIT E | −0.653 | −0.599 | −0.594 | −0.607 | −0.280 | 0.953 | −0.648 | 0.939 | 0.918 | −0.456 | −0.582 | −0.481 | −0.494 | 0.754 | −0.261 | −0.084 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muscolo, A.; Maffia, A.; Marra, F.; Battaglia, S.; Oliva, M.; Mallamaci, C.; Russo, M. Unlocking the Health Secrets of Onions: Investigating the Phytochemical Power and Beneficial Properties of Different Varieties and Their Parts. Molecules 2025, 30, 1758. https://doi.org/10.3390/molecules30081758
Muscolo A, Maffia A, Marra F, Battaglia S, Oliva M, Mallamaci C, Russo M. Unlocking the Health Secrets of Onions: Investigating the Phytochemical Power and Beneficial Properties of Different Varieties and Their Parts. Molecules. 2025; 30(8):1758. https://doi.org/10.3390/molecules30081758
Chicago/Turabian StyleMuscolo, Adele, Angela Maffia, Federica Marra, Santo Battaglia, Mariateresa Oliva, Carmelo Mallamaci, and Mariateresa Russo. 2025. "Unlocking the Health Secrets of Onions: Investigating the Phytochemical Power and Beneficial Properties of Different Varieties and Their Parts" Molecules 30, no. 8: 1758. https://doi.org/10.3390/molecules30081758
APA StyleMuscolo, A., Maffia, A., Marra, F., Battaglia, S., Oliva, M., Mallamaci, C., & Russo, M. (2025). Unlocking the Health Secrets of Onions: Investigating the Phytochemical Power and Beneficial Properties of Different Varieties and Their Parts. Molecules, 30(8), 1758. https://doi.org/10.3390/molecules30081758









