Transition Metal Catalysis for the Asymmetric Synthesis of 2-Arylethylamines: A Review of the New Millennium
Abstract
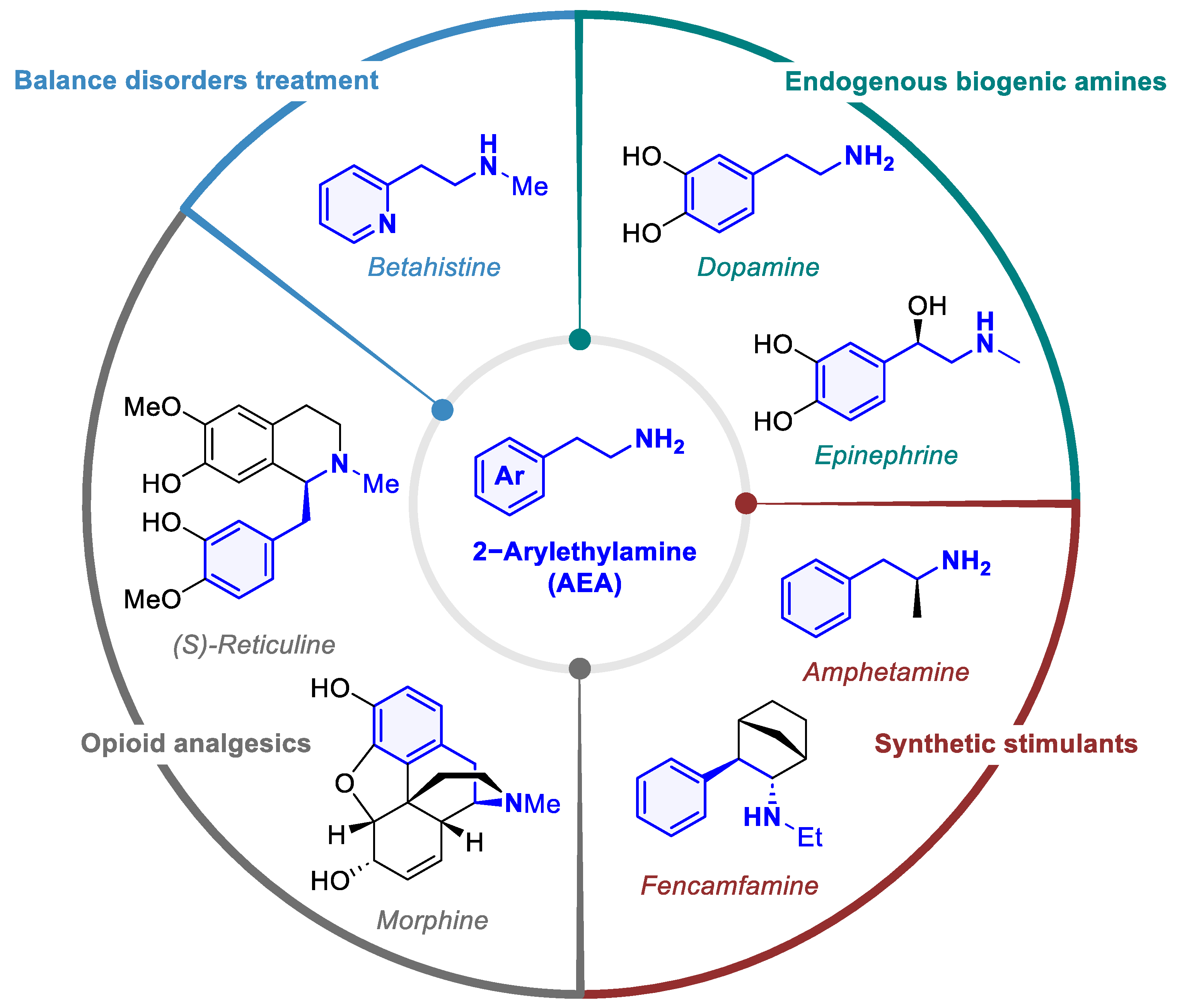
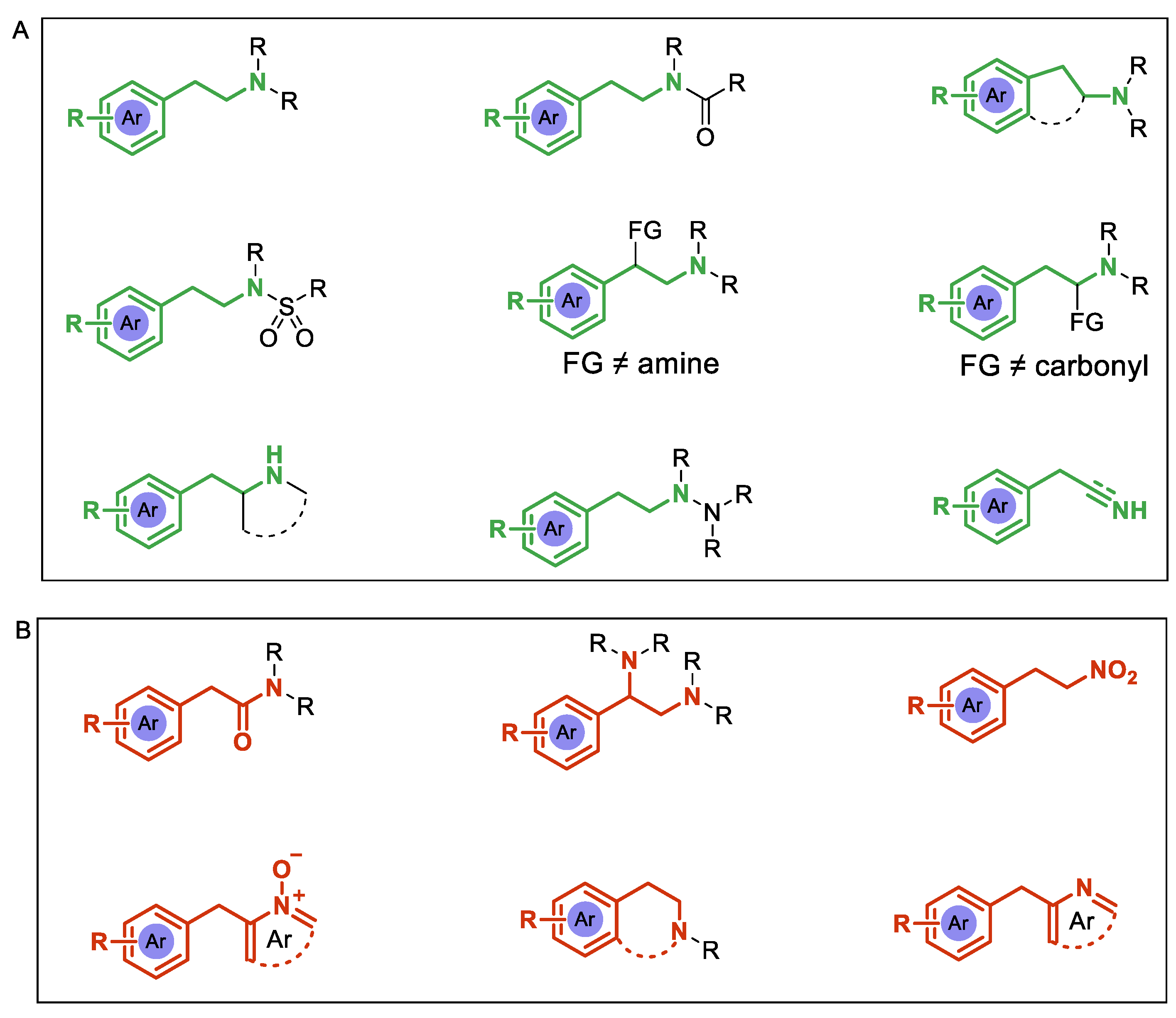
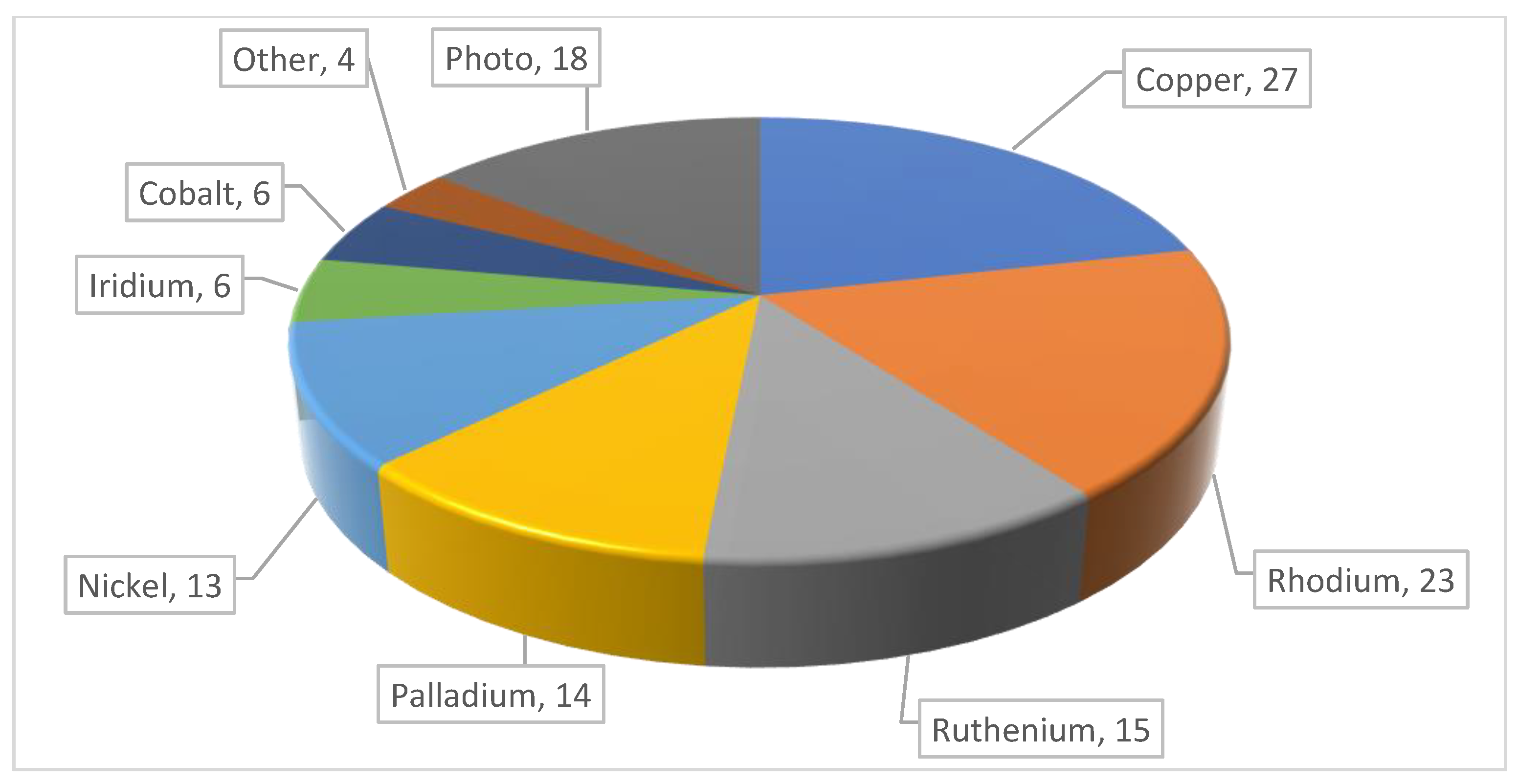
1. Introduction
2. Conventional Metal Catalysis
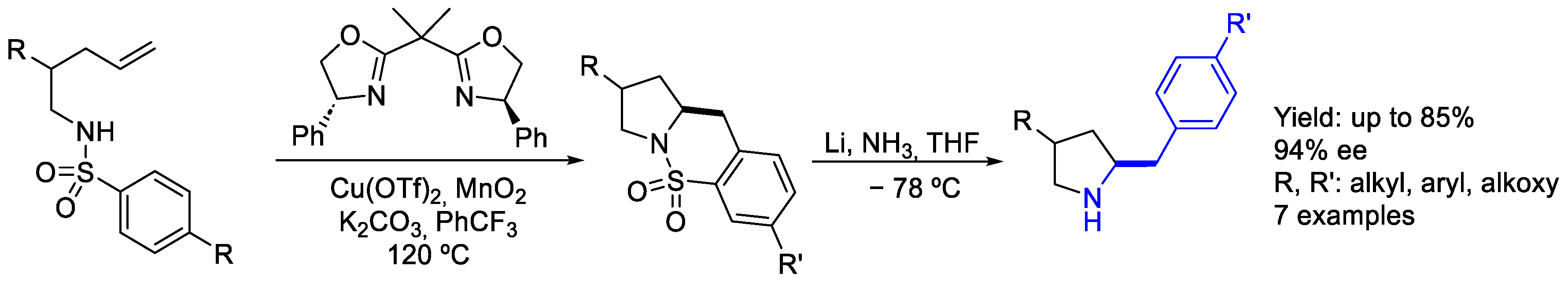
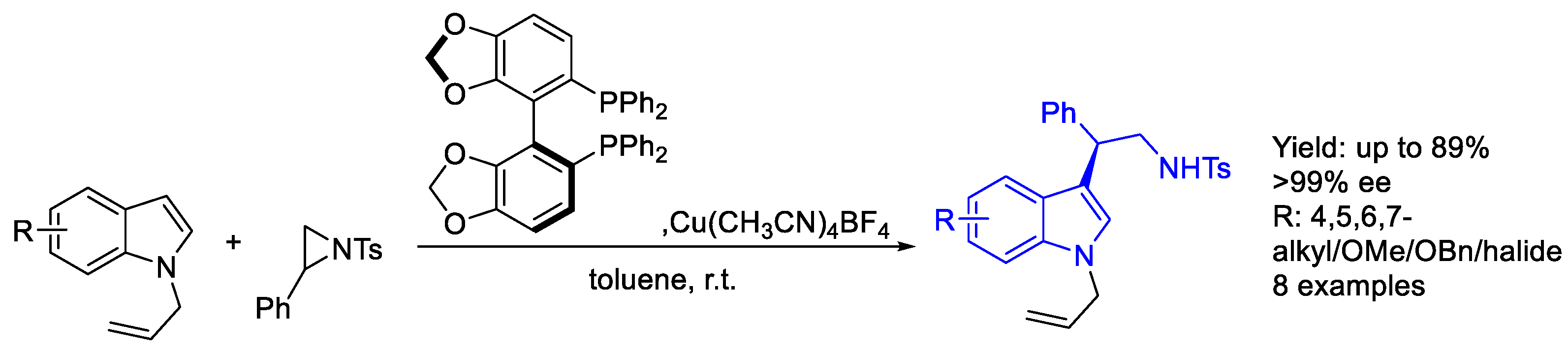
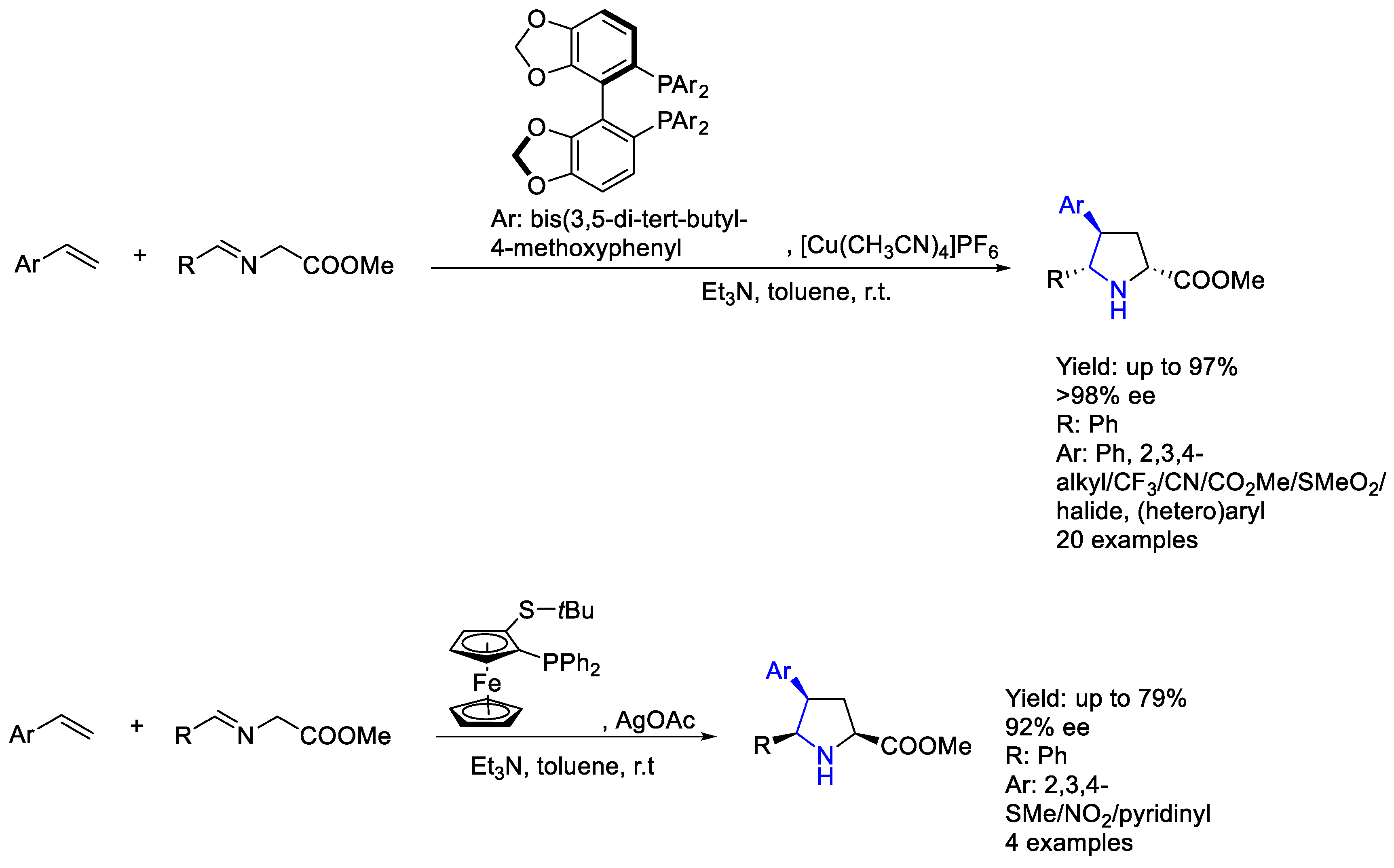
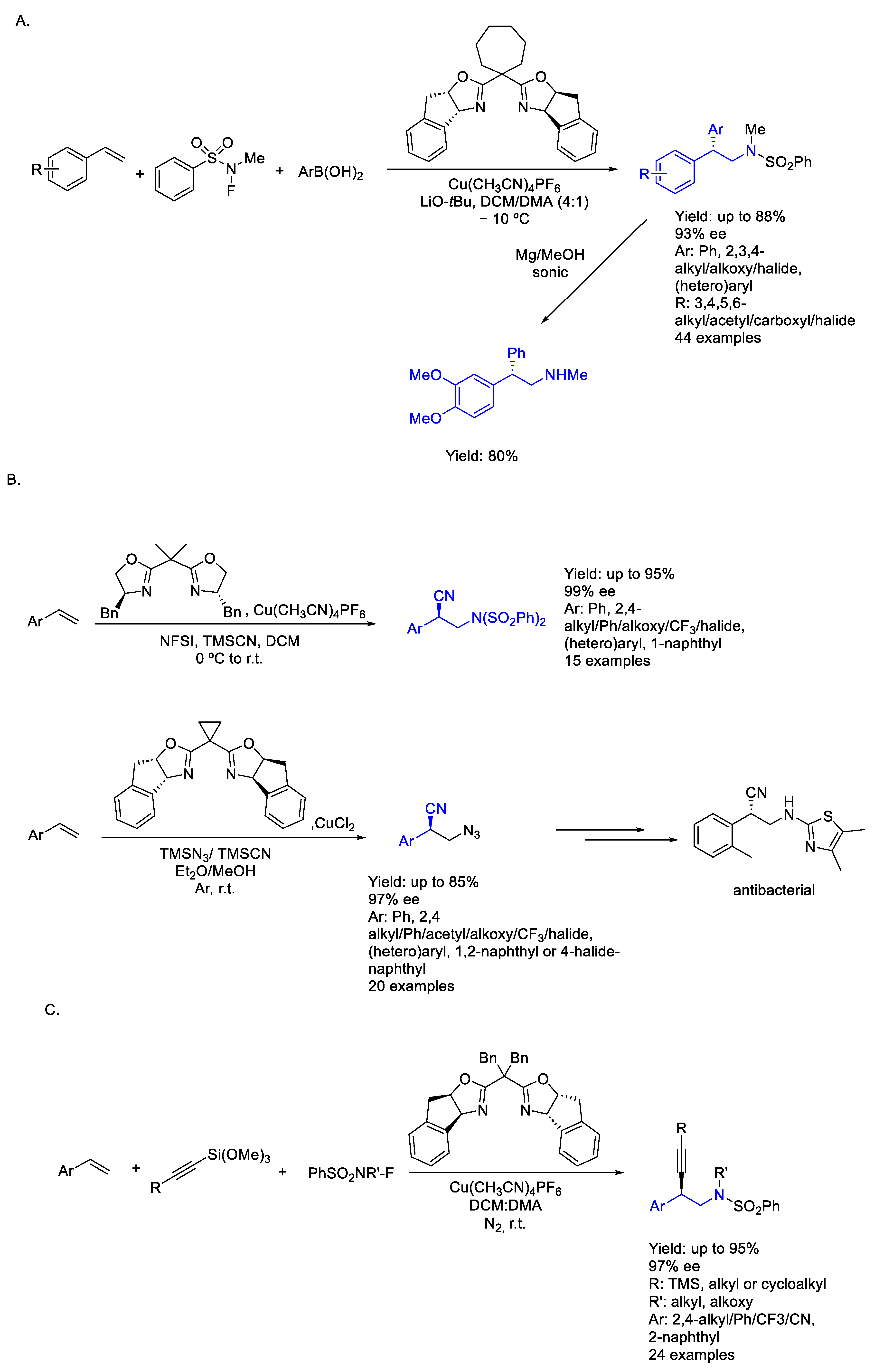
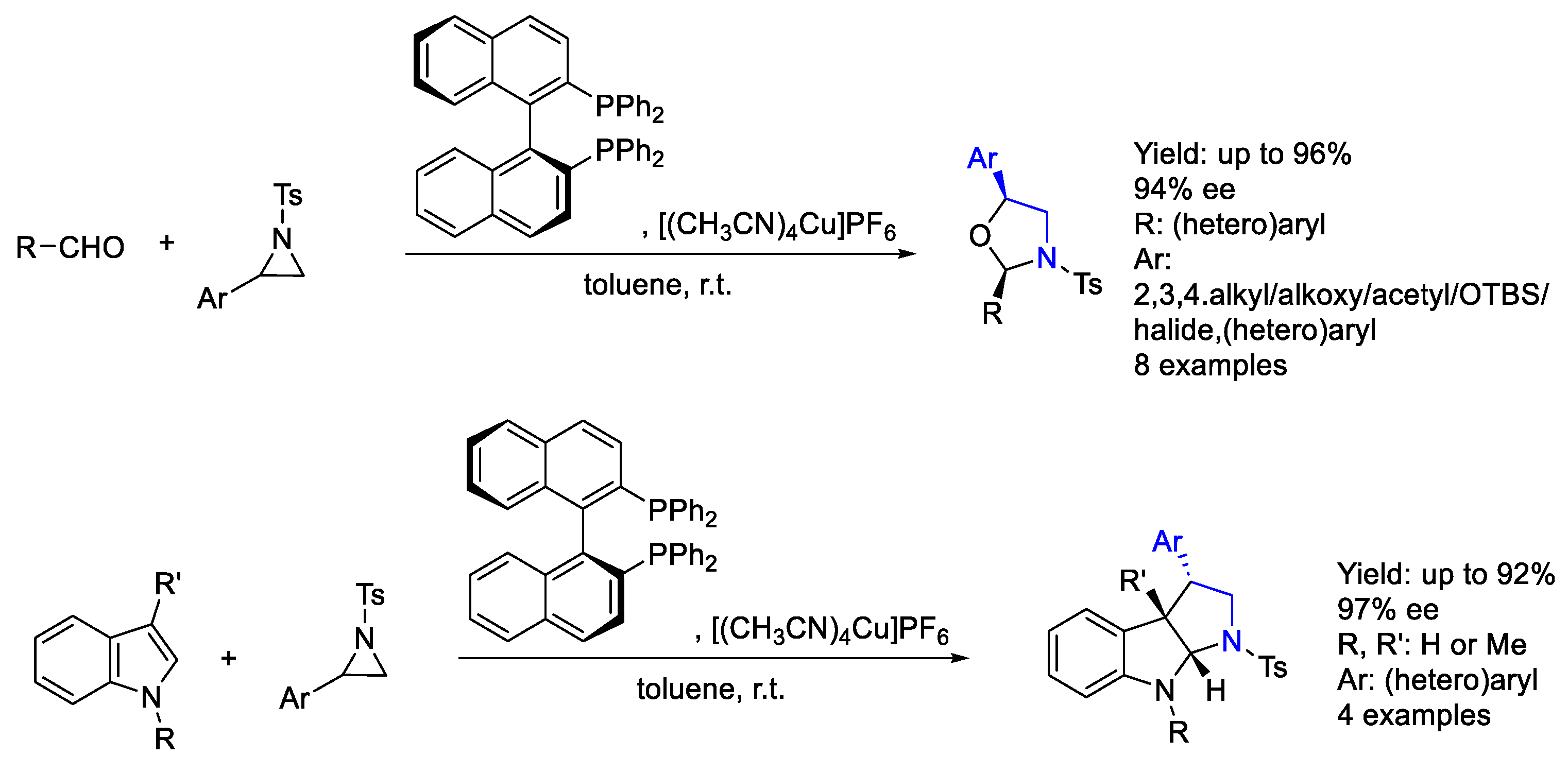
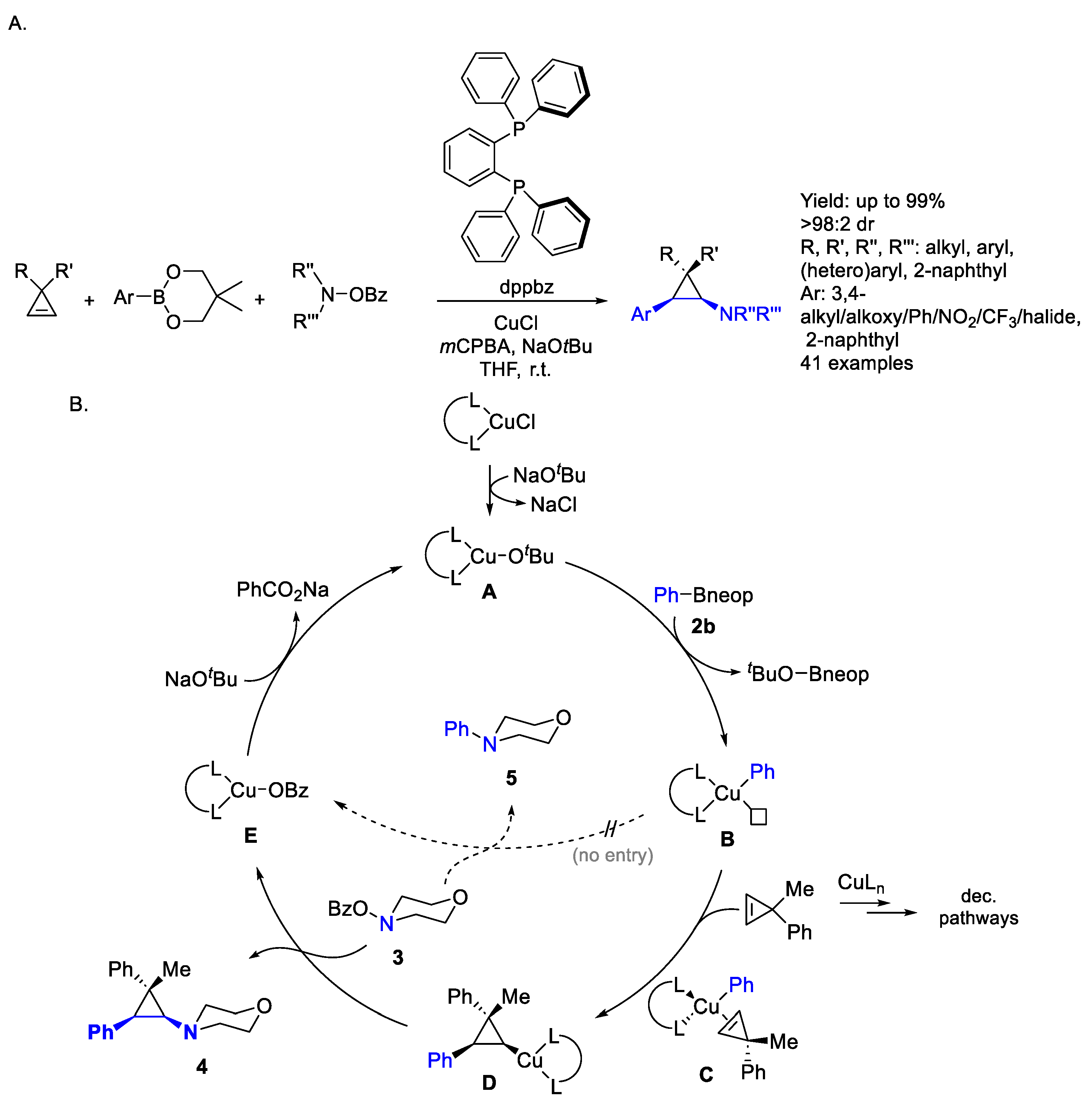
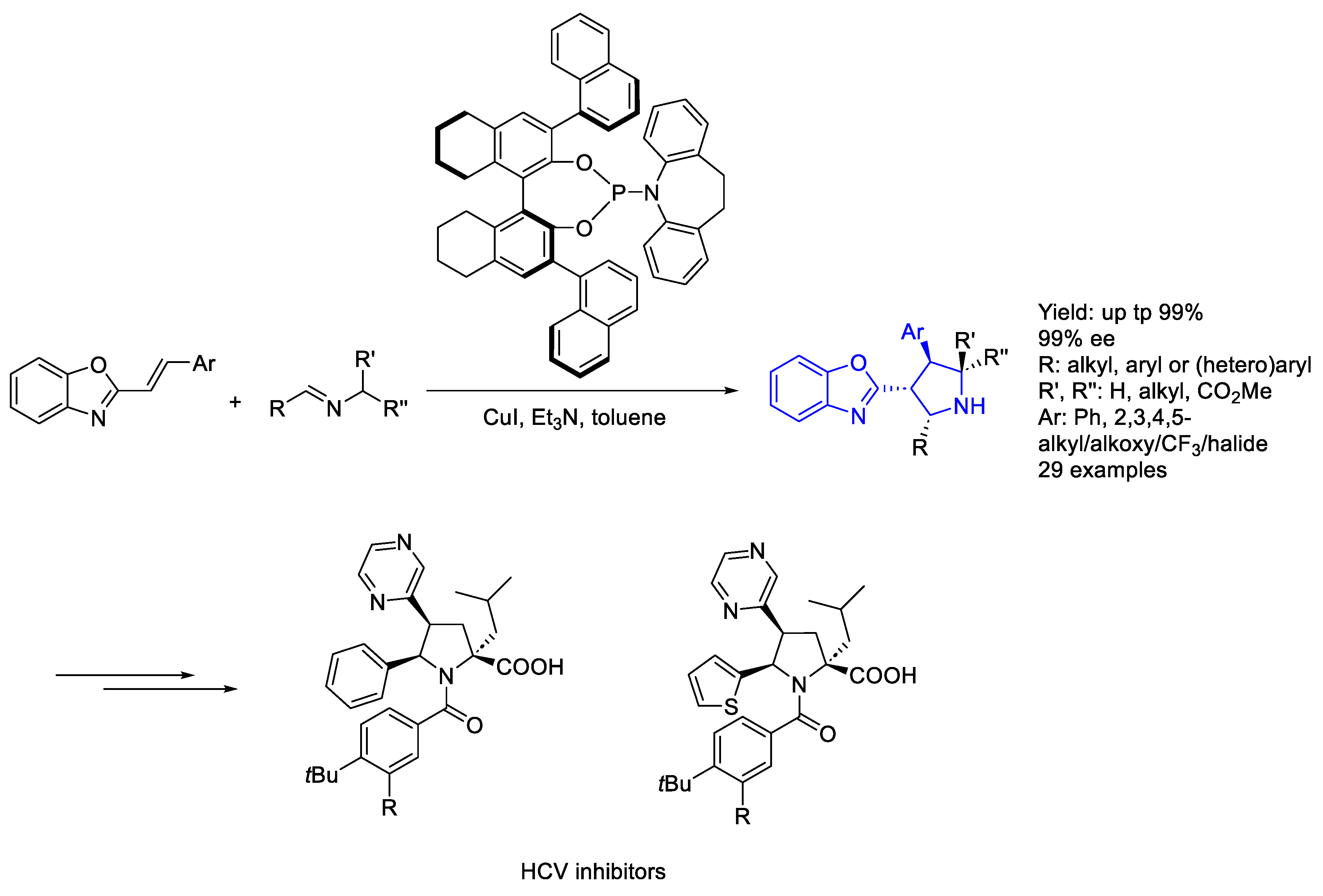
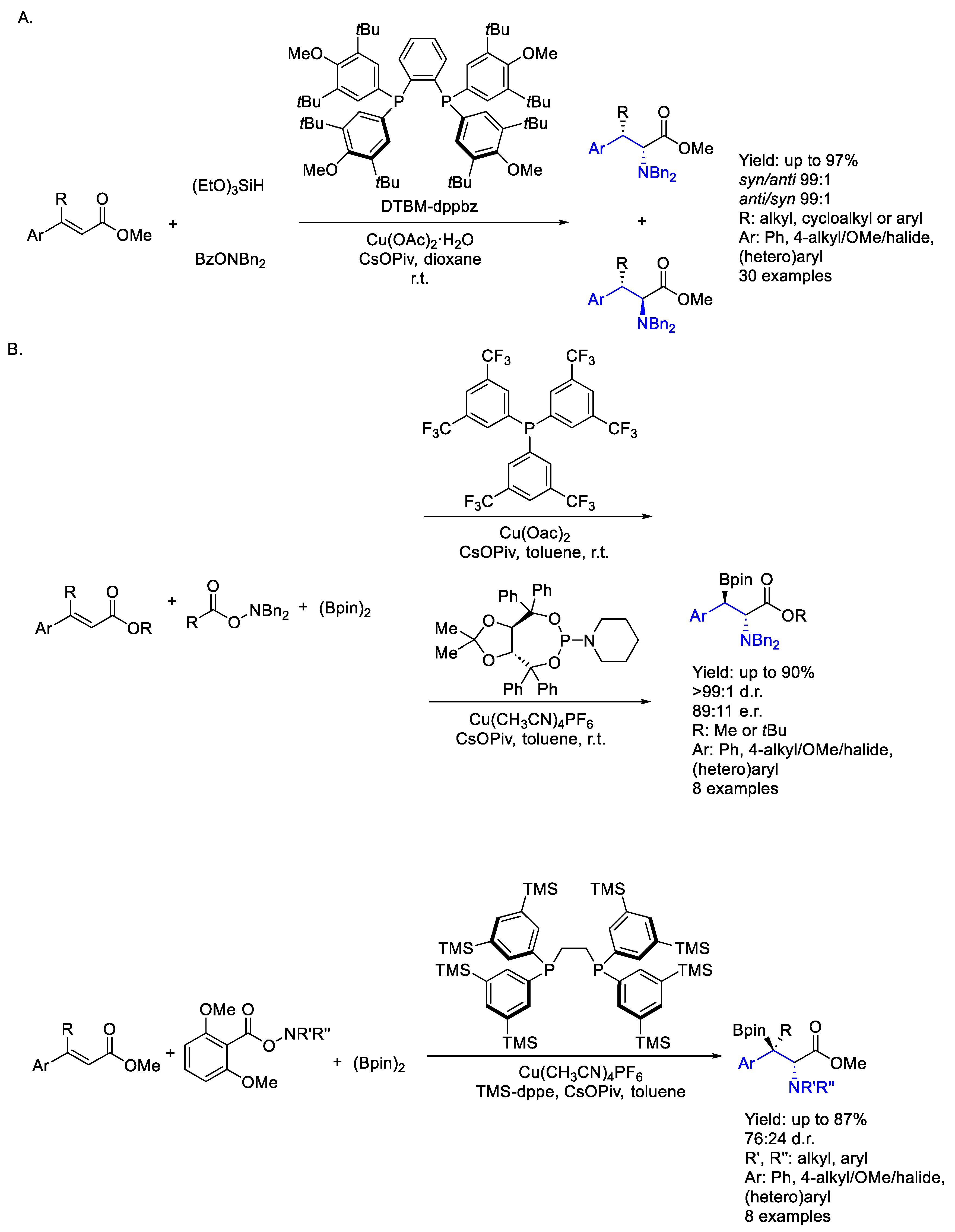
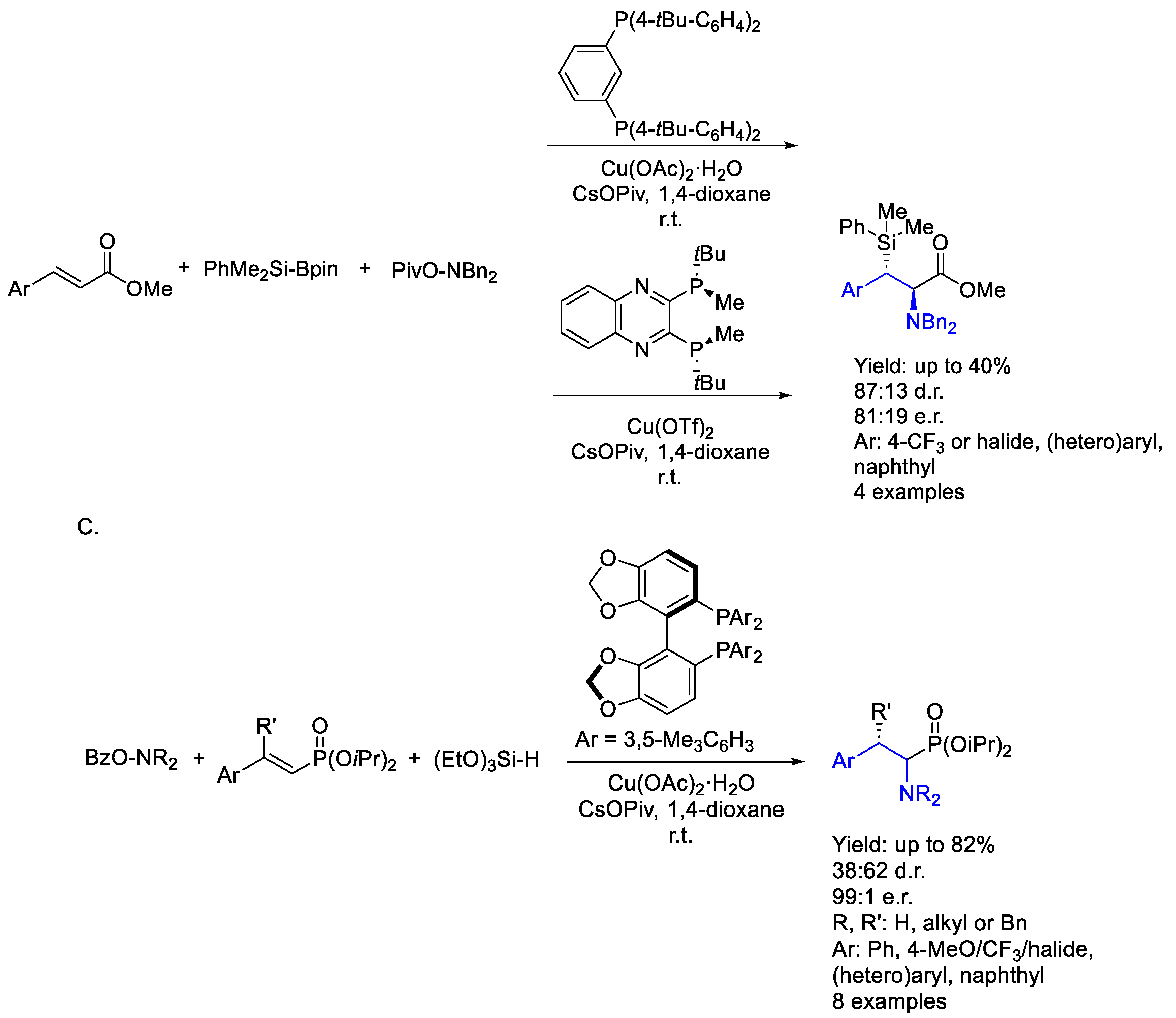
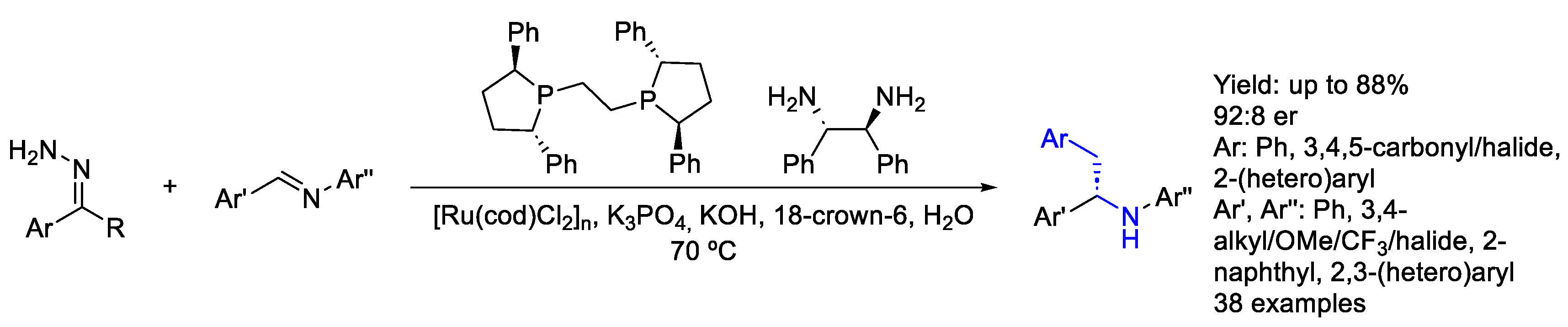
2.1. Copper
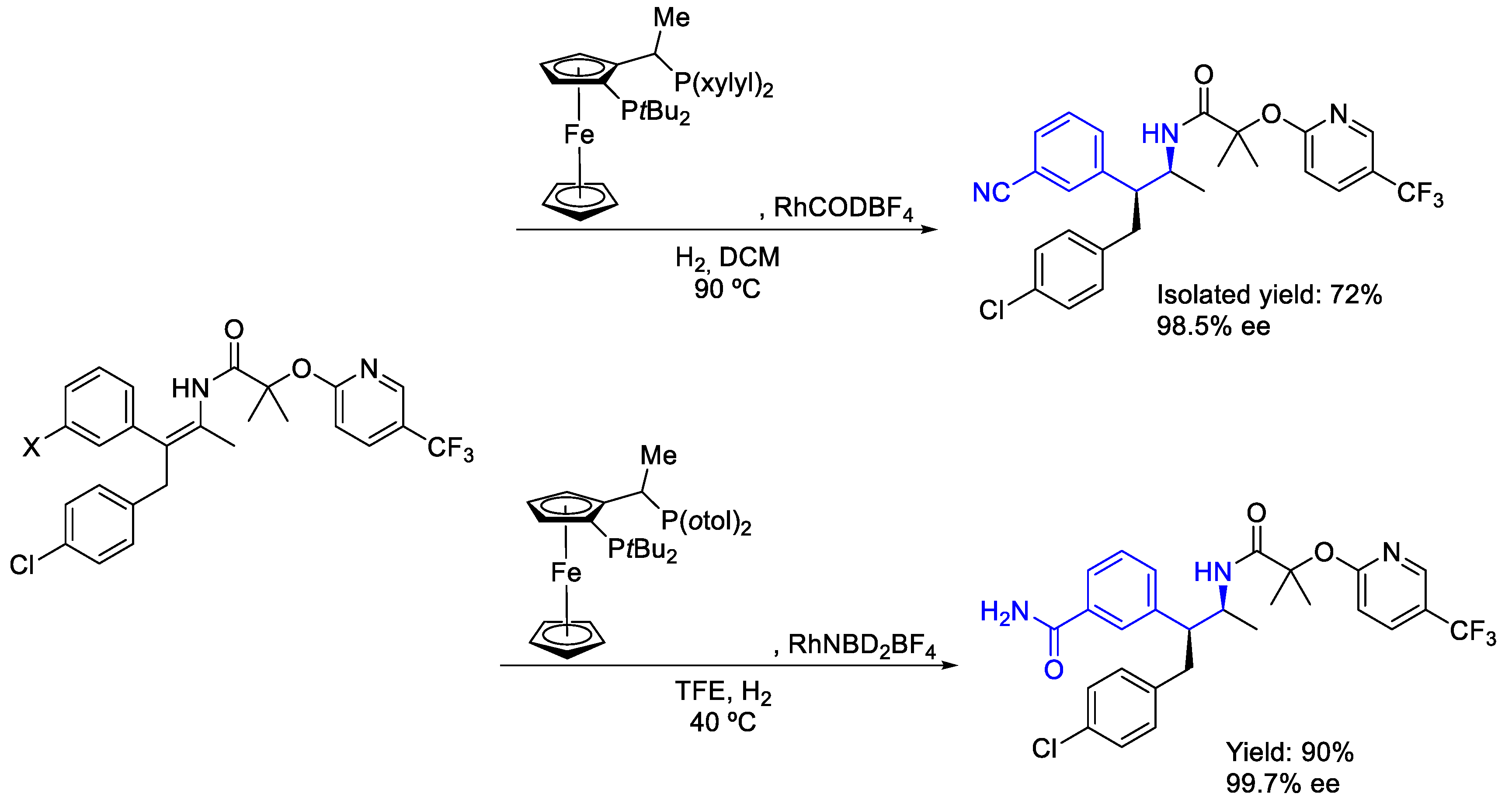
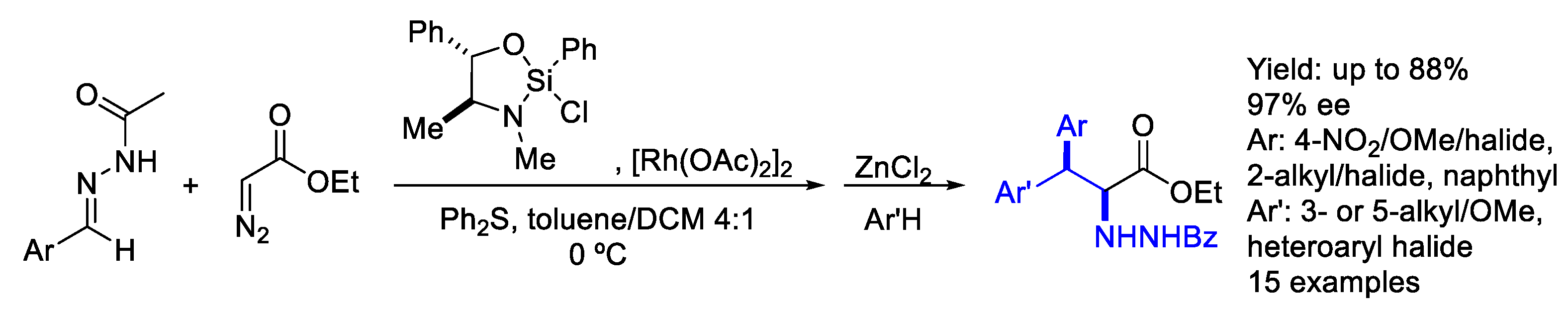
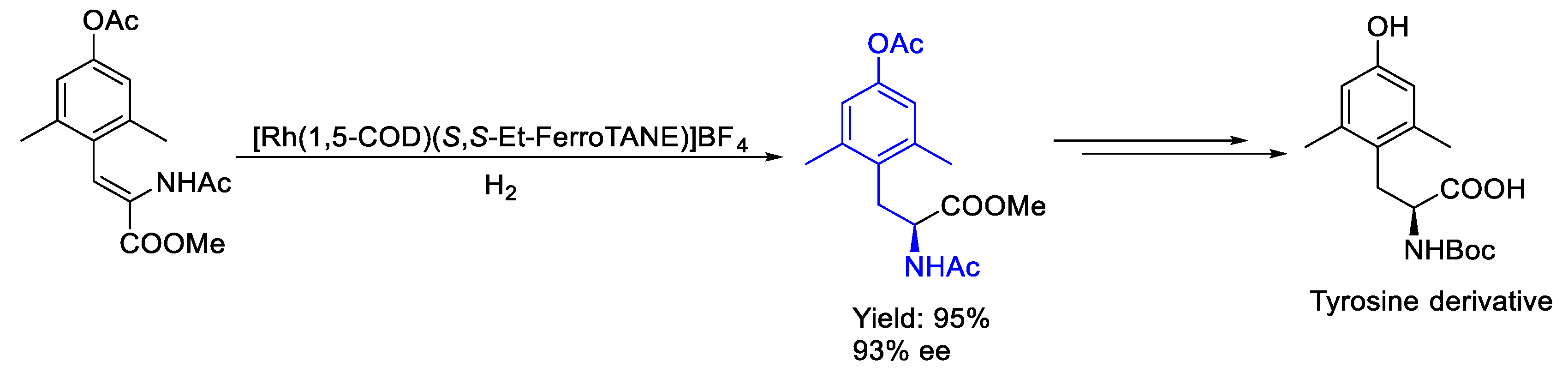
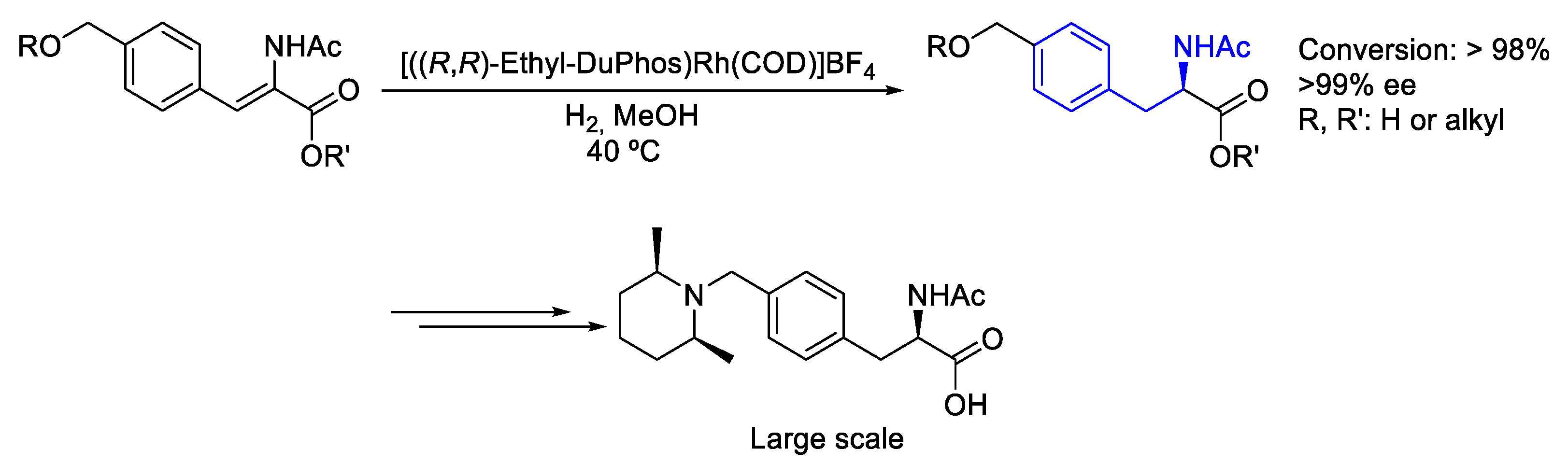
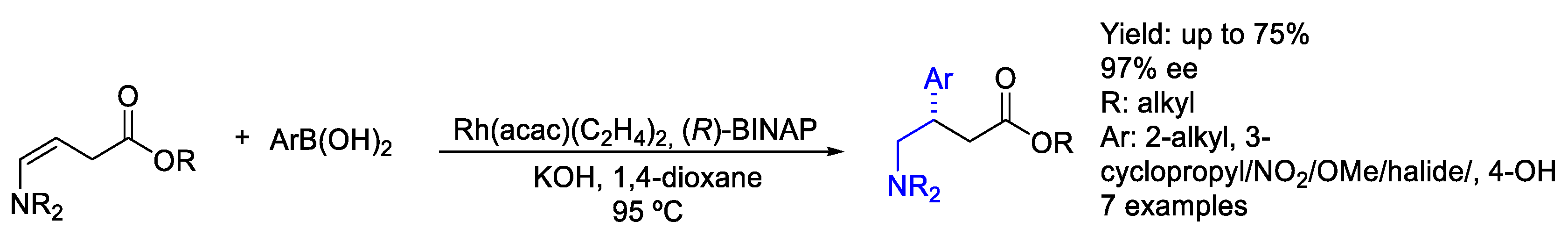
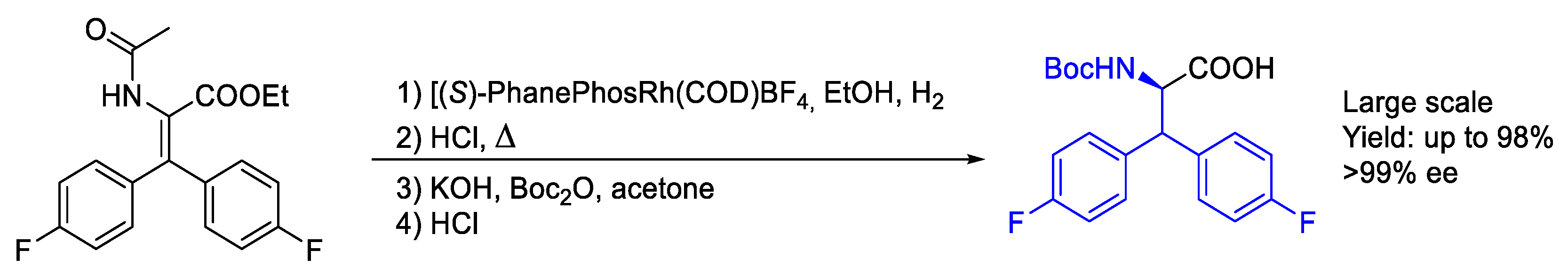
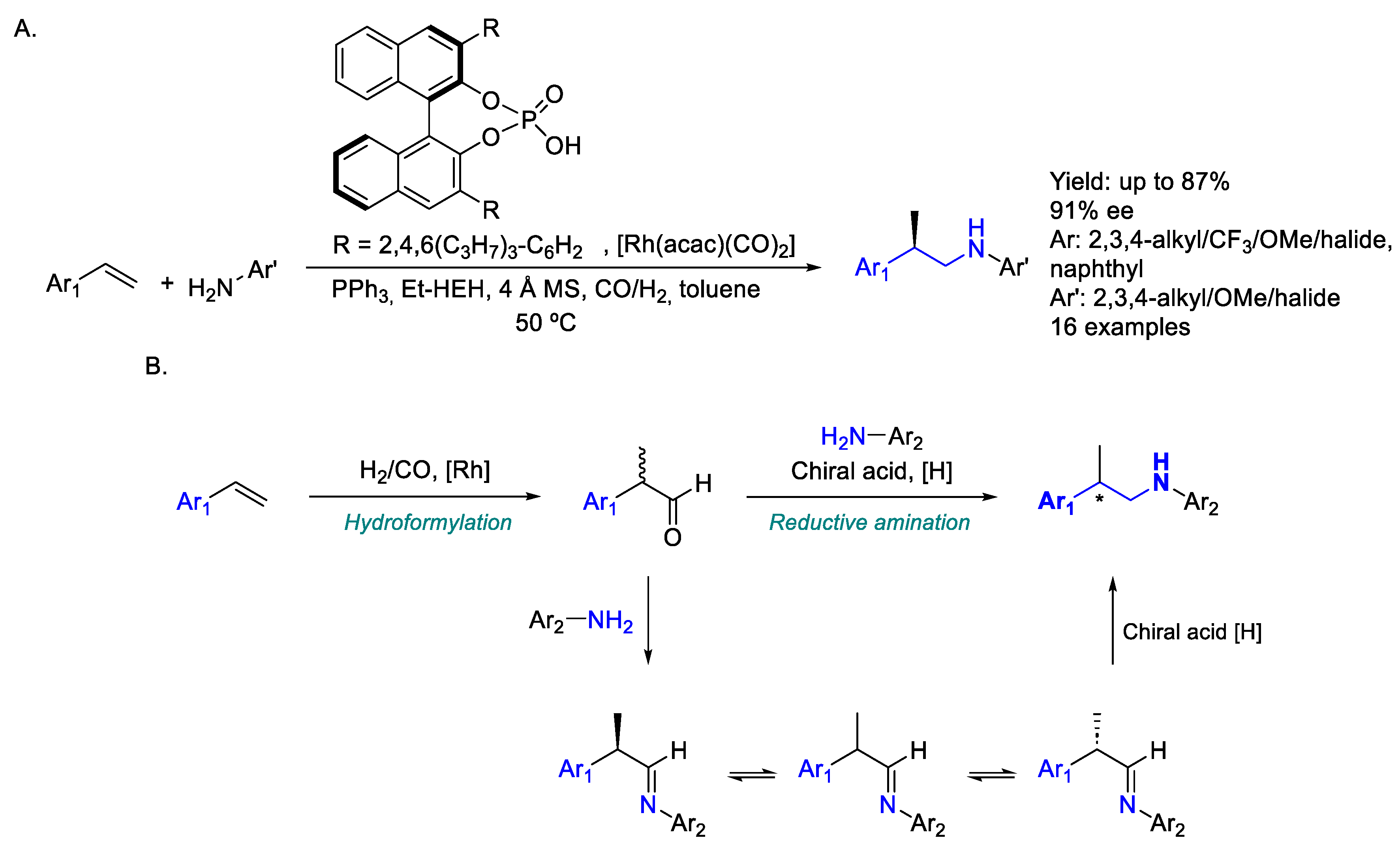
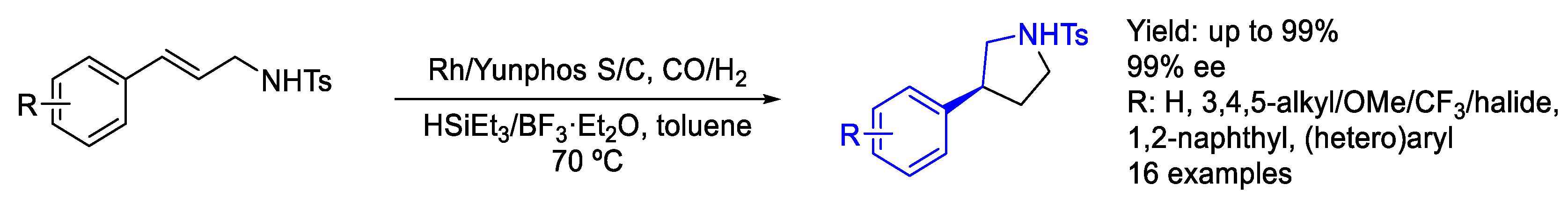
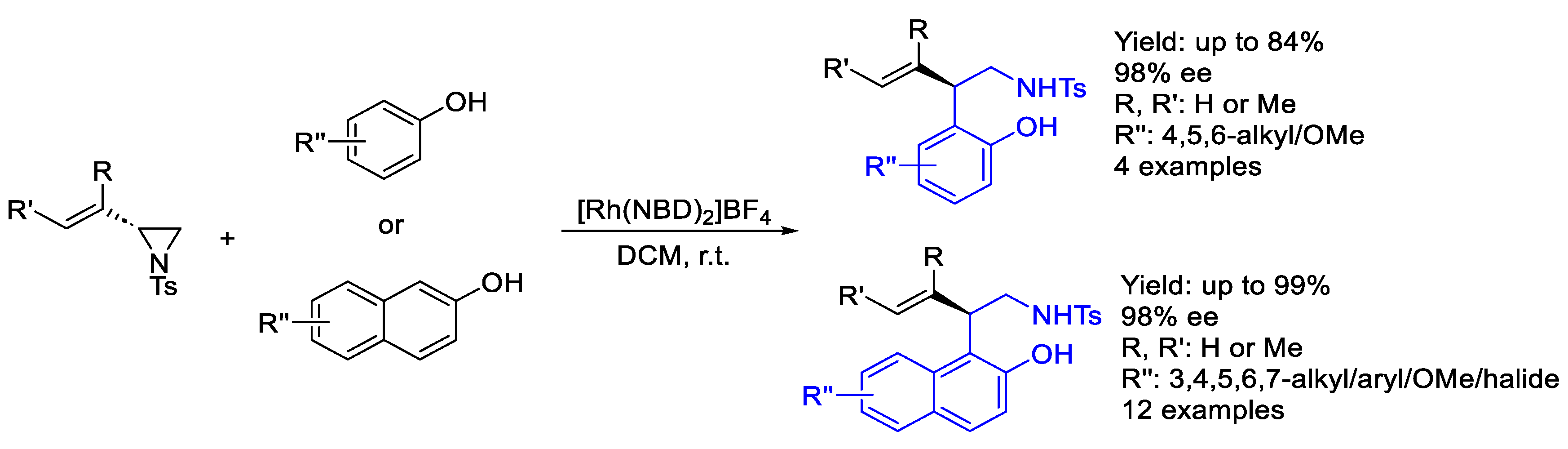
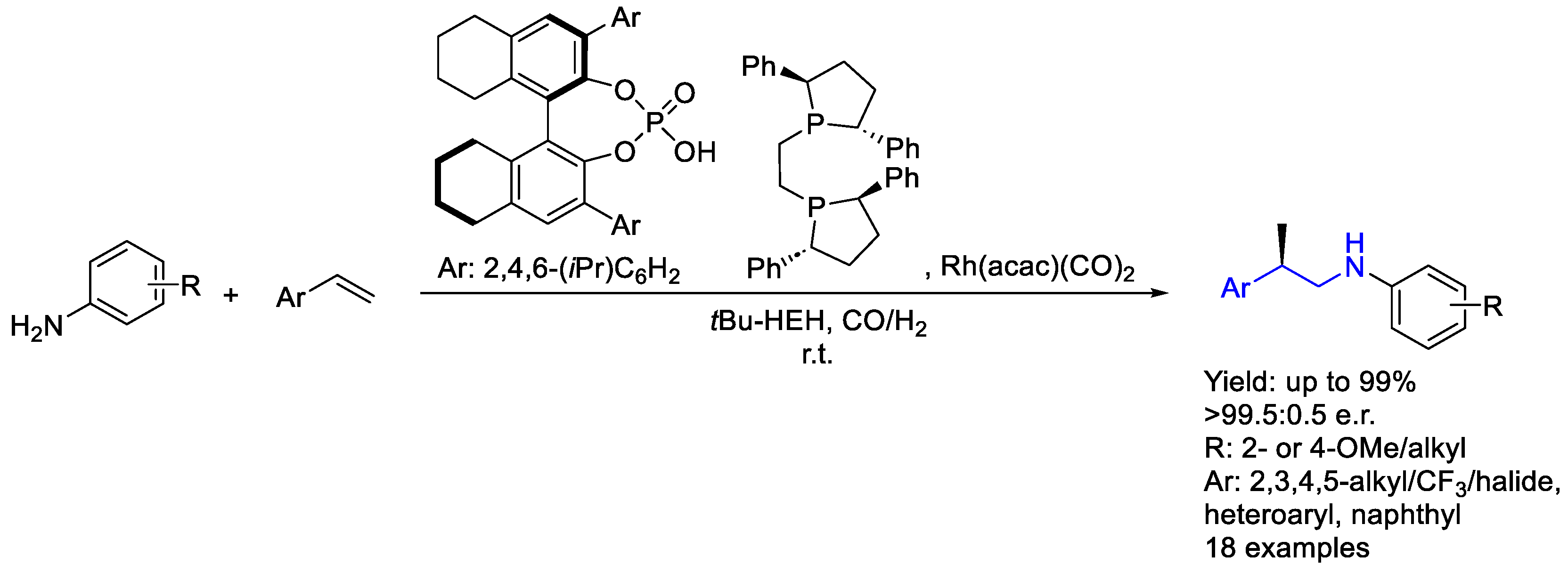
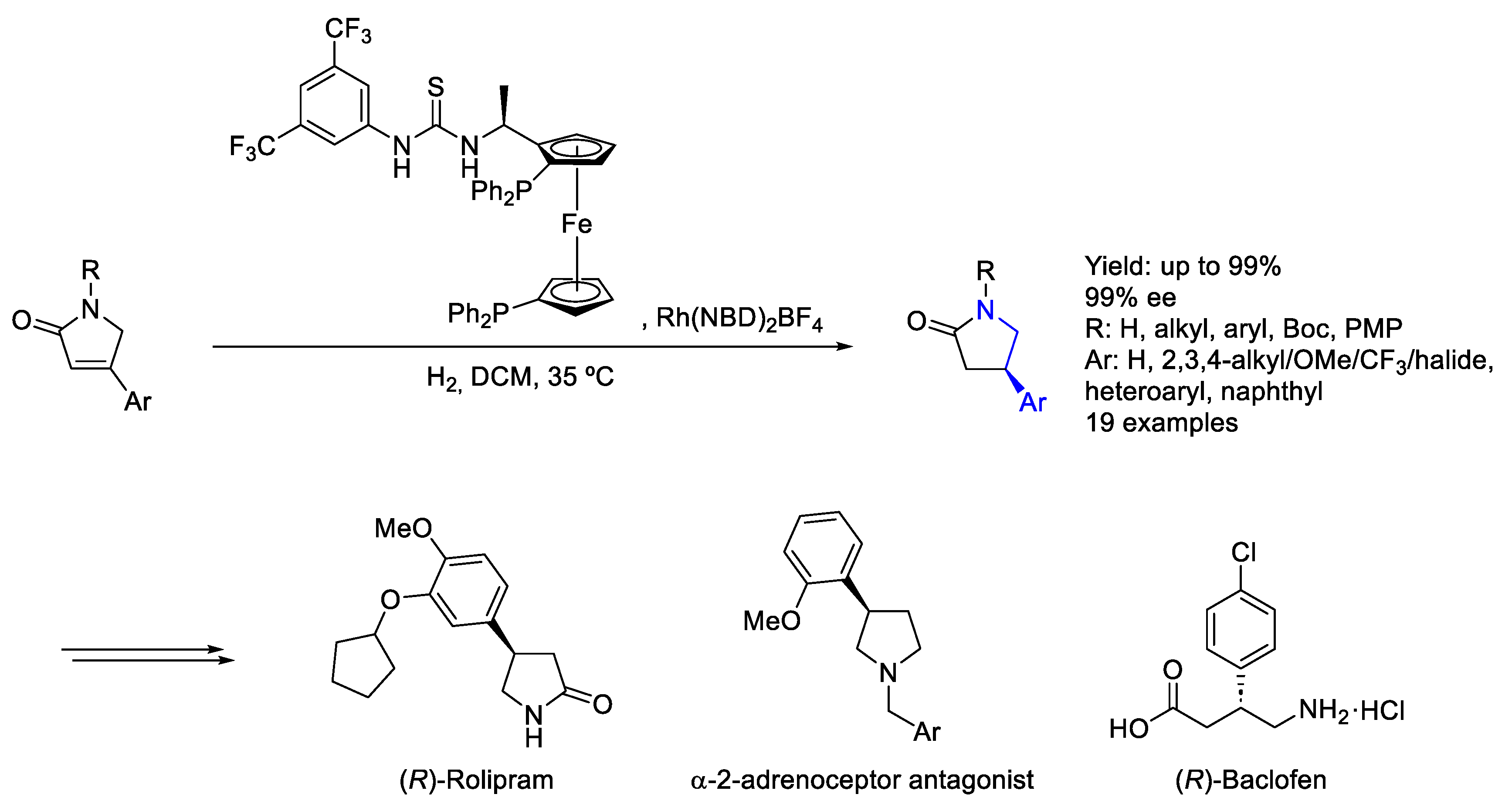
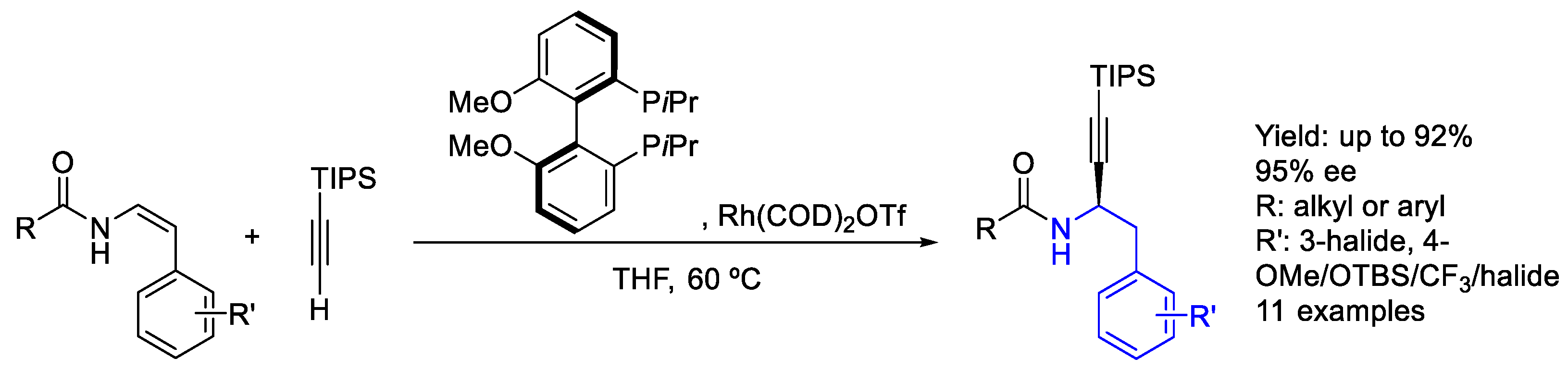
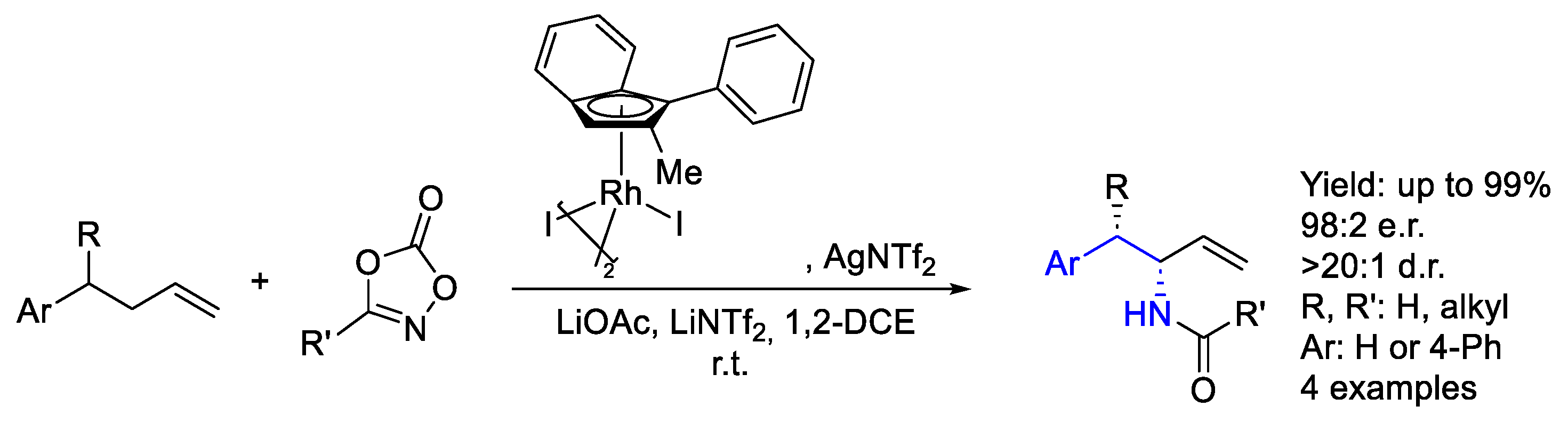
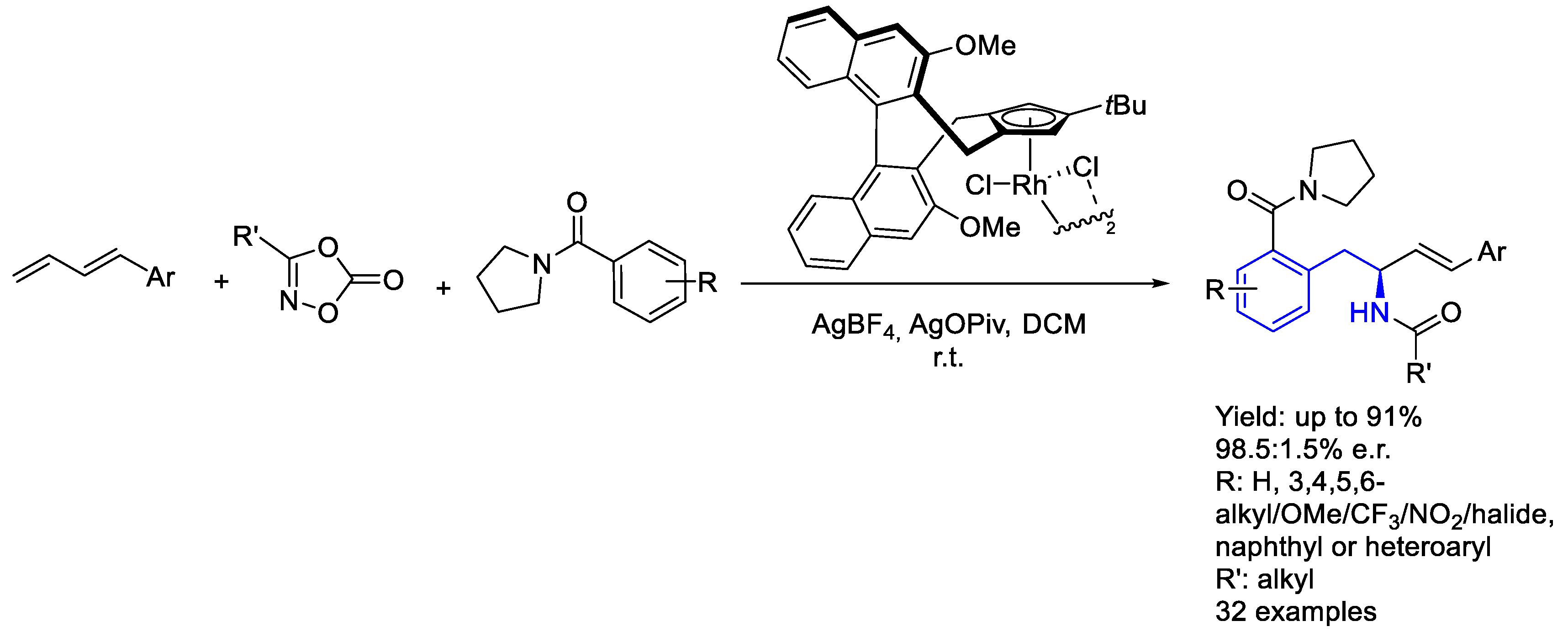
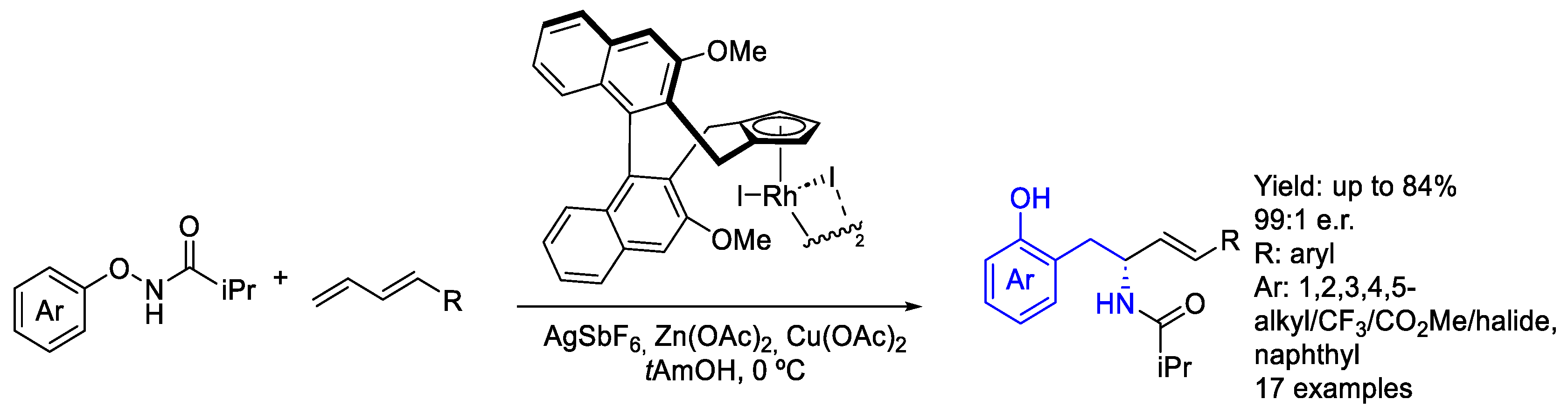
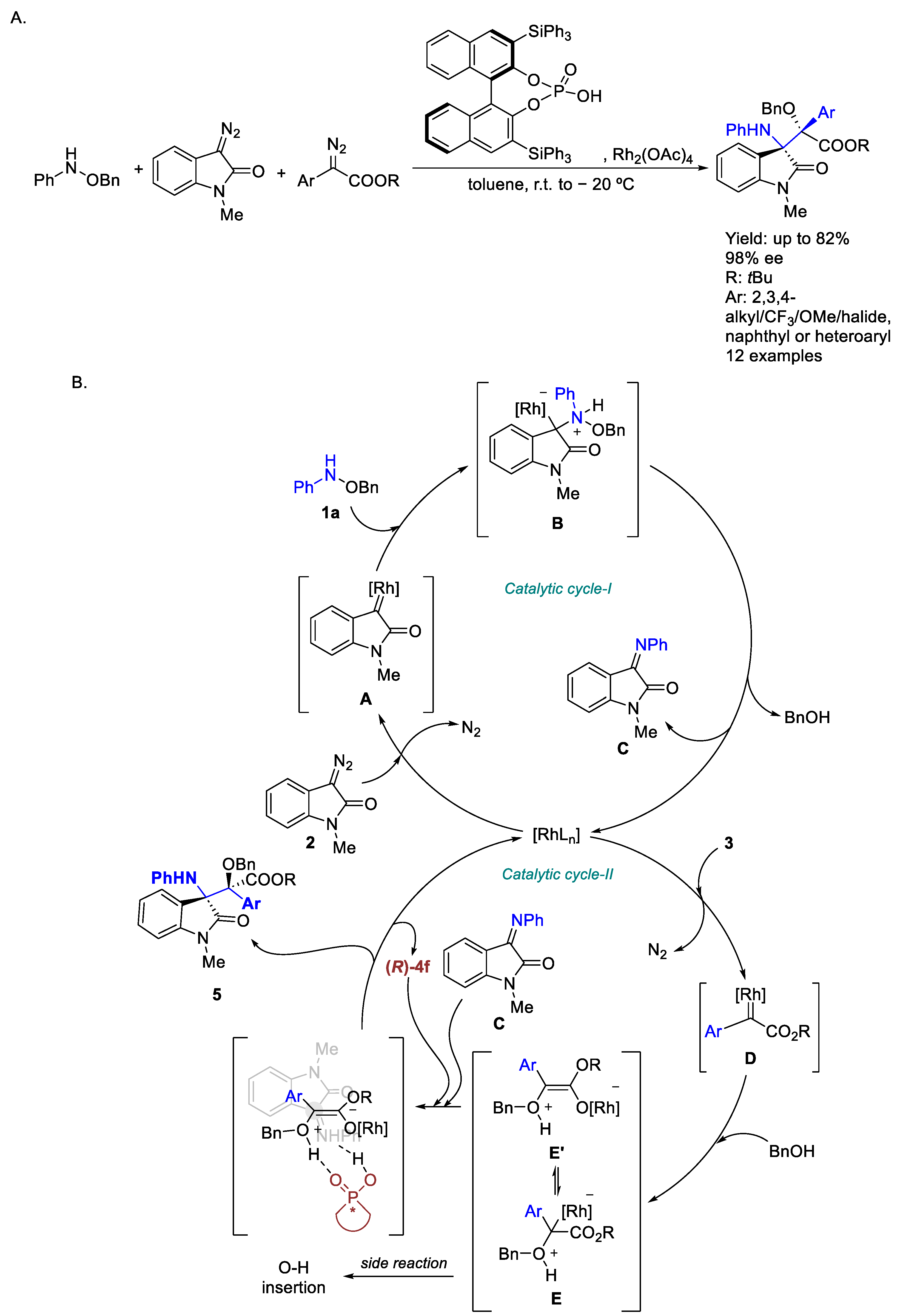
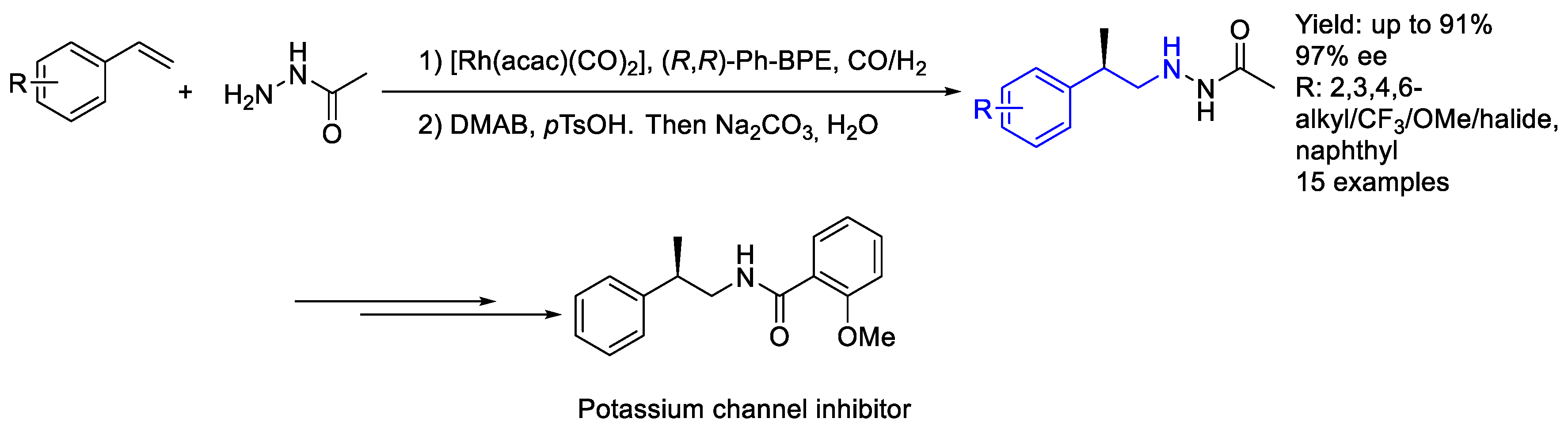
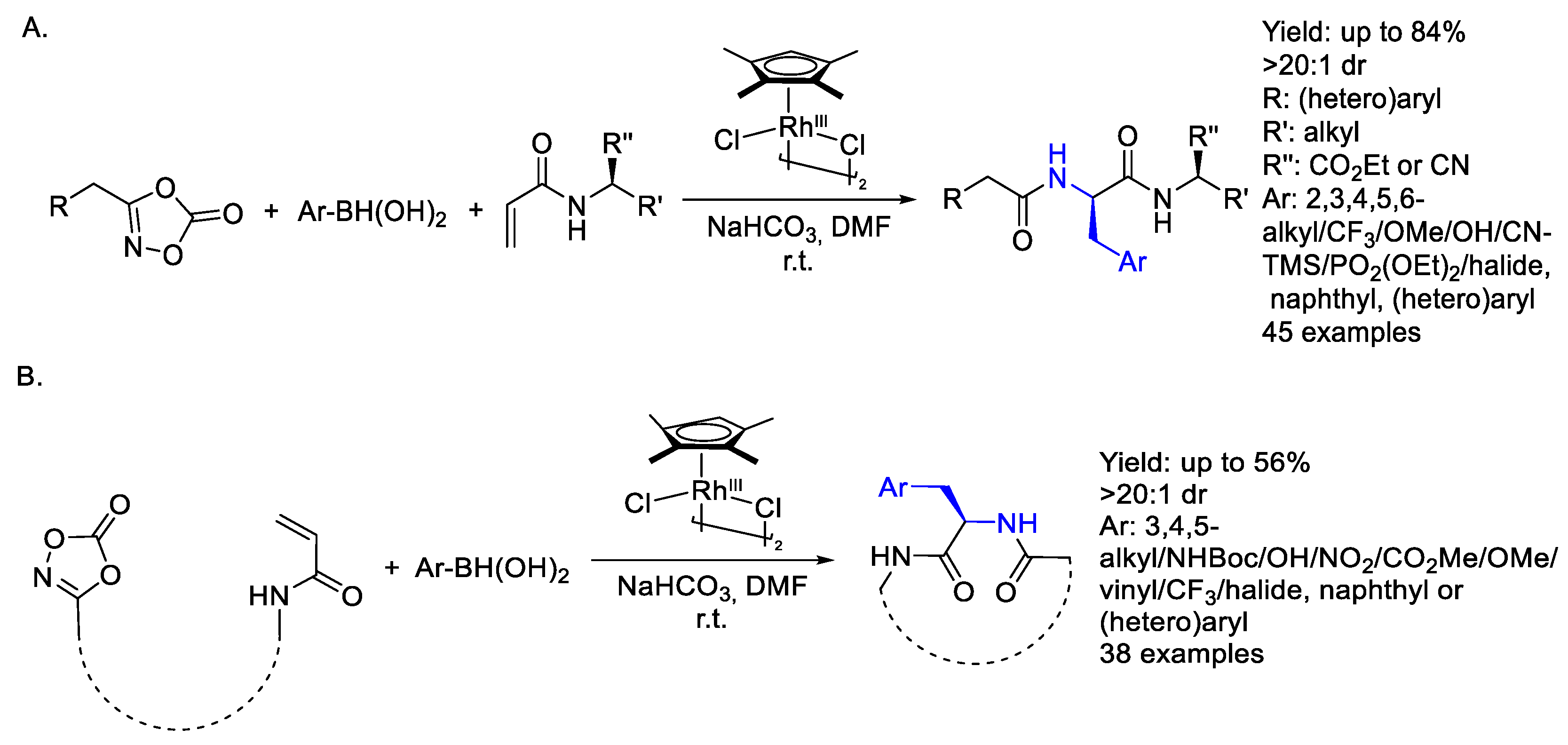
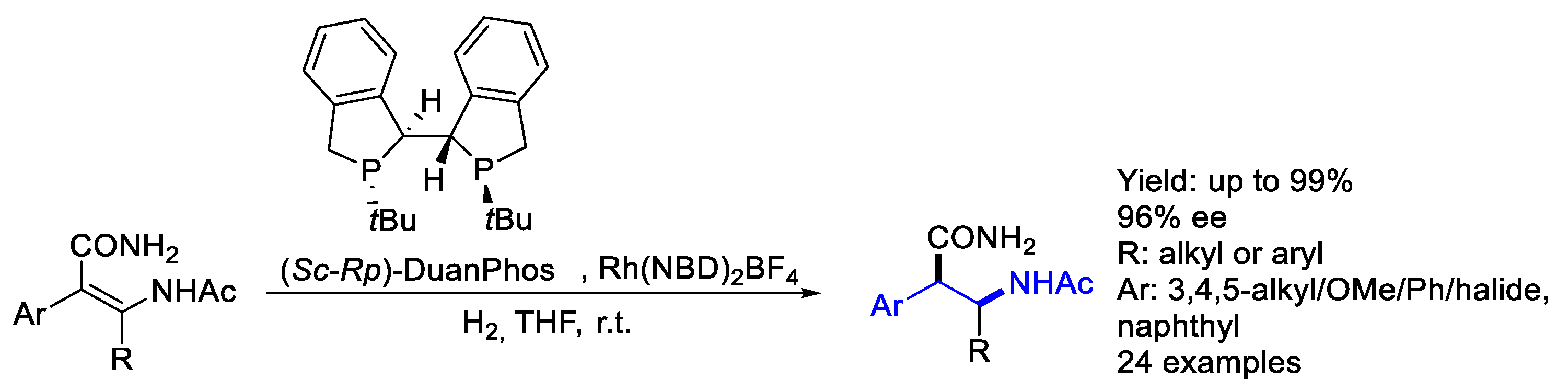
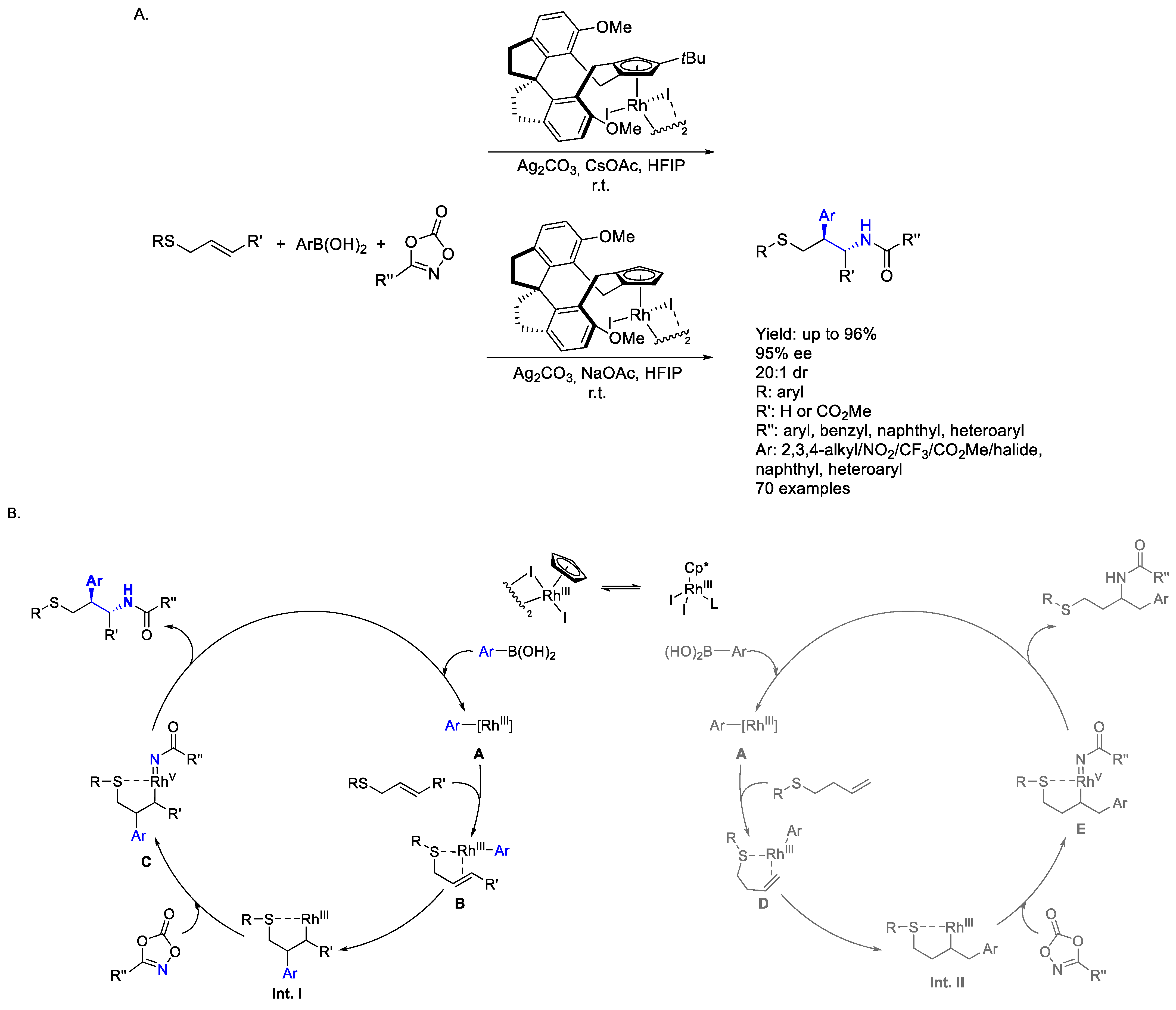
2.2. Rhodium
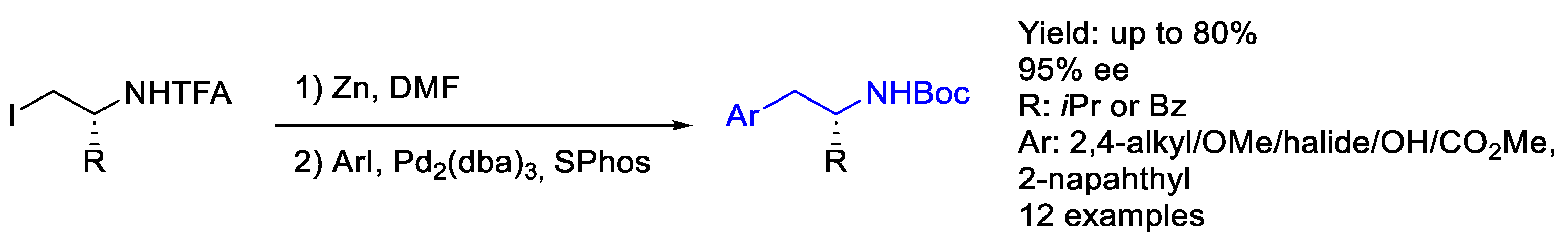
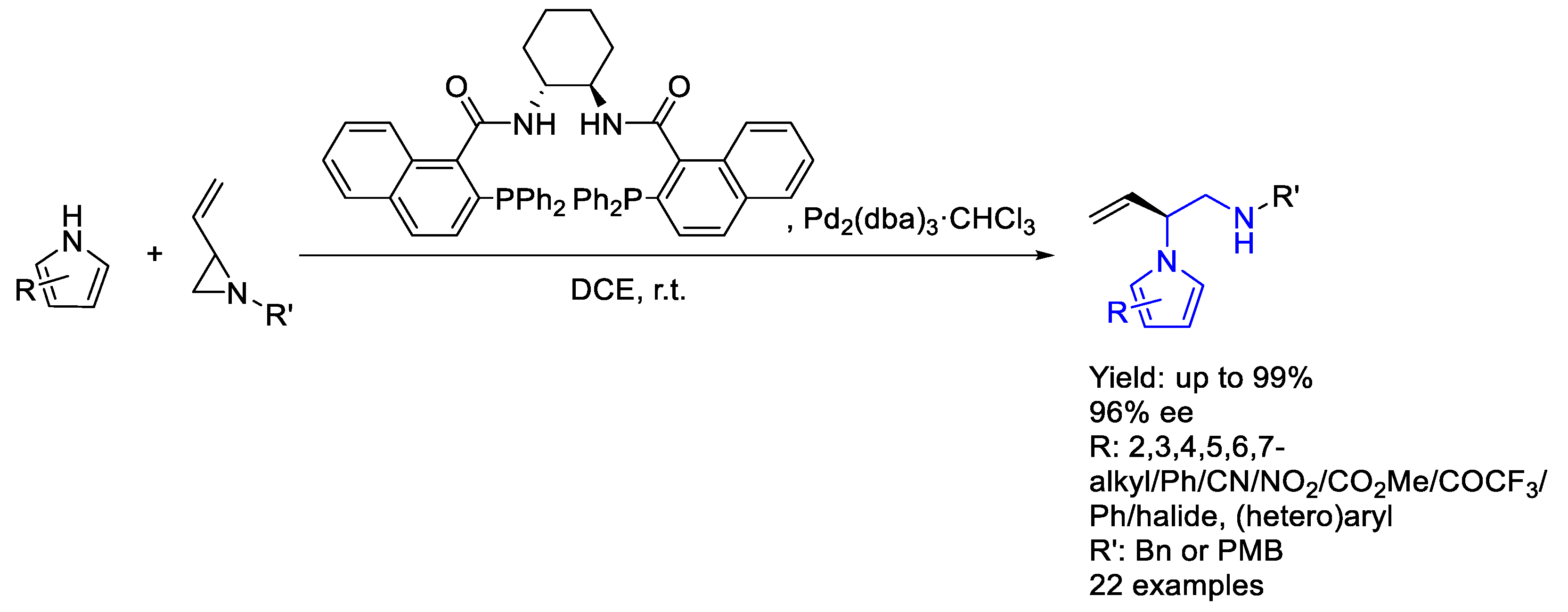
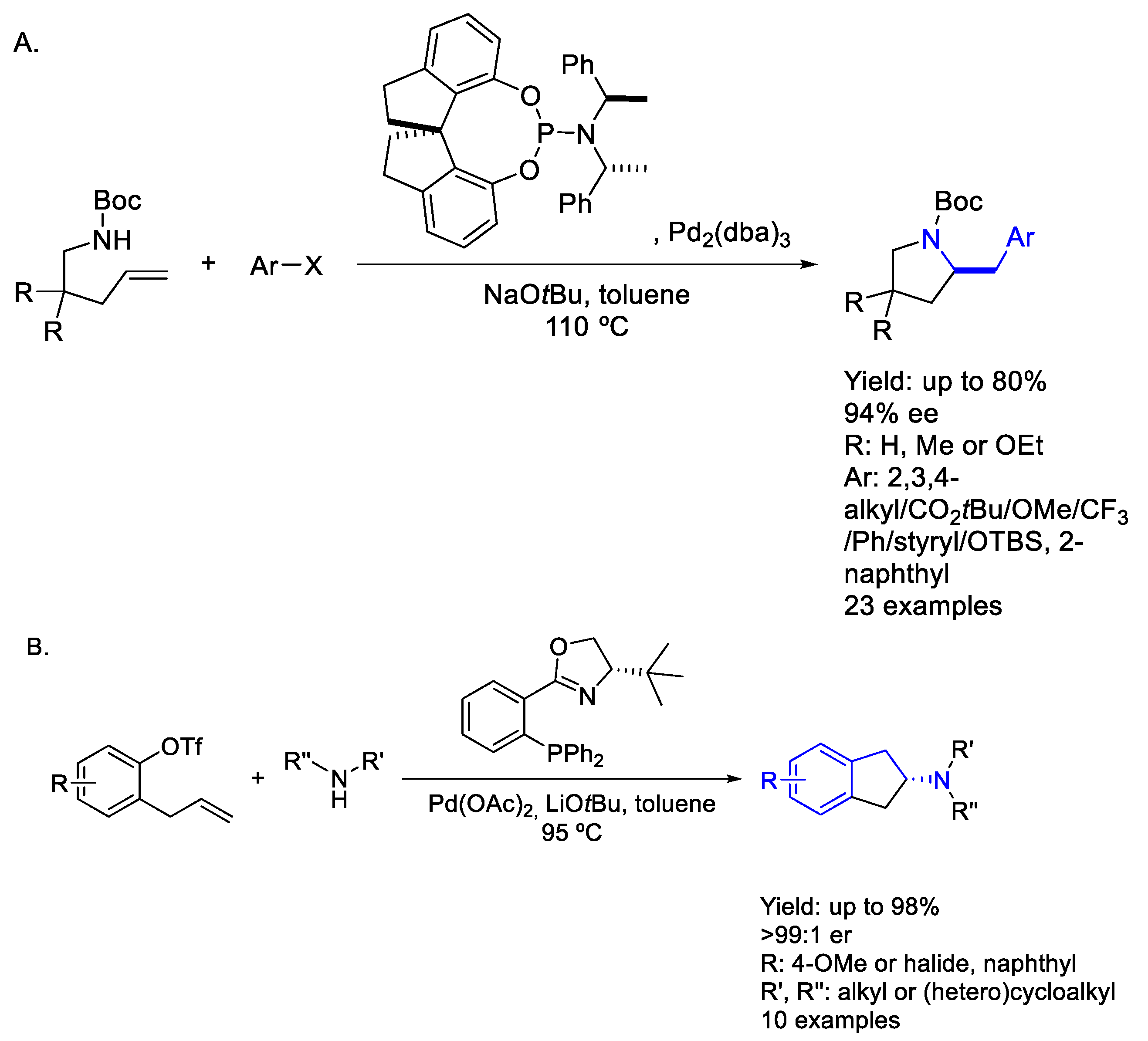
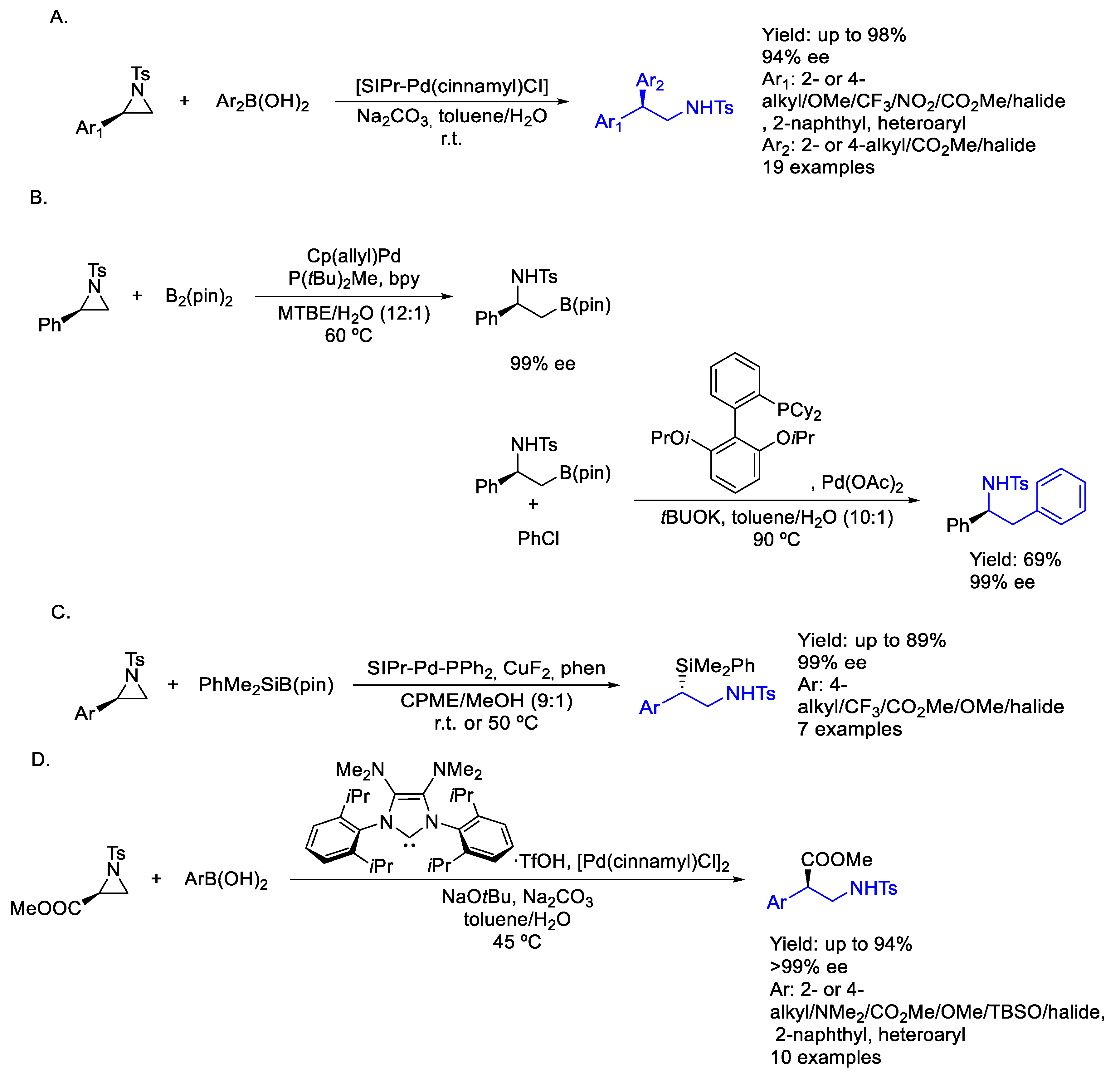
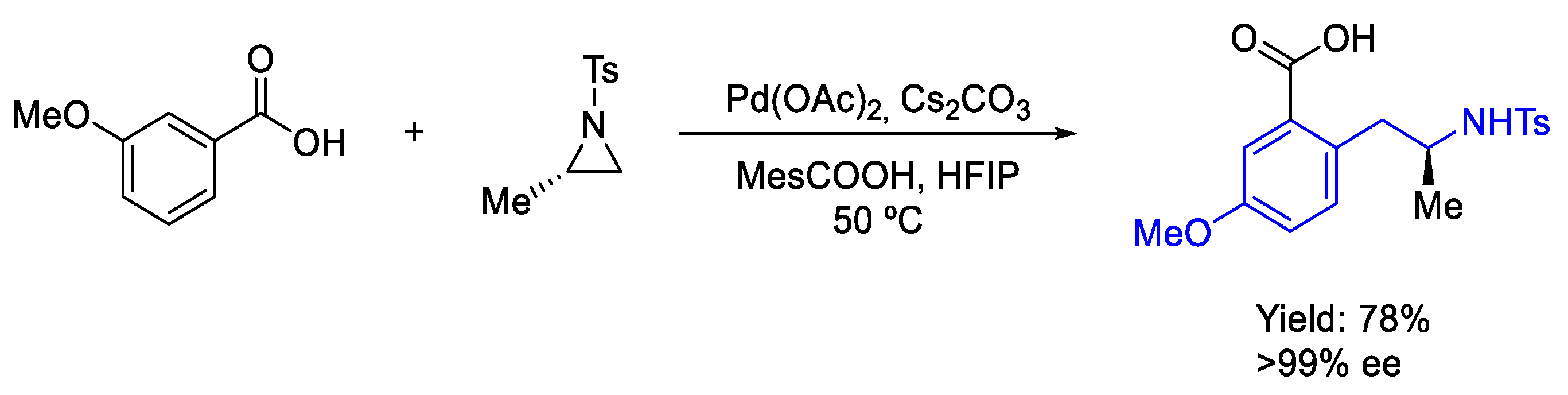
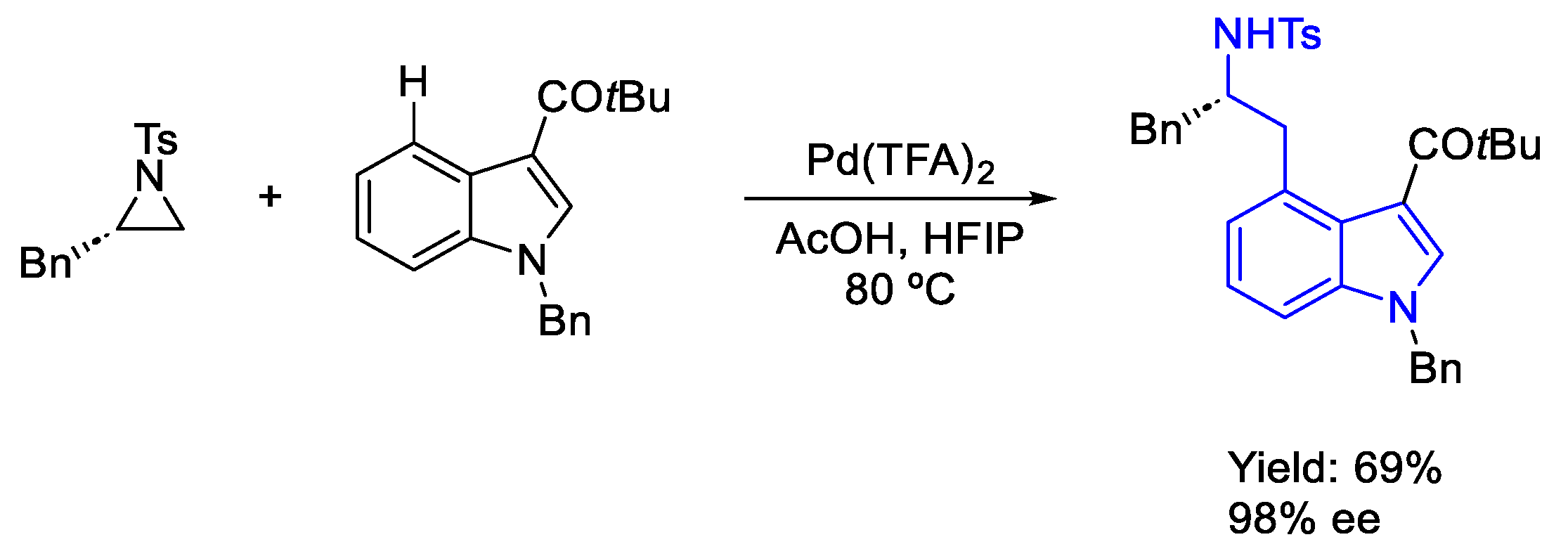
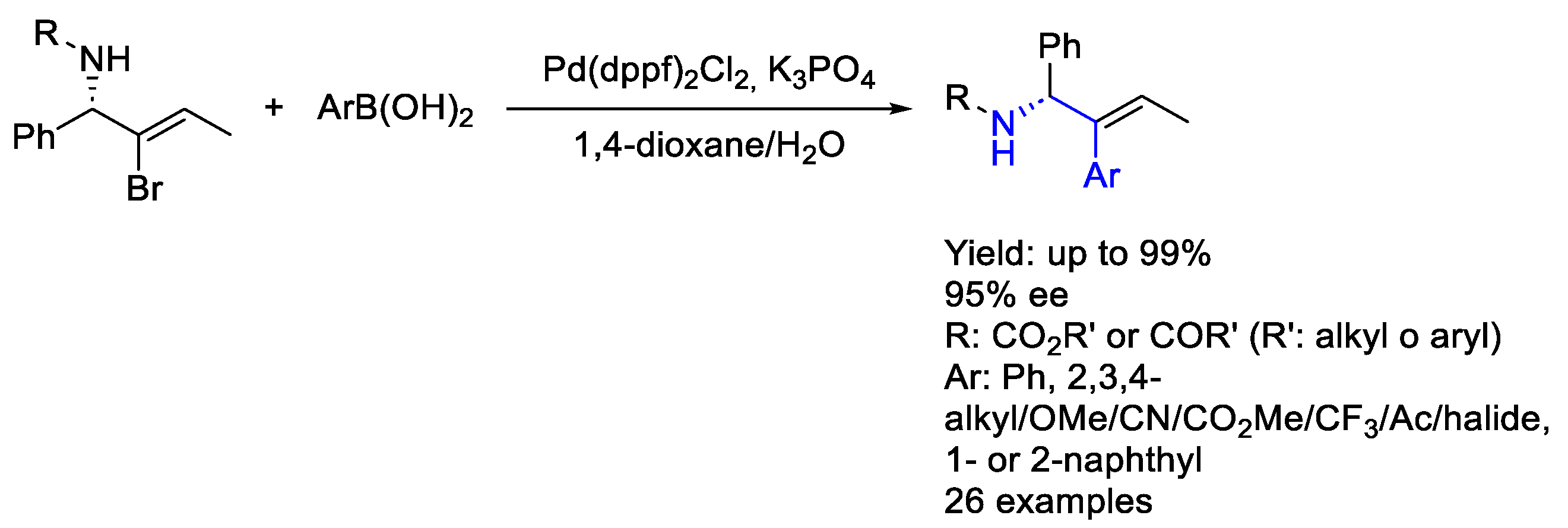
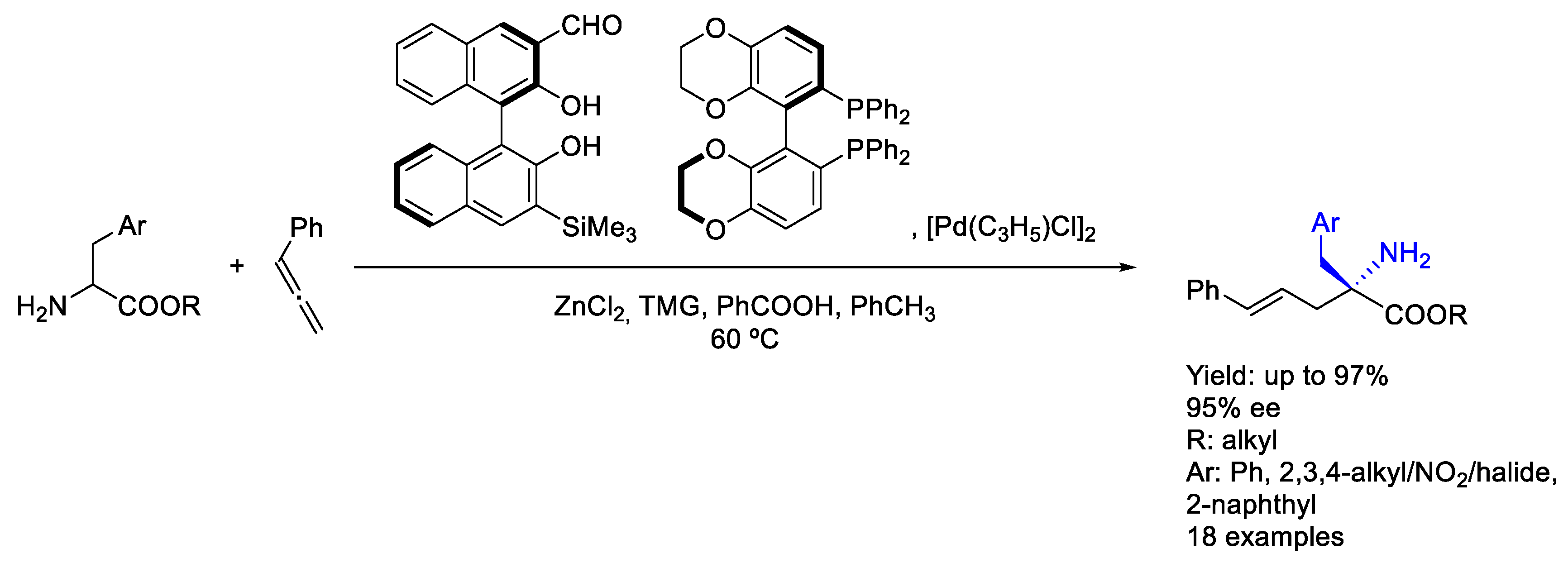
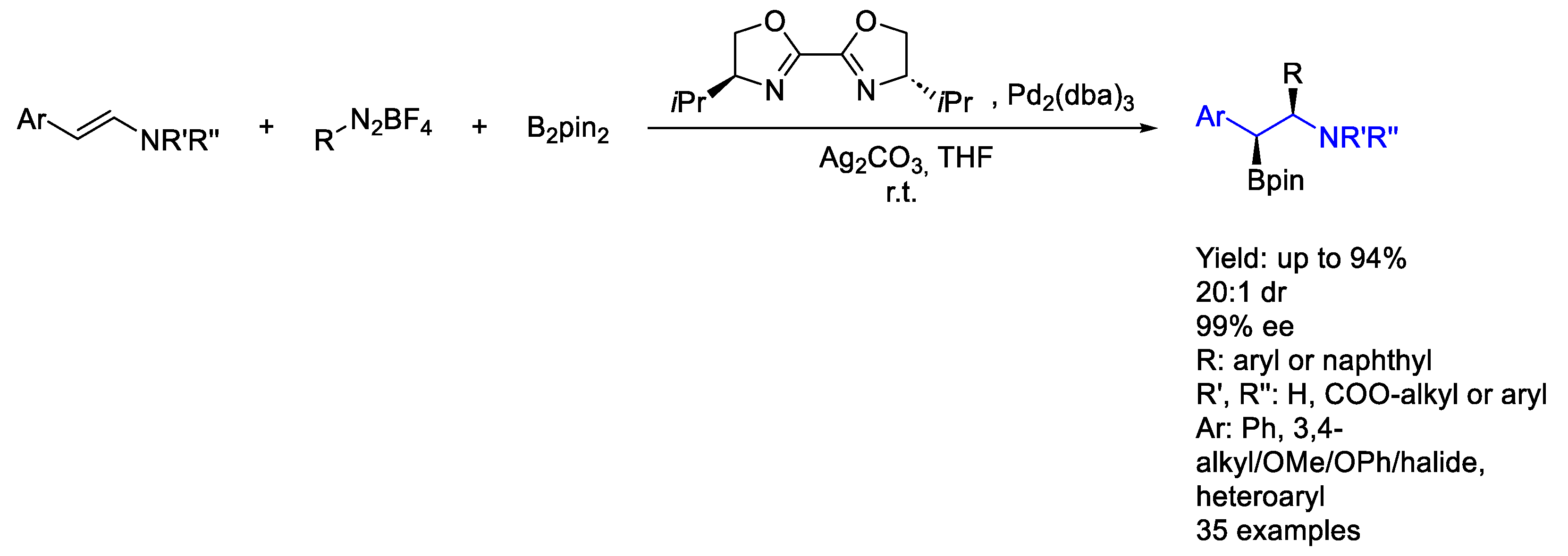
2.3. Palladium
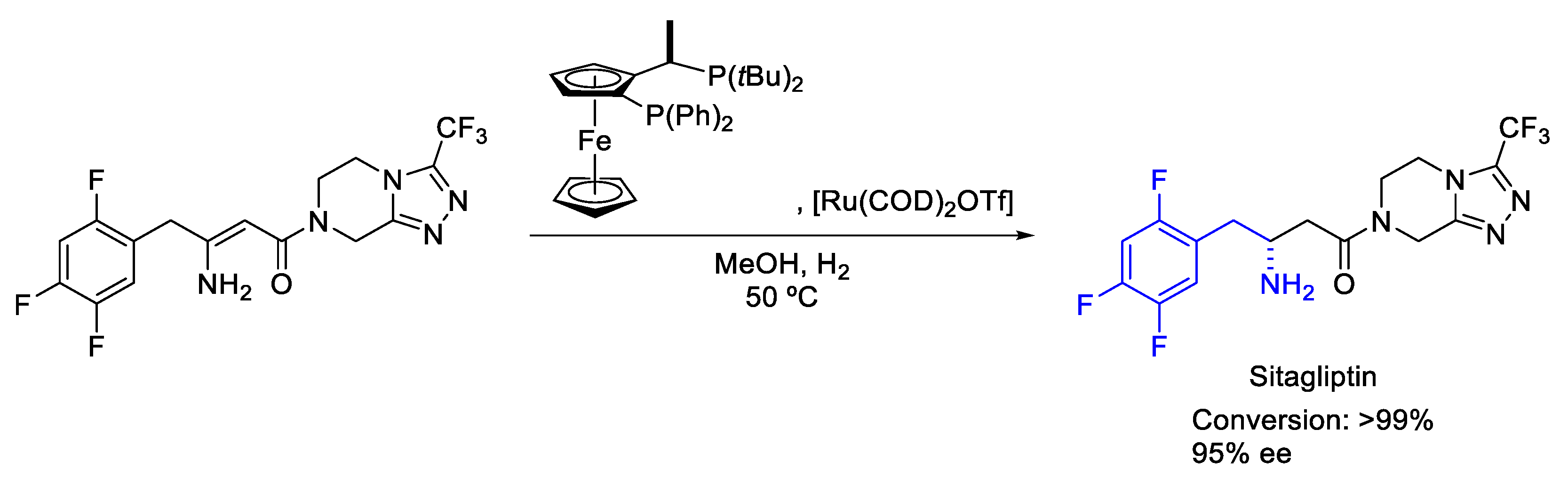
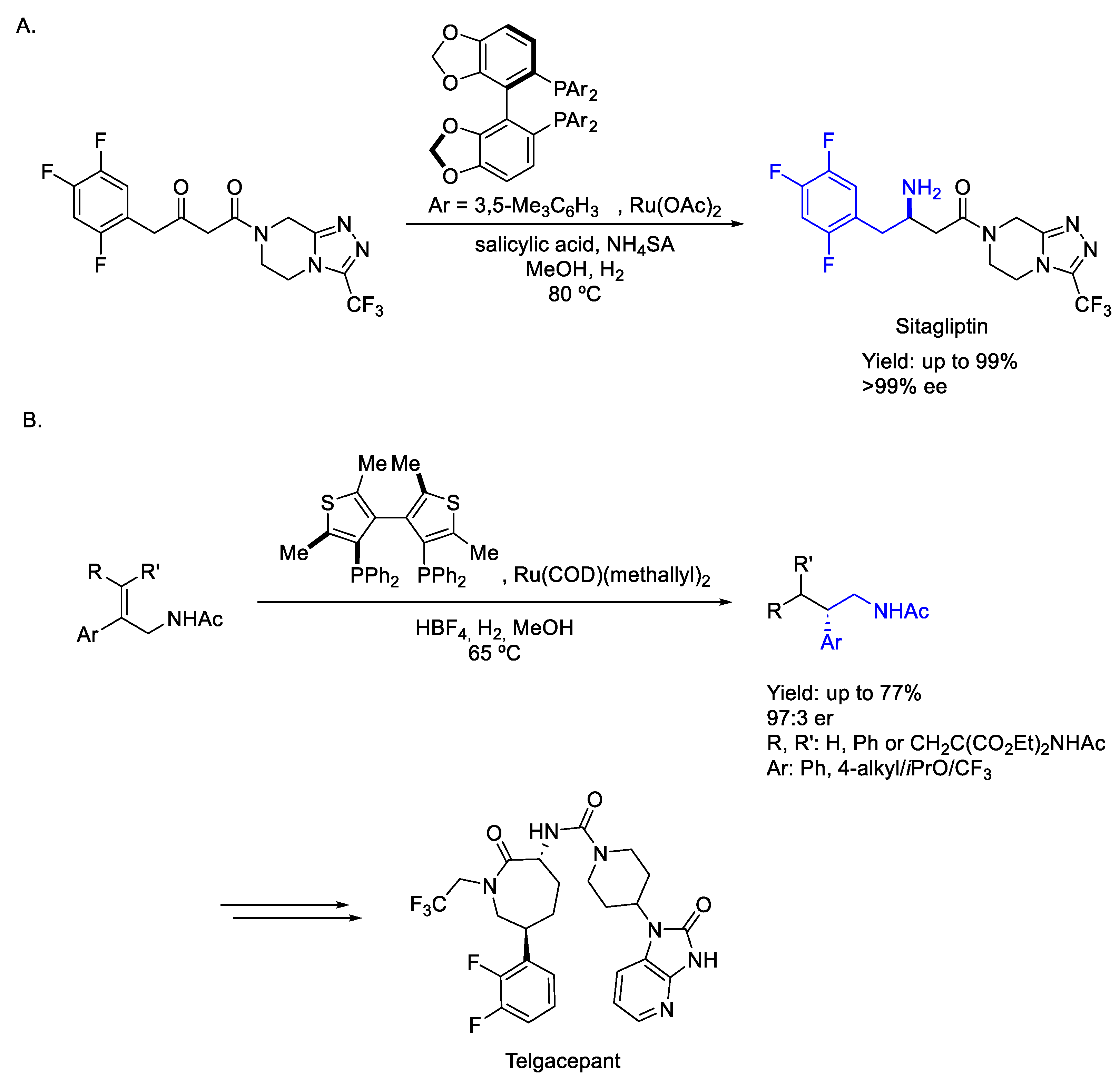
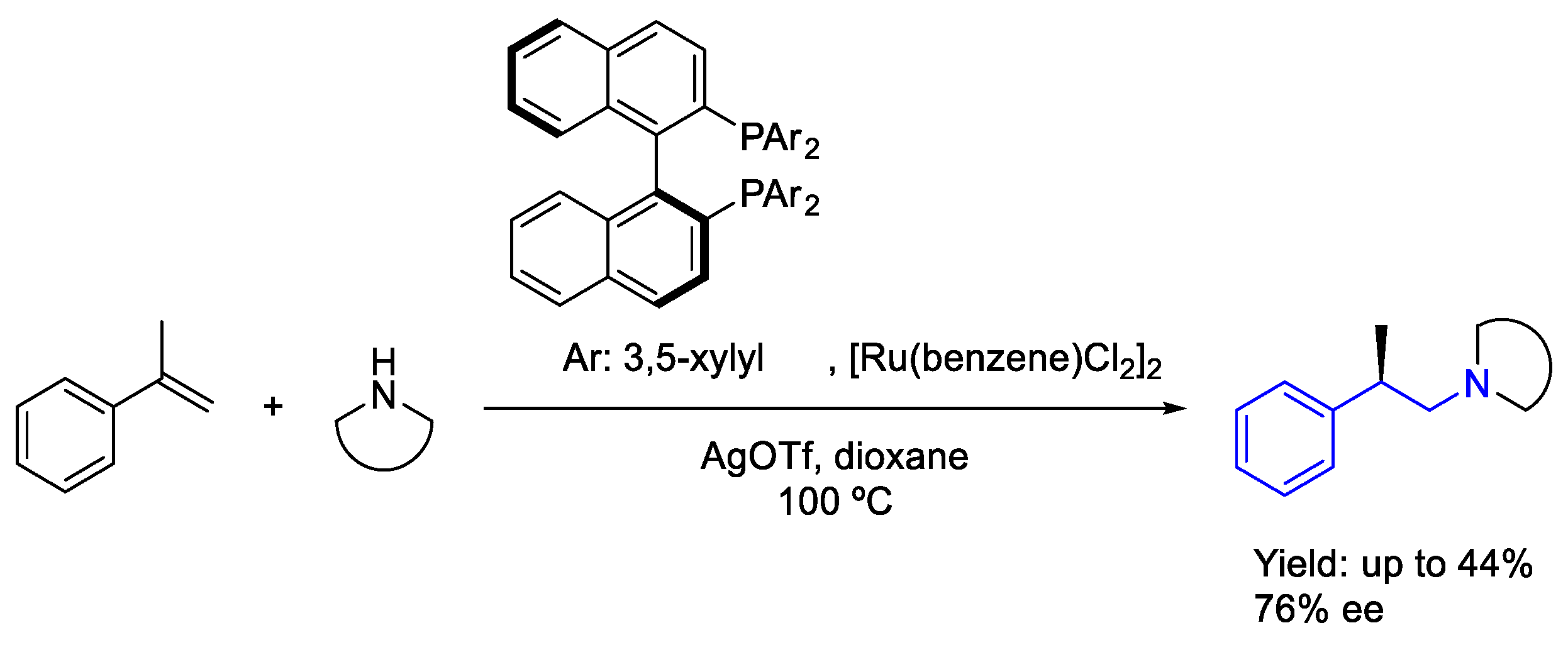
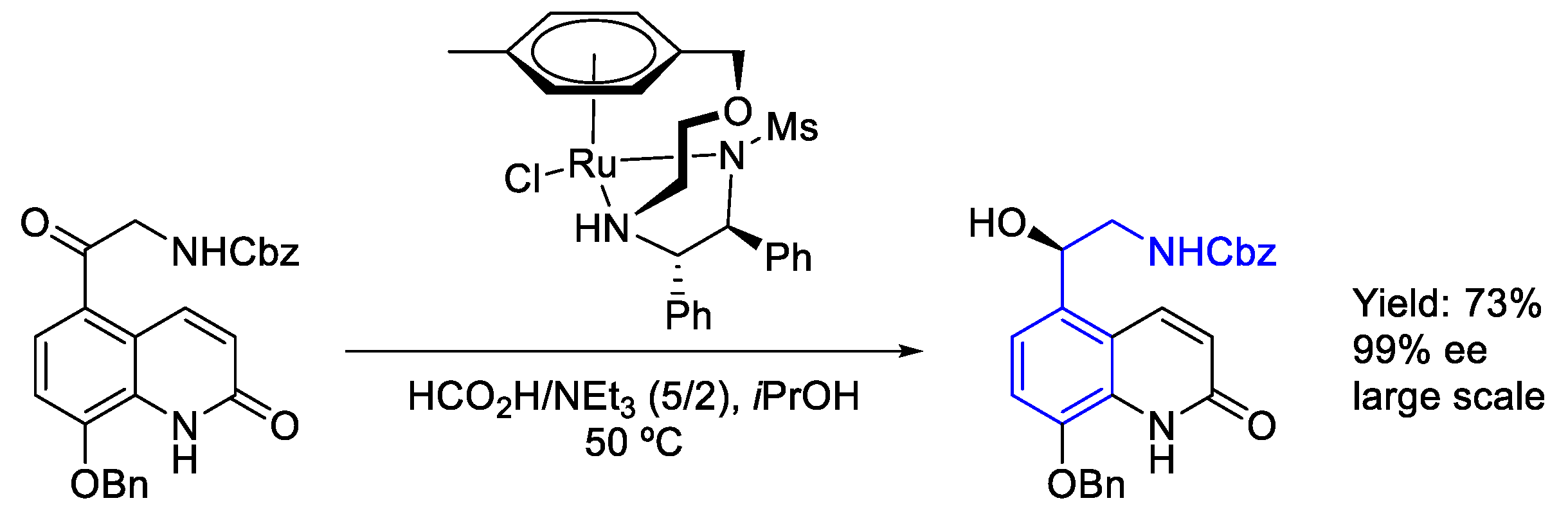
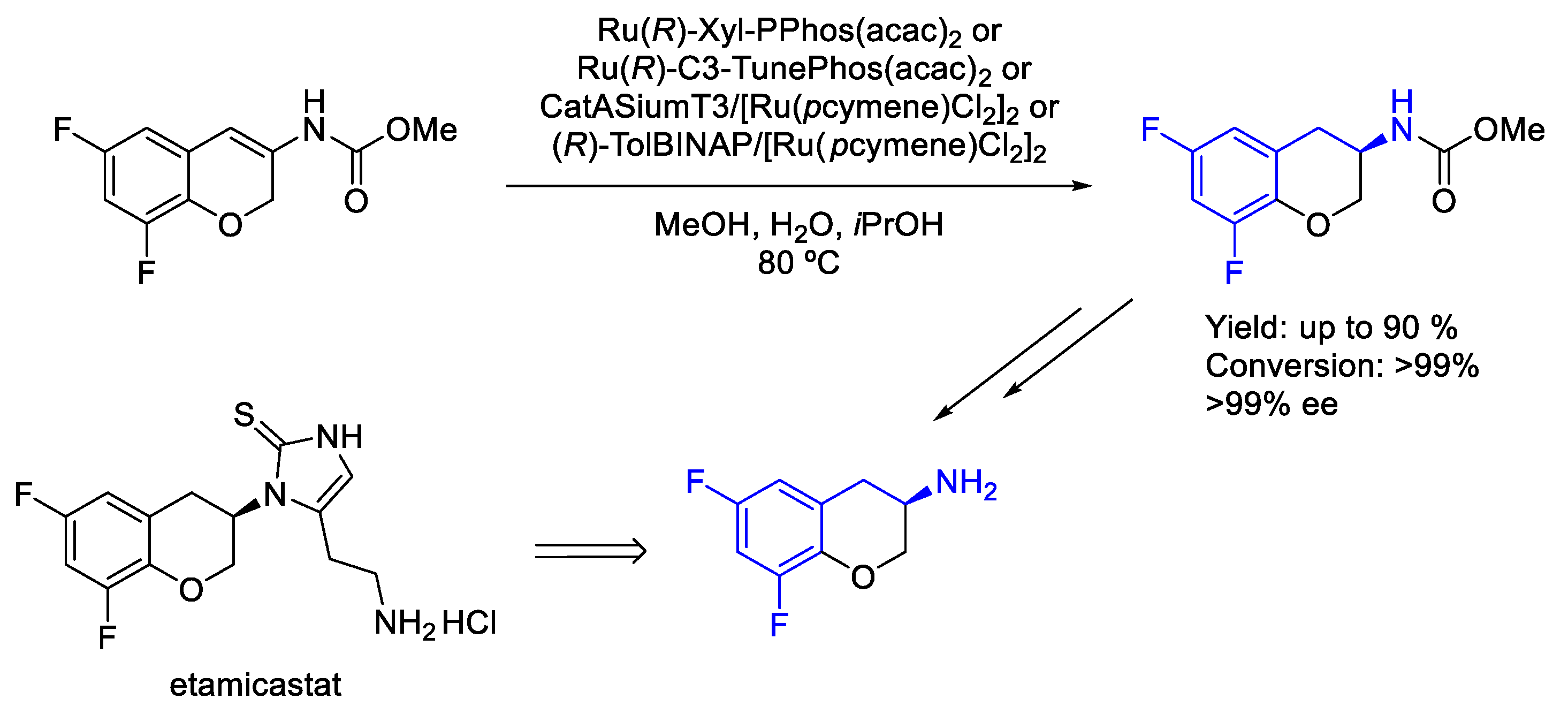
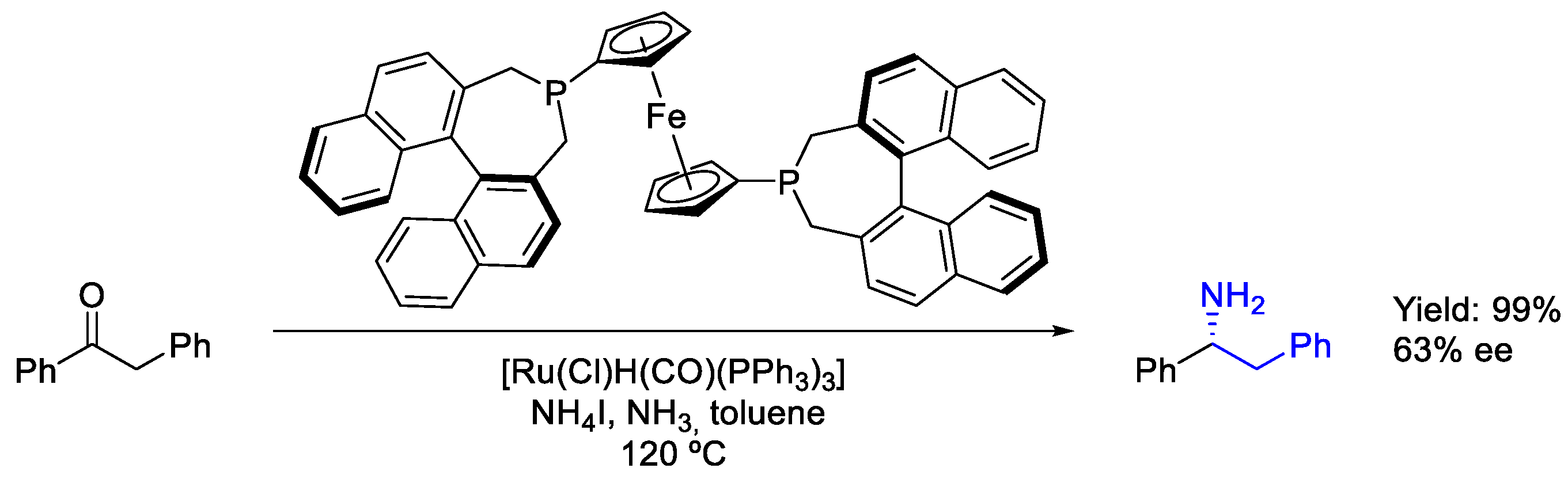
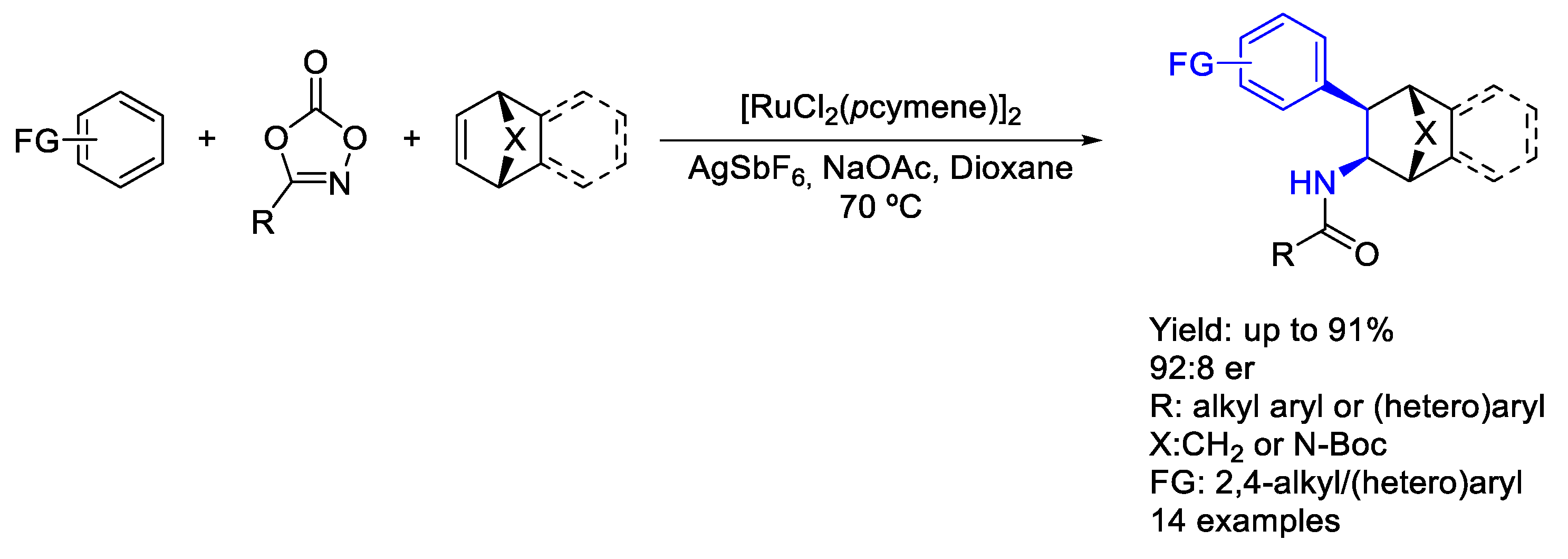
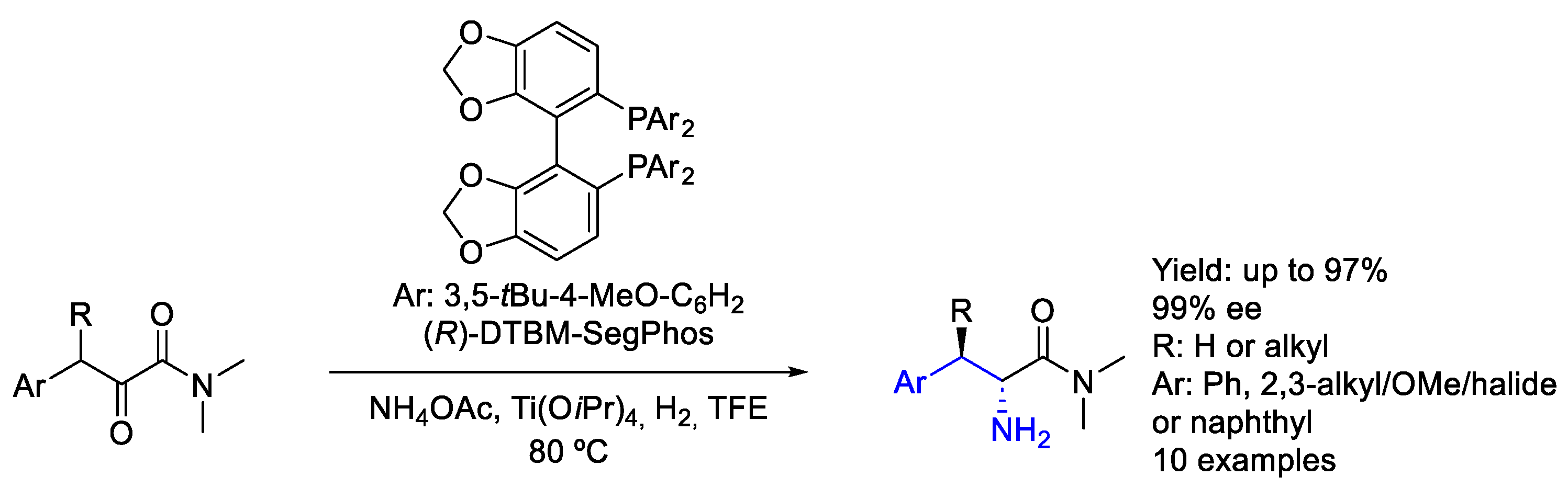
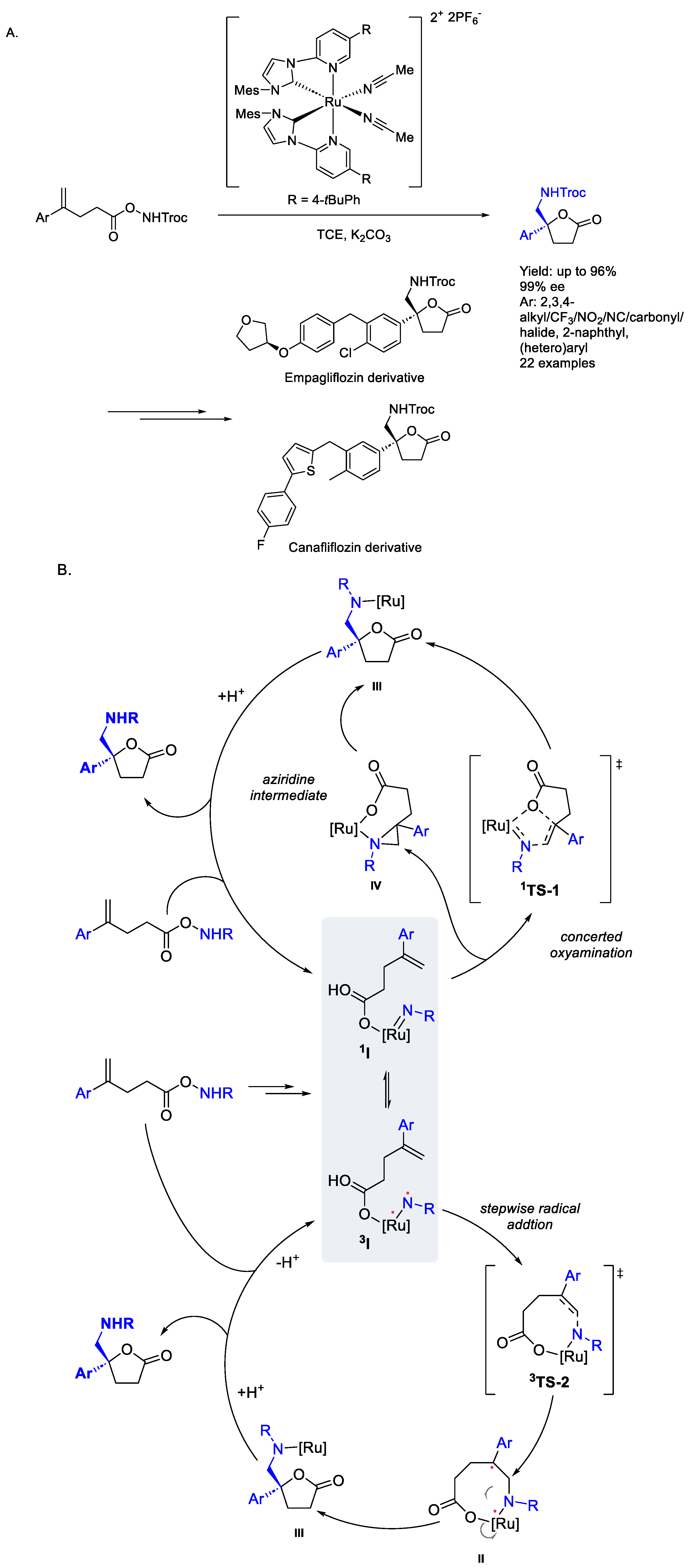
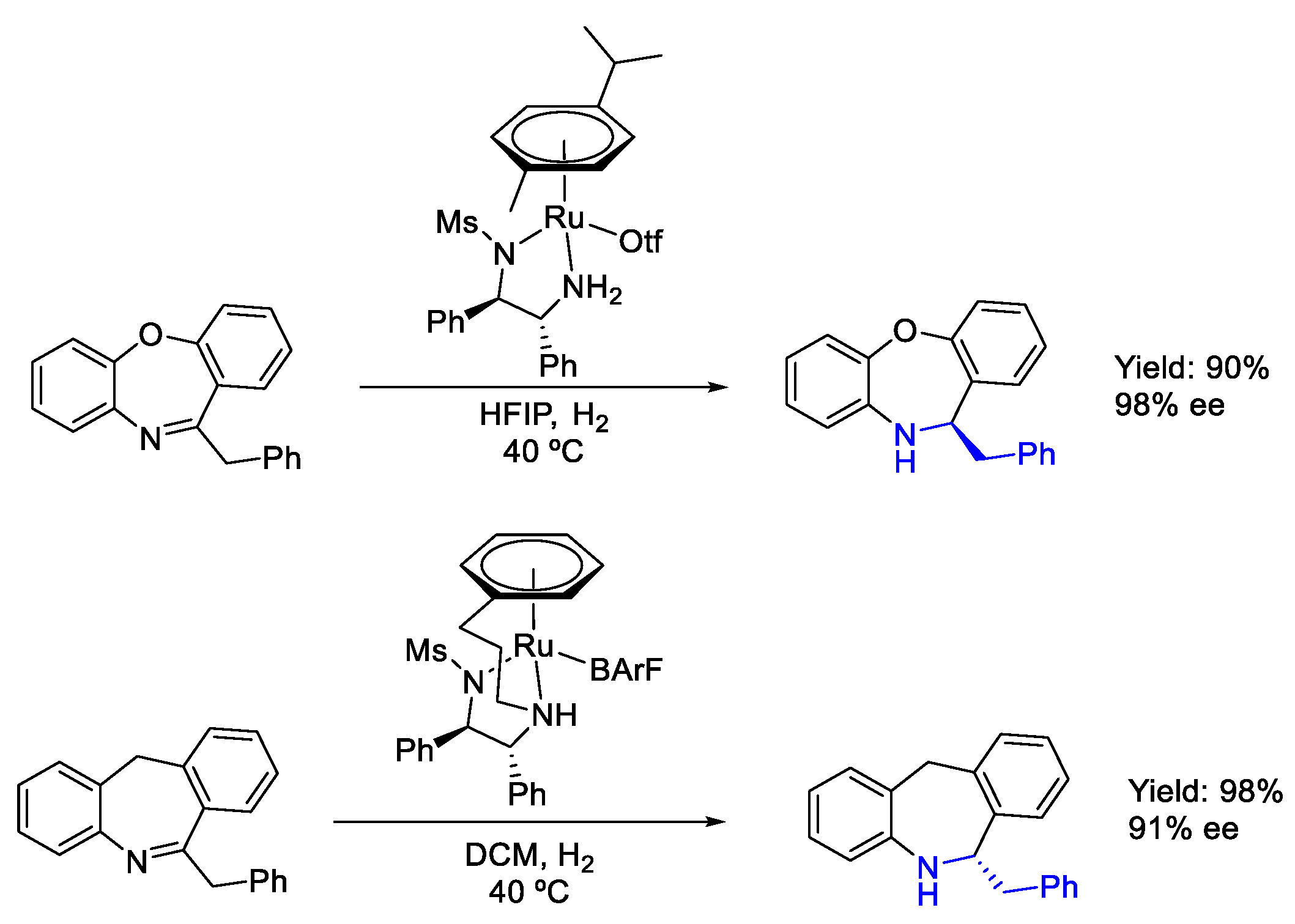
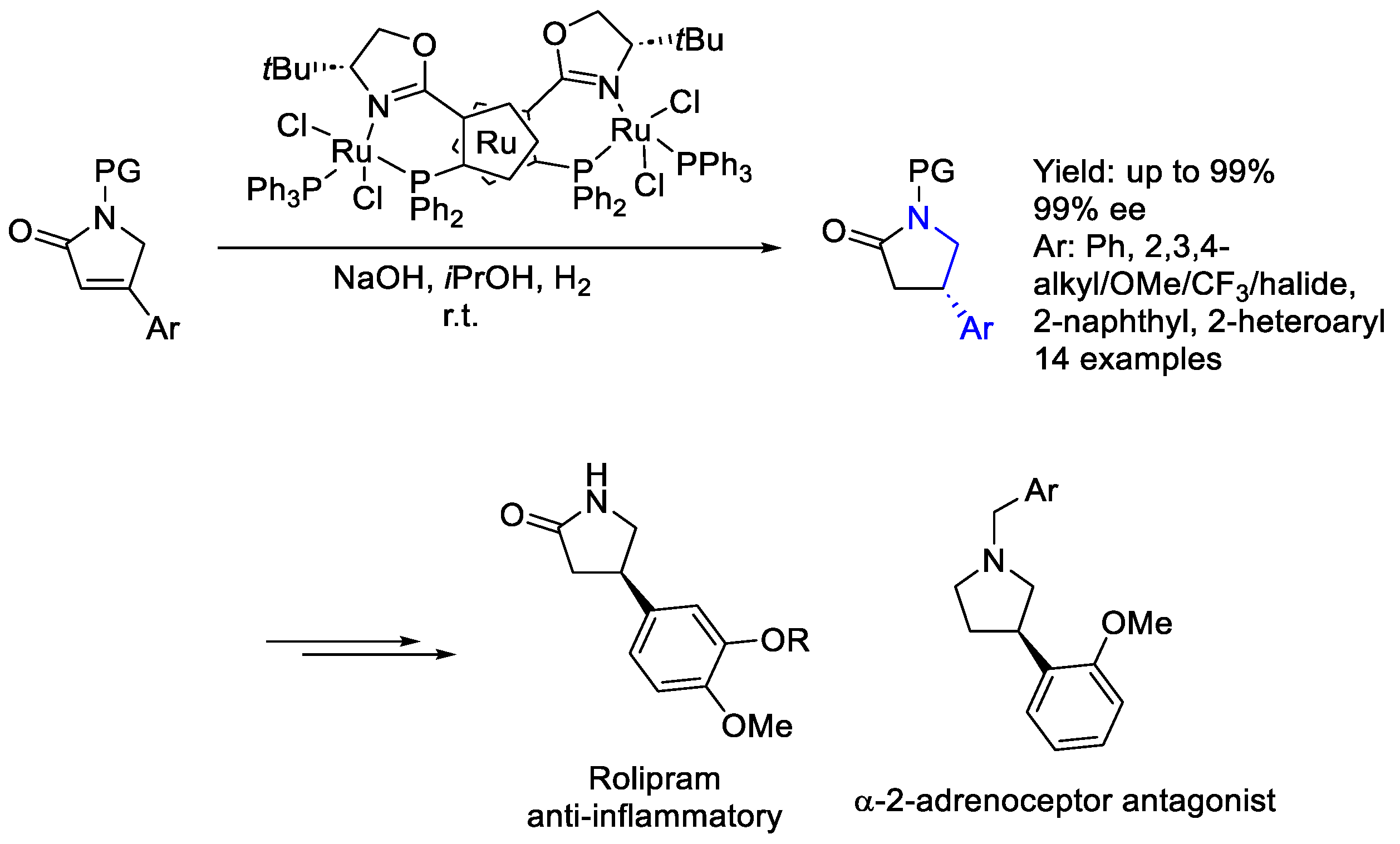
2.4. Ruthenium
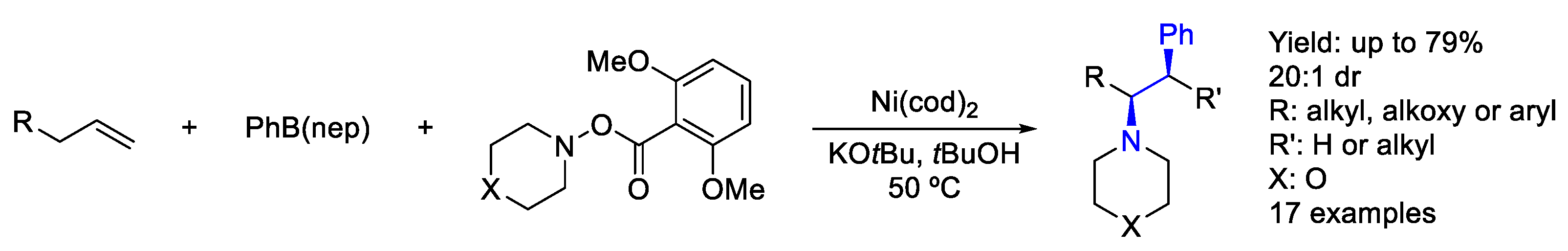
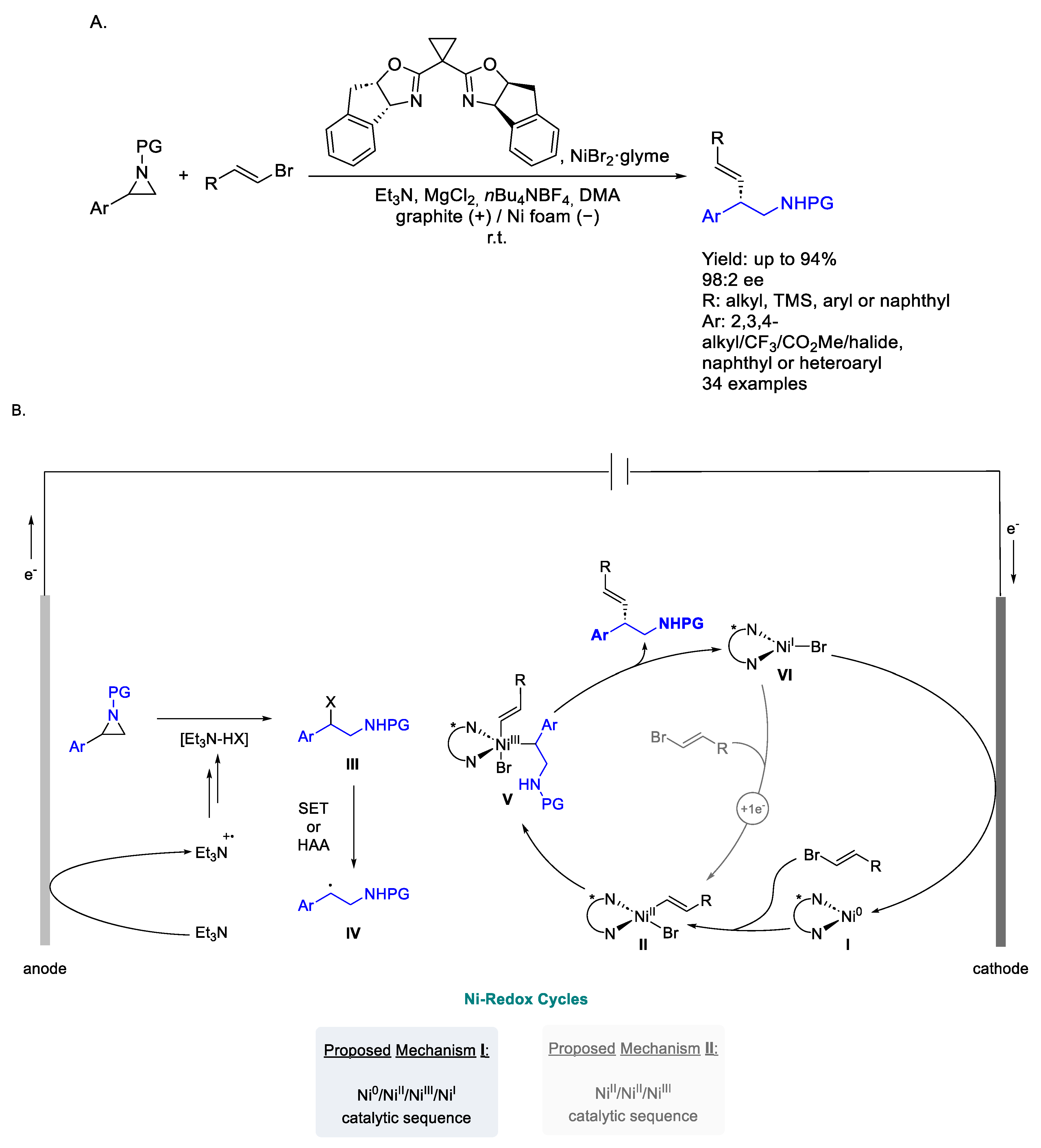
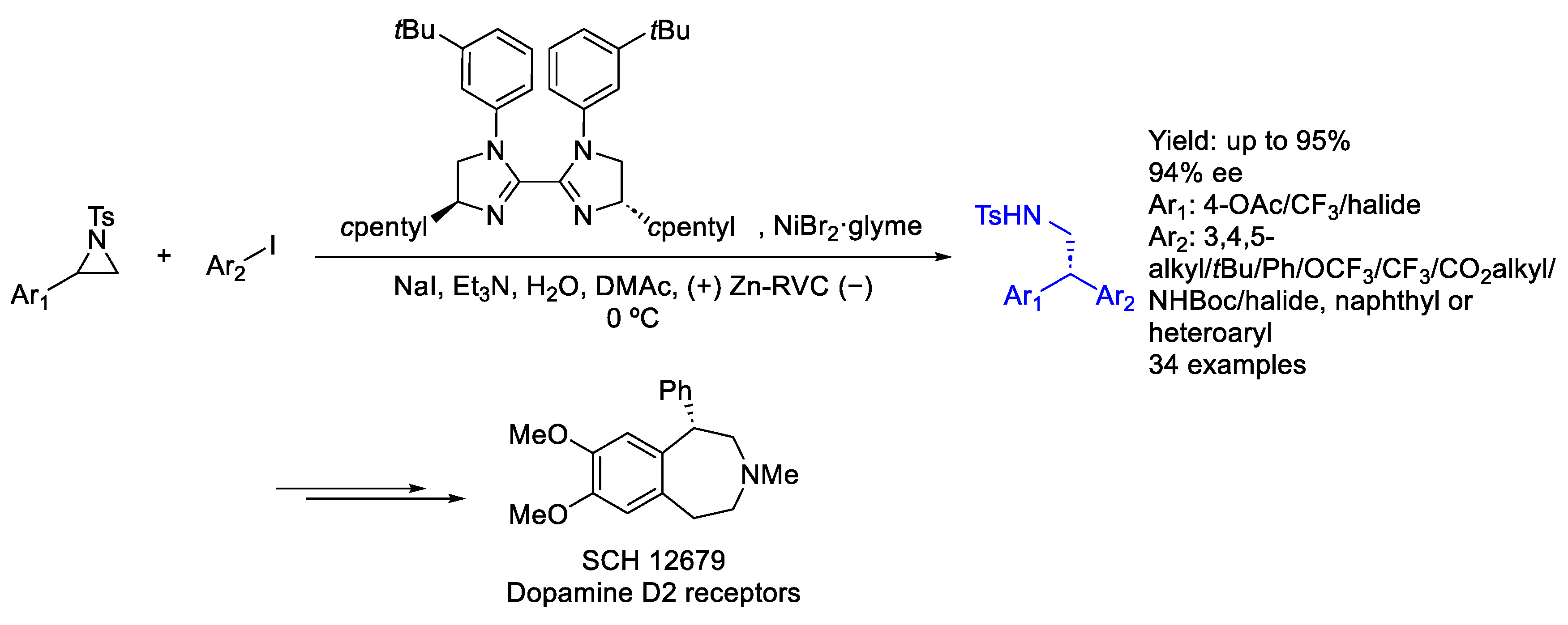
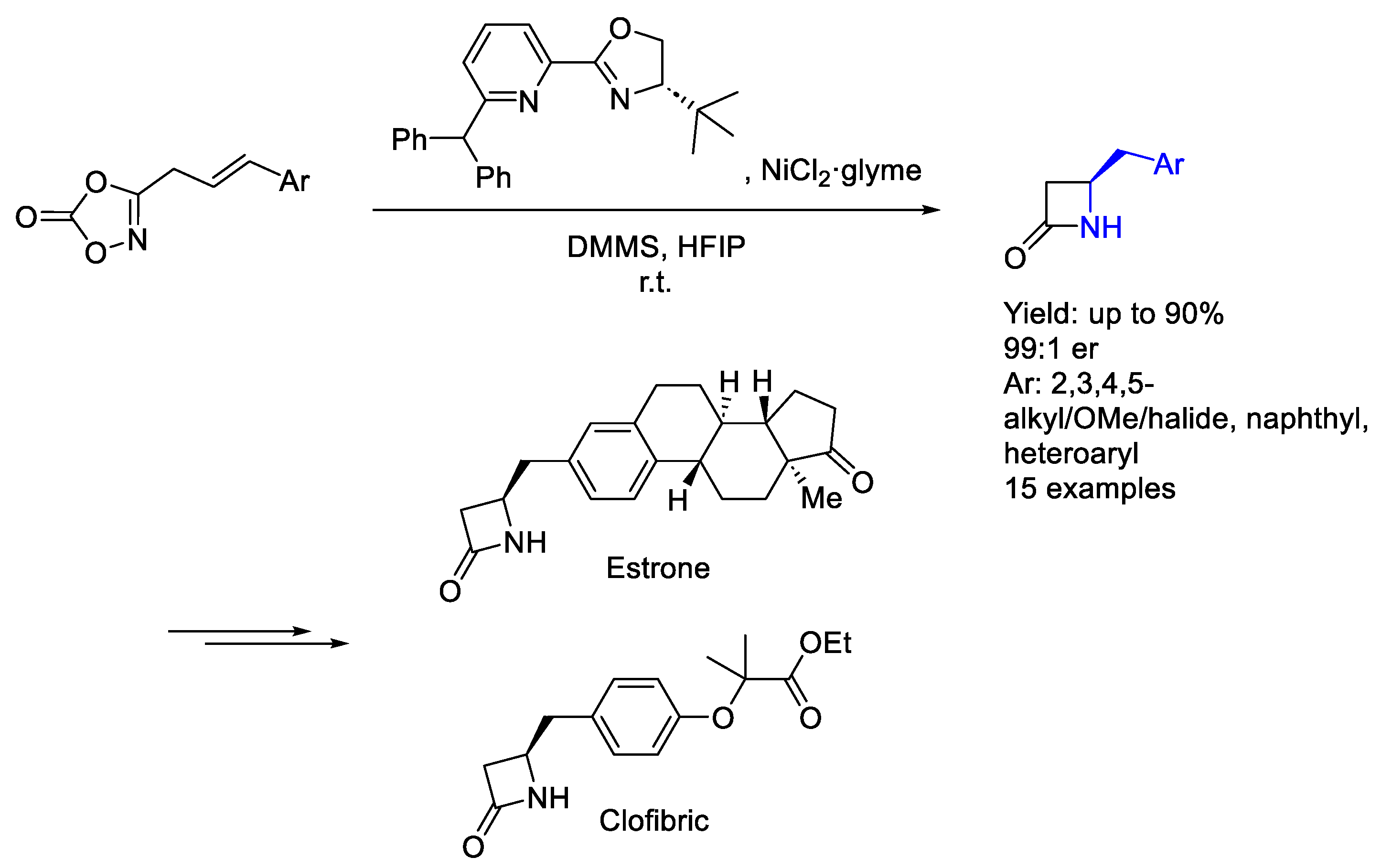
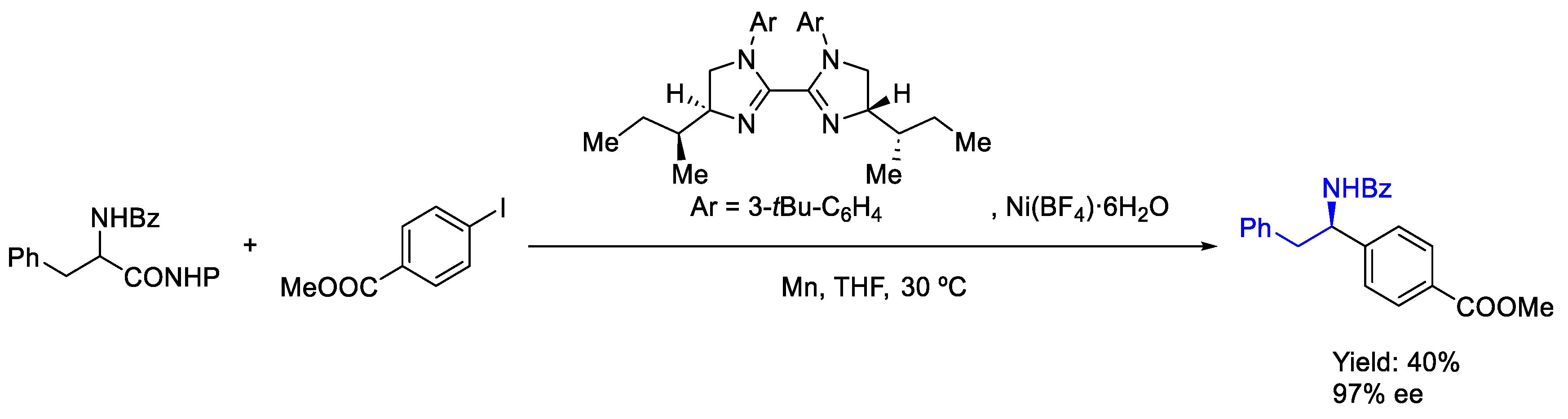
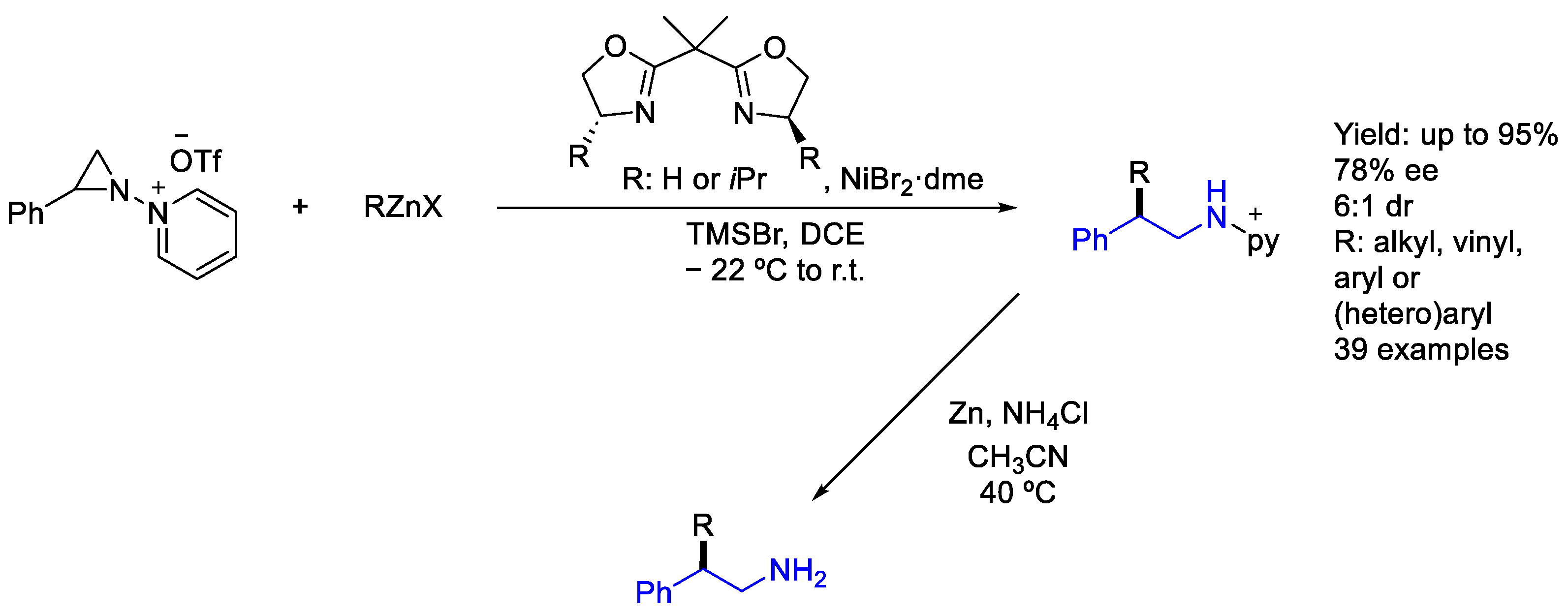
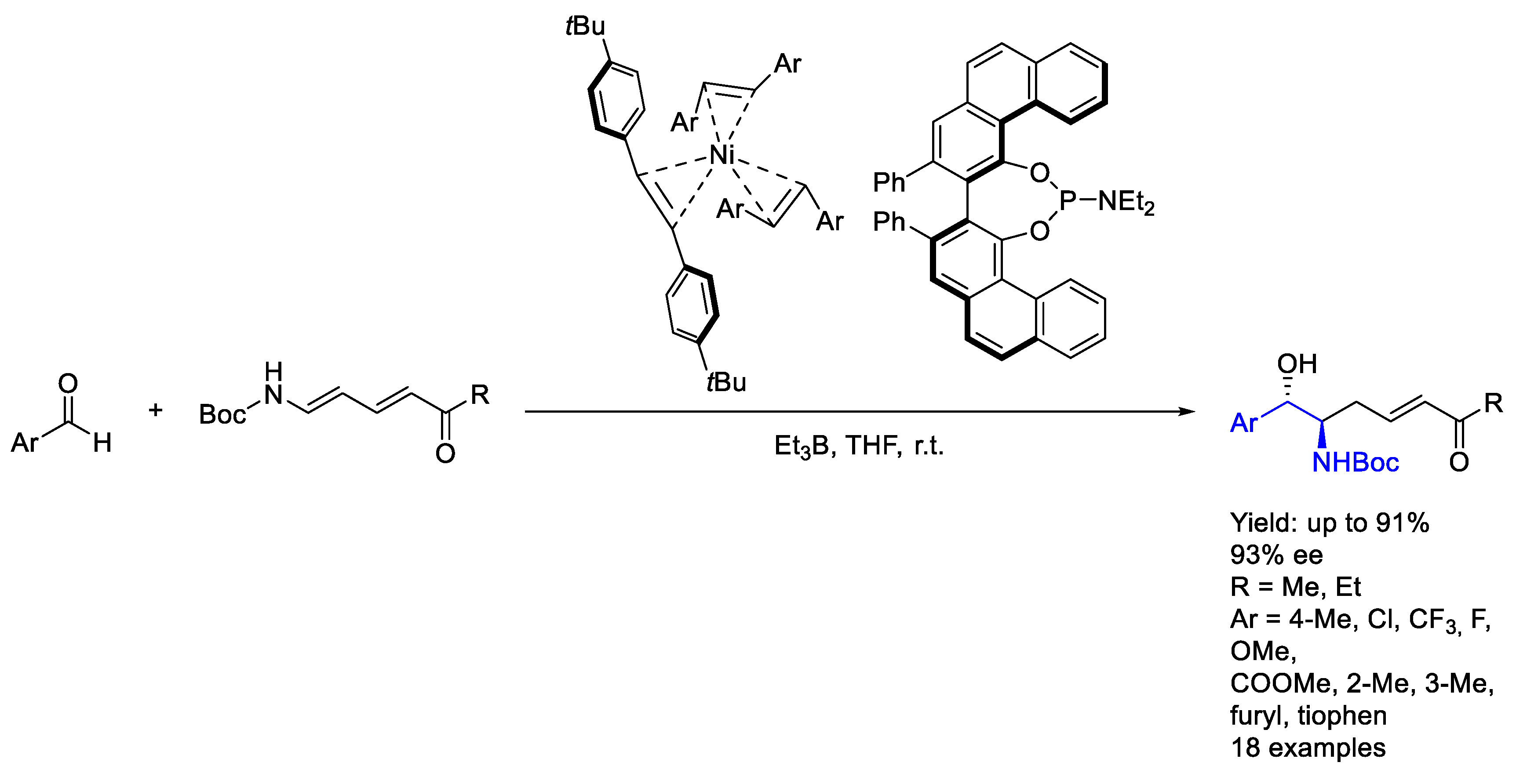
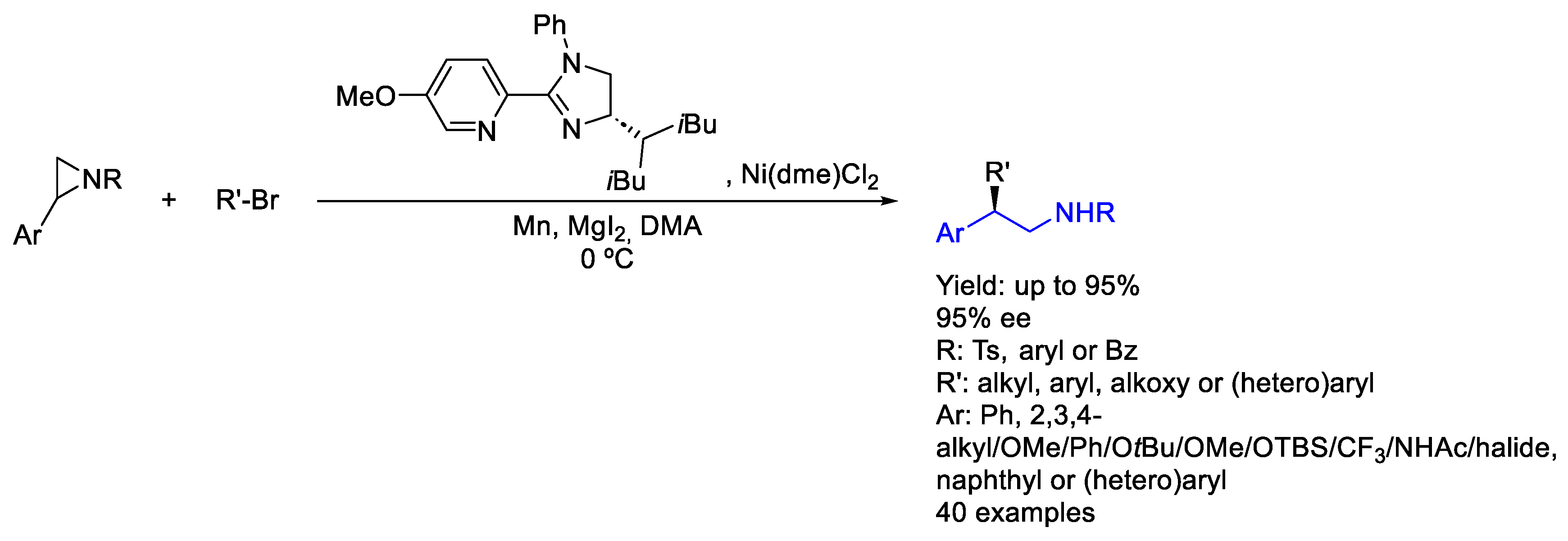
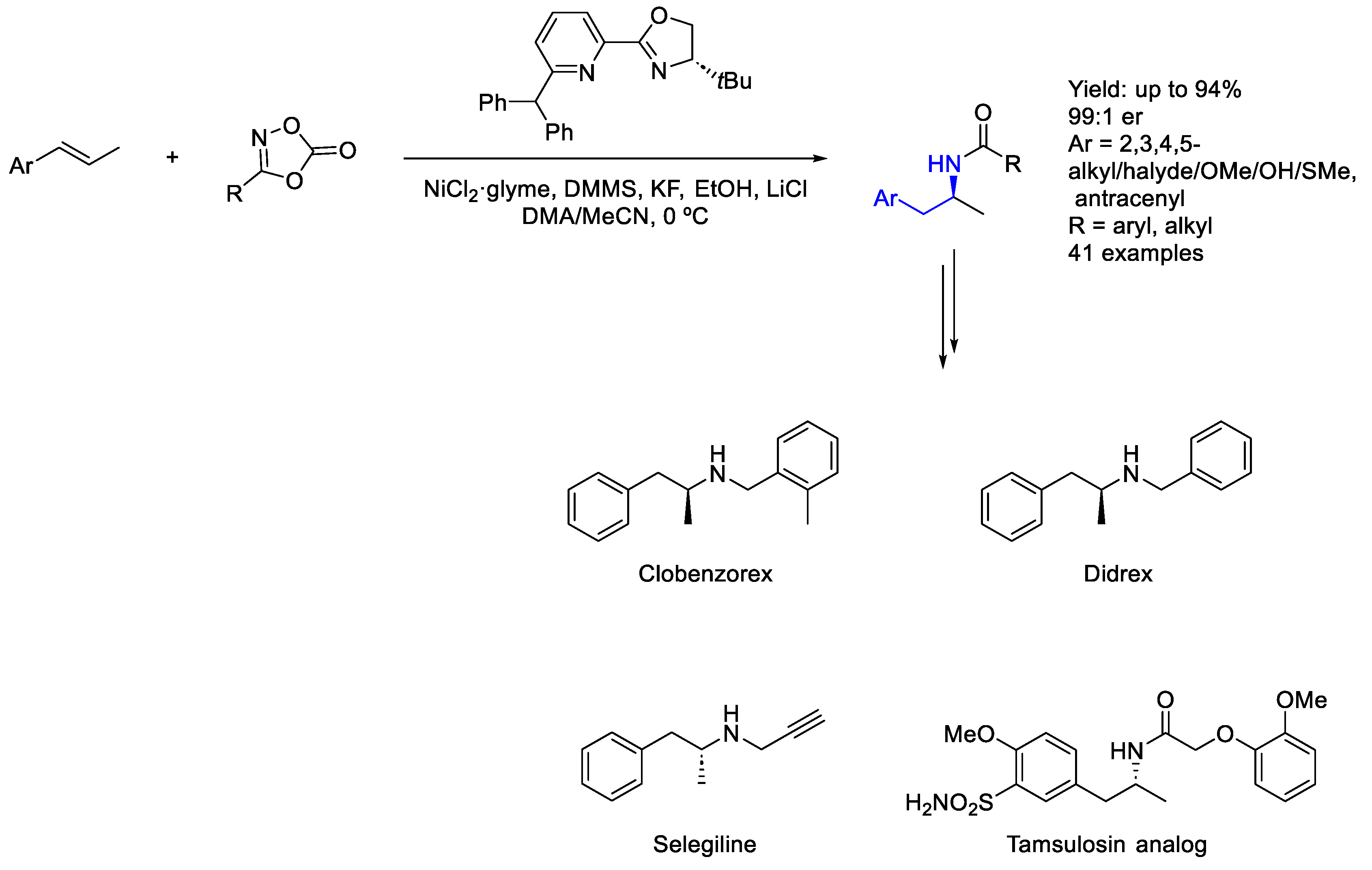
2.5. Nickel
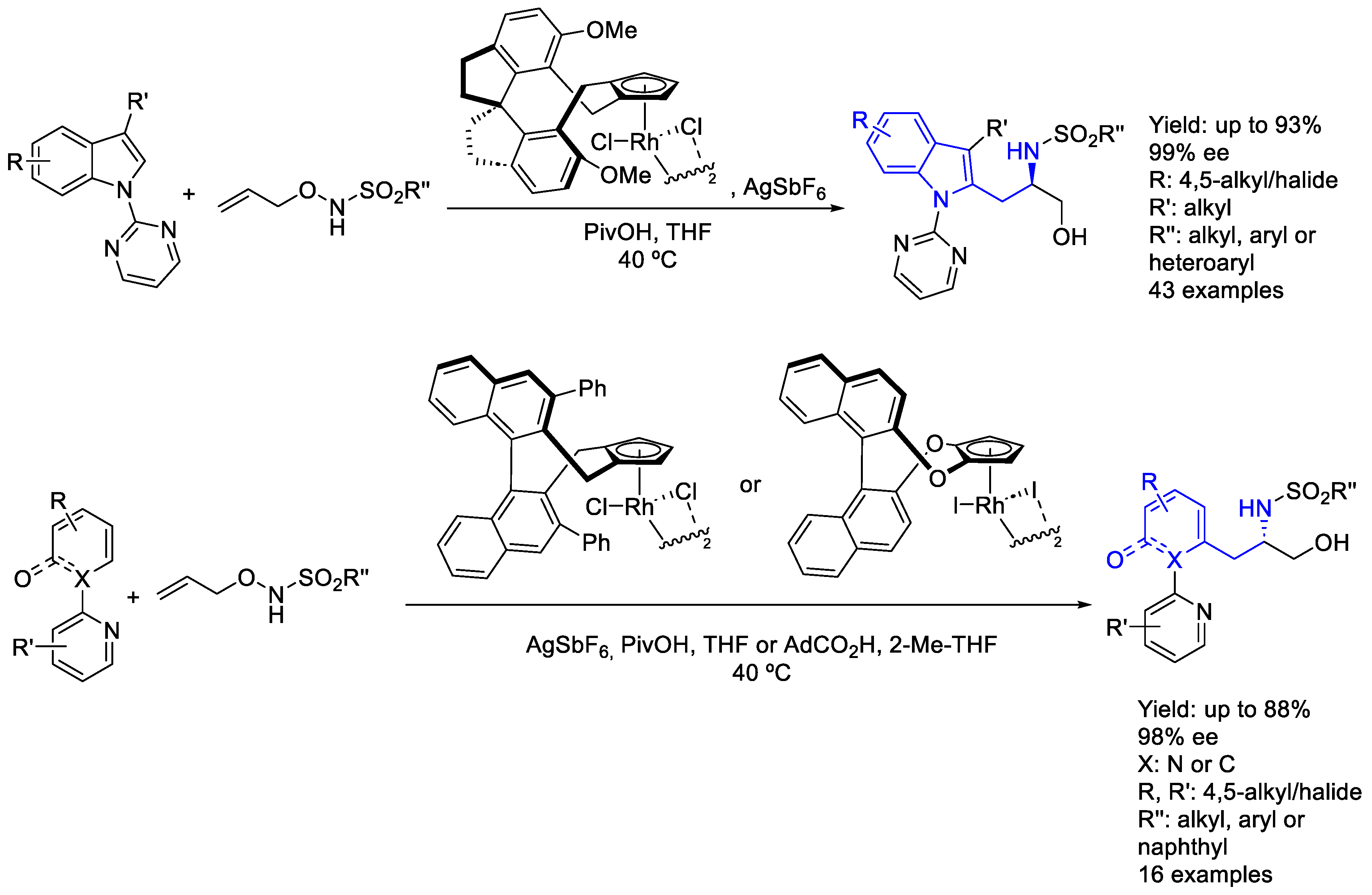
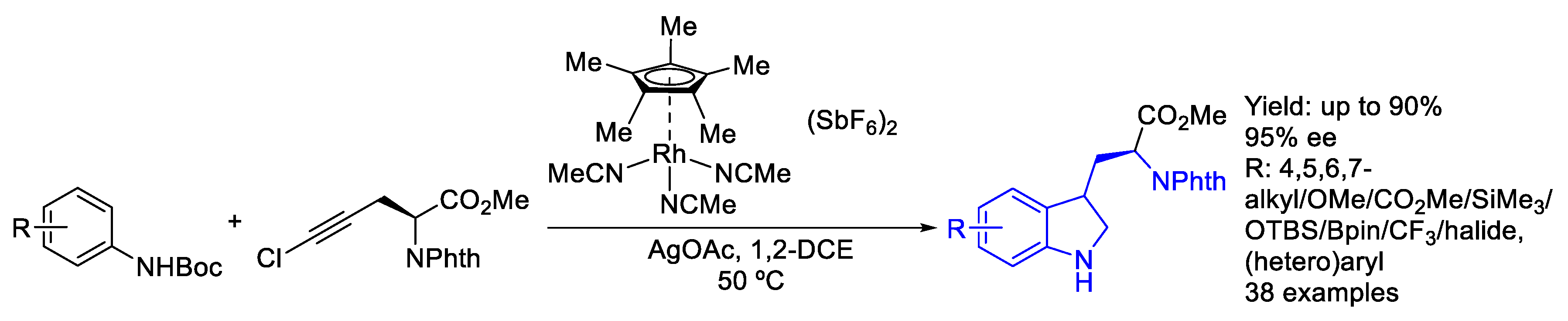
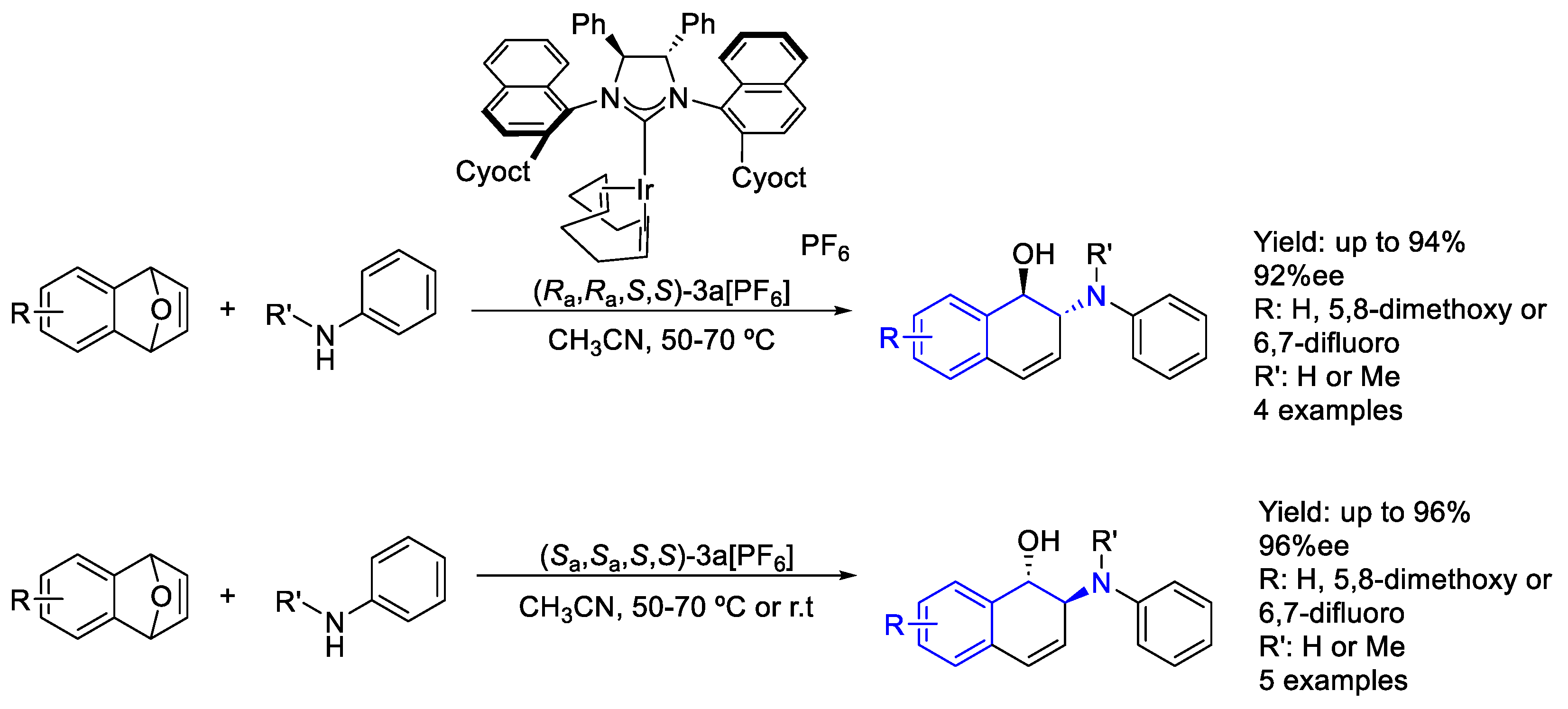
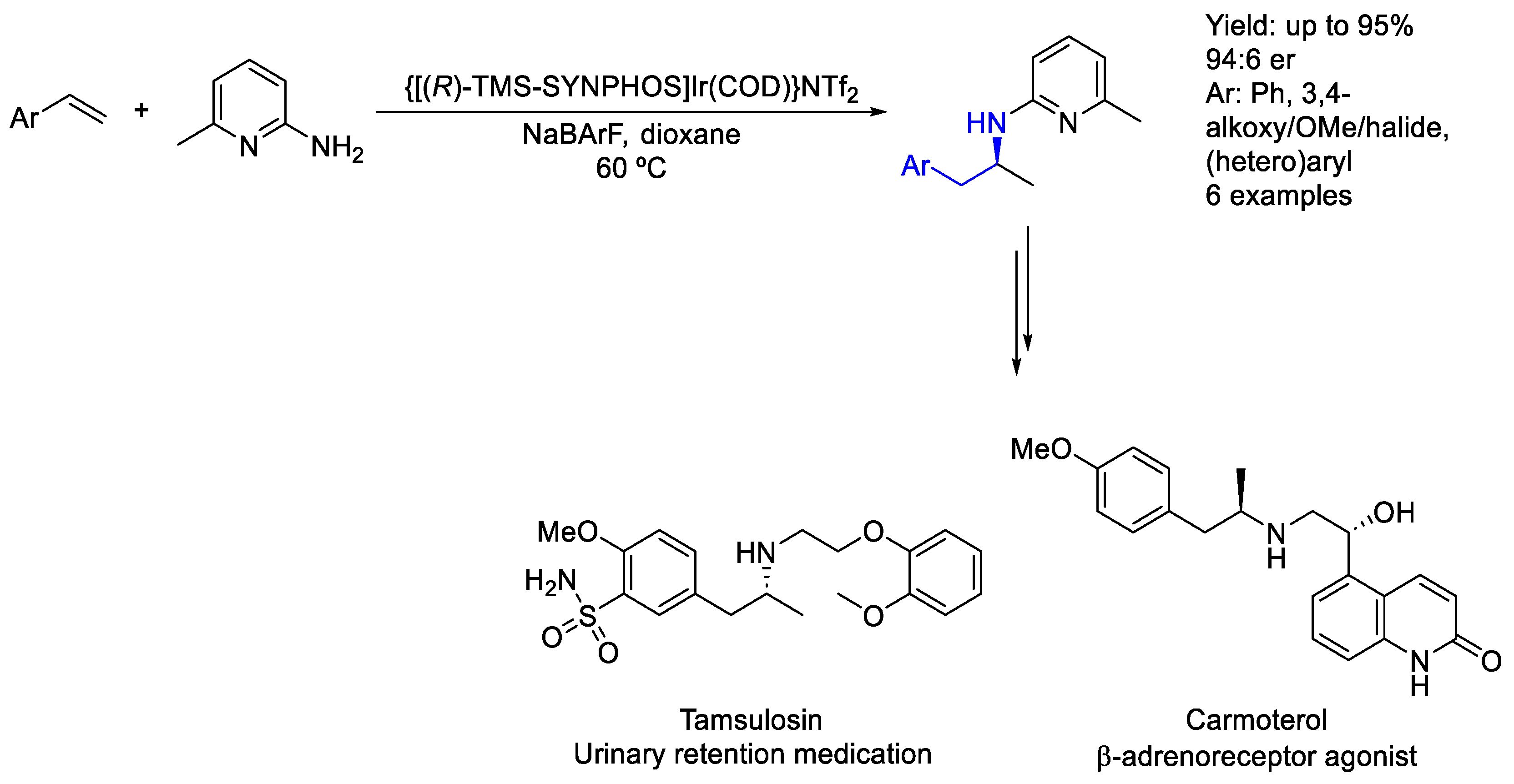
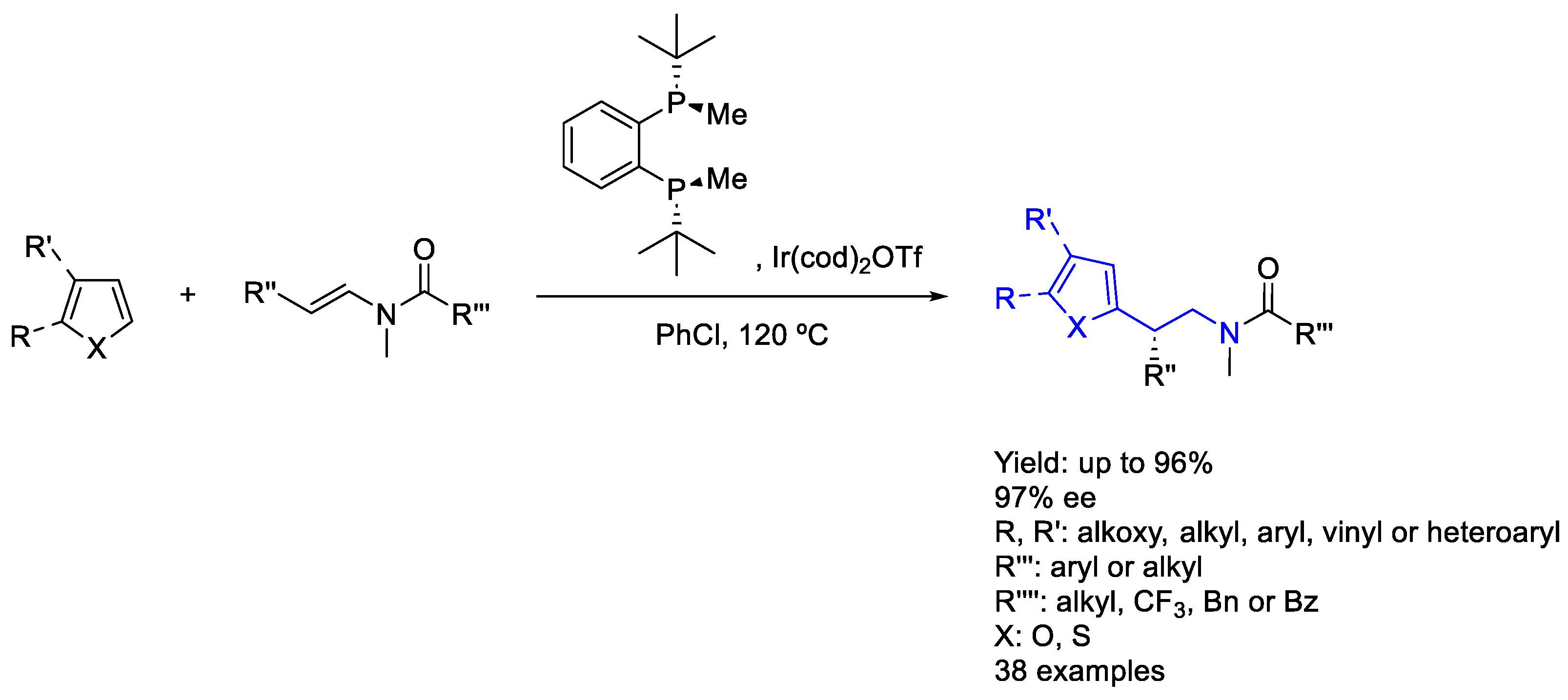
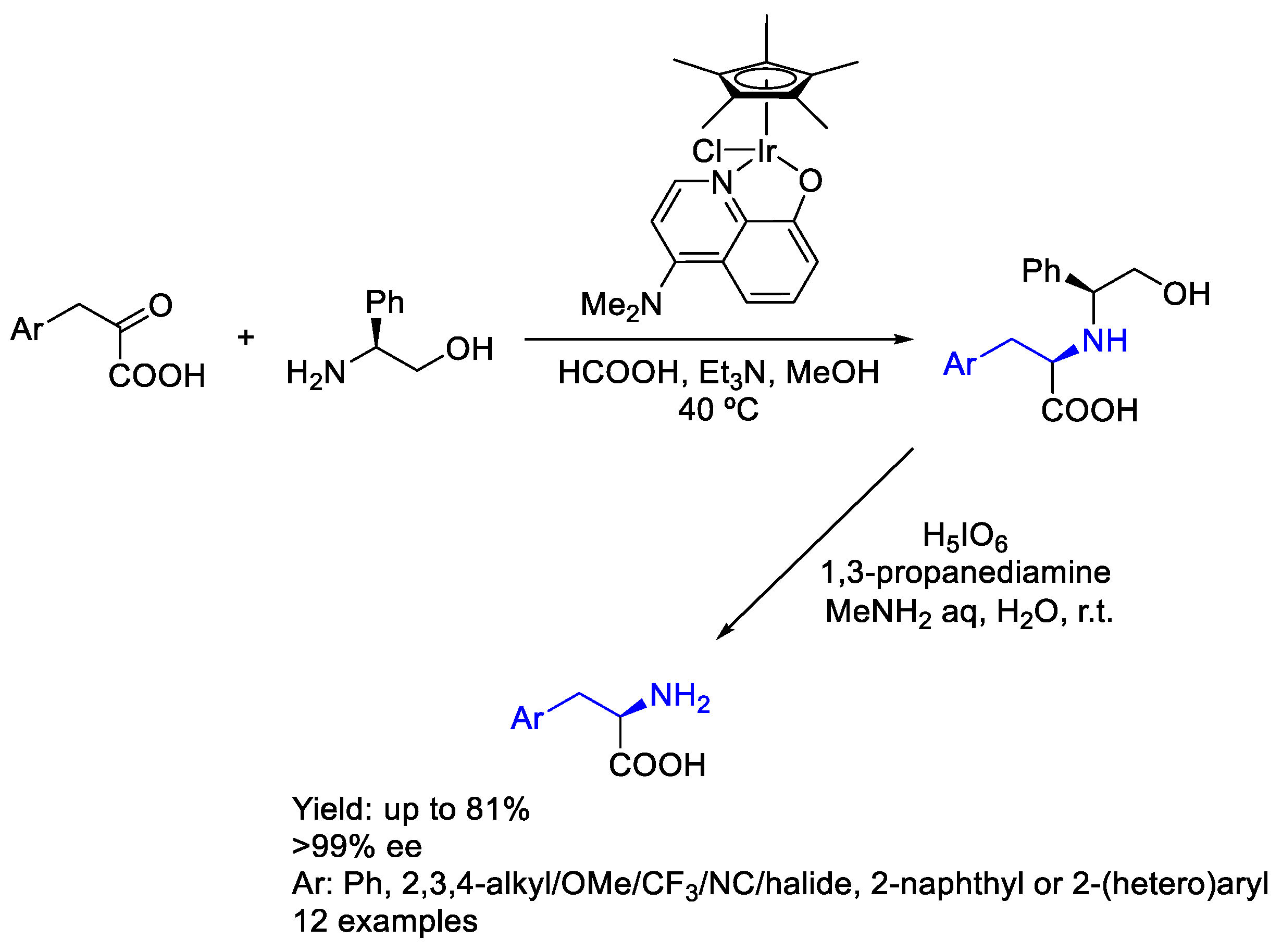
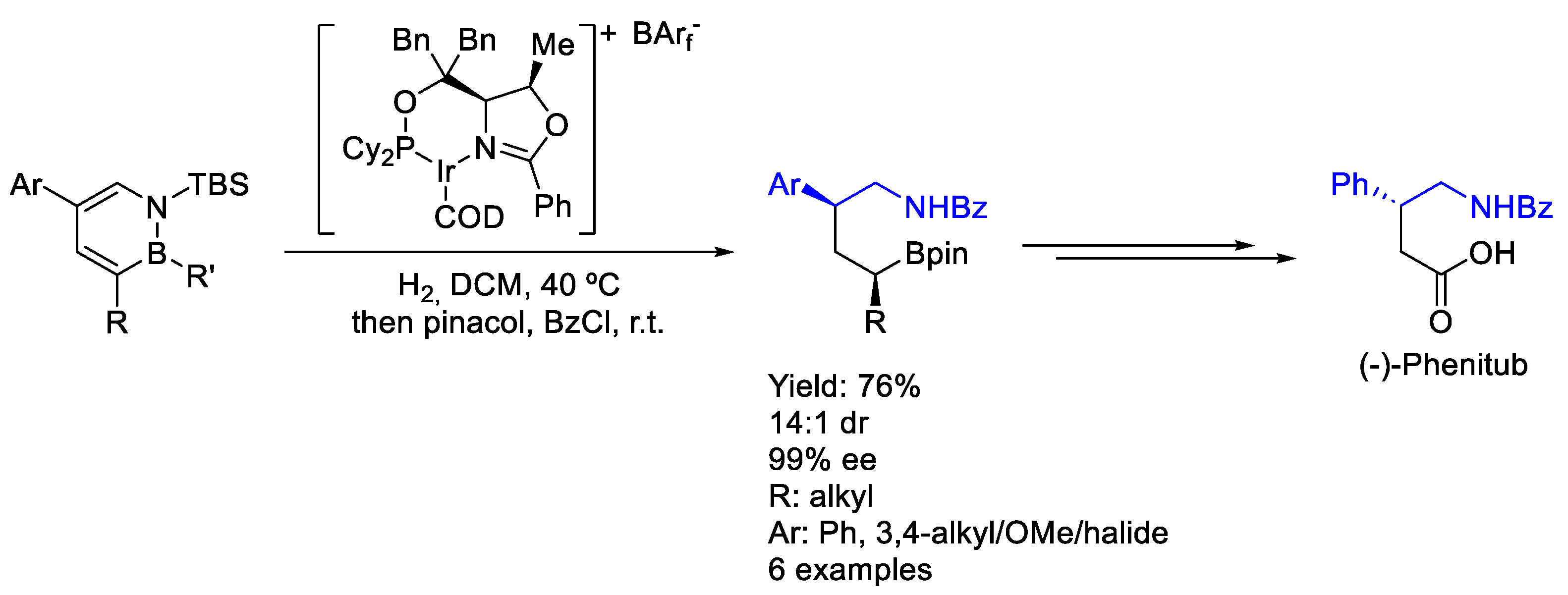
2.6. Iridium
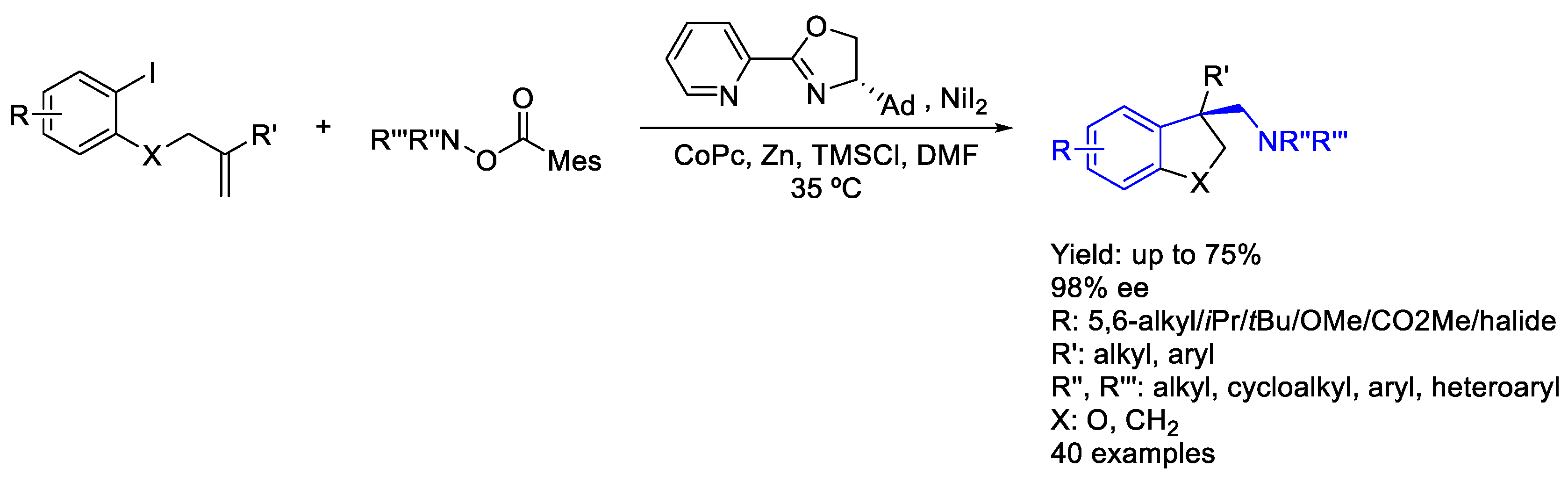
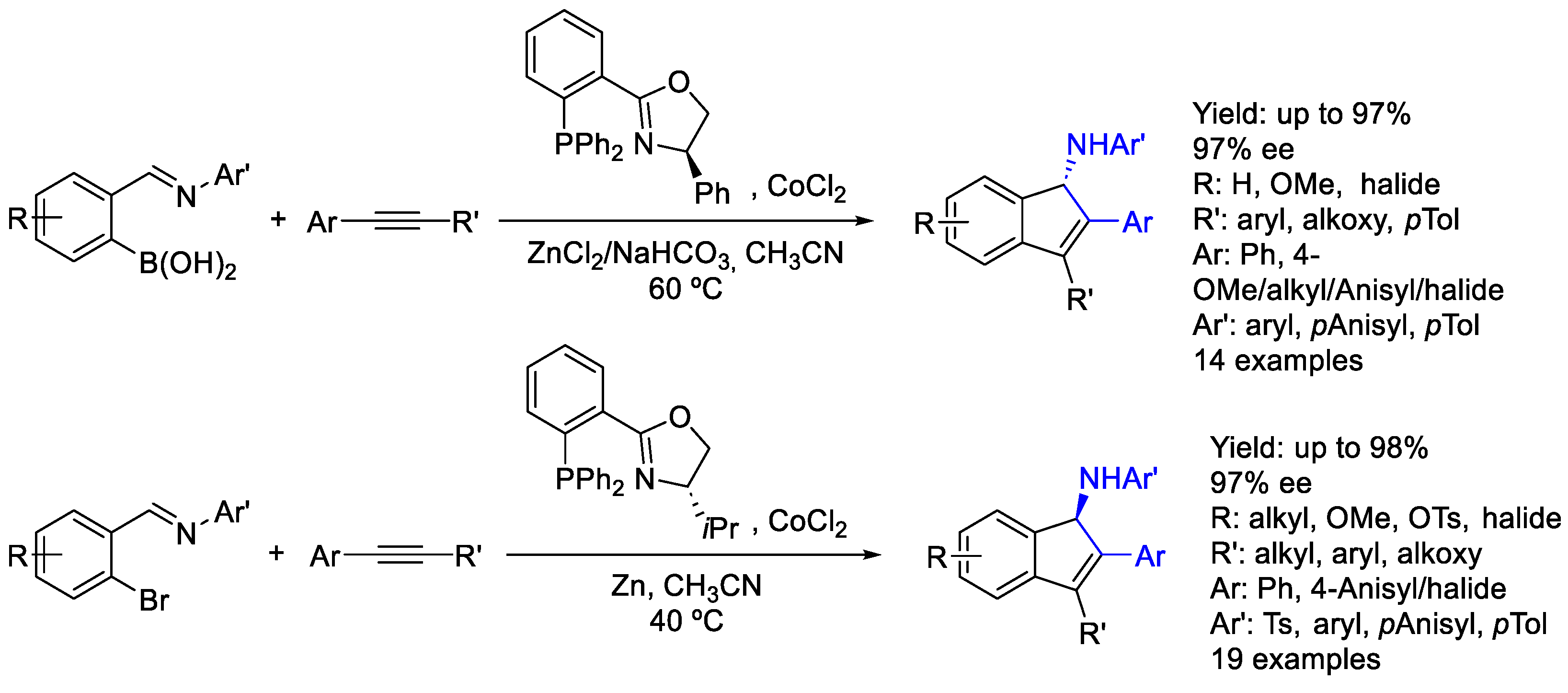
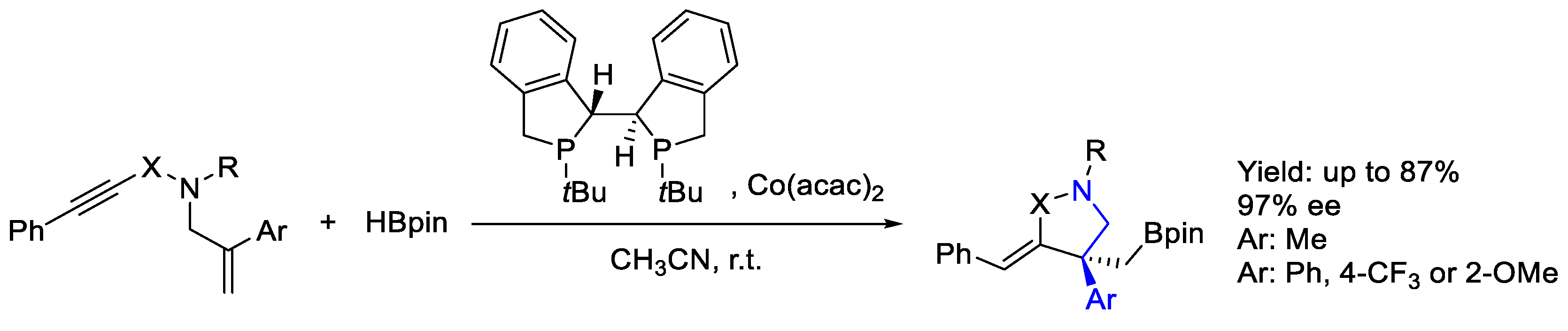
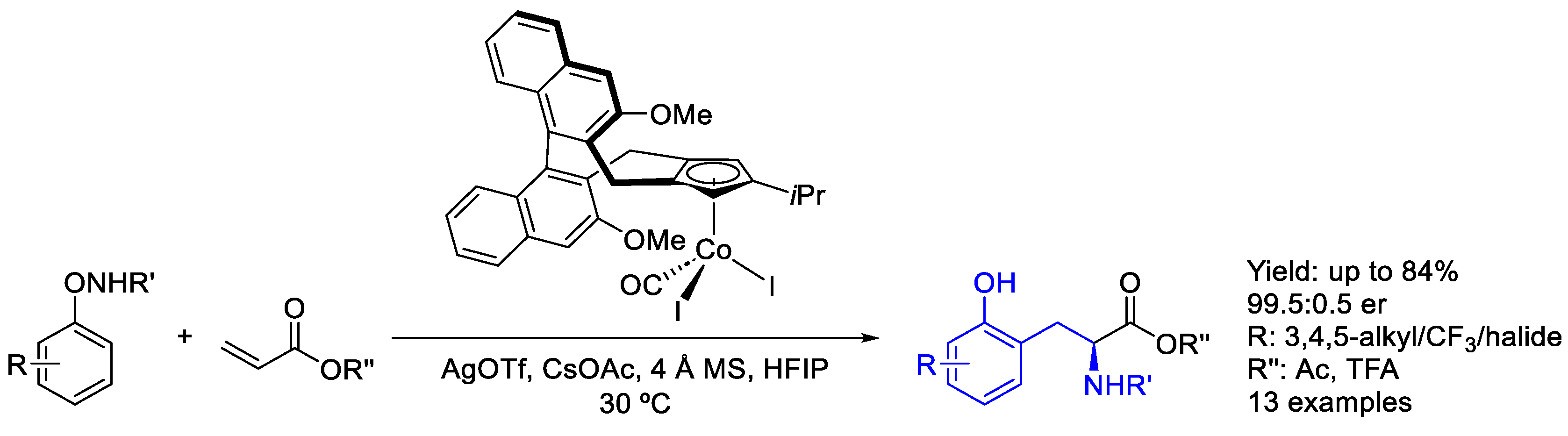
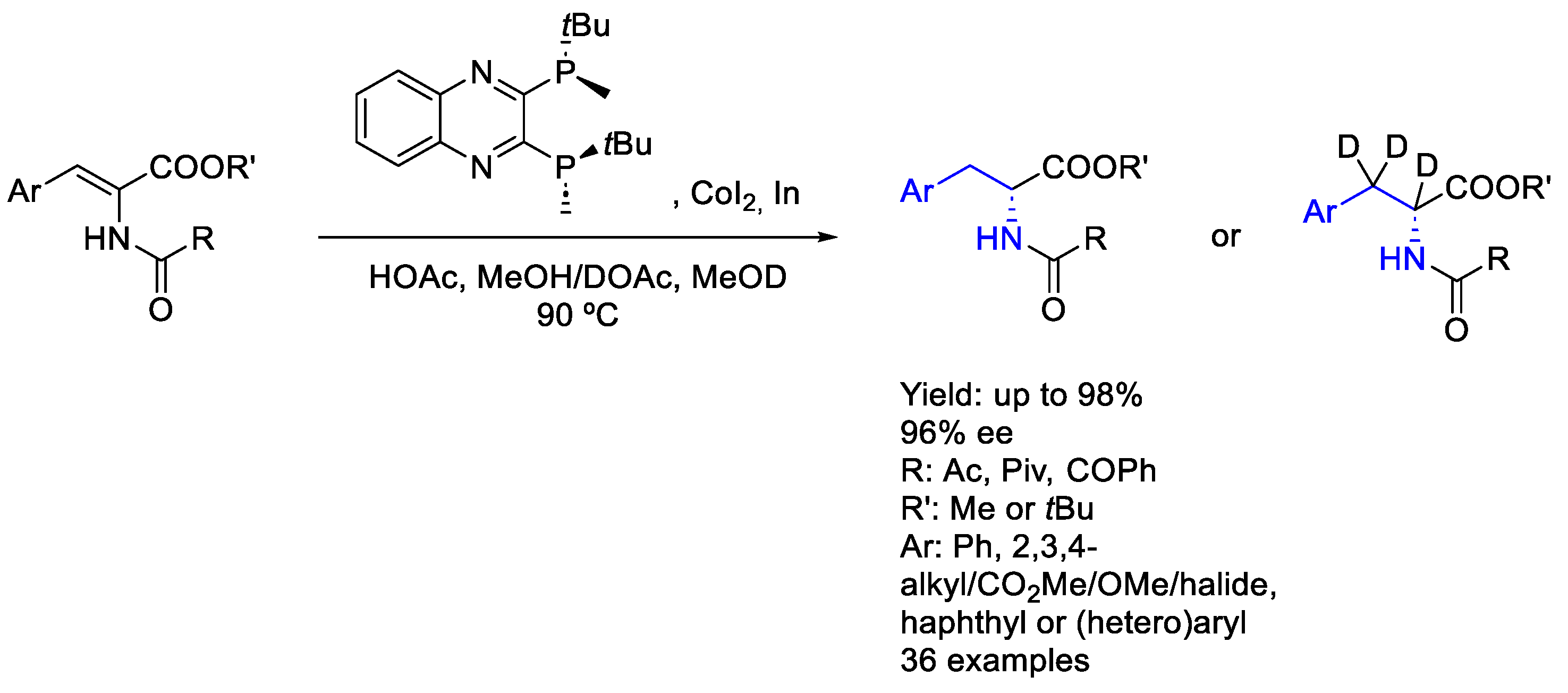
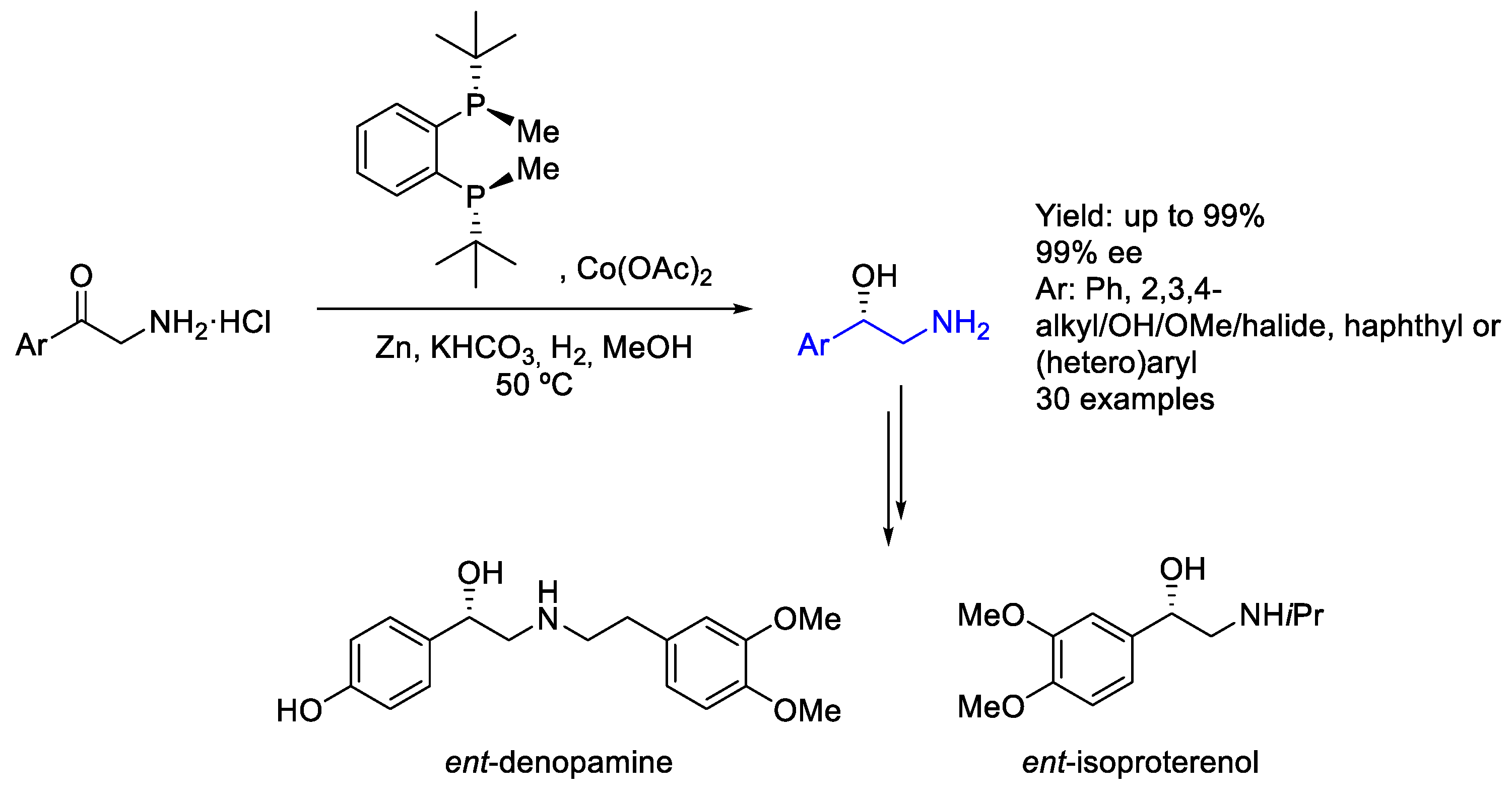
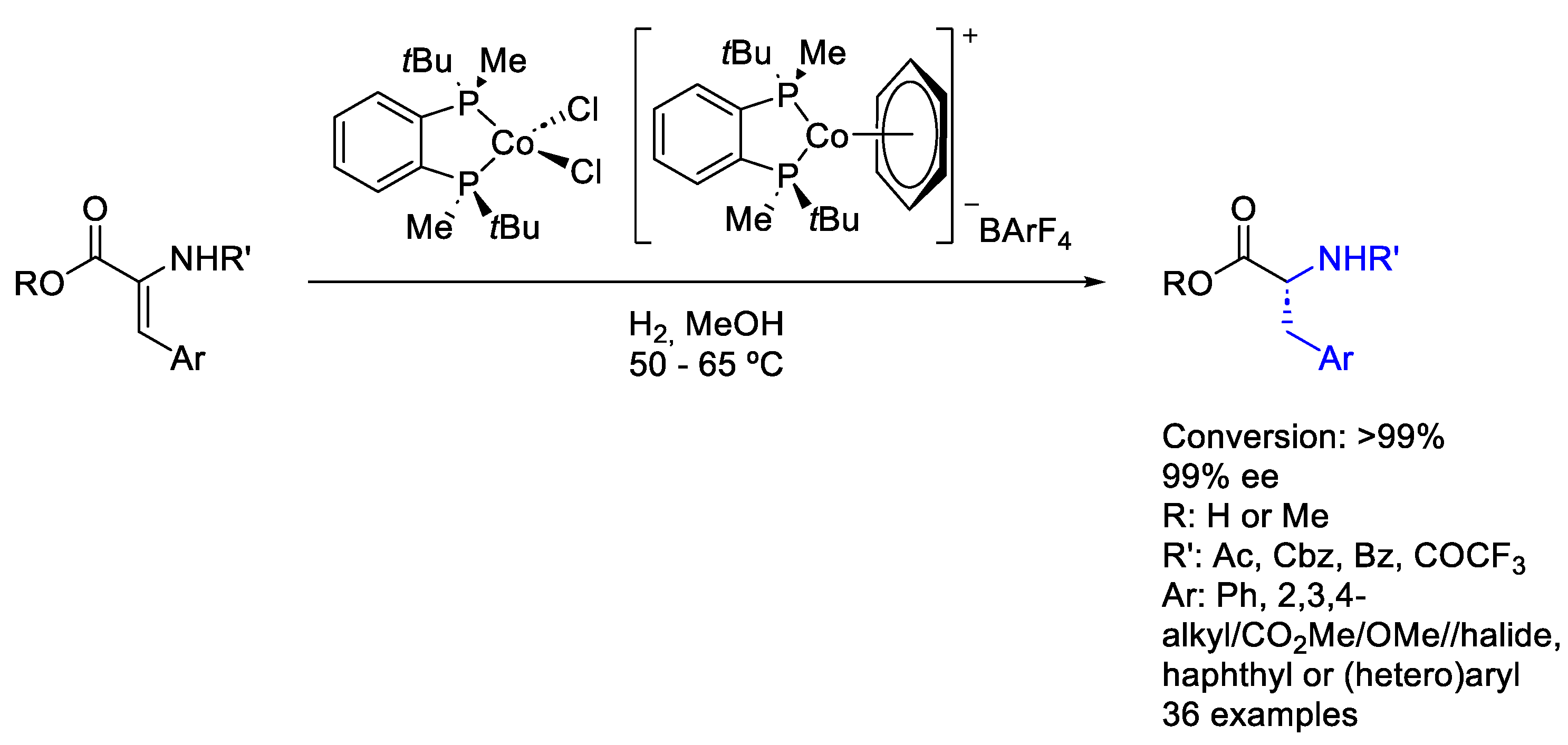
2.7. Cobalt
2.8. Others
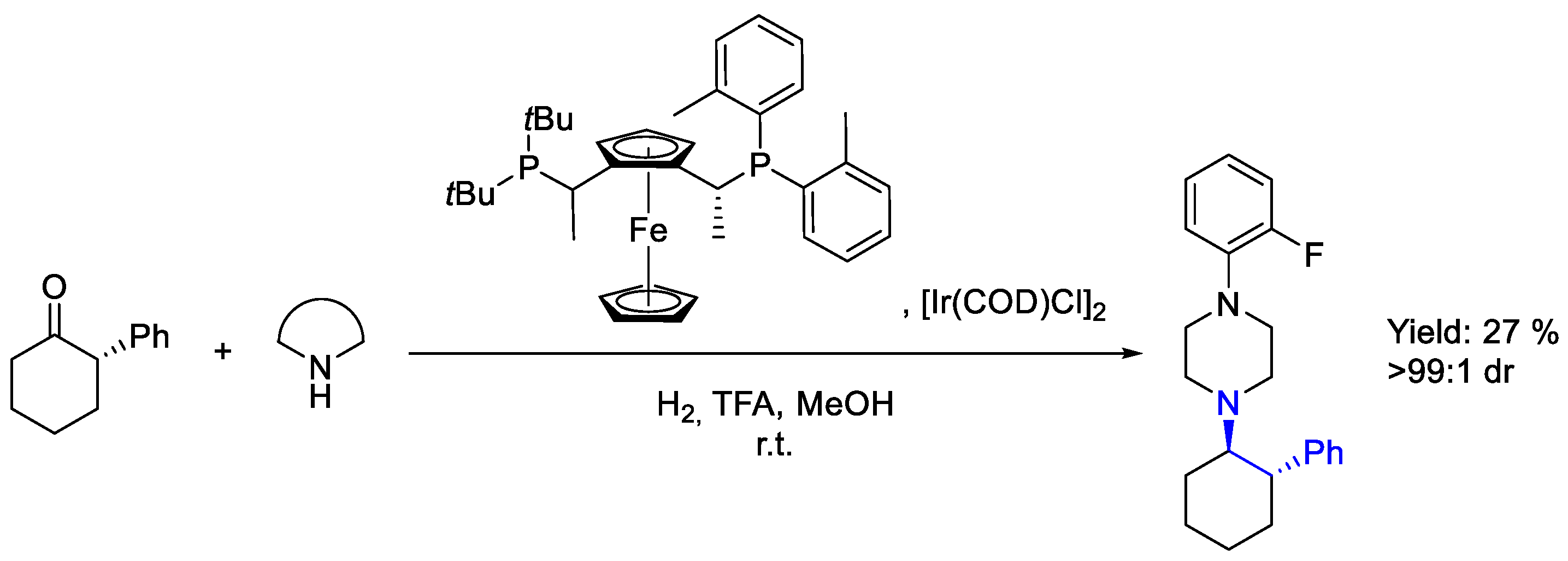
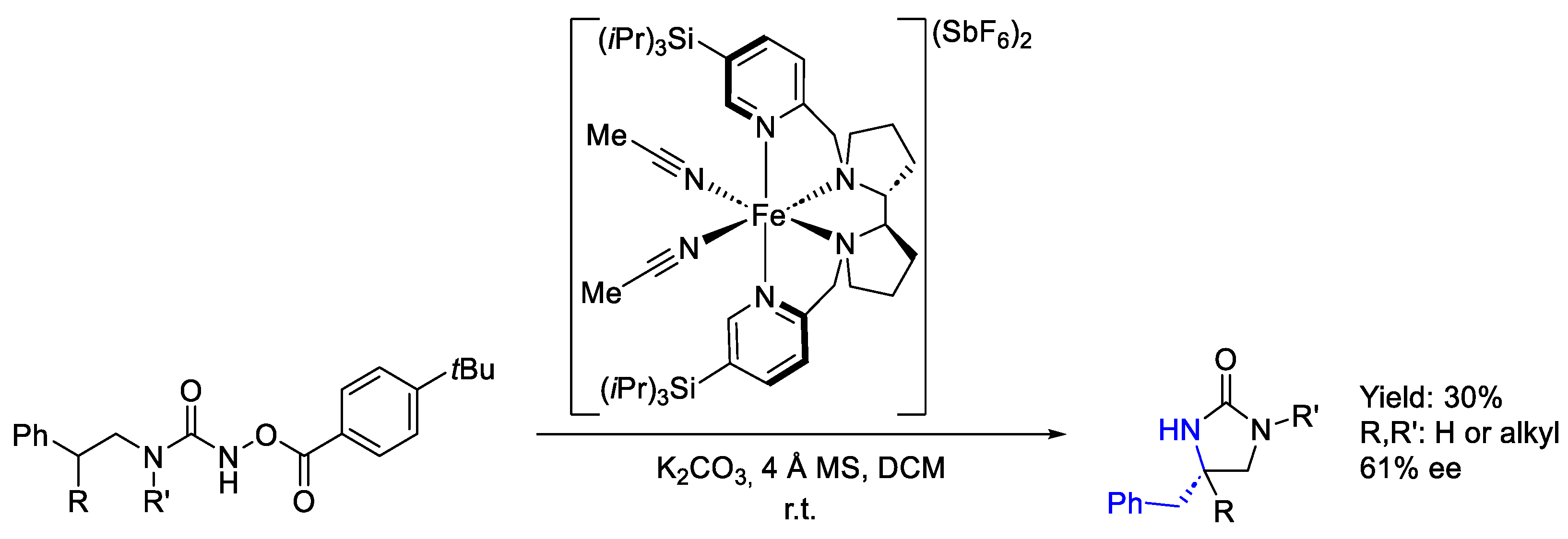
2.8.1. Iron
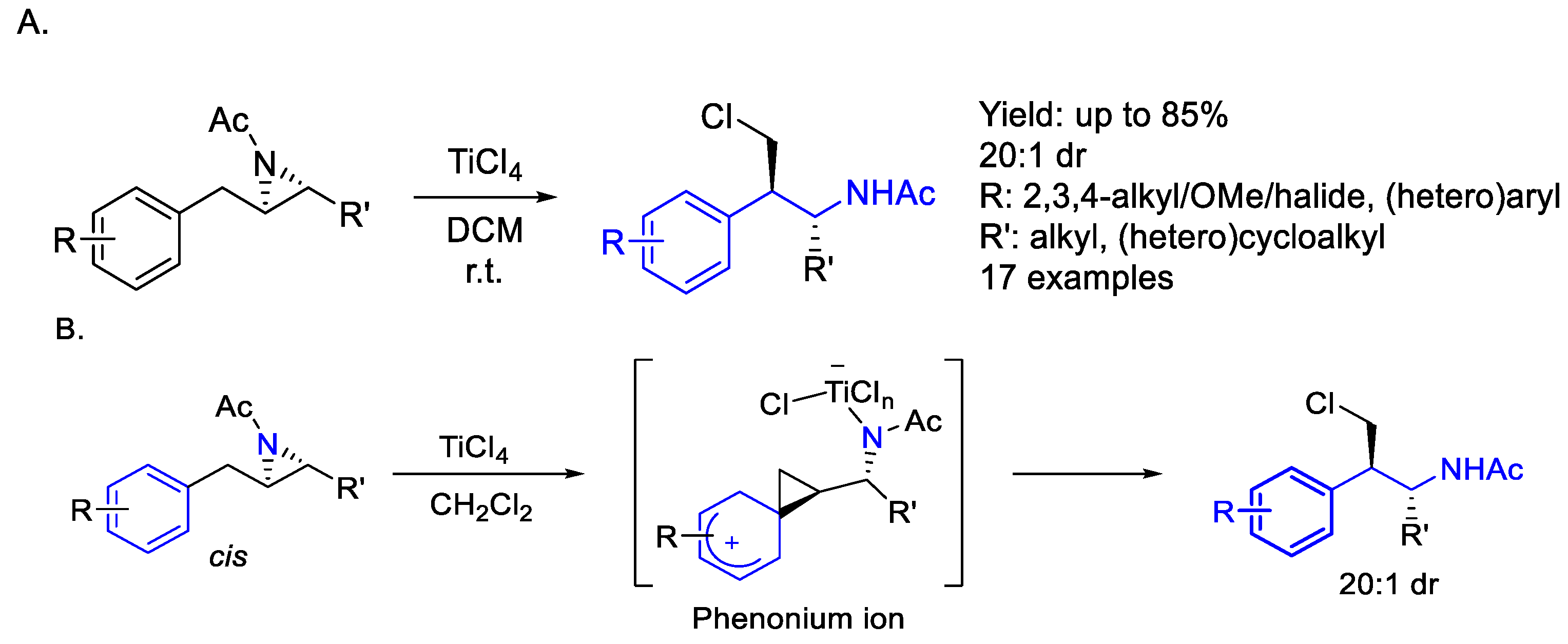
2.8.2. Titanium
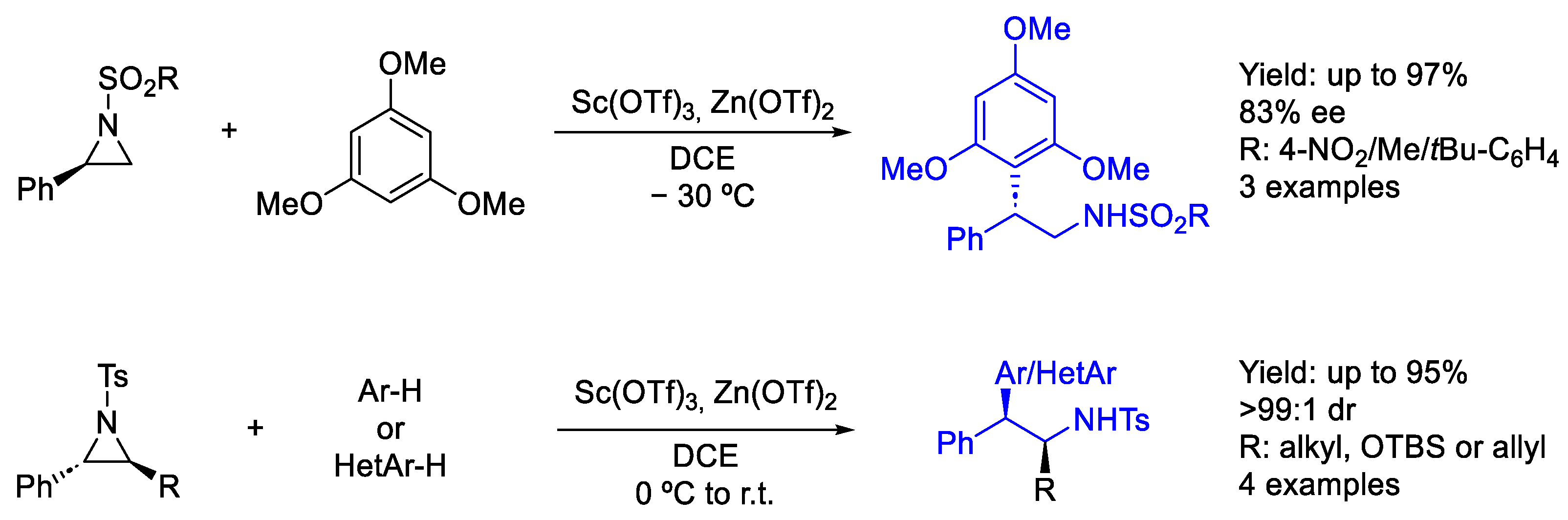
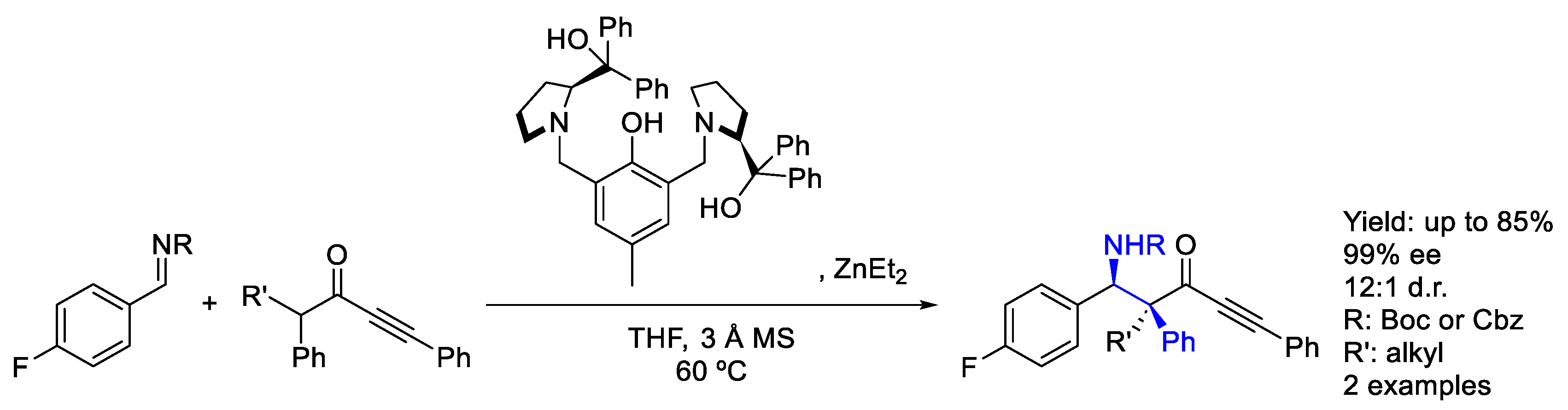
2.8.3. Zinc
3. Photocatalysis
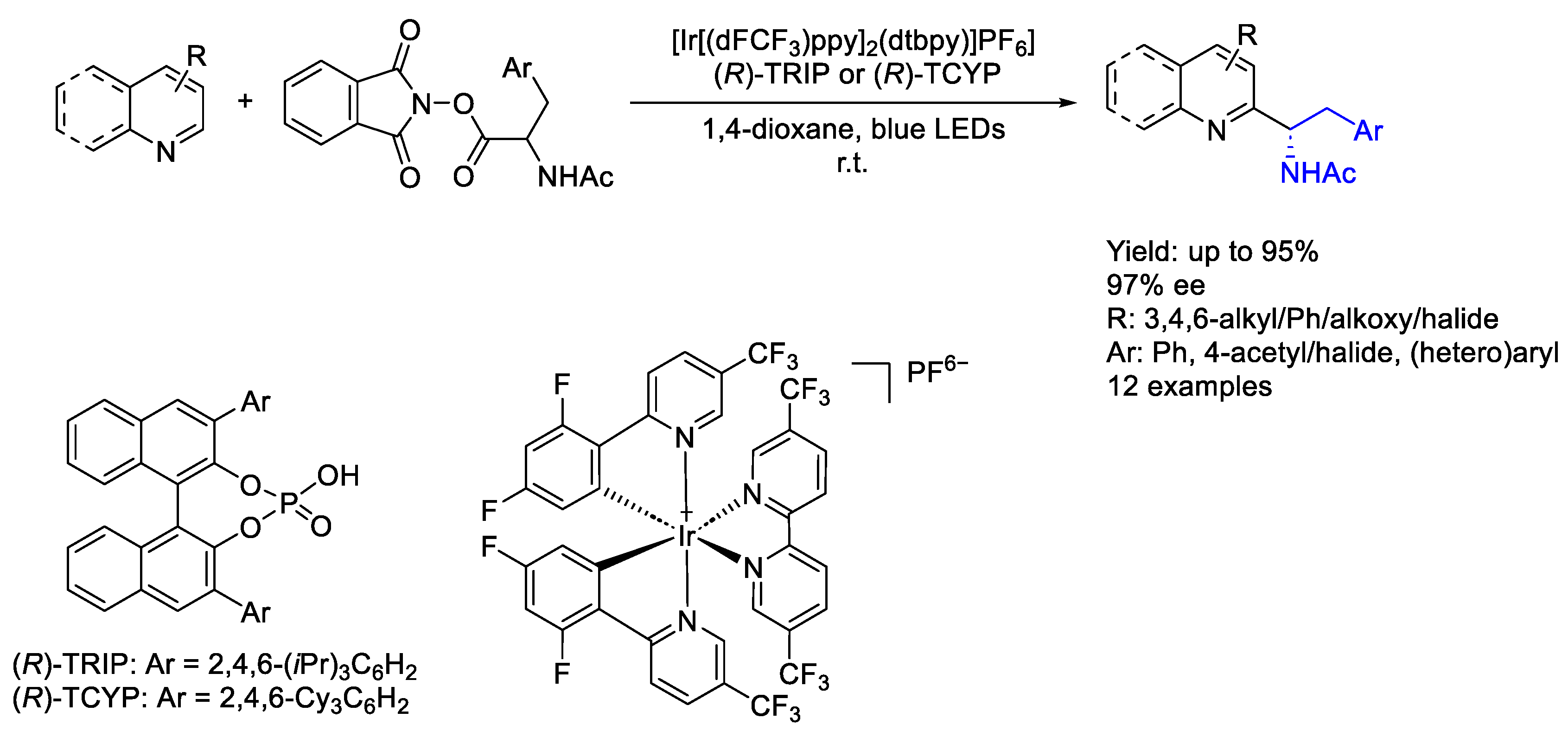
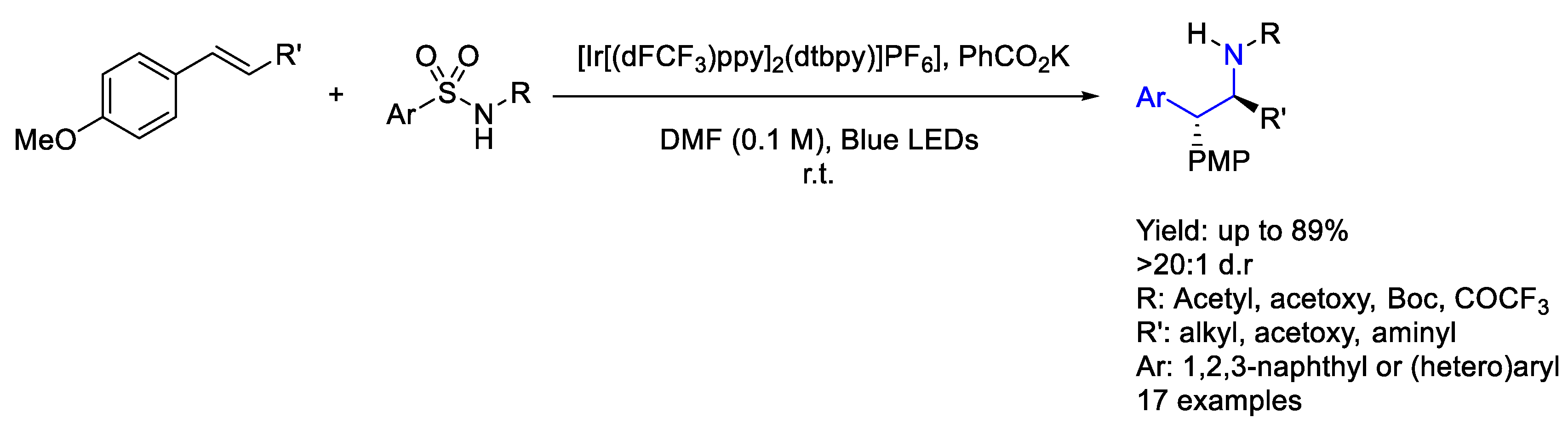
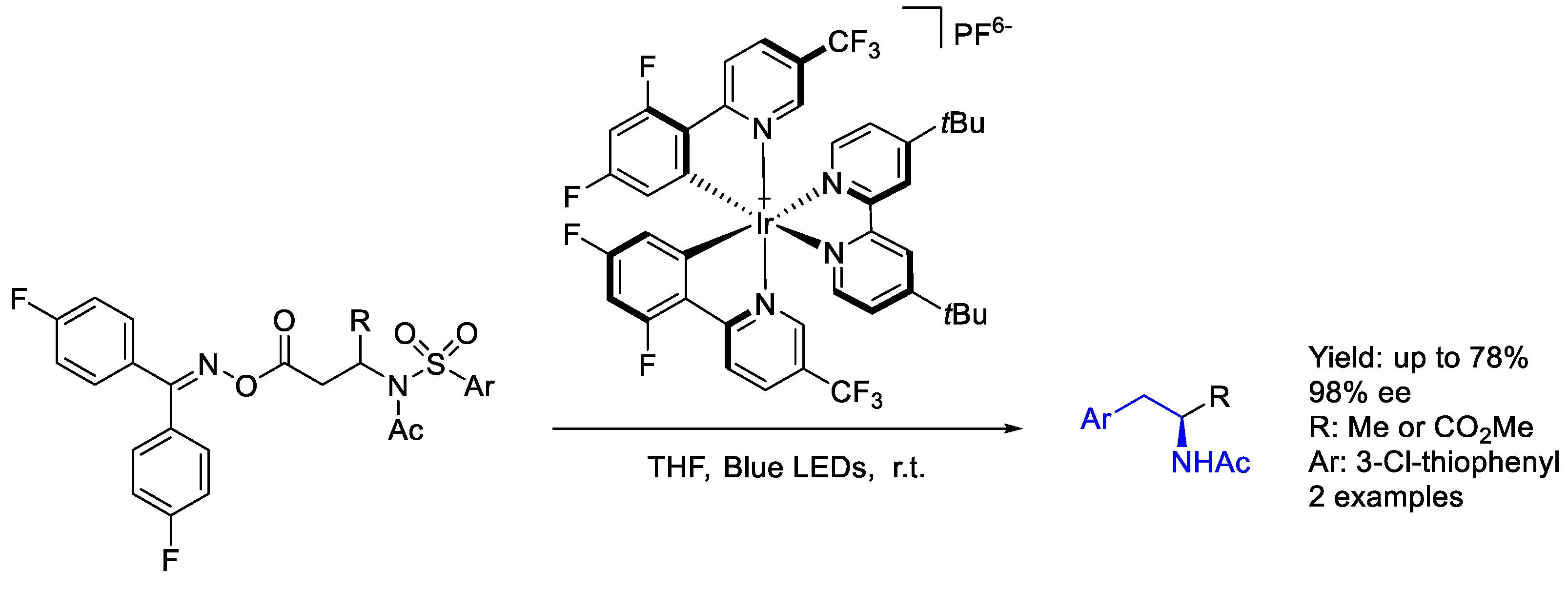
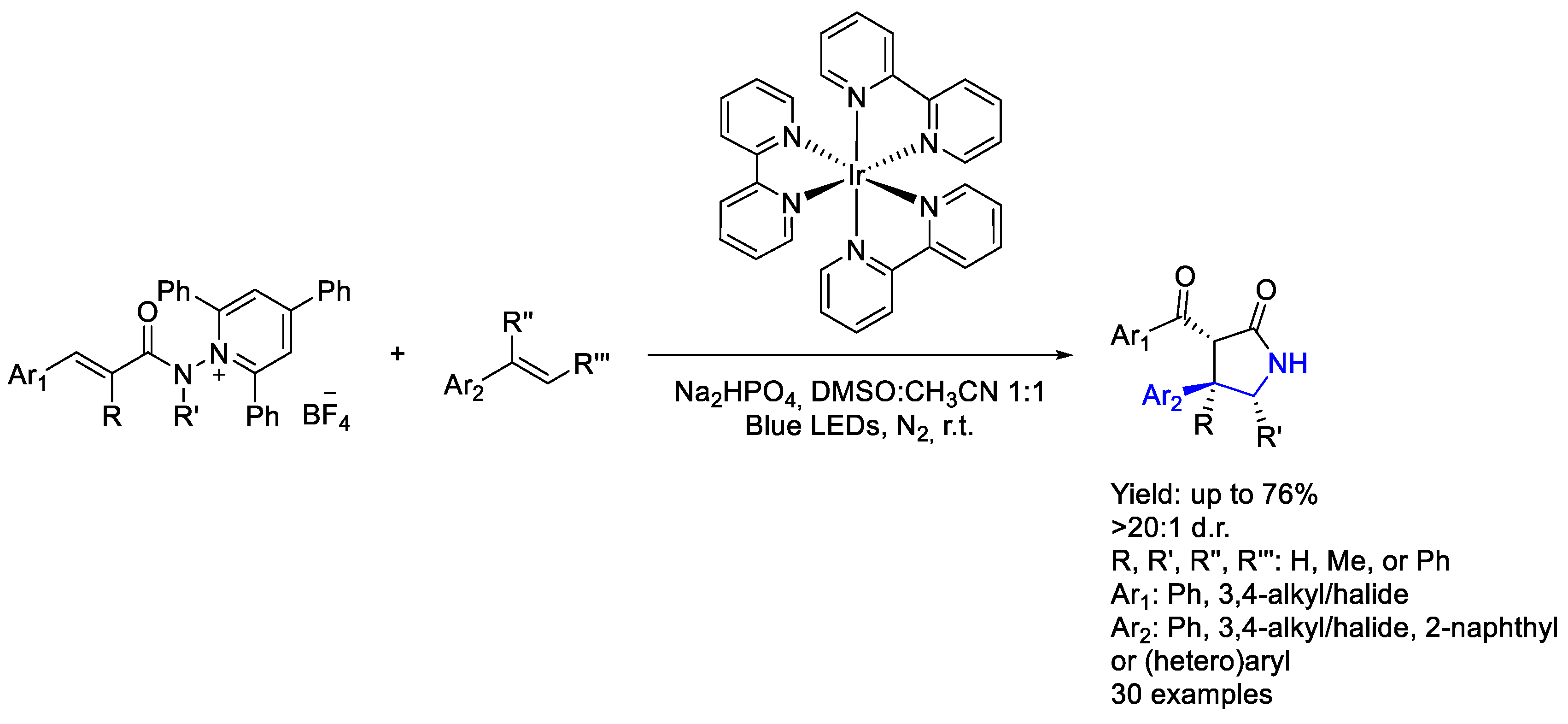
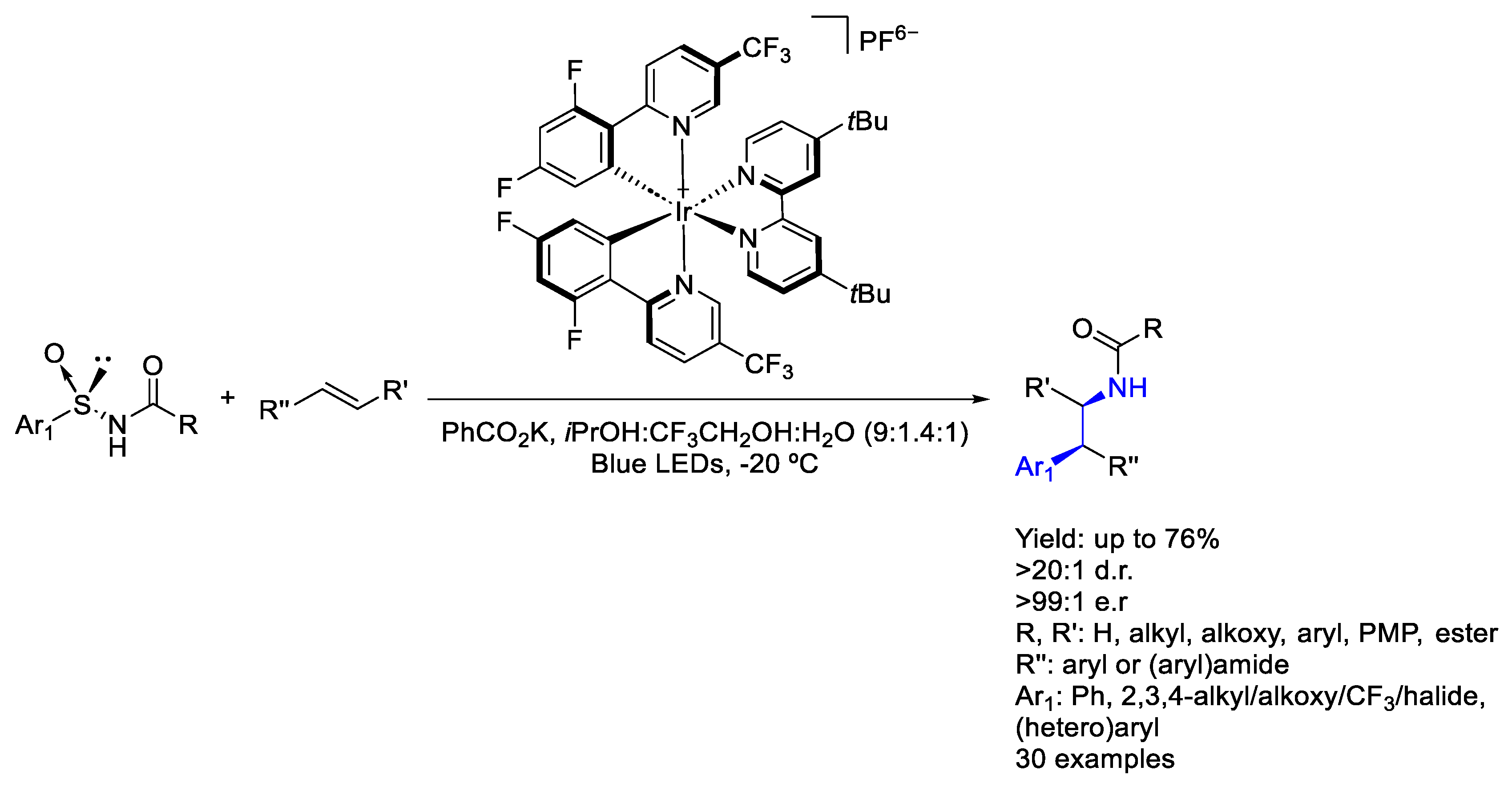
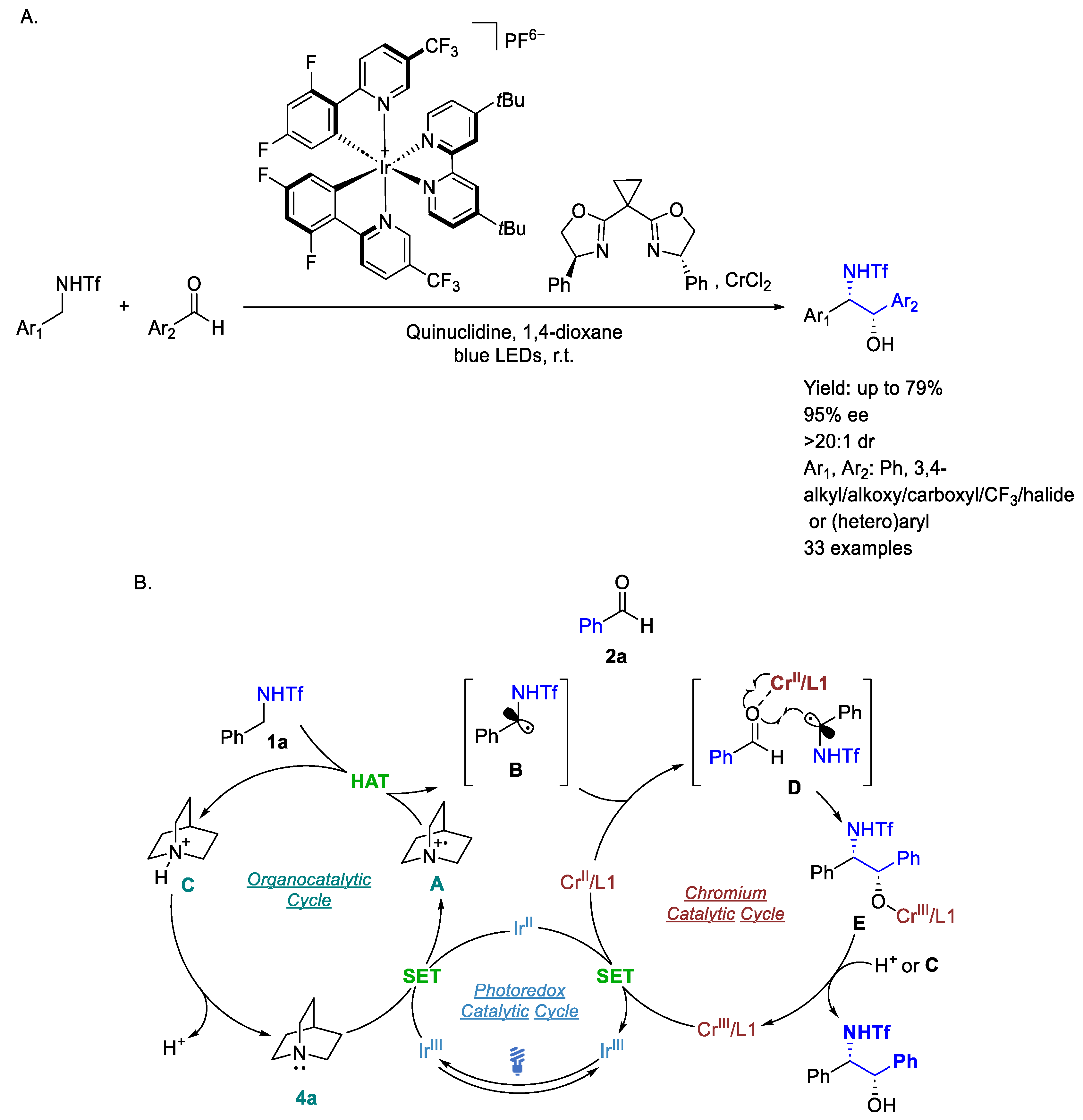
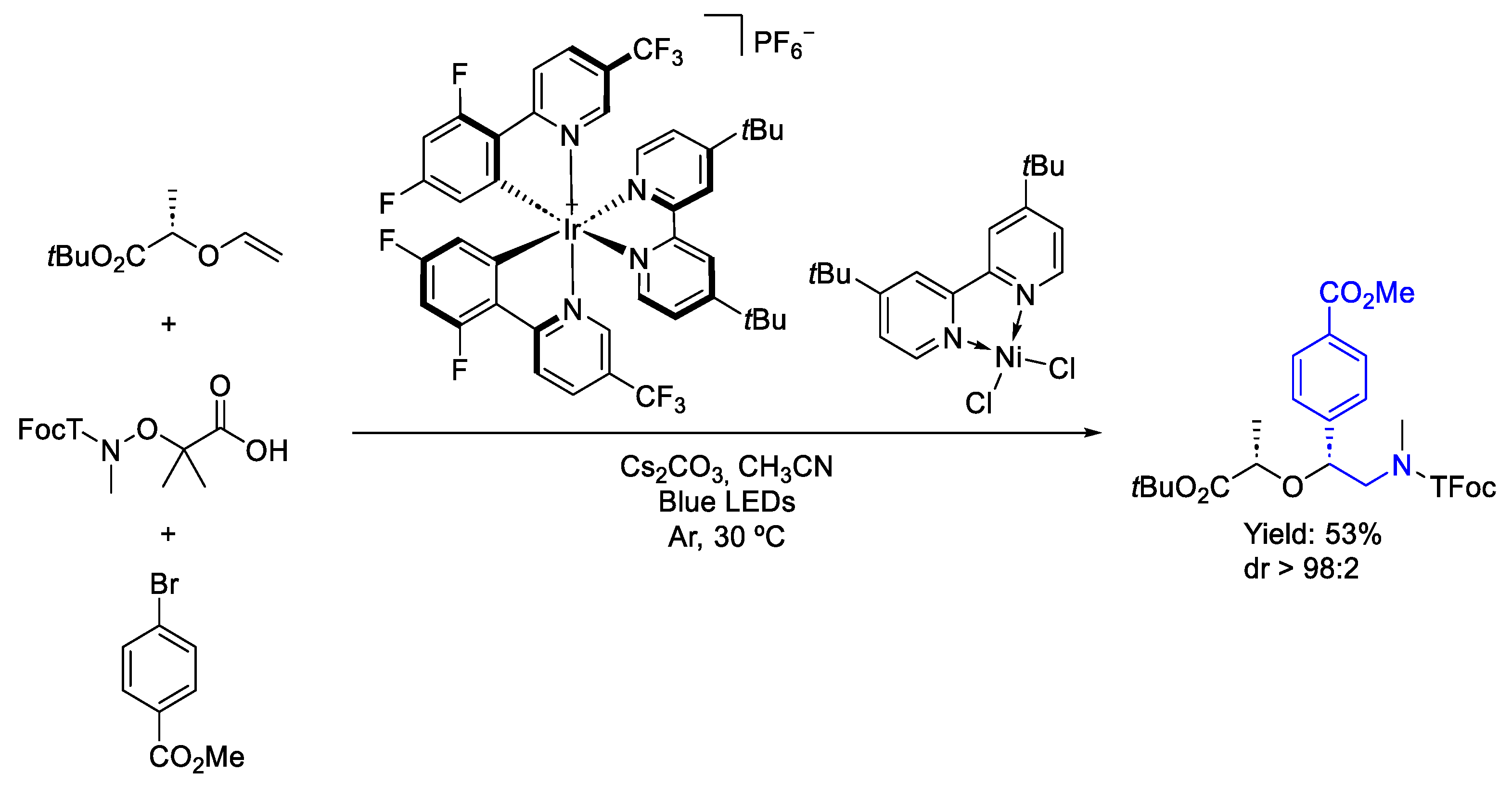
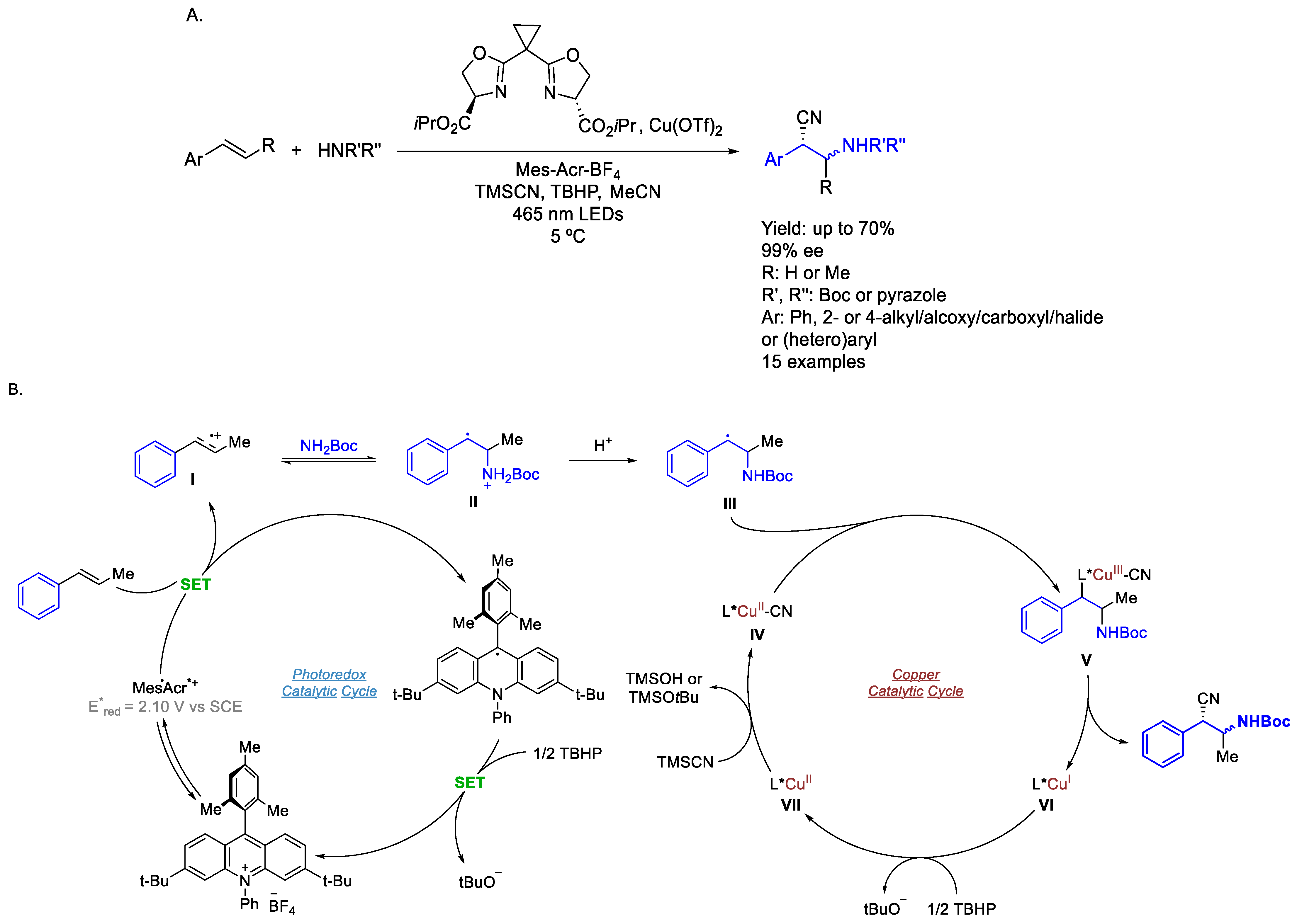
3.1. Iridium
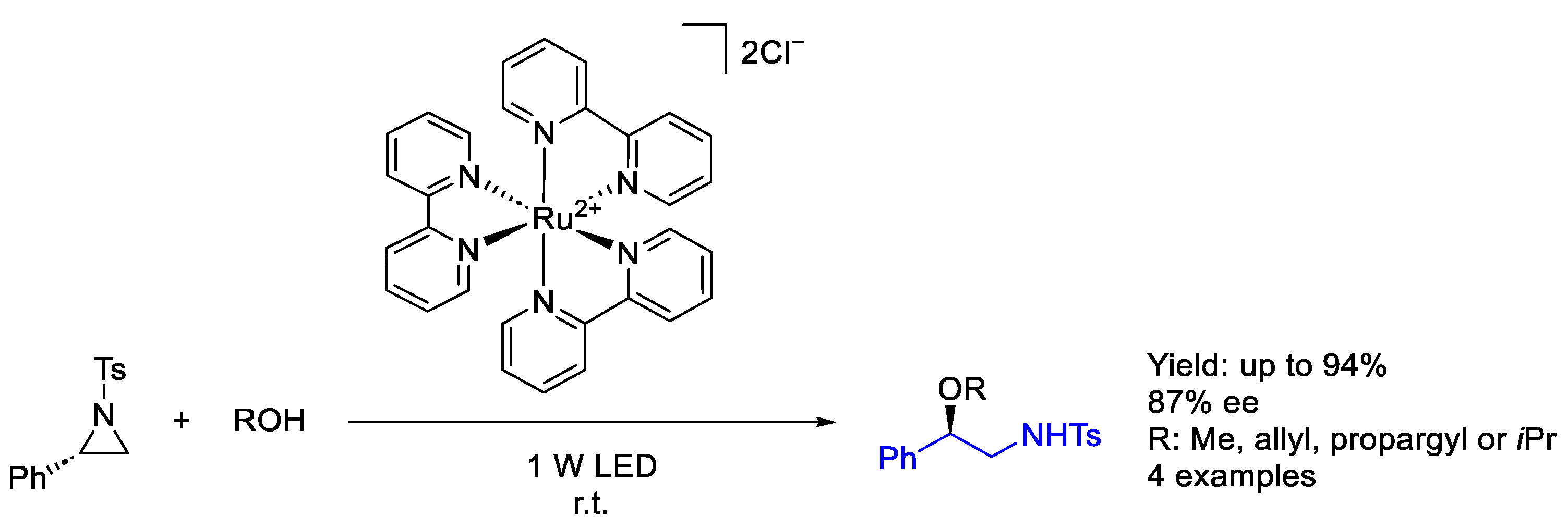
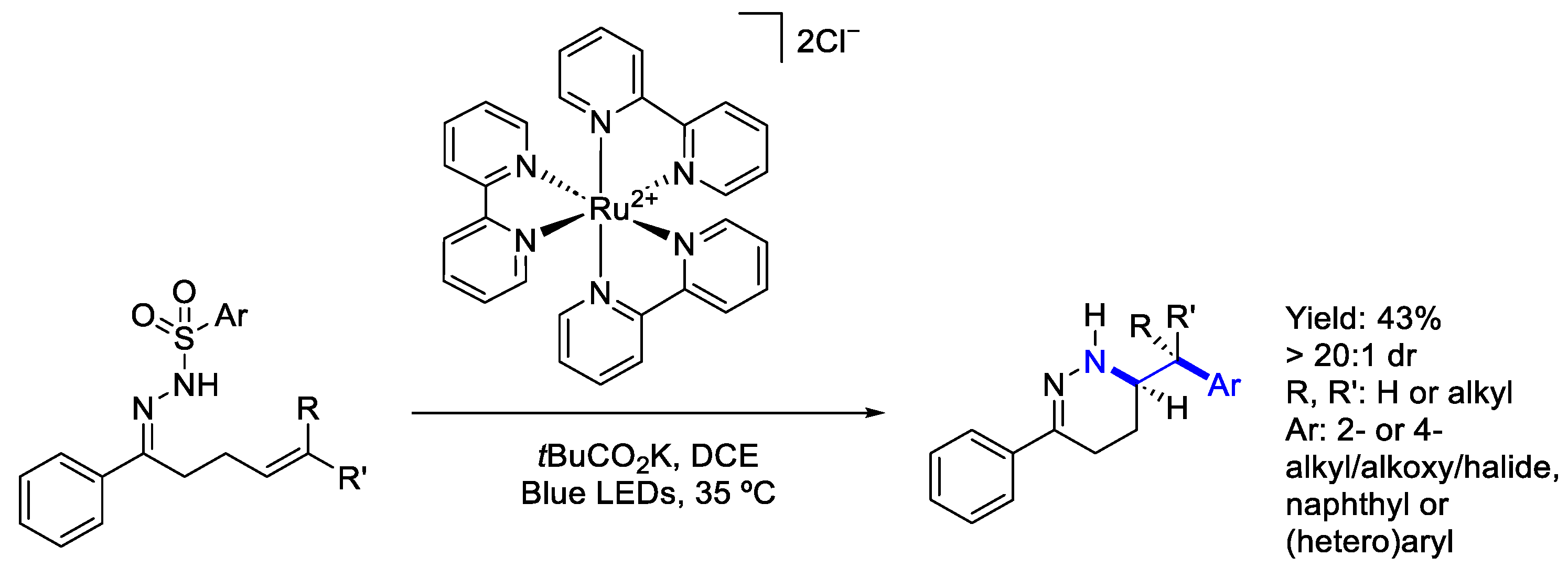
3.2. Ruthenium
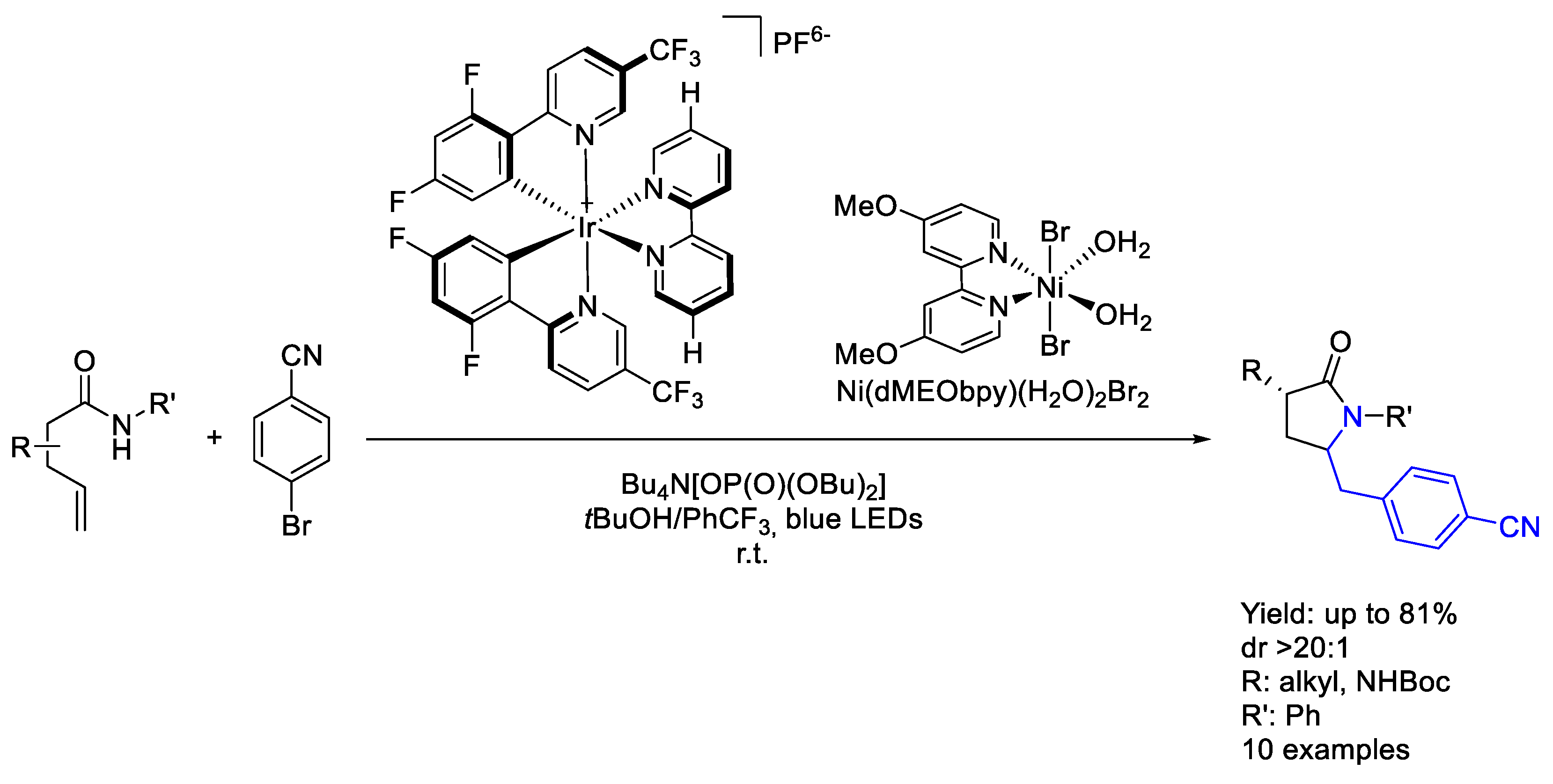
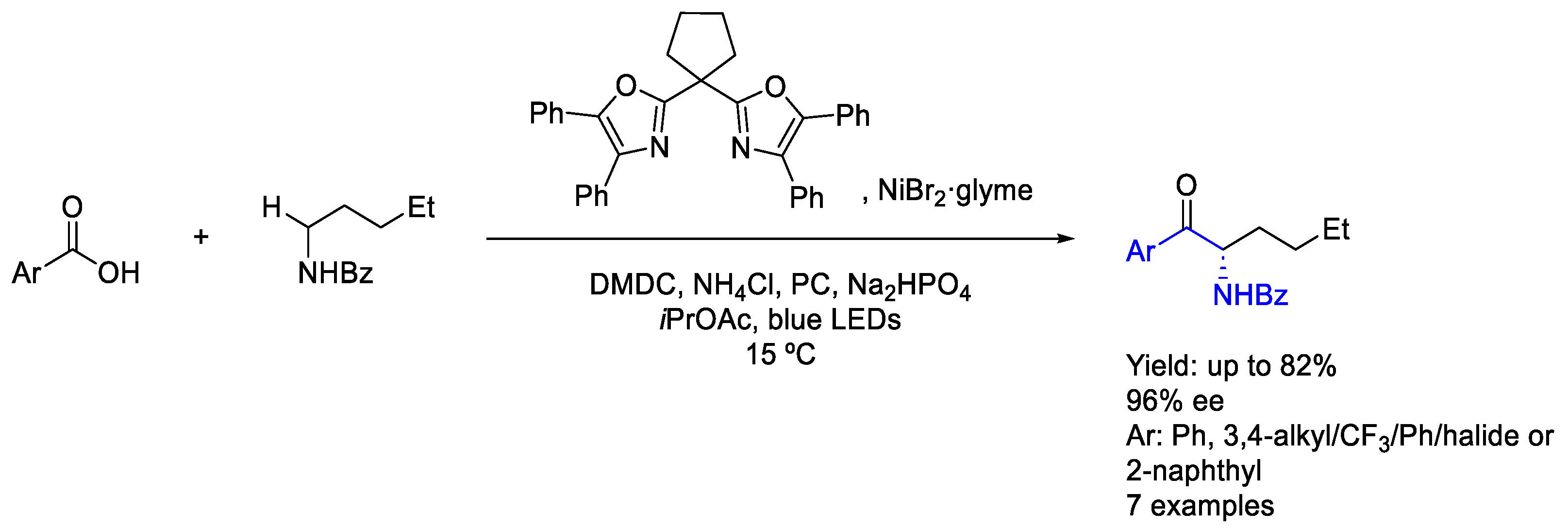
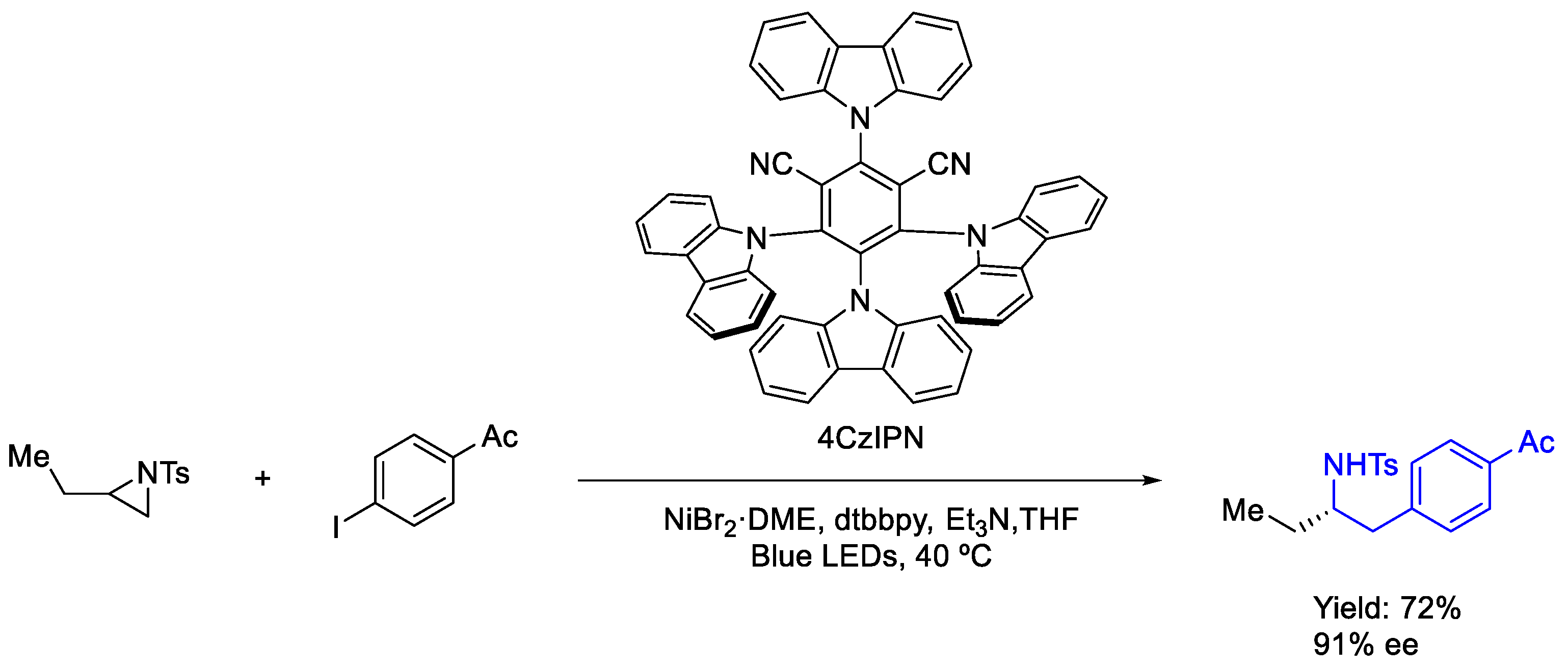
3.3. Nickel
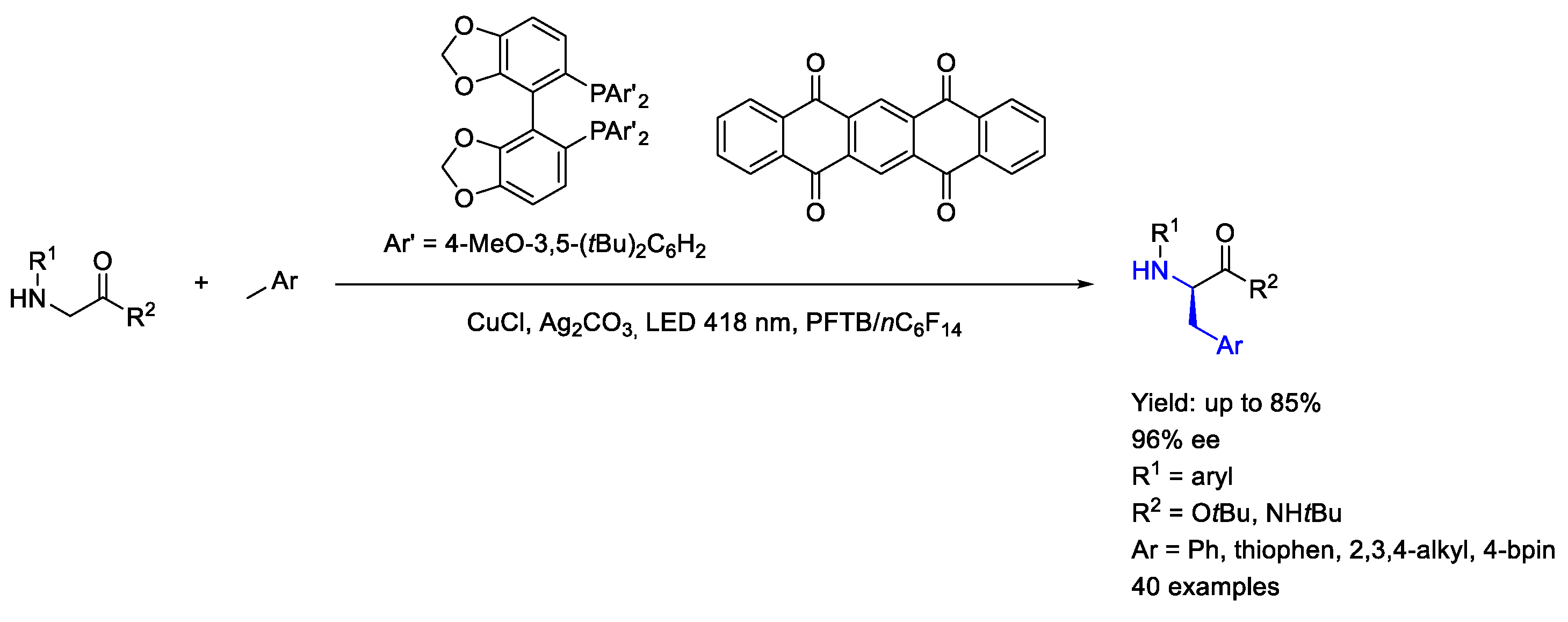
3.4. Copper
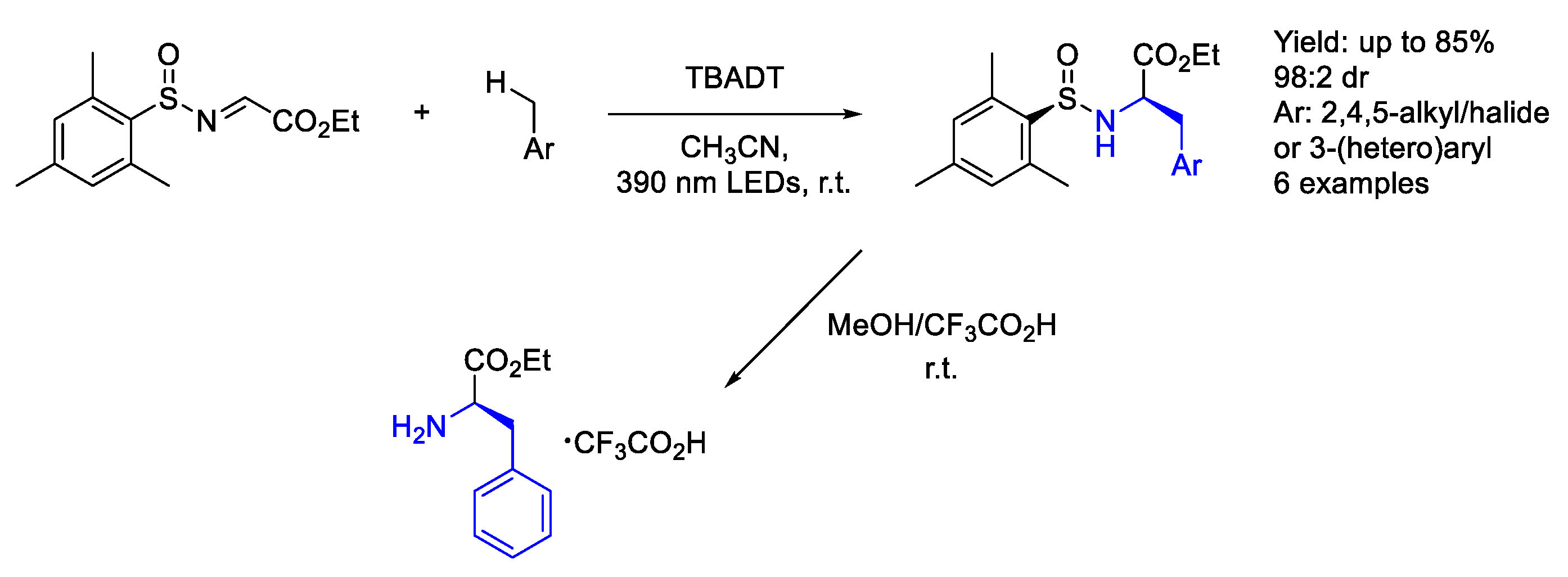
3.5. Wolframium
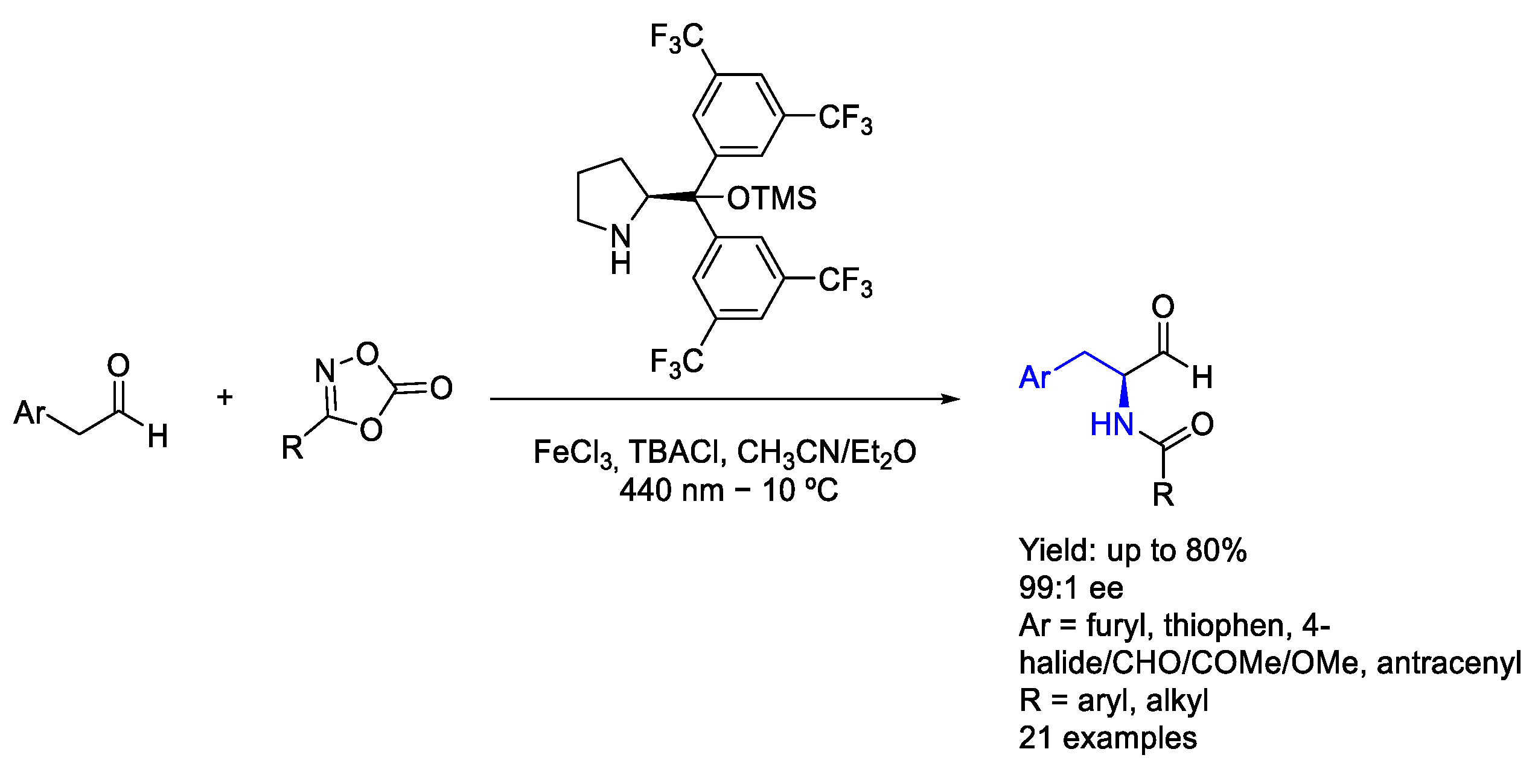
3.6. Iron
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sulzer, D.; Sonders, M.S.; Poulsen, N.W.; Galli, A. Mechanisms of Neurotransmitter Release by Amphetamines: A Review. Prog. Neurobiol. 2005, 75, 406–433. [Google Scholar] [CrossRef]
- Bentley, K.W. β-Phenylethylamines and the Isoquinoline Alkaloids. Nat. Prod. Rep. 2006, 23, 444–463. [Google Scholar] [CrossRef]
- Chinta, S.J.; Andersen, J.K. Dopaminergic Neurons. Int. J. Biochem. Cell Biol. 2005, 37, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.T.; Cascón, A.M.; Arroyo, L.B.; Martin, D.D.; Garrido, N.M. 2-Phenethylamines in Medicinal Chemistry: A Review. Molecules 2022, 28, 855. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.; Manchado, A.; García-González, Á.; Díez, D.; Garrido, N.M. 2-Heteroarylethylamines in Medicinal Chemistry: A Review of 2-Phenethylamine Satellite Chemical Space. Beilstein J. Org. Chem. 2024, 20, 1880–1893. [Google Scholar] [CrossRef] [PubMed]
- Manchado, A.; García-González, Á.; Nieto, C.T.; Díez, D.; Garrido, N.M. Asymmetric Synthesis of 2-Arylethylamines: A Metal-Free Review of the New Millennium. Molecules 2024, 29, 5729. [Google Scholar] [CrossRef]
- Abdine, R.A.A.; Hedouin, G.; Colobert, F.; Wencel-Delord, J. Metal-Catalyzed Asymmetric Hydrogenation of C=N Bonds. ACS Catal. 2021, 11, 215–247. [Google Scholar] [CrossRef]
- Cabré, A.; Verdaguer, X.; Riera, A. Recent Advances in the Enantioselective Synthesis of Chiral Amines via Transition Metal-Catalyzed Asymmetric Hydrogenation. Chem. Rev. 2022, 122, 269–339. [Google Scholar] [CrossRef]
- Mathew, S.; Renn, D.; Rueping, M. Advances in One-Pot Chiral Amine Synthesis Enabled by Amine Transaminase Cascades: Pushing the Boundaries of Complexity. ACS Catal. 2023, 13, 5584–5598. [Google Scholar] [CrossRef]
- Pozhydaiev, V.; Muller, C.; Moran, J.; Lebœuf, D. Catalytic Synthesis of β-(Hetero)Arylethylamines: Modern Strategies and Advances. Angew. Chem.-Int. Ed. 2023, 62, e202309289. [Google Scholar] [CrossRef]
- Kwon, Y.; Wang, Q. Recent Advances in 1,2-Amino(Hetero)Arylation of Alkenes. Chem. Asian J. 2022, 17, e202200215. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, G.; Zhang, G.; Li, Y.; Zhang, Q. Recent Advances in Enantioselective Construction of C-N Bonds Involving Radical Intermediates. Org. Chem. Front. 2025, 12, 1671–1694. [Google Scholar] [CrossRef]
- Ju, X.; Lee, M.; Leung, J.C.; Lee, J. Synthesis and Functionalization of Aziridines: A Perspective View from Pharmaceutical Industries. Eur. J. Org. Chem. 2025, e202401414. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, X.; Jiang, Z. Asymmetric Photocatalytic Synthesis of Enantioenriched Azaarene Derivatives. Chin. J. Org. Chem. 2022, 42, 1609–1625. [Google Scholar] [CrossRef]
- Zeng, W.; Chemler, S.R. Copper(II)-Catalyzed Enantioselective Intramolecular Carboamination of Alkenes. J. Am. Chem. Soc. 2007, 129, 12948–12949. [Google Scholar] [CrossRef]
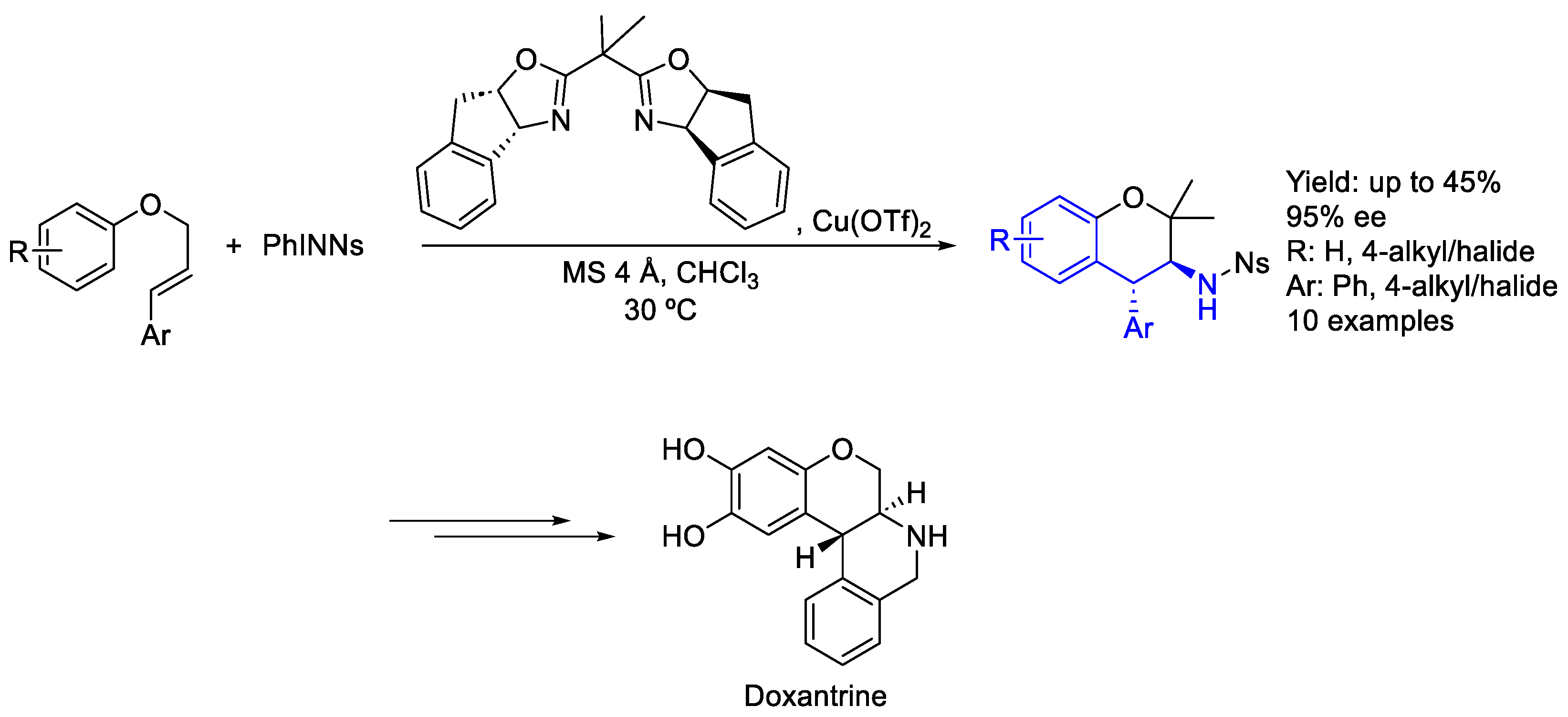
- Hajra, S.; Sinha, D. Catalytic Enantioselective Aziridoarylation of Aryl Cinnamyl Ethers toward Synthesis of Trans-3-Amino-4-Arylchromans. J. Org. Chem. 2011, 76, 7334–7340. [Google Scholar] [CrossRef] [PubMed]
- Sakae, R.; Matsuda, N.; Hirano, K.; Satoh, T.; Miura, M. Highly Stereoselective Synthesis of (Borylmethyl)Cyclopropylamines by Copper-Catalyzed Aminoboration of Methylenecyclopropanes. Org. Lett. 2014, 16, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
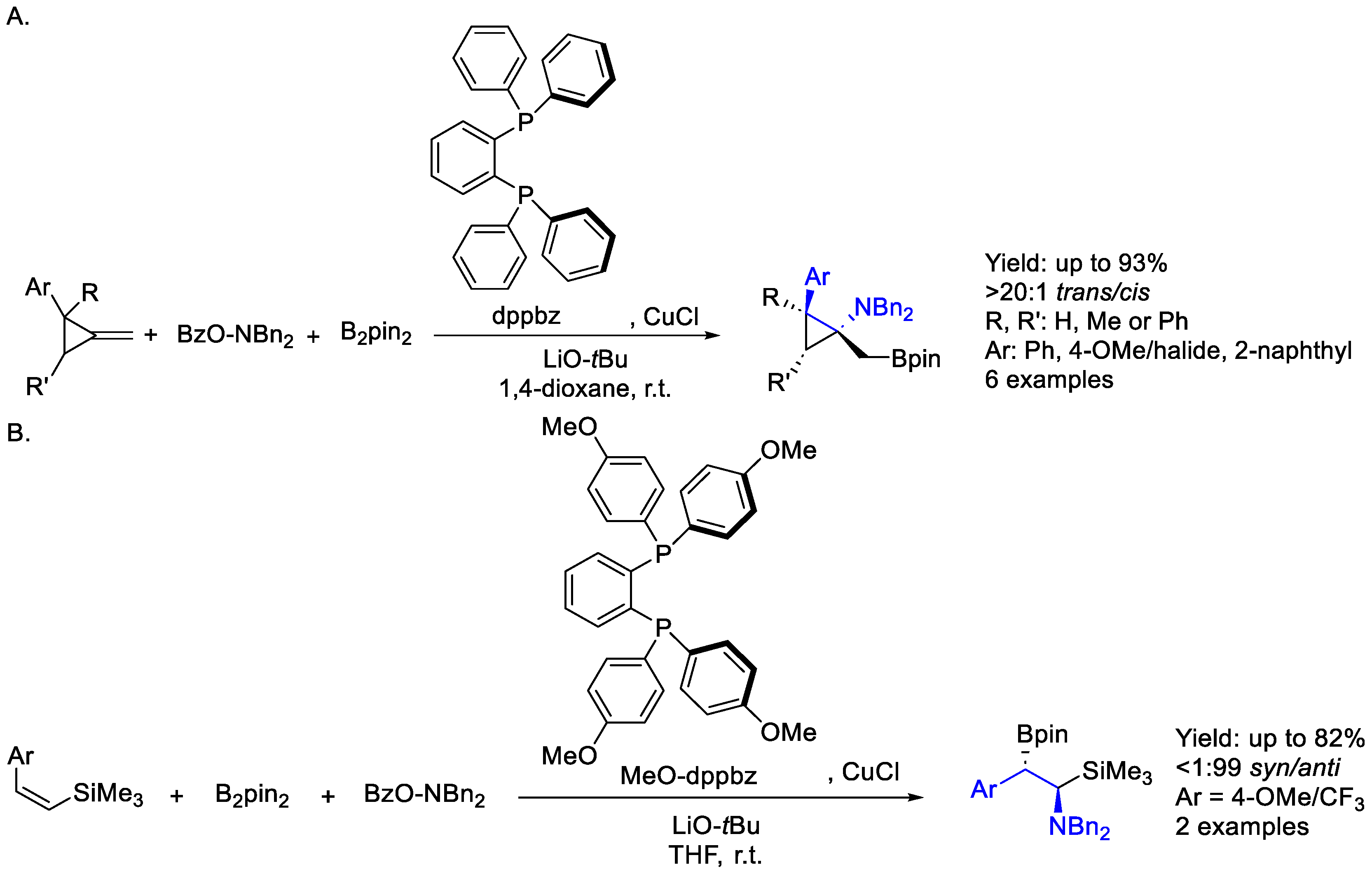
- Kato, K.; Hirano, K.; Miura, M. Synthesis of Β-Boryl-α-Aminosilanes by Copper-Catalyzed Aminoboration of Vinylsilanes. Angew. Chem. Int. Ed. 2016, 128, 14612–14616. [Google Scholar] [CrossRef]
- Parra, A.; Amenós, L.; Guisán-Ceinos, M.; López, A.; García Ruano, J.L.; Tortosa, M. Copper-Catalyzed Diastereo- and Enantioselective Desymmetrization of Cyclopropenes: Synthesis of Cyclopropylboronates. J. Am. Chem. Soc. 2014, 136, 15833–15836. [Google Scholar] [CrossRef]
- Yang, Y.; Perry, I.B.; Buchwald, S.L. Copper-Catalyzed Enantioselective Addition of Styrene-Derived Nucleophiles to Imines Enabled by Ligand-Controlled Chemoselective Hydrocupration. J. Am. Chem. Soc. 2016, 138, 9787–9790. [Google Scholar] [CrossRef]
- Ge, C.; Liu, R.R.; Gao, J.R.; Jia, Y.X. Cu(I)-Catalyzed Enantioselective Friedel-Crafts Alkylation of Indoles with 2-Aryl-N-Sulfonylaziridines as Alkylating Agents. Org. Lett. 2016, 18, 3122–3125. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huo, Y.; An, P.; Wang, X.; Song, C.; Ma, Y. Paracyclophane-Based N-Heterocyclic Carbene as Efficient Catalyst or as Ligand for Copper Catalyst for Asymmetric α-Silylation of: N -Tosylaldimines. Org. Chem. Front. 2016, 3, 1725–1737. [Google Scholar] [CrossRef]
- Pascual-Escudero, A.; de Cózar, A.; Cossío, F.P.; Adrio, J.; Carretero, J.C. Alkenyl Arenes as Dipolarophiles in Catalytic Asymmetric 1,3-Dipolar Cycloaddition Reactions of Azomethine Ylides. Angew. Chem.-Int. Ed. 2016, 55, 15334–15338. [Google Scholar] [CrossRef]
- Peng, F.; Chen, Y.; Chen, C.Y.; Dormer, P.G.; Kassim, A.; McLaughlin, M.; Reamer, R.A.; Sherer, E.C.; Song, Z.J.; Tan, L.; et al. Asymmetric Formal Synthesis of the Long-Acting DPP-4 Inhibitor Omarigliptin. J. Org. Chem. 2017, 82, 9023–9029. [Google Scholar] [CrossRef]
- Wang, D.; Wang, F.; Chen, P.; Lin, Z.; Liu, G. Enantioselective Copper-Catalyzed Intermolecular Amino- and Azidocyanation of Alkenes in a Radical Process. Angew. Chem. 2017, 129, 2086–2090. [Google Scholar] [CrossRef]
- Wang, D.; Wu, L.; Wang, F.; Wan, X.; Chen, P.; Lin, Z.; Liu, G. Asymmetric Copper-Catalyzed Intermolecular Aminoarylation of Styrenes: Efficient Access to Optical 2,2-Diarylethylamines. J. Am. Chem. Soc. 2017, 139, 6811–6814. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fu, L.; Chen, P.; Cao, W.; Liu, G. Enantioselective Intermolecular Aminoalkynylation of Styrenes via Copper-Catalyzed Radical Relay. Org. Lett. 2021, 23, 129–134. [Google Scholar] [CrossRef]
- Yang, P.J.; Qi, L.; Liu, Z.; Yang, G.; Chai, Z. Lewis Acid Catalyzed Dynamic Kinetic Asymmetric Transformation of Racemic N-Sulfonylaziridines. J. Am. Chem. Soc. 2018, 140, 17211–17217. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M.; Zhang, Y.; Liu, S.; Zhao, J.; Zhang, Q. Multicomponent Cyclopropane Synthesis Enabled by Cu-Catalyzed Cyclopropene Carbometalation with Organoboron Reagent: Enantioselective Modular Access to Polysubstituted 2-Arylcyclopropylamines. Org. Lett. 2019, 21, 5432–5437. [Google Scholar] [CrossRef]
- Chang, X.; Yang, Y.; Shen, C.; Xue, K.S.; Wang, Z.F.; Cong, H.; Tao, H.Y.; Chung, L.W.; Wang, C.J. β-Substituted Alkenyl Heteroarenes as Dipolarophiles in the Cu(I)-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides Empowered by a Dual Activation Strategy: Stereoselectivity and Mechanistic Insight. J. Am. Chem. Soc. 2021, 143, 3519–3535. [Google Scholar] [CrossRef]
- Nishino, S.; Miura, M.; Hirano, K. An Umpolung-Enabled Copper-Catalysed Regioselective Hydroamination Approach to α-Amino Acids. Chem. Sci. 2021, 12, 11525–11537. [Google Scholar] [CrossRef] [PubMed]
- Nishino, S.; Nishii, Y.; Hirano, K. Anti-Selective Synthesis of β-Boryl-α-Amino Acid Derivatives by Cu-Catalysed Borylamination of α,β-Unsaturated Esters. Chem. Sci. 2022, 13, 14387–14394. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Nishino, S.; Hirano, K. Synthesis of α-Aminophosphonates by Umpolung-Enabled Cu-Catalyzed Regioselective Hydroamination. J. Org. Chem. 2023, 88, 1270–1281. [Google Scholar] [CrossRef]
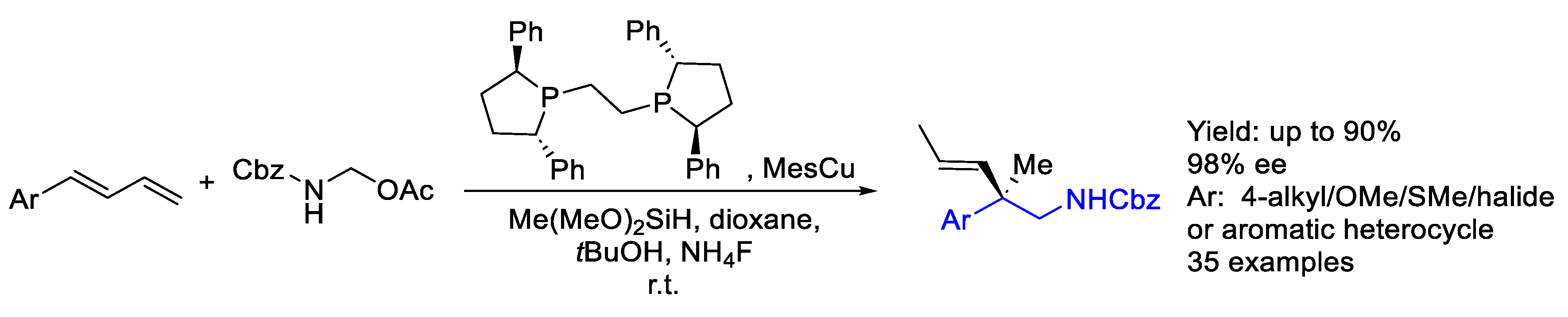
- Ren, K.; Yuan, R.; Gui, Y.Y.; Chen, X.W.; Min, S.Y.; Wang, B.Q.; Yu, D.G. Cu-Catalyzed Reductive Aminomethylation of 1,3-Dienes with N,O-Acetals: Facile Construction of β-Chiral Amines with Quaternary Stereocenters. Org. Chem. Front. 2022, 10, 467–472. [Google Scholar] [CrossRef]
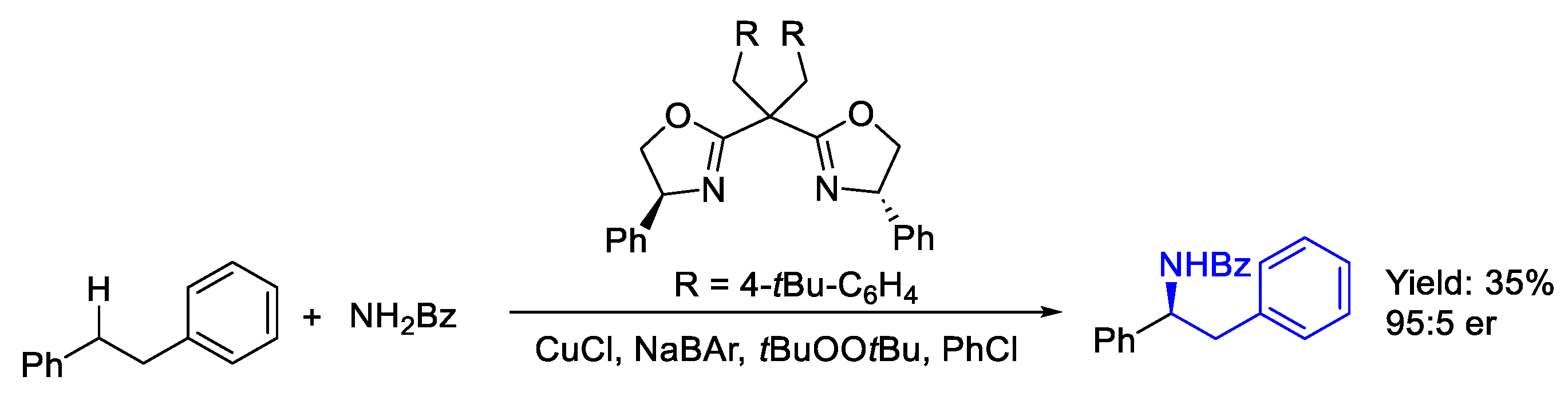
- Dai, L.; Chen, Y.-Y.; Xiao, L.-J.; Zhou, Q.-L. Intermolecular Enantioselective Benzylic C(Sp3)-H Amination via Cationic Copper Catalysis. Angew. Chem. 2023, 62, e202304427. [Google Scholar] [CrossRef] [PubMed]
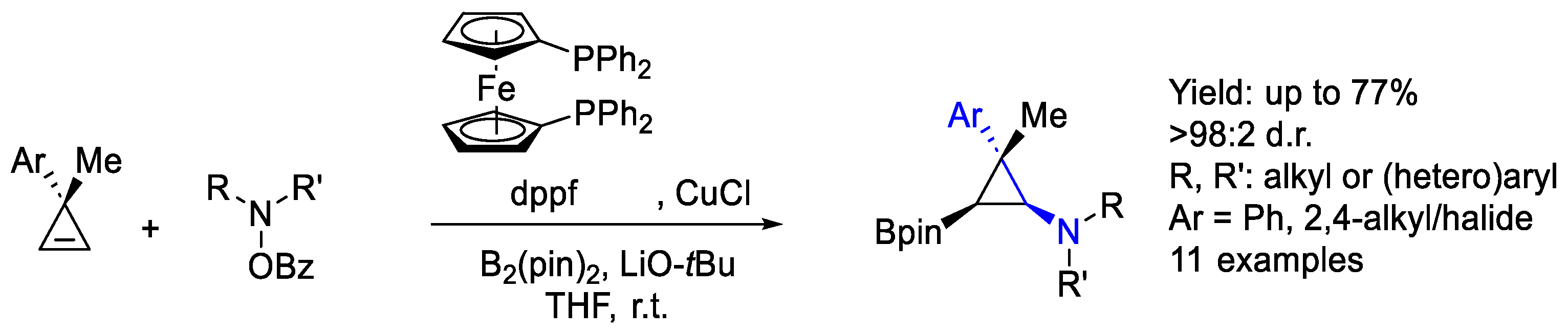
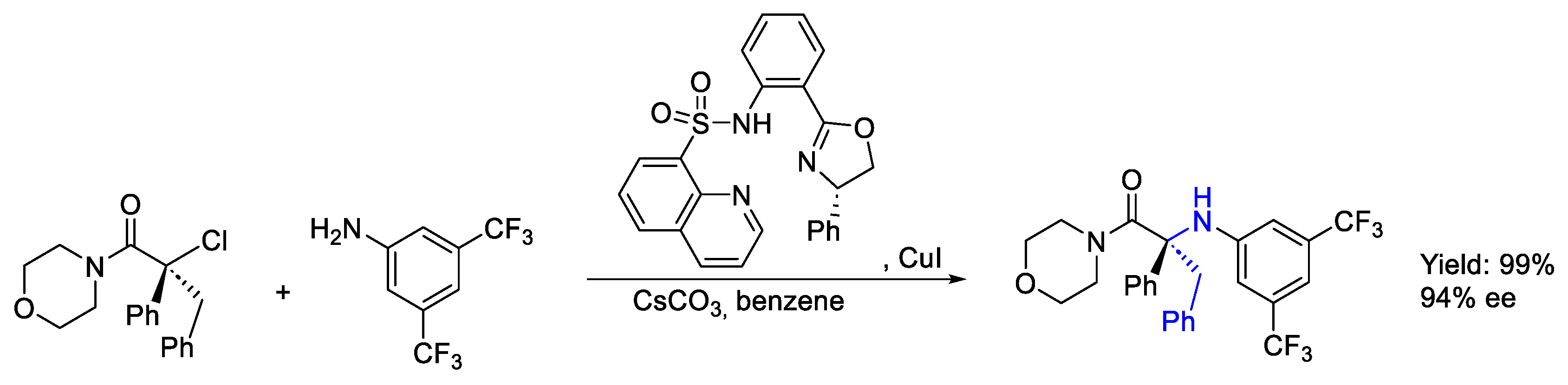
- Chen, J.J.; Zhang, J.Y.; Fang, J.H.; Du, X.Y.; Xia, H.D.; Cheng, B.; Li, N.; Yu, Z.L.; Bian, J.Q.; Wang, F.L.; et al. Copper-Catalyzed Enantioconvergent Radical C(Sp3)-N Cross-Coupling of Activated Racemic Alkyl Halides with (Hetero)Aromatic Amines under Ambient Conditions. J. Am. Chem. Soc. 2023, 145, 14686–14696. [Google Scholar] [CrossRef]
- Klake, R.K.; Sieber, J.D. Aminoalcohol Synthesis through Nonprecious Metal Catalysis: Enantioselective Cu-Catalyzed Reductive Coupling of Aldehydes and Allenamides. Org. Lett. 2023, 25, 4730–4734. [Google Scholar] [CrossRef] [PubMed]
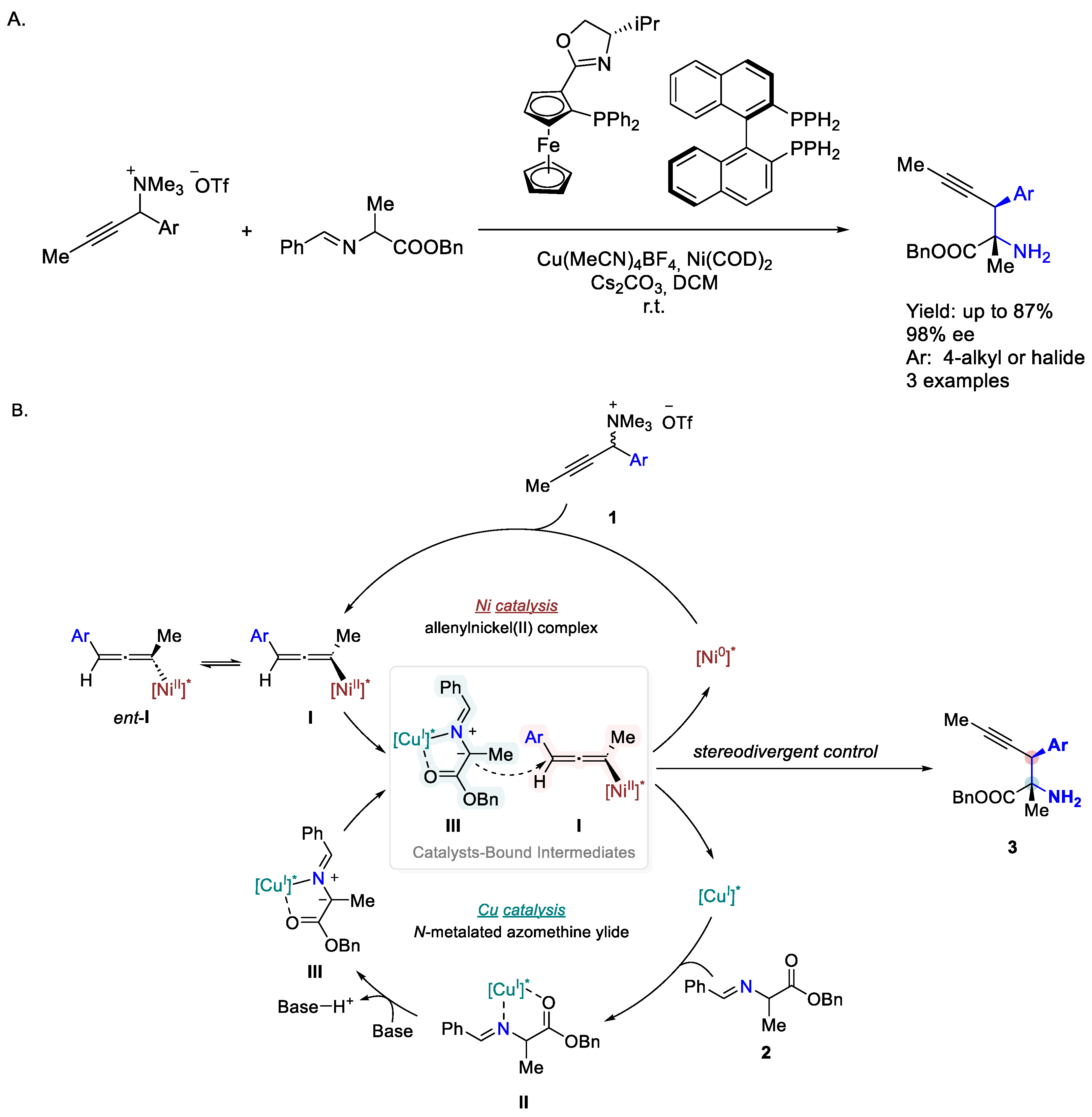
- Geng, R.; Peng, L.; Guo, C. Dynamic Kinetic Stereodivergent Transformations of Propargylic Ammonium Salts via Dual Nickel and Copper Catalysis. Chin. Chem. Lett. 2024, 35, 109433. [Google Scholar] [CrossRef]
- Zhu, J.; Rahim, F.; Zhou, P.; Zhang, A.; Malcolmson, S.J. Copper-Catalyzed Diastereo-, Enantio-, and (Z)-Selective Aminoallylation of Ketones through Reductive Couplings of Azatrienes for the Synthesis of Allylic 1,2-Amino Tertiary Alcohols. J. Am. Chem. Soc. 2024, 146, 20270–20278. [Google Scholar] [CrossRef]
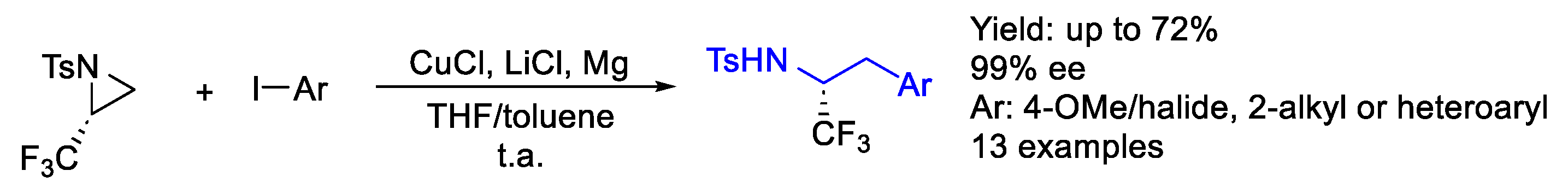
- Lee, M.; Sulwey, D.; Rotella, M.E.; Kim, W.S.; Ju, X.; Jiang, Q.; Kozlowski, M.C.; Lee, J. Mechanistic Insights of Copper Catalyzed Trifluoromethyl Aziridine Opening: Regioselective and Stereospecific Aryl Grignard Addition. Org. Lett. 2024, 26, 2713–2717. [Google Scholar] [CrossRef]
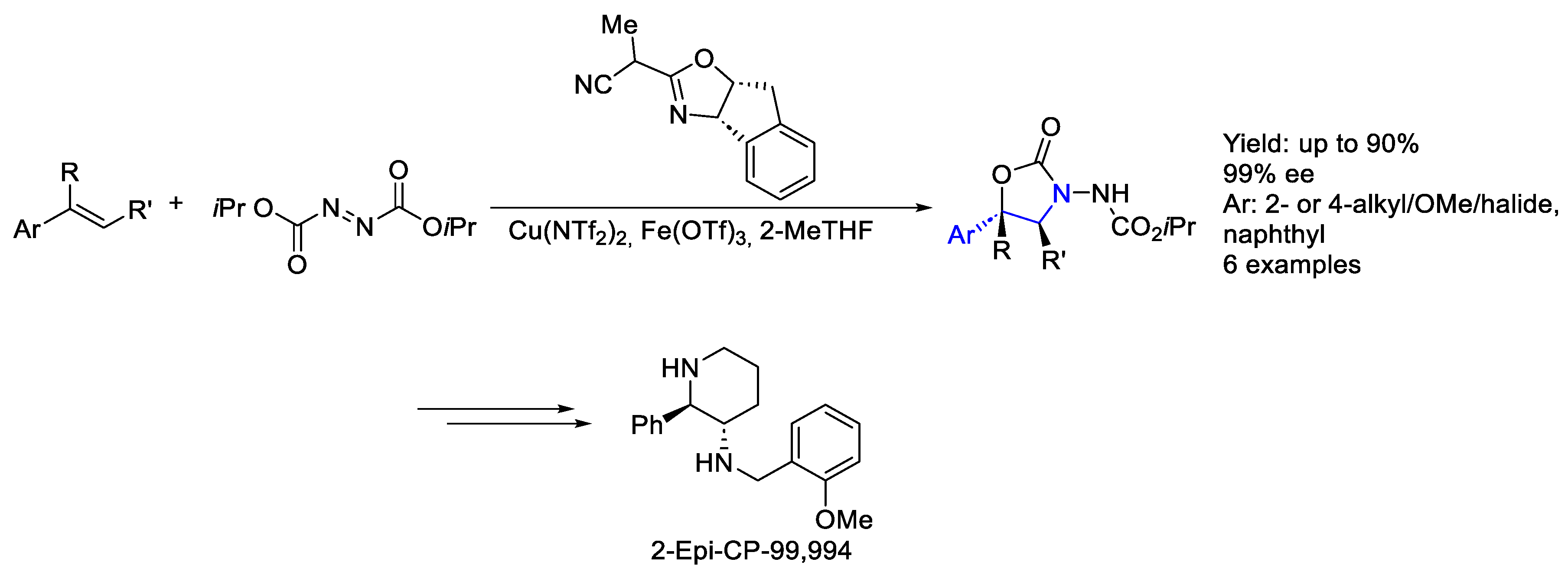
- Huang, N.; Luo, J.; Liao, L.; Zhao, X. Catalytic Enantioselective Aminative Difunctionalization of Alkenes. J. Am. Chem. Soc. 2024, 146, 7029–7038. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.J.; Campos, K.R.; Shultz, C.S.; Klapars, A.; Zewge, D.; Crump, B.R.; Phenix, B.D.; McWilliams, J.C.; Krska, S.; Sun, Y.; et al. New Efficient Asymmetric Synthesis of Taranabant, a CB1R Inverse Agonist for the Treatment of Obesity. Org. Process Res. Dev. 2009, 13, 84–90. [Google Scholar] [CrossRef]
- Valdez, S.C.; Leighton, J.L. Tandem Asymmetric Aza-Darzens/Ring-Opening Reactions: Dual Functionality from the Silane Lewis Acid. J. Am. Chem. Soc. 2009, 131, 14638–14639. [Google Scholar] [CrossRef]
- Praquin, C.F.B.; De Koning, P.D.; Peach, P.J.; Howard, R.M.; Spencer, S.L. Development of an Asymmetric Hydrogenation Route to (S)- N -Boc-2,6-Dimethyltyrosine. Org. Process Res. Dev. 2011, 15, 1124–1129. [Google Scholar] [CrossRef]
- Fox, M.E.; Jackson, M.; Meek, G.; Willets, M. Large-Scale Synthesis of a Substituted d -Phenylalanine Using Asymmetric Hydrogenation. Org. Process Res. Dev. 2011, 15, 1163–1171. [Google Scholar] [CrossRef]
- Anderson, N.A.; Fallon, B.J.; Valverde, E.; MacDonald, S.J.F.; Pritchard, J.M.; Suckling, C.J.; Watson, A.J.B. Asymmetric Rhodium-Catalysed Addition of Arylboronic Acids to Acyclic Unsaturated Esters Containing a Basic γ-Amino Group. Synlett 2012, 23, 2817–2821. [Google Scholar] [CrossRef]
- Appell, R.B.; Boulton, L.T.; Daugs, E.D.; Hansen, M.; Hanson, C.H.; Heinrich, J.; Kronig, C.; Lloyd, R.C.; Louks, D.; Nitz, M.; et al. The Large-Scale Synthesis of (S)-N-Boc-Bis(4-Fluorophenyl)Alanine. Org. Process Res. Dev. 2013, 17, 69–76. [Google Scholar] [CrossRef]
- Villa-Marcos, B.; Xiao, J. Metal and Organo-Catalysed Asymmetric Hydroaminomethylation of Styrenes. Cuihua Xuebao/Chin. J. Catal. 2015, 36, 106–112. [Google Scholar] [CrossRef]
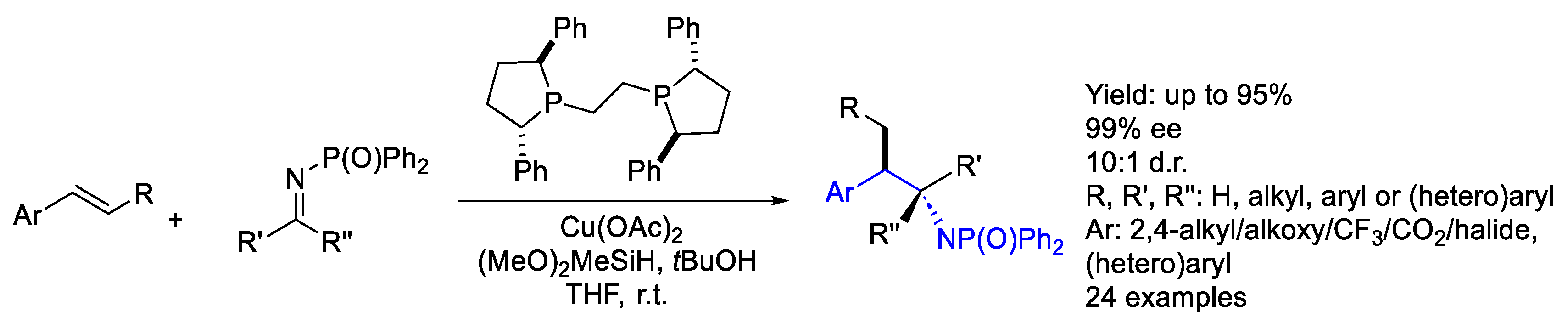
- Chen, C.; Jin, S.; Zhang, Z.; Wei, B.; Wang, H.; Zhang, K.; Lv, H.; Dong, X.Q.; Zhang, X. Rhodium/Yanphos-Catalyzed Asymmetric Interrupted Intramolecular Hydroaminomethylation of Trans-1,2-Disubstituted Alkenes. J. Am. Chem. Soc. 2016, 138, 9017–9020. [Google Scholar] [CrossRef]
- Lin, T.Y.; Wu, H.H.; Feng, J.J.; Zhang, J. Transfer of Chirality in the Rhodium-Catalyzed Chemoselective and Regioselective Allylic Alkylation of Hydroxyarenes with Vinyl Aziridines. Org. Lett. 2017, 19, 2897–2900. [Google Scholar] [CrossRef]
- Meng, J.; Li, X.H.; Han, Z.Y. Enantioselective Hydroaminomethylation of Olefins Enabled by Rh/Brønsted Acid Relay Catalysis. Org. Lett. 2017, 19, 1076–1079. [Google Scholar] [CrossRef]
- Lang, Q.; Gu, G.; Cheng, Y.; Yin, Q.; Zhang, X. Highly Enantioselective Synthesis of Chiral γ-Lactams by Rh-Catalyzed Asymmetric Hydrogenation. ACS Catal. 2018, 8, 4824–4828. [Google Scholar] [CrossRef]
- Bai, X.Y.; Zhang, W.W.; Li, Q.; Li, B.J. Highly Enantioselective Synthesis of Propargyl Amides through Rh-Catalyzed Asymmetric Hydroalkynylation of Enamides: Scope, Mechanism, and Origin of Selectivity. J. Am. Chem. Soc. 2018, 140, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Farr, C.M.B.; Kazerouni, A.M.; Park, B.; Poff, C.D.; Won, J.; Sharp, K.R.; Baik, M.H.; Blakey, S.B. Designing a Planar Chiral Rhodium Indenyl Catalyst for Regio- And Enantioselective Allylic C-H Amidation. J. Am. Chem. Soc. 2020, 142, 13996–14004. [Google Scholar] [CrossRef] [PubMed]
- Mi, R.; Zhang, X.; Wang, J.; Chen, H.; Lan, Y.; Wang, F.; Li, X. Rhodium-Catalyzed Regio-, Diastereo-, and Enantioselective Three-Component Carboamination of Dienes via C-H Activation. ACS Catal. 2021, 11, 6692–6697. [Google Scholar] [CrossRef]
- Wu, L.; Xu, H.; Gao, H.; Li, L.; Chen, W.; Zhou, Z.; Yi, W. Chiral Allylic Amine Synthesis Enabled by the Enantioselective CpXRh(III)-Catalyzed Carboaminations of 1,3-Dienes. ACS Catal. 2021, 11, 2279–2287. [Google Scholar] [CrossRef]
- Yang, X.; Hong, K.; Zhang, S.; Zhang, Z.; Zhou, S.; Huang, J.; Xu, X.; Hu, W. Asymmetric Three-Component Reaction of Two Diazo Compounds and Hyrdroxylamine Derivatives for the Access to Chiral α-Alkoxy-β-Amino-Carboxylates. ACS Catal. 2022, 12, 12302–12309. [Google Scholar] [CrossRef]
- Kliemann, M.N.; Teeuwen, S.; Weike, C.; Franciò, G.; Leitner, W. Rhodium-Catalyzed Asymmetric Hydrohydrazonemethylation of Styrenes: Access to Chiral Hydrazones, Hydrazides, Hydrazines and Amines. Adv. Synth. Catal. 2022, 364, 4006–4012. [Google Scholar] [CrossRef]
- Lamartina, C.W.; Chartier, C.A.; Lee, S.; Shah, N.H.; Rovis, T. Modular Synthesis of Unnatural Peptides via Rh(III)-Catalyzed Diastereoselective Three-Component Carboamidation Reaction. J. Am. Chem. Soc. 2023, 145, 1129–1135. [Google Scholar] [CrossRef]
- Lamartina, C.W.; Chartier, C.A.; Hirano, J.M.; Shah, N.H.; Rovis, T. Crafting Unnatural Peptide Macrocycles via Rh(III)-Catalyzed Carboamidation. J. Am. Chem. Soc. 2024, 146, 20868–20877. [Google Scholar] [CrossRef]
- Mi, R.; Ding, Z.; Yu, S.; Crabtree, R.H.; Li, X. O-Allylhydroxyamine: A Bifunctional Olefin for Construction of Axially and Centrally Chiral Amino Alcohols via Asymmetric Carboamidation. J. Am. Chem. Soc. 2023, 145, 8150–8162. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Z.; Ying, V.Y.; Seyedsayamdost, M.R. Synthesis of Non-canonical Tryptophan Variants via Rh-catalyzed C–H Functionalization of Anilines. Angew. Chem. Int. Ed. 2025, 64, e202414998. [Google Scholar] [CrossRef] [PubMed]
- Jiao, B.; Wang, F.; Lv, H. Rhodium-Catalyzed Asymmetric Hydrogenation of Tetrasubstituted α,β-Unsaturated Amides: Efficient Access to Chiral β-Amino Amides†. Chin. J. Chem. 2024, 42, 2641–2646. [Google Scholar] [CrossRef]
- Jia, X.; Hao, G.L.; Feng, M.; Jiang, H.; Wang, S.G.; Huang, L. Rh(III)-Catalyzed Diastereo- and Enantioselective Regiodivergent (Hetero)Arylamidation of (Homo)Allylic Sulfides. J. Am. Chem. Soc. 2024, 146, 9768–9778. [Google Scholar] [CrossRef]
- Goddard, C.M.L.; Massah, A.R.; Jackson, R.F.W. Improved Synthesis of Phenylethylamine Derivatives by Negishi Cross-Coupling Reactions. Tetrahedron 2010, 66, 9175–9181. [Google Scholar] [CrossRef]
- Trost, B.M.; Osipov, M.; Dong, G. Palladium-Catalyzed Dynamic Kinetic Asymmetric Transformations of Vinyl Aziridines with Nitrogen Heterocycles: Rapid Access to Biologically Active Pyrroles and Indoles. J. Am. Chem. Soc. 2010, 132, 15800–15807. [Google Scholar] [CrossRef]
- Mai, D.N.; Wolfe, J.P. Asymmetric Palladium-Catalyzed Carboamination Reactions for the Synthesis of Enantiomerically Enriched 2-(Arylmethyl)- and 2-(Alkenylmethyl)Pyrrolidines. J. Am. Chem. Soc. 2010, 132, 12157–12159. [Google Scholar] [CrossRef]
- White, D.R.; Hutt, J.T.; Wolfe, J.P. Asymmetric Pd-Catalyzed Alkene Carboamination Reactions for the Synthesis of 2-Aminoindane Derivatives. J. Am. Chem. Soc. 2015, 137, 11246–11249. [Google Scholar] [CrossRef]
- Duda, M.L.; Michael, F.E. Palladium-Catalyzed Cross-Coupling of n -Sulfonylaziridines with Boronic Acids. J. Am. Chem. Soc. 2013, 135, 18347–18349. [Google Scholar] [CrossRef]
- Takeda, Y.; Ikeda, Y.; Kuroda, A.; Tanaka, S.; Minakata, S. Pd/NHC-Catalyzed Enantiospecific and Regioselective Suzuki-Miyaura Arylation of 2-Arylaziridines: Synthesis of Enantioenriched 2-Arylphenethylamine Derivatives. J. Am. Chem. Soc. 2014, 136, 8544–8547. [Google Scholar] [CrossRef]
- Takeda, Y.; Kuroda, A.; Sameera, W.M.C.; Morokuma, K.; Minakata, S. Palladium-Catalyzed Regioselective and Stereo-Invertive Ring-Opening Borylation of 2-Arylaziridines with Bis(Pinacolato)Diboron: Experimental and Computational Studies. Chem. Sci. 2016, 7, 6141–6152. [Google Scholar] [CrossRef]
- Takeda, Y.; Shibuta, K.; Aoki, S.; Tohnai, N.; Minakata, S. Catalyst-Controlled Regiodivergent Ring-Opening C(Sp3)-Si Bond-Forming Reactions of 2-Arylaziridines with Silylborane Enabled by Synergistic Palladium/Copper Dual Catalysis. Chem. Sci. 2019, 10, 8642–8647. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Matsuno, T.; Sharma, A.K.; Sameera, W.M.C.; Minakata, S. Asymmetric Synthesis of Β2-Aryl Amino Acids through Pd-Catalyzed Enantiospecific and Regioselective Ring-Opening Suzuki–Miyaura Arylation of Aziridine-2-Carboxylates. Chem.-A Eur. J. 2019, 25, 10226–10231. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.L.; Fu, H.Y.; Yu, J.Q. Palladium(II)-Catalyzed Highly Enantioselective C-H Arylation of Cyclopropylmethylamines. J. Am. Chem. Soc. 2015, 137, 2042–2046. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhu, Y.; Fan, W.; Chen, Y.; Xu, X.; Zhang, J.; Zhao, Y. Late-Stage Functionalization of Aromatic Acids with Aliphatic Aziridines: Direct Approach to Form β-Branched Arylethylamine Backbones. ACS Catal. 2019, 9, 6738–6743. [Google Scholar] [CrossRef]
- Ahmad, A.; Dutta, H.S.; Kumar, M.; Raziullah; Gangwar, M.K.; Koley, D. Directing Group Guided Site-Selective Diversification of Indoles by Aziridine: Synthesis of β-Indolylethylamines. Org. Lett. 2022, 24, 2783–2787. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhou, Q.; Lin, Y.; Wu, Z.L.; Cai, T.; Wen, W.; Huang, Y.M.; Guo, Q.X. Modular Chiral-Aldehyde/Palladium Catalysis Enables Atom-Economical α-Allylation of N-Unprotected Amino Acid Esters with 1,3-Dienes and Allenes. ACS Catal. 2023, 13, 6013–6022. [Google Scholar] [CrossRef]
- Wang, C.; Xi, Y.; Xia, T.; Qu, J.; Chen, Y. Pd(0)-Catalyzed Diastereoselective and Enantioselective Intermolecular Heck-Miyaura Borylation of Internal Enamides for the β-Aminoboronate Ester Synthesis. ACS Catal. 2024, 14, 418–425. [Google Scholar] [CrossRef]
- Hansen, K.B.; Hsiao, Y.; Xu, F.; Rivera, N.; Clausen, A.; Kubryk, M.; Krska, S.; Rosner, T.; Simmons, B.; Balsells, J.; et al. Highly Efficient Asymmetric Synthesis of Sitagliptin. J. Am. Chem. Soc. 2009, 131, 8798–8804. [Google Scholar] [CrossRef]
- Steinhuebel, D.; Sun, Y.; Matsumura, K.; Sayo, N.; Saito, T. Direct Asymmetric Reductive Amination. J. Am. Chem. Soc. 2009, 131, 11316–11317. [Google Scholar] [CrossRef]
- Steinhuebel, D.P.; Krska, S.W.; Alorati, A.; Baxter, J.M.; Belyk, K.; Bishop, B.; Palucki, M.; Sun, Y.; Davies, I.W. Asymmetric Hydrogenation of Protected Allylic Amines. Org. Lett. 2010, 12, 4201–4203. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Yokoyama, H.; Endo, K.; Shibata, T. Ru-Catalyzed β-Selective and Enantioselective Addition of Amines to Styrenes Initiated by Direct Arene-Exchange. Org. Biomol. Chem. 2012, 10, 3815–3818. [Google Scholar] [CrossRef]
- Komiyama, M.; Itoh, T.; Takeyasu, T. Scalable Ruthenium-Catalyzed Asymmetric Synthesis of a Key Intermediate for the Β2-Adrenergic Receptor Agonist. Org. Process Res. Dev. 2015, 19, 315–319. [Google Scholar] [CrossRef]
- Wysocki, J.; Schlepphorst, C.; Glorius, F. Asymmetric Homogeneous Hydrogenation of 2-Pyridones. Synlett 2015, 26, 1557–1562. [Google Scholar] [CrossRef]
- Beliaev, A. Development of the Asymmetric Hydrogenation Step for Multikilogram Production of Etamicastat. Org. Process Res. Dev. 2016, 20, 724–732. [Google Scholar] [CrossRef]
- Gallardo-Donaire, J.; Hermsen, M.; Wysocki, J.; Ernst, M.; Rominger, F.; Trapp, O.; Hashmi, A.S.K.; Schäfer, A.; Comba, P.; Schaub, T. Direct Asymmetric Ruthenium-Catalyzed Reductive Amination of Alkyl-Aryl Ketones with Ammonia and Hydrogen. J. Am. Chem. Soc. 2018, 140, 355–361. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Zhang, Q.; Yin, Q.; Zhang, X. Ruthenium-Catalyzed Direct Asymmetric Reductive Amination of Diaryl and Sterically Hindered Ketones with Ammonium Salts and H2. Angew. Chem. 2020, 132, 5359–5363. [Google Scholar] [CrossRef]
- Brandes, D.S.; Sirvent, A.; Mercado, B.Q.; Ellman, J.A. Three-Component 1,2-Carboamidation of Bridged Bicyclic Alkenes via RhIII-Catalyzed Addition of C-H Bonds and Amidating Reagents. Org. Lett. 2021, 23, 2836–2840. [Google Scholar] [CrossRef]
- Hu, L.; Wang, Y.Z.; Xu, L.; Yin, Q.; Zhang, X. Highly Enantioselective Synthesis of N-Unprotected Unnatural α-Amino Acid Derivatives by Ruthenium-Catalyzed Direct Asymmetric Reductive Amination. Angew. Chem.-Int. Ed. 2022, 61, e202202552. [Google Scholar] [CrossRef]
- Nie, X.; Ritter, C.W.; Hemming, M.; Ivlev, S.I.; Xie, X.; Chen, S.; Meggers, E. Nitrene-Mediated Enantioselective Intramolecular Olefin Oxyamination to Access Chiral γ-Aminomethyl-γ-Lactones. Angew. Chem.-Int. Ed. 2023, 62, e202314398. [Google Scholar] [CrossRef]
- Yi, Z.Q.; Deng, B.W.; Chen, F.; He, Y.M.; Fan, Q.H. Asymmetric Hydrogenation of Dibenzo-Fused Azepines with Chiral Cationic Ruthenium Diamine Catalysts. New J. Chem. 2023, 47, 11492–11497. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Liu, S.D.; Cheng, L.; Liu, L.; Li, C.J. Asymmetric Addition of Hydrazones as Alkyl Carbanion Equivalents with Aryl Imines in Water. Org. Chem. Front. 2023, 10, 3021–3026. [Google Scholar] [CrossRef]
- Ding, Z.; Luo, Y.; Yuan, Q.; Wang, G.; Yu, Z.; Zhao, M.; Liu, D.; Zhang, W. Ru-Catalyzed Asymmetric Hydrogenation of α,β-Unsaturated γ-Lactams. J. Am. Chem. Soc. 2024, 146, 25312–25320. [Google Scholar] [CrossRef]
- Huang, C.Y.; Doyle, A.G. Nickel-Catalyzed Negishi Alkylations of Styrenyl Aziridines. J. Am. Chem. Soc. 2012, 134, 9541–9544. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Doyle, A.G. Electron-Deficient Olefin Ligands Enable Generation of Quaternary Carbons by Ni-Catalyzed Cross-Coupling. J. Am. Chem. Soc. 2015, 137, 5638–5641. [Google Scholar] [CrossRef]
- Woods, B.P.; Orlandi, M.; Huang, C.Y.; Sigman, M.S.; Doyle, A.G. Nickel-Catalyzed Enantioselective Reductive Cross-Coupling of Styrenyl Aziridines. J. Am. Chem. Soc. 2017, 139, 5688–5691. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xue, Y.; Han, B.; Zhang, C.; Wang, Y.; Zhu, S. Nickel-Catalyzed Asymmetric Reductive 1,2-Carboamination of Unactivated Alkenes. Angew. Chem. 2020, 132, 2348–2352. [Google Scholar] [CrossRef]
- Kang, T.; Kim, N.; Cheng, P.T.; Zhang, H.; Foo, K.; Engle, K.M. Nickel-Catalyzed 1,2-Carboamination of Alkenyl Alcohols. J. Am. Chem. Soc. 2021, 143, 13962–13970. [Google Scholar] [CrossRef]
- Hu, X.; Cheng-Sánchez, I.; Cuesta-Galisteo, S.; Nevado, C. Nickel-Catalyzed Enantioselective Electrochemical Reductive Cross-Coupling of Aryl Aziridines with Alkenyl Bromides. J. Am. Chem. Soc. 2023, 145, 6270–6279. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Wang, Z.H.; Eshel, I.L.; Sun, B.; Liu, D.; Gu, Y.C.; Milo, A.; Mei, T.S. Nickel/Biimidazole-Catalyzed Electrochemical Enantioselective Reductive Cross-Coupling of Aryl Aziridines with Aryl Iodides. Nat. Commun. 2023, 14, 2322. [Google Scholar] [CrossRef]
- Lyu, X.; Seo, C.; Faber, T.; Kim, D.; Seo, S.; Chang, S. Intramolecular Hydroamidation of Alkenes Enabling Asymmetric Synthesis of β-Lactams via Unprecedented Mechanism of NiH Catalysis. Nat. Cat. 2023, 6, 784–795. [Google Scholar] [CrossRef]
- Wang, C.Y.; Huang, Y.L.; Xu, W.C.; Gao, Q.; Liu, P.; Bi, Y.X.; Liu, G.K.; Wang, X.S. Nickel-Catalyzed Asymmetric Decarboxyarylation with NHP Esters of α-Amino Acid to Chiral Benzylamines. Org. Lett. 2023, 25, 6964–6968. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Biswas, P.; O’Bannon, B.C.; Powers, D.C. β-Phenethylamine Synthesis: N-Pyridinium Aziridines as Latent Dual Electrophiles. Angew. Chem.-Int. Ed. 2024, 63, e202406335. [Google Scholar] [CrossRef]
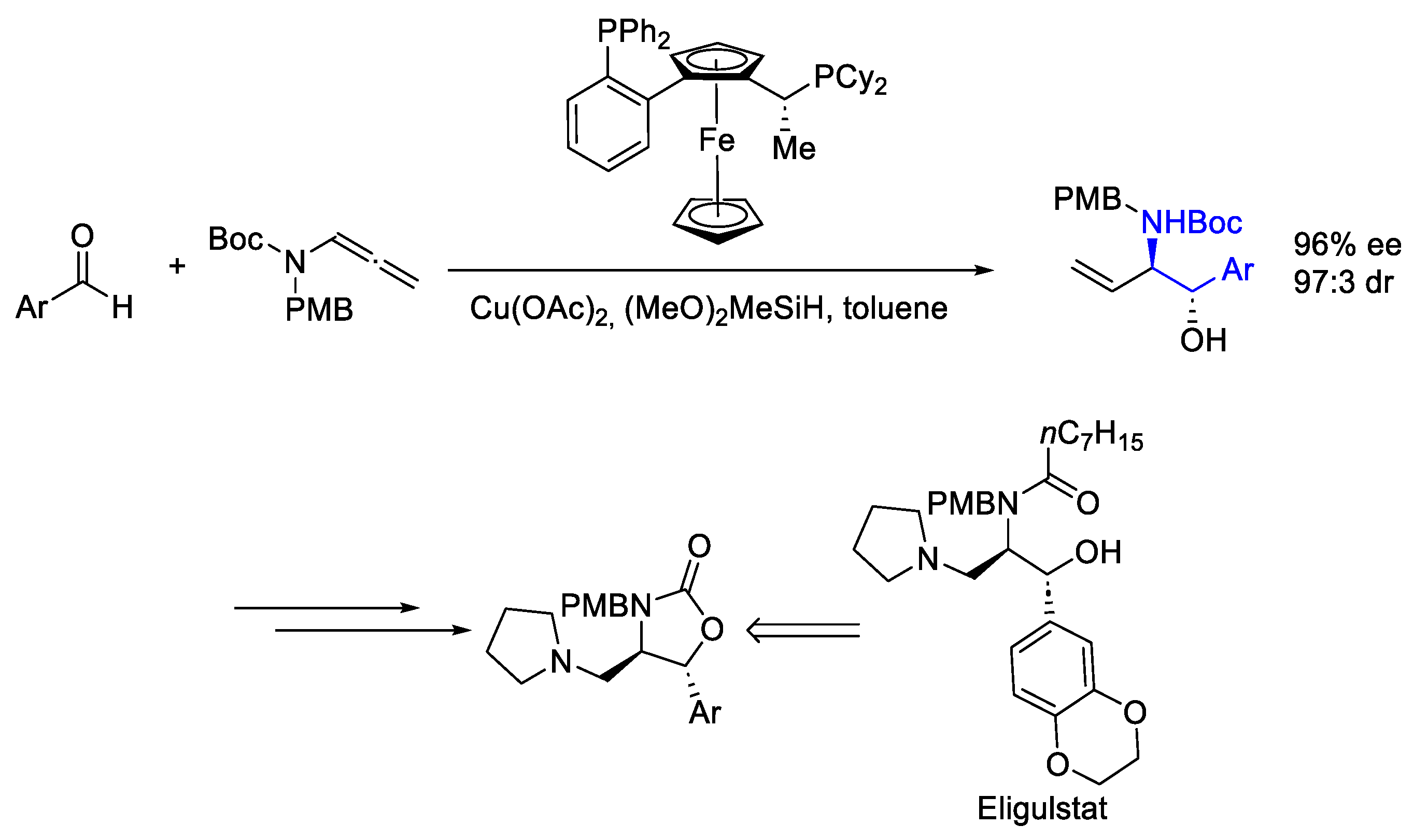
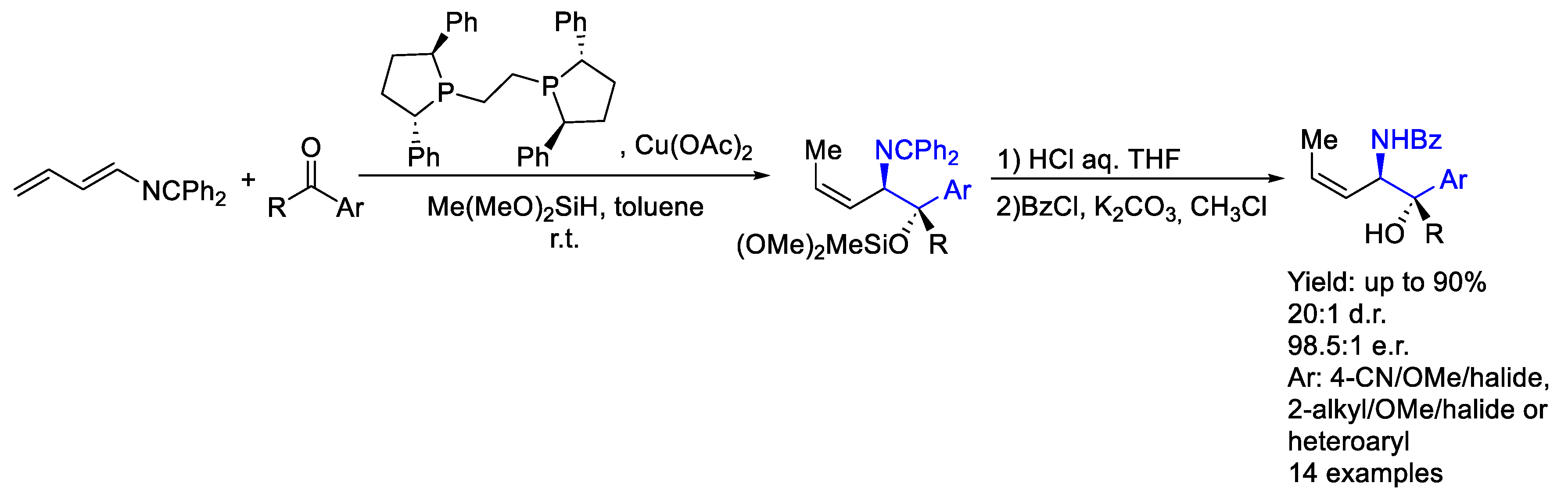
- Bender, T.; Fürstner, A. Enantioselective Synthesis of Vic-Aminoalcohol Derivatives by Nickel-Catalyzed Reductive Coupling of Aldehydes with Protected Amino-Pentadienoates. J. Am. Chem. Soc. 2024, 146, 33295–33301. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Han, Q.; Liao, P.; Chen, R.; Fan, F.; Zhao, X.; Liu, W. Nickel-Catalyzed Enantioselective C(Sp3)-C(Sp3) Cross-Electrophile Coupling of N-Sulfonyl Styrenyl Aziridines with Alkyl Bromides. J. Am. Chem. Soc. 2024, 146, 25426–25432. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Jeon, E.; Seo, C.; Kim, D.; Chang, S. Nickel-Catalyzed Asymmetric Homobenzylic Hydroamidation of Aryl Alkenes to Access Chiral β-Arylamides. J. Am. Chem. Soc. 2025, 147, 8928–8938. [Google Scholar] [CrossRef]
- Gao, P.; Foster, D.; Sipos, G.; Skelton, B.W.; Sobolev, A.N.; Dorta, R. Chiral NHC-Iridium Complexes and Their Performance in Enantioselective Intramolecular Hydroamination and Ring-Opening Amination Reactions. Organometallics 2020, 39, 556–573. [Google Scholar] [CrossRef]
- Jouffroy, M.; Nguyen, T.M.; Cordier, M.; Blot, M.; Roisnel, T.; Gramage-Doria, R. Iridium-Catalyzed Direct Reductive Amination of Ketones and Secondary Amines: Breaking the Aliphatic Wall. Chemistry 2022, 28, e202201078. [Google Scholar] [CrossRef]
- Ma, S.; Xi, Y.; Fan, H.; Roediger, S.; Hartwig, J.F. Enantioselective Hydroamination of Unactivated Terminal Alkenes. Chem 2022, 8, 532–542. [Google Scholar] [CrossRef]
- Zhao, W.; Li, B.J. Directing Group Repositioning Strategy Enabled Site- and Enantioselective Addition of Heteroaromatic C-H Bonds to Acyclic Internal Alkenes. J. Am. Chem. Soc. 2023, 145, 6861–6870. [Google Scholar] [CrossRef]
- Yajima, T.; Katayama, A.; Ito, T.; Kawada, T.; Yabushita, K.; Yasuda, T.; Ohta, T.; Katayama, T.; Utsumi, N.; Kayaki, Y.; et al. Asymmetric Reductive Amination of α-Keto Acids Using Ir-Based Hydrogen Transfer Catalysts: An Access to Unprotected Unnatural α-Amino Acids. Org. Lett. 2024, 26, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Robinson, D.; Li, B.; Liu, S.-Y. Synthesis of Chiral δ-Aminoboronic Esters by Enantioselec- Tive Hydrogenation of 1,2-Azaborines. Chem. Rxiv 2024. [Google Scholar] [CrossRef]
- Chen, M.H.; Hsieh, J.C.; Lee, Y.H.; Cheng, C.H. Controlled Synthesis of Enantioselective 1-Aminoindenes via Cobalt-Catalyzed [3 + 2] Annulation Reaction. ACS Catal. 2018, 8, 9364–9369. [Google Scholar] [CrossRef]
- Wang, C.; Ge, S. Versatile Cobalt-Catalyzed Enantioselective Entry to Boryl-Functionalized All-Carbon Quaternary Stereogenic Centers. J. Am. Chem. Soc. 2018, 140, 10687–10690. [Google Scholar] [CrossRef] [PubMed]
- Ozols, K.; Onodera, S.; Woźniak, Ł.; Cramer, N. Cobalt(III)-Catalyzed Enantioselective Intermolecular Carboamination by C–H Functionalization. Angew. Chem.-Int. Ed. 2021, 60, 655–659. [Google Scholar] [CrossRef]
- Li, A.; Song, X.; Ren, Q.; Bao, P.; Long, X.; Huang, F.; Yuan, L.; Zhou, J.S.; Qin, X. Cobalt-Catalyzed Asymmetric Deuteration of α-Amidoacrylates for Stereoselective Synthesis of α,β-Dideuterated α-Amino Acids. Angew. Chem.-Int. Ed. 2023, 62, e202301091. [Google Scholar] [CrossRef]
- Yang, H.; Hu, Y.; Zou, Y.; Zhang, Z.; Zhang, W. Cobalt-Catalyzed Efficient Asymmetric Hydrogenation of α-Primary Amino Ketones. JACS Au 2023, 3, 2981–2986. [Google Scholar] [CrossRef]
- Mendelsohn, L.N.; MacNeil, C.S.; Esposito, M.R.; Pabst, T.P.; Leahy, D.K.; Davies, I.W.; Chirik, P.J. Asymmetric Hydrogenation of Indazole-Containing Enamides Relevant to the Synthesis of Zavegepant Using Neutral and Cationic Cobalt Precatalysts. Org. Lett. 2024, 26, 2718–2723. [Google Scholar] [CrossRef]
- Cui, T.; Ye, C.X.; Thelemann, J.; Jenisch, D.; Meggers, E. Enantioselective and Enantioconvergent Iron-Catalyzed C(Sp3)-H Aminations to Chiral 2-Imidazolidinones. Chin. J. Chem. 2023, 41, 2065–2070. [Google Scholar] [CrossRef]
- Holst, H.M.; Floreancig, J.T.; Ritts, C.B.; Race, N.J. Aziridine Opening via a Phenonium Ion Enables Synthesis of Complex Phenethylamine Derivatives. Org. Lett. 2022, 24, 501–505. [Google Scholar] [CrossRef]
- Ghorai, M.K.; Tiwari, D.P.; Jain, N. Lewis Acid Catalyzed SN2-Type Ring Opening of N -Activated Aziridines with Electron-Rich Arenes/Heteroarenes. J. Org. Chem. 2013, 78, 7121–7130. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Hung, C.I.J.; Gnanamani, E. Tuning the Reactivity of Ketones through Unsaturation: Construction of Cyclic and Acyclic Quaternary Stereocenters via Zn-ProPhenol Catalyzed Mannich Reactions. ACS Catal. 2019, 9, 1549–1557. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Amani, A.M. Photocatalytic Systems: Reactions, Mechanism, and Applications. RSC Adv. 2024, 14, 20609–20645. [Google Scholar] [CrossRef]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Photokatalyse Mit Sichtbarem Licht: Welche Bedeutung Hat Sie Für Die Organische Synthese? Angew. Chem. 2018, 130, 10188–10228. [Google Scholar] [CrossRef]
- König, B. Photocatalysis in Organic Synthesis—Past, Present, and Future. Eur. J. Org. Chem. 2017, 2017, 1979–1981. [Google Scholar] [CrossRef]
- David, A.N.; David, W.C. MacMillan Merging Photoredox Catalysis with Organocatalysis: The Direct Alkylation of Aldehydes. Science (1979) 2008, 322, 77–80. [Google Scholar] [CrossRef]
- Pitre, S.P.; Overman, L.E. Strategic Use of Visible-Light Photoredox Catalysis in Natural Product Synthesis. Chem. Rev. 2022, 122, 1717–1751. [Google Scholar] [CrossRef]
- Großkopf, J.; Kratz, T.; Rigotti, T.; Bach, T. Enantioselective Photochemical Reactions Enabled by Triplet Energy Transfer. Chem. Rev. 2022, 122, 1626–1653. [Google Scholar] [CrossRef] [PubMed]
- Weinstain, R.; Slanina, T.; Kand, D.; Klán, P. Visible-to-NIR-Light Activated Release: From Small Molecules to Nanomaterials. Chem. Rev. 2020, 120, 13135–13272. [Google Scholar] [CrossRef]
- Proctor, R.S.J.; Davis, H.J.; Phipps, R.J. Catalytic Enantioselective Minisci-Type Addition to Heteroarenes. Science 2018, 360, 419–422. [Google Scholar] [CrossRef]
- Monos, T.M.; Mcatee, R.C.; Stephenson, C.R.J. Arylsulfonylacetamides as Bifunctional Reagents for Alkene Aminoarylation. Science 2018, 361, 1369–1373. [Google Scholar] [CrossRef]
- Zheng, S.; Gutiérrez-Bonet, Á.; Molander, G.A. Merging Photoredox PCET with Ni-Catalyzed Cross-Coupling: Cascade Amidoarylation of Unactivated Olefins. Chem 2019, 5, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Whalley, D.M.; Duong, H.A.; Greaney, M.F. A Visible Light-Mediated, Decarboxylative, Desulfonylative Smiles Rearrangement for General Arylethylamine Syntheses. Chem. Commun. 2020, 56, 11493–11496. [Google Scholar] [CrossRef]
- Noten, E.A.; McAtee, R.C.; Stephenson, C.R.J. Catalytic Intramolecular Aminoarylation of Unactivated Alkenes with Aryl Sulfonamides. Chem. Sci. 2022, 13, 6942–6949. [Google Scholar] [CrossRef] [PubMed]
- Noten, E.A.; Ng, C.H.; Wolesensky, R.M.; Stephenson, C.R.J. A General Alkene Aminoarylation Enabled by N-Centered Radical Reactivity of Sulfinamides. Nat. Chem. 2024, 16, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Guo, L.; Gao, H.; Luo, M.; Yang, C.; Xia, W. Highly Diastereoselective Synthesis of γ-Lactams Enabled by Photoinduced Deaminative [3 + 2] Annulation Reaction. Org. Lett. 2022, 24, 4365–4370. [Google Scholar] [CrossRef]
- Hervieu, C.; Kirillova, M.S.; Hu, Y.; Cuesta-Galisteo, S.; Merino, E.; Nevado, C. Chiral Arylsulfinylamides as Reagents for Visible Light-Mediated Asymmetric Alkene Aminoarylations. Nat. Chem. 2024, 16, 607–614. [Google Scholar] [CrossRef]
- Hu, H.; Shi, Z.; Guo, X.; Zhang, F.H.; Wang, Z. A Radical Approach for Asymmetric α-C-H Addition of N-Sulfonyl Benzylamines to Aldehydes. J. Am. Chem. Soc. 2024, 146, 5316–5323. [Google Scholar] [CrossRef]
- Sun, H.; Yang, C.; Lin, R.; Xia, W. Regioselective Ring-Opening Nucleophilic Addition of Aziridines through Photoredox Catalyst. Adv. Synth. Catal. 2014, 356, 2775–2780. [Google Scholar] [CrossRef]
- Tu, J.L.; Tang, W.; Liu, F.; Liu, F. Photoredox-Neutral Alkene Aminoarylation for the Synthesis of 1,4,5,6-Tetrahydropyridazines. Org. Chem. Front. 2021, 8, 3712–3717. [Google Scholar] [CrossRef]
- Shu, X.; Huan, L.; Huang, Q.; Huo, H. Direct Enantioselective C(Sp3)-H Acylation for the Synthesis of α-Amino Ketones. J. Am. Chem. Soc. 2020, 142, 19058–19064. [Google Scholar] [CrossRef] [PubMed]
- Steiman, T.J.; Liu, J.; Mengiste, A.; Doyle, A.G. Synthesis of β-Phenethylamines via Ni/Photoredox Cross-Electrophile Coupling of Aliphatic Aziridines and Aryl Iodides. J. Am. Chem. Soc. 2020, 142, 7598–7605. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yu, X.; Daniliuc, C.G.; Studer, A. Three-Component Aminoarylation of Electron-Rich Alkenes by Merging Photoredox with Nickel Catalysis. Angew. Chem.-Int. Ed. 2021, 60, 14399–14404. [Google Scholar] [CrossRef]
- Qian, S.; Lazarus, T.M.; Nicewicz, D.A. Enantioselective Amino- and Oxycyanation of Alkenes via Organic Photoredox and Copper Catalysis. J. Am. Chem. Soc. 2023, 145, 18247–18252. [Google Scholar] [CrossRef]
- Yang, F.; Chi, L.; Ye, Z.; Gong, L. Photoinduced Regiodivergent and Enantioselective Cross-Coupling of Glycine Derivatives with Hydrocarbon Feedstocks. J. Am. Chem. Soc. 2025, 147, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Milton, J.P.; Gryko, D.; Neuville, L.; Masson, G. TBADT-Mediated Photocatalytic Stereoselective Radical Alkylation of Chiral N-Sulfinyl Imines: Towards Efficient Synthesis of Diverse Chiral Amines. Chem.-A Eur. J. 2024, 30, e202400363. [Google Scholar] [CrossRef]
- Hore, S.; Jeong, J.; Kim, D.; Chang, S. Visible-Light-Promoted Enantioselective α-Amidation of Aldehydes by Harnessing Organo-Iron Dual Catalysis. J. Am. Chem. Soc. 2024, 146, 22172–22179. [Google Scholar] [CrossRef]


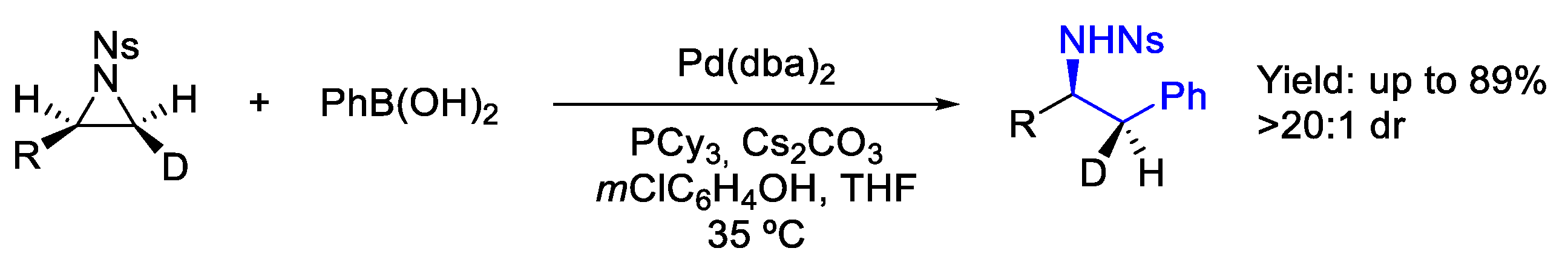
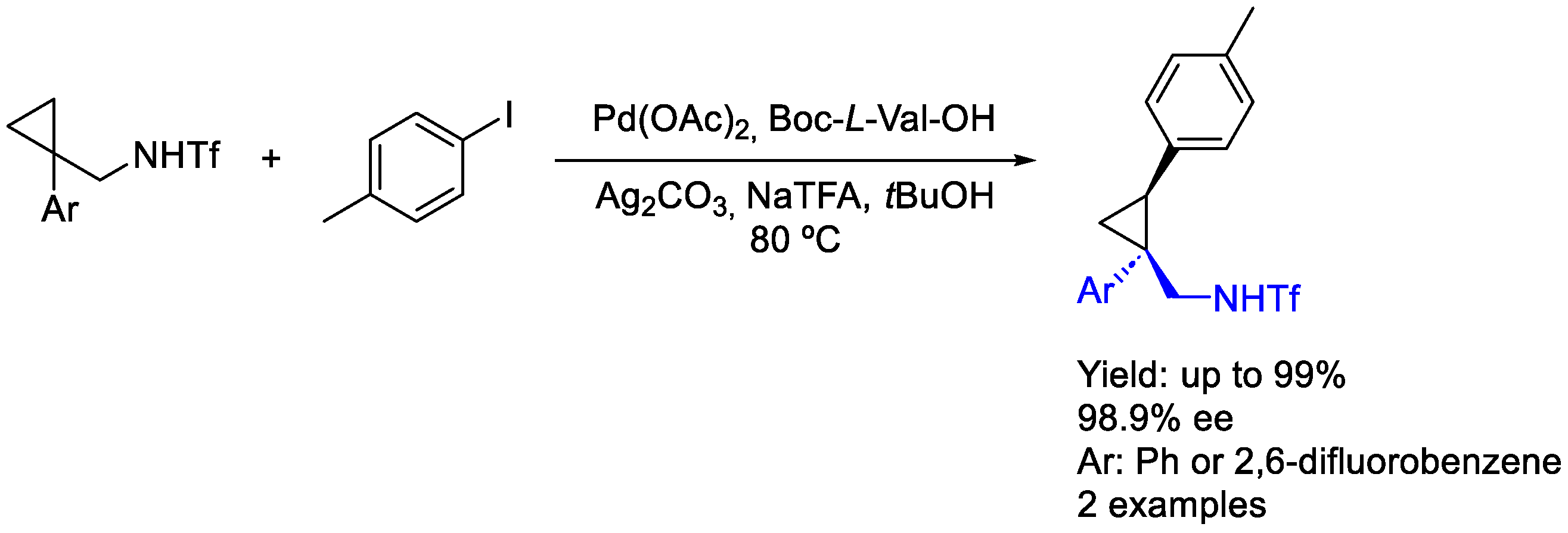
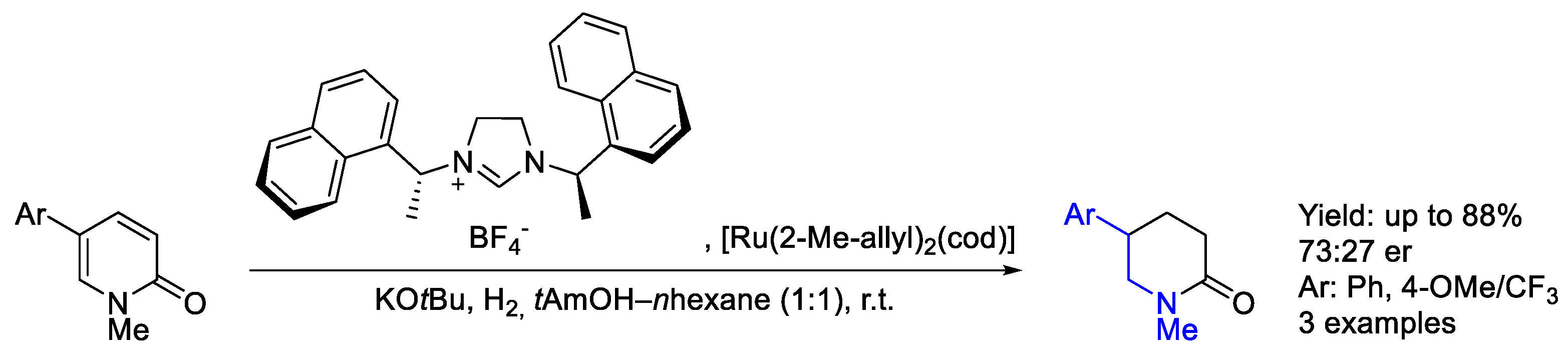
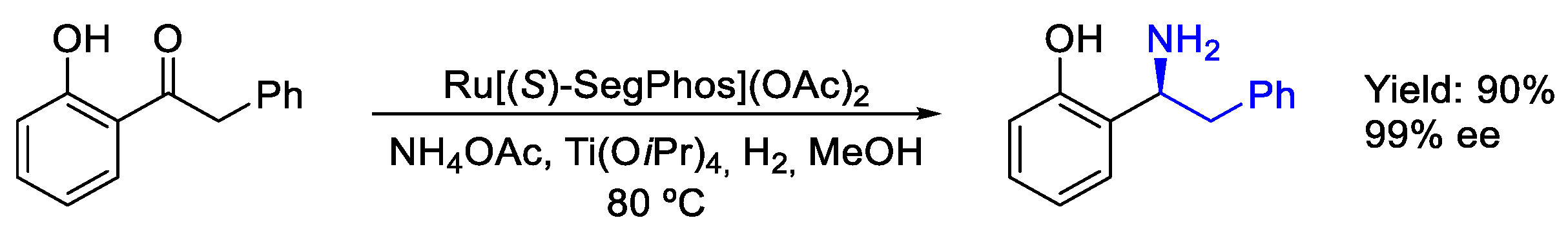


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manchado, A.; García-González, Á.; Nieto, C.T.; Ledesma, N.G.; Díez, D.; Garrido, N.M. Transition Metal Catalysis for the Asymmetric Synthesis of 2-Arylethylamines: A Review of the New Millennium. Molecules 2025, 30, 1721. https://doi.org/10.3390/molecules30081721
Manchado A, García-González Á, Nieto CT, Ledesma NG, Díez D, Garrido NM. Transition Metal Catalysis for the Asymmetric Synthesis of 2-Arylethylamines: A Review of the New Millennium. Molecules. 2025; 30(8):1721. https://doi.org/10.3390/molecules30081721
Chicago/Turabian StyleManchado, Alejandro, Ángel García-González, Carlos T. Nieto, Nieves G. Ledesma, David Díez, and Narciso M. Garrido. 2025. "Transition Metal Catalysis for the Asymmetric Synthesis of 2-Arylethylamines: A Review of the New Millennium" Molecules 30, no. 8: 1721. https://doi.org/10.3390/molecules30081721
APA StyleManchado, A., García-González, Á., Nieto, C. T., Ledesma, N. G., Díez, D., & Garrido, N. M. (2025). Transition Metal Catalysis for the Asymmetric Synthesis of 2-Arylethylamines: A Review of the New Millennium. Molecules, 30(8), 1721. https://doi.org/10.3390/molecules30081721






