The Potential Therapeutic Role of Beta-Caryophyllene as a Chemosensitizer and an Inhibitor of Angiogenesis in Cancer
Abstract
1. Introduction
2. Plant Species That Contain Exceptionally High Concentrations of BCP
3. Cytotoxicity of Beta-Caryophyllene in Normal and Cancer Cells
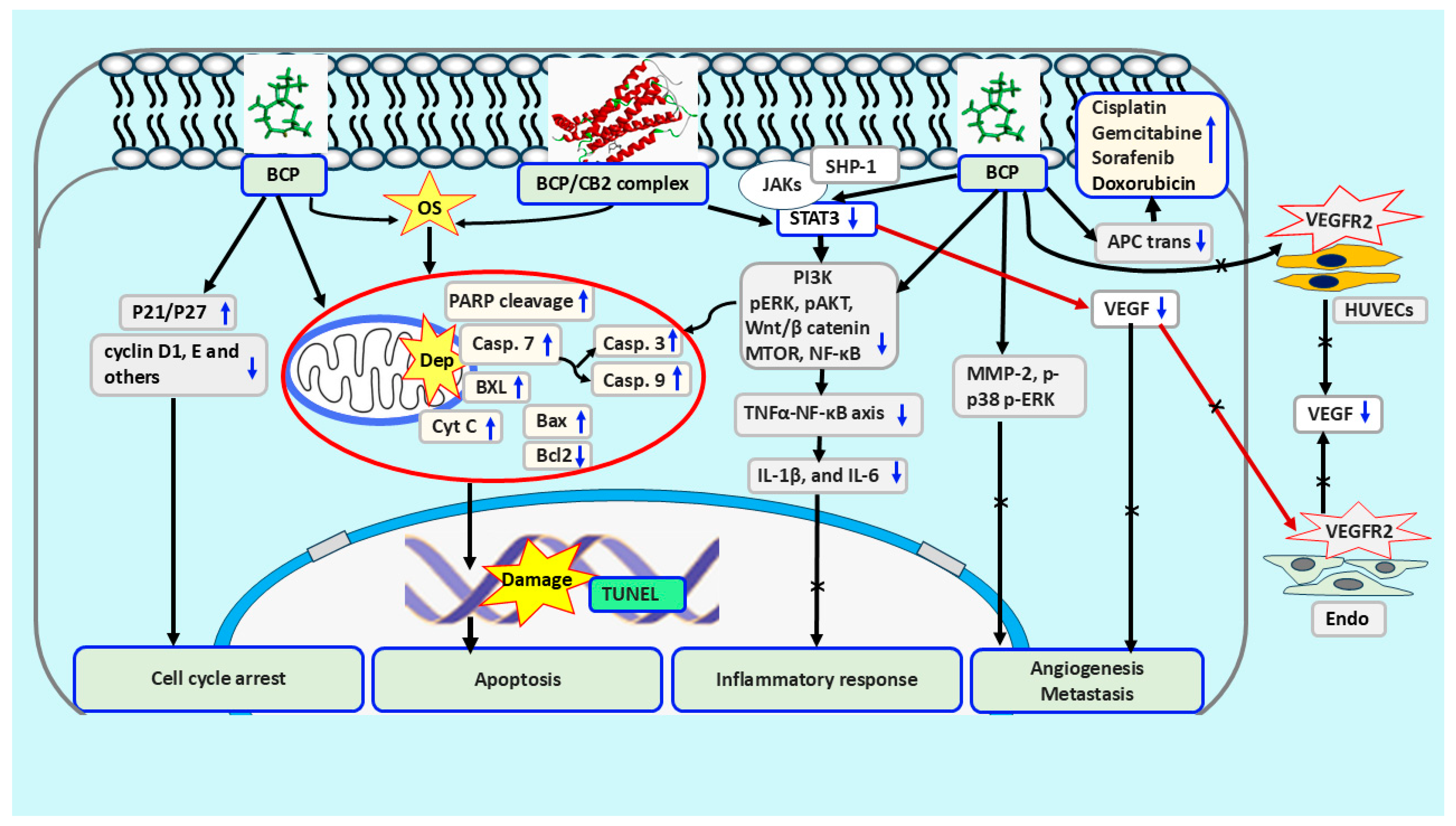
4. Beta-Caryophyllene Enhances Oxidative Stress and Activates Cell Cycle Checkpoints to Promote Apoptosis in Cancer Cells
5. Beta-Caryophyllene Reduces Angiogenesis and Cancer Cells’ Ability to Invade and Metastasize
6. Chemo-Sensitization Properties of Beta-Caryophyllene in Cancer Treatment
7. Signaling Mechanisms Involved in BCP Enhancing Chemo-Sensitization Alone or in Combination in Cancer Therapy
7.1. The Lipophilic Nature of BCP and Cancer Treatment
7.2. Selective Interaction of Beta-Caryophyllene with CB2 and Cancer Suppression
7.3. Virtual Interaction of Beta-Caryophyllene with Cancer Involved Signaling Molecules
7.4. STAT3 Works as a Downstram Effector of Beta-Caryophyllene Regulating a Complex Network in Cancer
7.5. BCP’s Effects on Transporters in Cancer in Relation to Its Synergetic and Chemosensitization Properties
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kubo, I.; Chaudhuri, S.K.; Kubo, Y.; Sanchez, Y.; Ogura, T.; Saito, T.; Ishikawa, H.; Haraguchi, H. Cytotoxic and antioxidative sesquiterpenoids from Heterotheca inuloides. Planta Med. 1996, 62, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.S.; Hong, J.Y.; Lee, J.H.; Lee, H.J.; Park, J.Y.; Choi, J.H.; Park, H.J.; Hong, J.; Lee, K.T. β-Caryophyllene in the Essential Oil from Chrysanthemum Boreale Induces G Phase Cell Cycle Arrest in Human Lung Cancer Cells. Molecules 2019, 24, 3754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, E.A.; Abu Zahra, H.; Ammar, R.B.; Mohamed, M.E.; Ibrahim, H.M. Beta-Caryophyllene Enhances the Anti-Tumor Activity of Cisplatin in Lung Cancer Cell Lines through Regulating Cell Cycle and Apoptosis Signaling Molecules. Molecules 2022, 27, 8354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shabana, S.M.; Gad, N.S.; Othman, A.I.; Mohamed, A.F.; El-Missiry, M.A. β-caryophyllene oxide induces apoptosis and inhibits proliferation of A549 lung cancer cells. Med. Oncol. 2023, 40, 189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wittig, F.; Koch, F.; Pannenberg, L.; Bekeschus, S.; Ramer, R.; Hinz, B. β-Caryophyllene Inhibits Endothelial Tube Formation by Modulating the Secretome of Hypoxic Lung Cancer Cells-Possible Role of VEGF Downregulation. Int. J. Mol. Sci. 2024, 25, 810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dahham, S.S.; Tabana, Y.; Asif, M.; Ahmed, M.; Babu, D.; Hassan, L.E.; Ahamed, M.B.K.; Sandai, D.; Barakat, K.; Siraki, A.; et al. β-Caryophyllene Induces Apoptosis and Inhibits Angiogenesis in Colorectal Cancer Models. Int. J. Mol. Sci. 2021, 22, 10550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arul, S.; Rajagopalan, H.; Ravi, J.; Dayalan, H. Beta-Caryophyllene Suppresses Ovarian Cancer Proliferation by Inducing Cell Cycle Arrest and Apoptosis. Anticancer Agents Med. Chem. 2020, 20, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liao, B.; Zhu, P.; Cheng, S.; Du, Z.; Jiang, G. β-Caryophyllene induces apoptosis and inhibits cell proliferation by deregulation of STAT-3/mTOR/AKT signaling in human bladder cancer cells: An in vitro study. J. Biochem. Mol. Toxicol. 2021, 35, e22863. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.; Zhu, Y.; Han, J.; Li, Y.; Yang, X.; Yang, G.; Song, G.; Li, S.; Li, Y.; Cheng, C.; et al. Caryophyllene Oxide Induces Ferritinophagy by Regulating the NCOA4/FTH1/LC3 Pathway in Hepatocellular Carcinoma. Front. Pharmacol. 2022, 13, 930958. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmad, Z.; Jain, S.K.; Mishra, S.K. Beta-Caryophyllene attenuates experimental hepatocellular carcinoma through downregulation of oxidative stress and inflammation. J. Biochem. Mol. Toxicol. 2024, 38, e23850. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Gullì, M.; Facchinetti, R.; Minacori, M.; Mancinelli, R.; Percaccio, E.; Scuderi, C.; Eufemi, M.; Di Sotto, A. Sorafenib Chemosensitization by Caryophyllane Sesquiterpenes in Liver, Biliary, and Pancreatic Cancer Cells: The Role of STAT3/ABC Transporter Axis. Pharmaceutics 2022, 14, 1264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanušová, V.; Caltová, K.; Svobodová, H.; Ambrož, M.; Skarka, A.; Murínová, N.; Králová, V.; Tomšík, P.; Skálová, L. The effects of β-caryophyllene oxide and trans-nerolidol on the efficacy of doxorubicin in breast cancer cells and breast tumor-bearing mice. Biomed. Pharmacother. 2017, 95, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Gullì, M.; Minacori, M.; Mancinelli, R.; Garzoli, S.; Percaccio, E.; Incocciati, A.; Romaniello, D.; Mazzanti, G.; Eufemi, M.; et al. β-Caryophyllene Counteracts Chemoresistance Induced by Cigarette Smoke in Triple-Negative Breast Cancer MDA-MB-468 Cells. Biomedicines 2022, 10, 2257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Zhang, H.; Li, Y.; Wang, M.; Qian, F. Beta-Caryophyllene attenuates lipopolysaccharide-induced acute lung injury via inhibition of the MAPK signalling pathway. J. Pharm. Pharmacol. 2021, 73, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.W.; Kim, M.M. β-Caryophyllene oxide inhibits metastasis by downregulating MMP-2, p-p38 and p-ERK in human fibrosarcoma cells. J. Food Biochem. 2022, 46, e14468. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Di Sotto, A.; Mazzanti, G.; Wink, M. Chemosensitizing Properties of β-Caryophyllene and β-Caryophyllene Oxide in Combination with Doxorubicin in Human Cancer Cells. Anticancer Res. 2017, 37, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Synergistic interaction of β-caryophyllene with aromadendrene oxide 2 and phytol induces apoptosis on skin epidermoid cancer cells. Phytomedicine 2018, 47, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.W.; Mahmoud, V.L.; Wang, X.; Teoh, M.L.; Loh, K.M.; Ng, C.H.; Wong, W.F.; Looi, C.Y. Chemical profiling and cytotoxicity screening of agarwood essential oil (Aquilaria sinensis) in brine shrimp nauplii and cancer cell lines. PLoS ONE 2024, 19, e0310770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. Beta-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Wang, Q.; Li, G.; Li, Y.; Zhang, P.; Xu, G. β-Caryophyllene from Chilli Pepper Inhibits the Proliferation of Non-Small Cell Lung Cancer Cells by Affecting miR-659-3p-Targeted Sphingosine Kinase 1 (SphK1). Int. J. Gen. Med. 2021, 14, 9599–9613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ambrož, M.; Boušová, I.; Skarka, A.; Hanušová, V.; Králová, V.; Matoušková, P.; Szotáková, B.; Skálová, L. The Influence of Sesquiterpenes from Myrica rubra on the Antiproliferative and Pro-Oxidative Effects of Doxorubicin and Its Accumulation in Cancer Cells. Molecules 2015, 20, 15343–15358. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhan, M.L.; Tang, Y.; Xiao, M.; Li, M.; Li, Q.S.; Yang, L.; Li, X.; Chen, W.W.; Wang, Y.L. Effects of β-caryophyllene on arginine ADP-ribosyltransferase 1-mediated regulation of glycolysis in colorectal cancer under high-glucose conditions. Int. J. Oncol. 2018, 53, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Irannejad, H.; Eufemi, M.; Mancinelli, R.; Abete, L.; Mammola, C.L.; Altieri, F.; Mazzanti, G.; Di Giacomo, S. Potentiation of Low-Dose Doxorubicin Cytotoxicity by Affecting P-Glycoprotein through Caryophyllane Sesquiterpenes in HepG2 Cells:an in Vitro and in Silico Study. Int. J. Mol. Sci. 2020, 21, 633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Giacomo, S.; Briz, O.; Monte, M.J.; Sanchez-Vicente, L.; Abete, L.; Lozano, E.; Mazzanti, G.; Di Sotto, A.; Marin, J.J.G. Chemosensitization of hepatocellular carcinoma cells to sorafenib by β-caryophyllene oxide-induced inhibition of ABC export pumps. Arch. Toxicol. 2019, 93, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rivero, S.; Peleteiro-Vigil, A.; Abete, L.; Lozano, E.; Hammer, H.S.; Giacomo, S.D.; Abad, M.; Boix, L.; Forner, A.; Reig, M.; et al. Sensitization of cholangiocarcinoma cells to chemotherapy through BCRP inhibition with β-caryophyllene oxide. Biomed. Pharmacother. 2024, 170, 116038. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Mendez-Callejas, G.; Celis, C. Caryophyllene Oxide, the Active Compound Isolated from Leaves of Hymenaea courbaril L. (Fabaceae) with Antiproliferative and Apoptotic Effects on PC-3 Androgen-Independent Prostate Cancer Cell Line. Molecules 2021, 26, 6142. [Google Scholar] [CrossRef]
- Mboge, M.Y.; Ramirez-Mata, A.; Bullock, A.; O’Donnell, R.; Mathias, J.V.; Davila, J.; Frost, C.J.; Frost, S.C. β-caryophyllene enhances the transcriptional upregulation of cholesterol biosynthesis in breast cancer cells. Curr. Top Biochem. Res. 2019, 20, 1–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frost, C.J.; Ramirez-Mata, A.; Khattri, R.B.; Merritt, M.E.; Frost, S.C. Effects of β-caryophyllene and oxygen availability on cholesterol and fatty acids in breast cancer cells. PLoS ONE 2023, 18, e0281396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, X.; Roseblade, A.; Rawling, T.; Ung, A.T. Antiproliferative activities of tricyclic amides derived from β-caryophyllene the Ritter reaction against MDA-MB-231 breast cancer cells. RSC Med. Chem. 2019, 11, 118–124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yano, T.; Nishimura, O. Essential oil composition of Cananga odorata (Ylang-ylang). J. Essent. Oil Res. 2011, 23, 1–4. [Google Scholar]
- Mohapatra, S.; Nanda, S. Chemical composition of essential oil from leaves of Spondias pinnata. J. Med. Plants Res. 2013, 7, 300–304. [Google Scholar]
- Koyama, S.; Purk, A.; Kaur, M.; Soini, H.A.; Novotny, M.V.; Davis, K.; Kao, C.C.; Matsunami, H.; Mescher, A. Beta-Caryophyllene enhances wound healing through multiple routes. PLoS ONE 2019, 14, e0216104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gülçin, İ.; Kültür, K. Antioxidant properties of Pimpinella kotschyana. Food Chem. 2007, 103, 885–893. [Google Scholar]
- Ezzat, S.M.; Dorman, H.J.D. Chemical composition and biological activities of the essential oil of Rhus coriaria. J. Essent. Oil Res. 2016, 28, 337–344. [Google Scholar]
- Babu, K.S.; Jayaprakasha, G.K. Evaluation of essential oil composition and antimicrobial activity of black pepper (Piper nigrum) fruits. Food Chem. 2013, 138, 303–310. [Google Scholar]
- Bakkali, F.; Averbeck, S. Biological effects of clove essential oil: A review. Food Chem. Toxicol. 2008, 46, 1724–1733. [Google Scholar] [CrossRef]
- Russo, E.B.; Guy, G.W. Cannabis and cannabinoids: Pharmacology and therapeutic applications. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 72–107. [Google Scholar]
- Gupta, S.C.; Mishra, S. Bioactive components from Annona squamosa: Implications in cancer treatment. Phytomedicine 2015, 22, 721–727. [Google Scholar]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective Effects of -β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Annamalai, V.; Kotakonda, M.; Periyannan, V. JAK1/STAT3 regulatory effect of β-caryophyllene on MG-63 osteosarcoma cells via ROS-induced apoptotic mitochondrial pathway by DNA fragmentation. J. Biochem. Mol. Toxicol. 2020, 34, e22514. [Google Scholar] [CrossRef] [PubMed]
- Basheer, I.; Wang, H.; Li, G.; Jehan, S.; Raza, A.; Du, C.; Ullah, N.; Li, D.; Sui, G. β-caryophyllene sensitizes hepatocellular carcinoma cells to chemotherapeutics and inhibits cell malignancy through targeting MAPK signaling pathway. Front. Pharmacol. 2024, 15, 1492670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Legault, J.; Pichette, A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Di Giacomo, S.; Rubini, E.; Macone, A.; Gulli, M.; Mammola, C.L.; Eufemi, M.; Mancinelli, R.; Mazzanti, G. Modulation of STAT3 Signaling, Cell Redox Defenses and Cell Cycle Checkpoints by β-Caryophyllene in Cholangiocarcinoma Cells: Possible Mechanisms Accounting for Doxorubicin Chemosensitization and Chemoprevention. Cells 2020, 9, 858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, C.; Cho, S.K.; Kim, K.D.; Nam, D.; Chung, W.S.; Jang, H.J.; Lee, S.G.; Shim, B.S.; Sethi, G.; Ahn, K.S. β-Caryophyllene oxide potentiates TNFα-induced apoptosis and inhibits invasion through down-modulation of NF-κB-regulated gene products. Apoptosis 2014, 19, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Q.; Wu, P.; Dong, X.M.; Cheng, L.H.; Lu, H.Q.; Lin, Y.Y.; Tang, W.Y.; Xie, T.; Zhou, J.L. Elemene induces cell apoptosis via inhibiting glutathione synthesis in lung adenocarcinoma. J. Ethnopharmacol. 2023, 311, 116409. [Google Scholar] [CrossRef] [PubMed]
- Mannino, F.; Pallio, G.; Corsaro, R.; Minutoli, L.; Altavilla, D.; Vermiglio, G.; Allegra, A.; Eid, A.H.; Bitto, A.; Squadrito, F.; et al. Beta-Caryophyllene Exhibits Anti-Proliferative Effects through Apoptosis Induction and Cell Cycle Modulation in Multiple Myeloma Cells. Cancers 2021, 13, 5741. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, S.K.; Kapoor, S.; Kumar, A.; Vali, S.; Abbasi, T.; Kim, S.H.; Sethi, G.; Ahn, K.S. β-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 2014, 53, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Kim, E.J.; Kwon, G.T.; Jung, Y.J.; Park, T.; Kim, Y.; Yu, R.; Choi, M.S.; Chun, H.S.; Kwon, S.H.; et al. β-Caryophyllene potently inhibits solid tumor growth and lymph node metastasis of B16F10 melanoma cells in high-fat diet-induced obese C57BL/6N mice. Carcinogenesis 2015, 36, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Maffei, F.; Hrelia, P.; Castelli, F.; Sarpietro, M.G.; Mazzanti, G. Genotoxicity assessment of β-caryophyllene oxide. Regul. Toxicol. Pharmacol. 2013, 66, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Mariano, A.; Gullì, M.; Fraschetti, C.; Vitalone, A.; Filippi, A.; Mannina, L.; Scotto d’Abusco, A.; Di Sotto, A. Role of Caryophyllane Sesquiterpenes in the Entourage Effect of Felina 32 Hemp Inflorescence Phytocomplex in Triple Negative MDA-MB-468 Breast Cancer Cells. Molecules 2021, 26, 6688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramírez-Santos, J.; Calzada, F.; García-Hernández, N.; Barbosa, E.; Velázquez, C.; Valdes, M. Caryophyllene Oxide, a Bicyclic Terpenoid Isolated from Annona macroprophyllata with Antitumor Activity: In Vivo, In Vitro, and In Silico Studies. Int. J. Mol. Sci. 2024, 25, 13355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.Q.; Man, Q.W.; Huo, F.Y.; Gao, X.; Lin, H.; Li, S.R.; Wang, J.; Su, F.C.; Cai, L.; Shi, Y.; et al. STAT3 pathway in cancers: Past, present, and future. MedComm 2022, 3, 124. [Google Scholar] [CrossRef]
| Cancer Type | Mechanism of Action | Reference |
|---|---|---|
| Lung Cancer | Enhances cisplatin efficacy by promoting apoptosis and A549 cell cycle arrest | [3] |
| Induces apoptosis and suppresses proliferative activity of lung cancer cells | [4] | |
| Induces G1 cell cycle arrest by dysregulating cyclins and other molecules | [2] | |
| Suppresses angiogenesis via modulating the secretome in hypoxic lung cancer cells | [5] | |
| Inhibits NSCLC growth to increase their apoptotic rate | [20] | |
| Glioblastoma | Inhibits cell proliferation via direct targeting of CB2 receptors | [19] |
| Works as a potential radiosensitizer for improving RT outcomes by inhibiting DNA repair, inducing apoptosis, and suppressing anti-apoptotic and survival pathways | [18] | |
| Colorectal Cancer | Inhibits angiogenesis via suppressing the vascular endothelial growth factor (VEGF) signaling, reduces oxidative stress, and promotes apoptosis | [6] |
| Activates initiator caspases via disruption of the mitochondrial membrane potential | [21] | |
| Inhibits the action of mono-ADP-ribosyltransferase 1 (ART1) by targeting nuclear factor-κB | [22] | |
| Ovarian Cancer | Induces S-phase arrest in ovarian OAW 42 cells that display a high rate of apoptosis mediated by PARP cleavage and active caspase-3 | [7] |
| Liver and Pancreatic Cancer | Enhances chemosensitization in liver, biliary, and pancreatic cancer cells treated with Sorafenib/doxorubicin using the STAT3/ABC transporter axis | [11,23,24] |
| Enhances liver cancer cells apoptosis via ferritinophagy-mediated ferroptosis mechanisms | [9] | |
| Enhances sensitization of cholangiocarcinoma cells to chemotherapy via BCRP inhibition | [25] | |
| Suppresses tumor incidence by downregulating inflammation and inducing caspase-3-mediated apoptosis in a hepatocellular carcinoma | [10] | |
| Prostate Cancer | Enhances mitochondrial membrane depolarization and thus activates caspase-7 dependent apoptosis | [26] |
| Breast Cancer | Enhances sensitization and promotes the cigarette smoke condensate (CSC)-induced apoptosis in MDA-MB-468 cells, mainly by triggering oxidative stress and inhibition of STAT3 | [13] |
| Enhances cholesterol biosynthesis transcriptional upregulation in breast cancer cells | [27,28] | |
| Combines therapy of DOX with BCPO synergistically and enhances the anti-proliferative effect on MDA-MB-231 breast cancer cells | [12] | |
| Inhibits tumor growth by activating CB2 receptors and modulating inflammatory pathways | [29,30] | |
| Bladder Cancer | Inhibits bladder cancer cell proliferation and induces apoptosis via deregulation of STAT-3/mTOR/AKT signaling | [8] |
| Cell Type | IC50 (μM) | Reference | Notes |
|---|---|---|---|
| Normal Cells (Control) | |||
| Skin Fibroblasts | >100 | [33] | Low cytotoxicity in normal cells |
| Peripheral Blood Mononuclear Cells (PBMCs) | >100 | [40] | Minimal toxicity in immune cells |
| Normal Human Fibroblasts | >200 | [7] | Highest IC50 in fibroblasts |
| Normal Human Lymphocytes | <100 | [41] | Safe at low concentrations |
| Normal Fibroblasts (comparison) | 195 μg/mL | [42] | Paired with hepatoma cells (see below) |
| 3T3-L1 (Mouse Embryonic Fibroblasts) | >100 | [43] | Requires ≥ 2× higher dose vs. cancer cells |
| RGC-5 (Retinal Ganglion Cells) | >100 | [43] | Requires ≥ 2× higher dose vs. cancer cells |
| Cancer Cells | |||
| Bladder Cancer (T24 & 5637 cells) | 40 µg/mL (~64 μM) | [8] | Dose- and time-dependent cytotoxicity |
| Colon Cancer (HCT-116) | 19 | [6,42] | Most sensitive cancer line |
| Bone Cancer (MG-63) | 20 | [6,42] | IC50 within typical range (19–64 μM) |
| Pancreatic Cancer (PANC-1) | 27 | [6,42] | IC50 within typical range (19–64 μM) |
| Hepatocellular Carcinoma (HepG2) | 44.7 µg/mL (~72.8 μM) | [23] | Pure compound administration |
| Breast Cancer (MDA-MB-468) | 19.2 µg/mL (~31.5 μM) | [23] | Pure compound administration |
| Cervical Cancer (ME-180) | Requires higher doses for toxicity | [44] | ≥2× lower sensitivity vs. normal cells |
| Triple Negative Breast Cancer (MCF-7) | Requires higher doses for toxicity | [44] | ≥2× lower sensitivity vs. normal cells |
| Hepatoma Cells | 63.7 μg/mL | [43] | One-third of the value required to induce toxicity in fibroblasts, under same conditions. |
| Chemotherapeutic Agent | Mechanism of Action | References |
|---|---|---|
| Paclitaxel | Reduces tumor growth by increasing the accumulation of Paclitaxel and facilitating its passage through the membrane to potentiate its anticancer activity. The paclitaxel anticancer activity, in colorectal adenocarcinoma cell line, increased to about 10-fold when combined with BCP. | [45] |
| Doxorubicin | Enhances tumor suppression by increasing the drug accumulation; inhibiting the efflux pumps; reducing P-gp; facilitating cycle arrest; and inducing STAT3 activation. | [12,16,21,23,46] |
| Cisplatin | Regulates cell cycle and apoptosis signaling molecules, and targets the placental growth factor gene and MAPK signaling pathways, and inhibits ABC proteins (such as MRP1, MRP2, and BCRP) that are very relevant in chemoresistance | [3,25,43] |
| 5-Fluorouracil (5-FU) | Enhances apoptosis and reduces tumor proliferation by disrupting the mitochondrial membrane potential and activating initiator caspases | [21] |
| Alpha-Humulene | Reduces tumor growth and enhances BCP’s therapeutic effects. | [47] |
| Isocaryophyllene | Potentiates the anticancer activity by facilitating drug accumulation within the cells. | [47] |
| Gemcitabine | Improves tumor suppression, reduces drug resistance, and inhibits the ABC proteins | [21] |
| Sorafenib | Enhances tumor suppression and reduces chemoresistance to sorafenib through the modulation of AMRP and MDR1 (or Pgp) transporters and regulation of STAT3/ABC-transporters axis. | [11,16,25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, E.A. The Potential Therapeutic Role of Beta-Caryophyllene as a Chemosensitizer and an Inhibitor of Angiogenesis in Cancer. Molecules 2025, 30, 1751. https://doi.org/10.3390/molecules30081751
Ahmed EA. The Potential Therapeutic Role of Beta-Caryophyllene as a Chemosensitizer and an Inhibitor of Angiogenesis in Cancer. Molecules. 2025; 30(8):1751. https://doi.org/10.3390/molecules30081751
Chicago/Turabian StyleAhmed, Emad A. 2025. "The Potential Therapeutic Role of Beta-Caryophyllene as a Chemosensitizer and an Inhibitor of Angiogenesis in Cancer" Molecules 30, no. 8: 1751. https://doi.org/10.3390/molecules30081751
APA StyleAhmed, E. A. (2025). The Potential Therapeutic Role of Beta-Caryophyllene as a Chemosensitizer and an Inhibitor of Angiogenesis in Cancer. Molecules, 30(8), 1751. https://doi.org/10.3390/molecules30081751





