Synthesis of the Pentasaccharide Unit of the Pseudomonas aeruginosa Exopolysaccharide Psl Conjugation with CRM197, and Evaluation of Antigenicity in a QS-21/Pam3CSK4-Liposomal Formulation
Abstract
1. Introduction
2. Results and Discussion
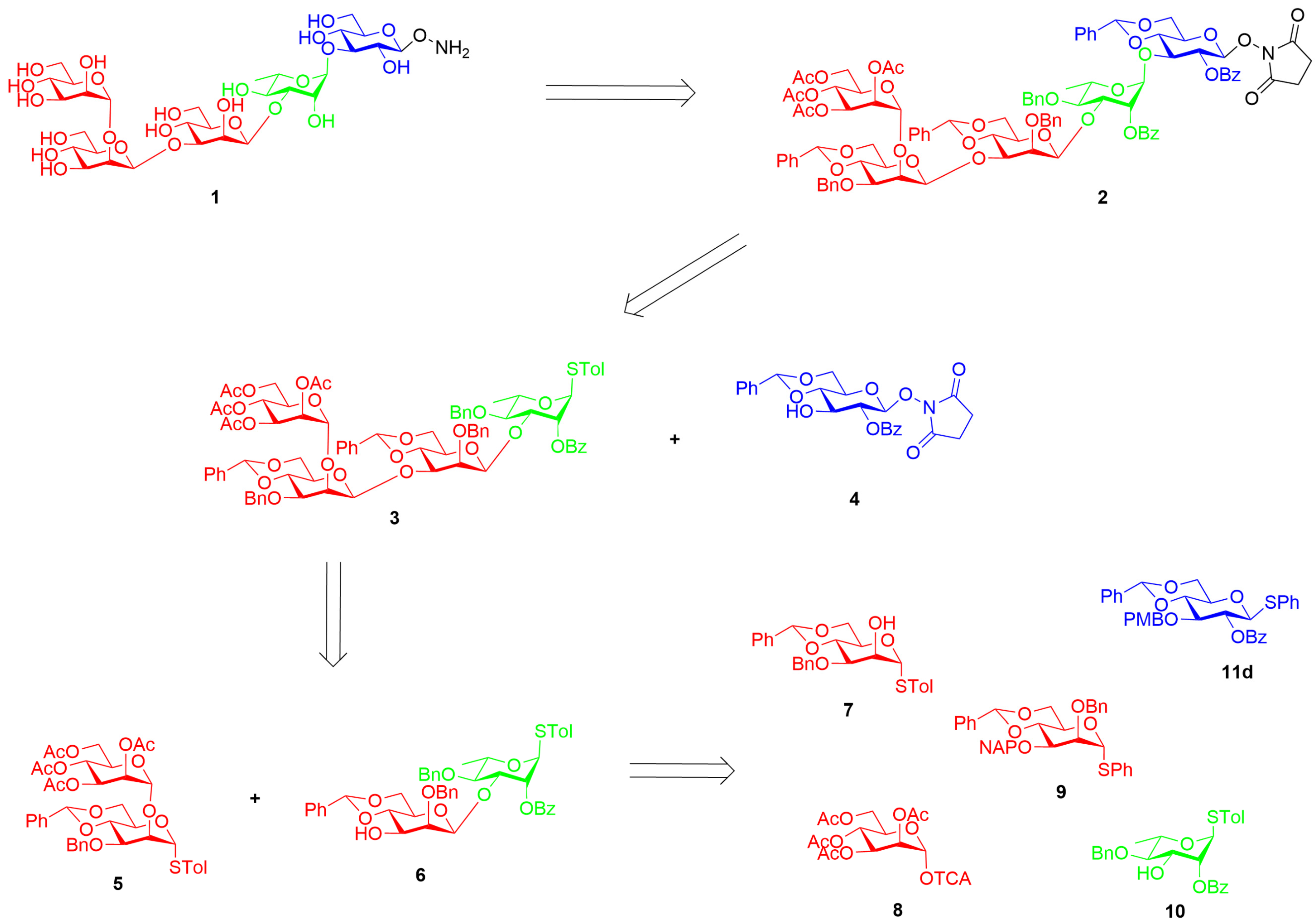
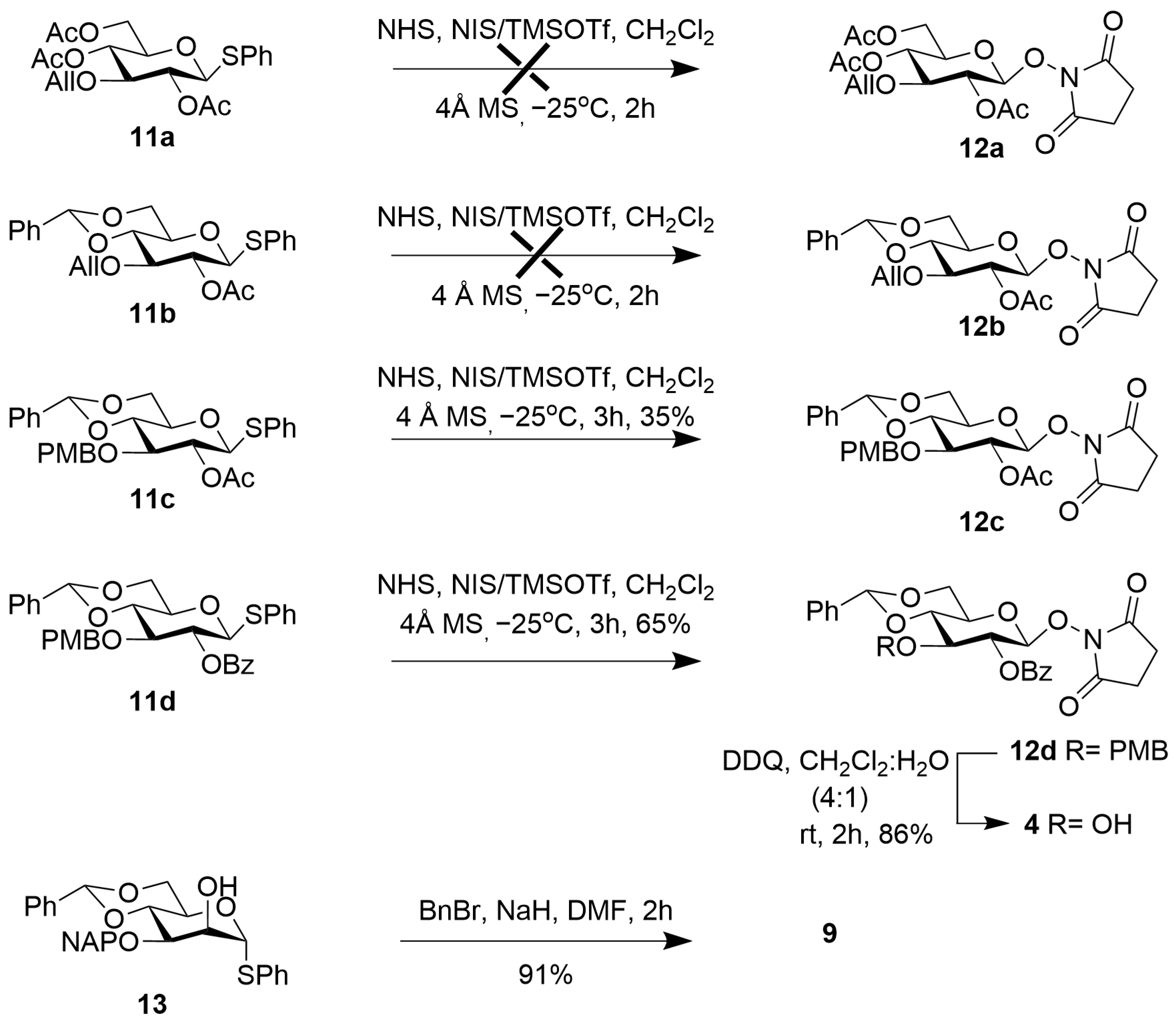
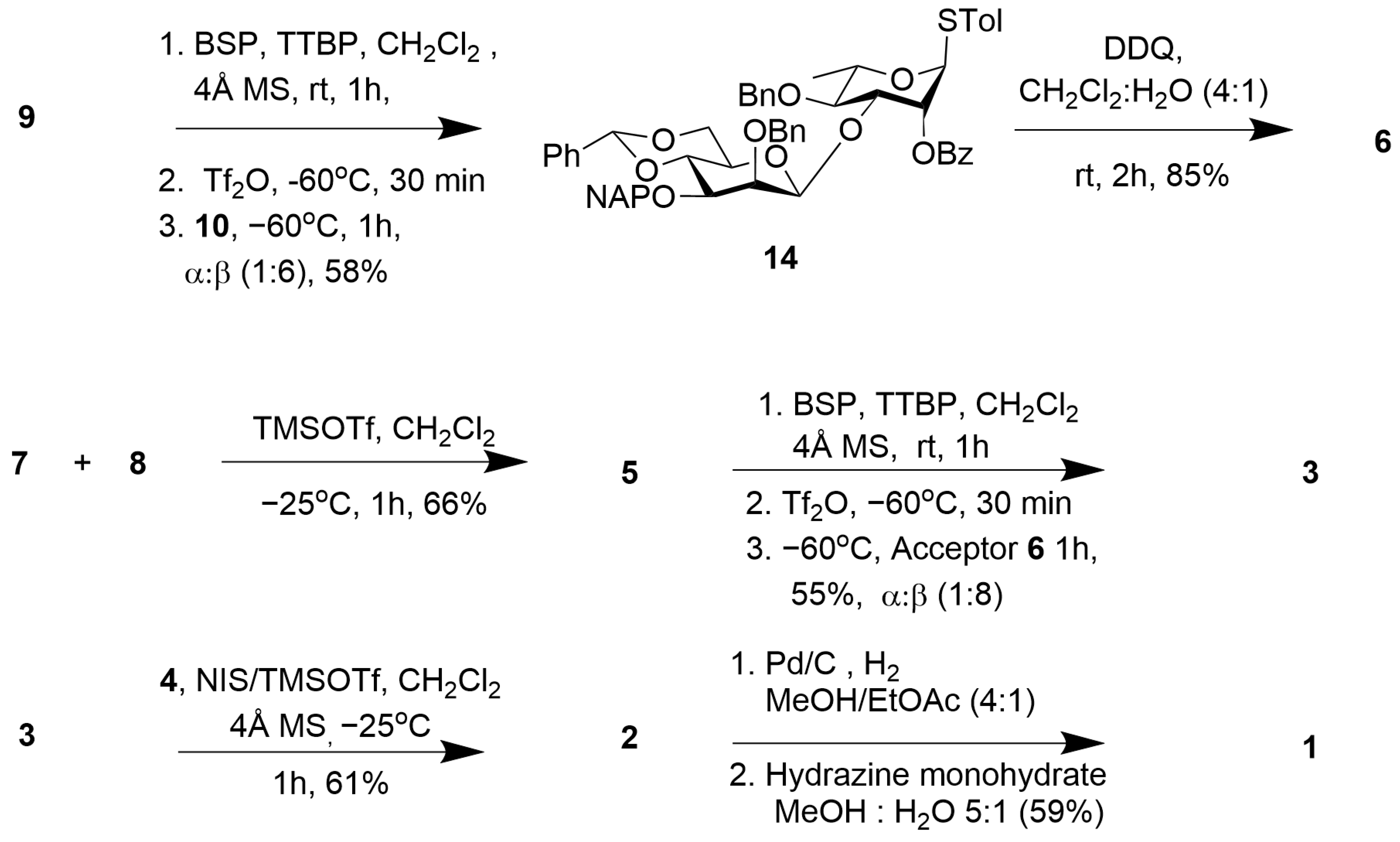
2.1. Synthesis of the Aminooxy Pentasaccharide 1
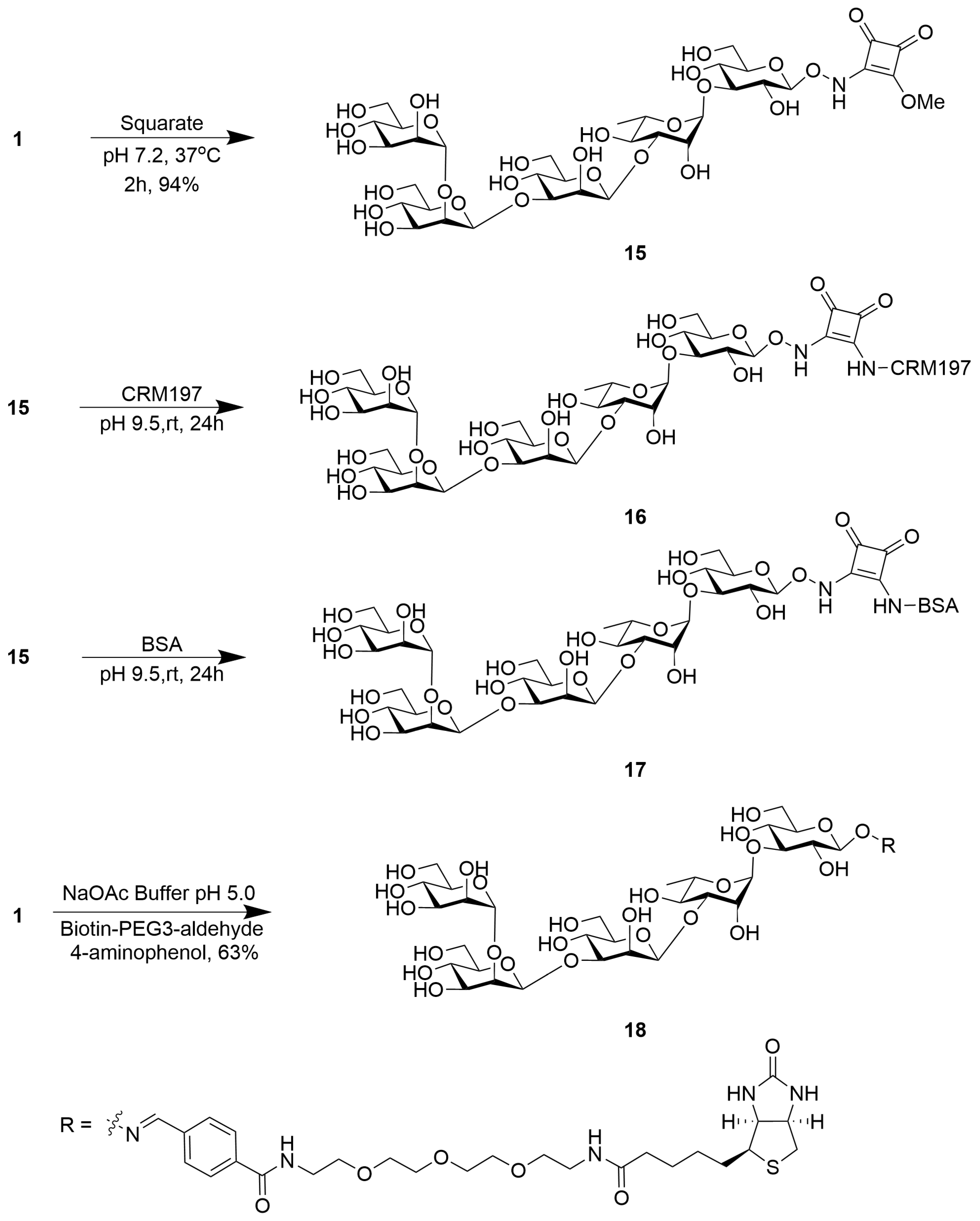
2.2. Synthesis of Psl Conjugates with CRM197, BSA, and Biotin
2.3. Vaccine Preparation Using Pam3CysSK4-QS-21 Liposomes and Psl-CRM197 Conjugate (16)
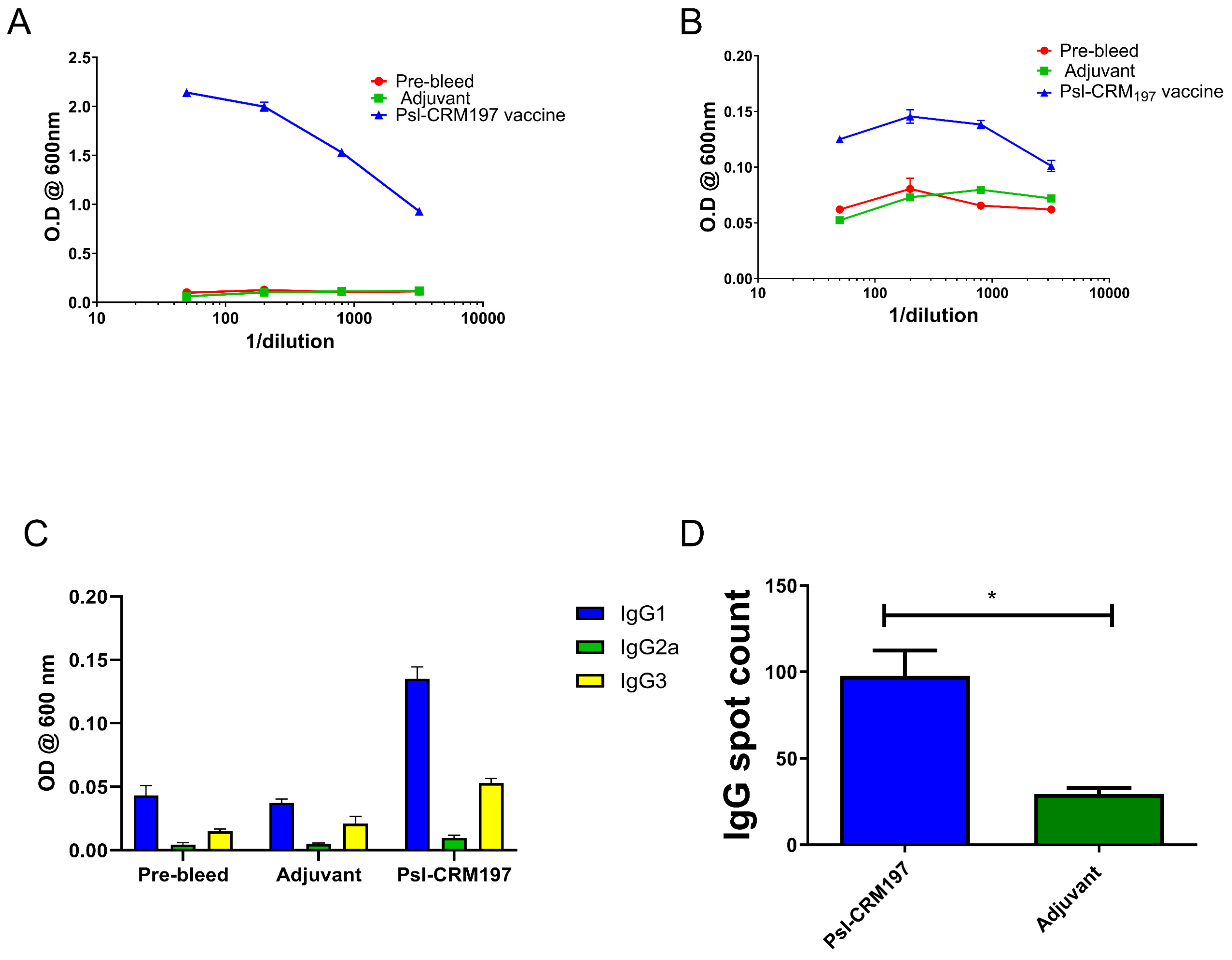
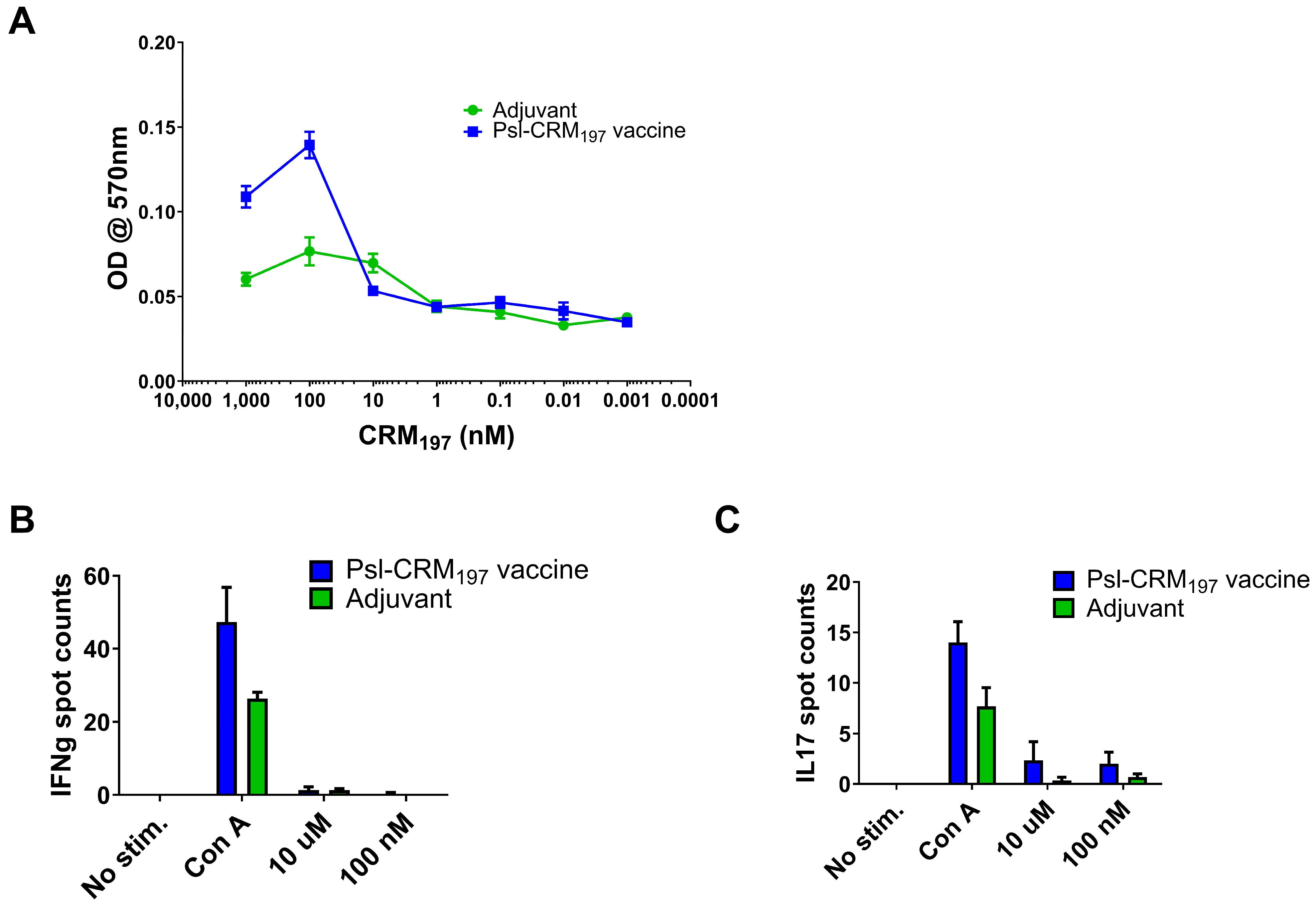
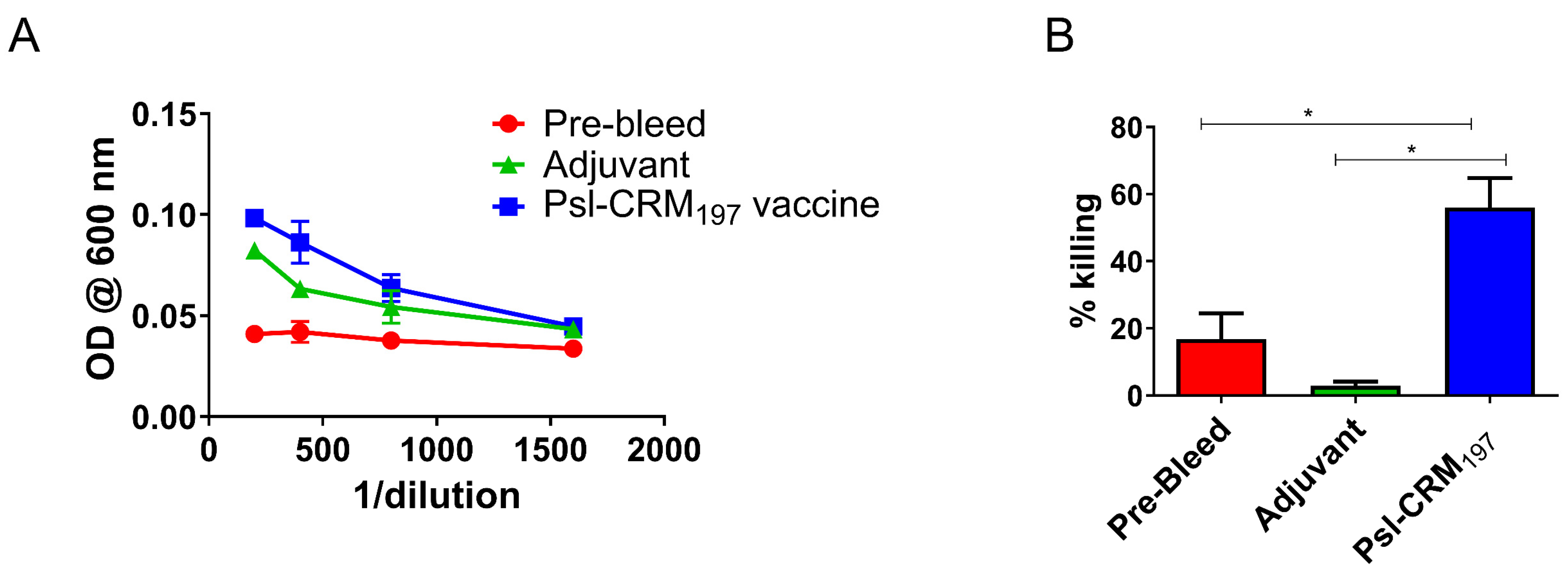
2.4. Vaccination Results
3. Experimental Section
3.1. General Methods
3.2. O-Succinimidyl-3-O-(4-Methoxybenzyl)-4,6-O-Benzylidene-2-Benzoyl-β-D-Glucopyranoside (12d)
3.3. O-Succinimidyl-4,6-O-Benzylidene-2-Benzoyl-β-D-Glucopyranoside (4)
3.4. Tolyl-2-O-Benzyl-4,6-O-Benzylidene-3-O-Napthyl-1-Thio-α-D-Mannoyranoside (9)
3.5. Tolyl-[2-O-Benzyl-4,6-O-Benzylidene-3-O-Napthyl-β-D-Mannopyranosyl]-(1→3)-2-O-Benzoyl-4-O-Benzyl-1-Thio-α-L-Rhamnopyranoside (14)
3.6. Tolyl-[2-O-Benzyl-4,6-O-Benzylidene-β-D-Mannopyranosyl]-(1→3)-2-O-Benzoyl-4-O-Benzyl-1-Thio-α-L-Rhamnopyranoside (6)
3.7. Tolyl-(2,3,4,6 Tetra-O-Acetyl)-α-D-Mannopyranosyl)-(1→2)-2-O-Benzyl-4,6-O-Benzylidene 1-Thio-α-D-Mannoyranoside (5)
3.8. Tolyl-(2,3,4,6 Tetra-O-Acetyl)-α-D-Mannopyranosyl)-(1→2)-2-O-Benzyl-4,6-O-Benzylidene (1→3)-[2-O-Benzyl-4,6-O-Benzylidene-β-D-Mannopyranosyl]-(1→3)-2-O-Benzoyl-4-o-Benzyl-1-Thio-α-L-Rhamnopyranoside (3)
3.9. N-Succinimidyl-(2,3,4,6 Tetra-O-Acetyl)-α-D-Mannopyranosyl)-(1→2)-2-O-Benzyl-4,6-O-Benzylidene (1→3)-[2-O-Benzyl-4,6-O-Benzylidene-β-D-Mannopyranosyl]-(1→3)-2-O-Benzoyl-4-O-Benzyl-1-Thio-α-L-Rhamnopyranosyl]-(1→3)-2-O-Benzoyl-4,6-O-Benzylidene-β-D-Glucopyranoside (2)
3.10. Aminooxy-(α-D-Mannopyranosyl)-(1→2)-(β-D-Mannopyranosyl)-(1→3)-(β-D-Mannopyranosyl)-(1→3)-(α-L-Rhamnopyranosyl)-(1→3)-β-D-Glucopyranoside (1)
4. Synthesis of Psl-Conjugates
4.1. Methoxy-Squaramido-(α-D-Mannopyranosyl)-(1→2)-(β-D-Mannopyranosyl)-(1→3)-(β-D-Mannopyranosyl)-(1→3)-(α-L-Rhamnopyranosyl)-(1→3)-β-D-Glucopyranoside (15)
4.2. Synthesis of Psl-CRM197 Conjugate (16)
4.3. Synthesis of Psl-Biotin Conjugate for ELISA Coating (18)
4.4. Mice and Vaccination
4.5. Spleen Cell Isolation
4.6. ELISA
4.7. Whole Cell ELISA Assay
4.8. Opsonophagocytic Killing Assay
4.9. Cell Proliferation Using the MTT Assay
4.10. Single-Color Murine IgG ELISPOT
4.11. Double-Color Murine IFN-γ/IL-17 ELISPOT
5. Statistics
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, W.R.; Arias, C.A. ESKAPE Pathogens: Antimicrobial Resistance, Epidemiology, Clinical Impact and Therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wei, X.; Xu, G.; Zhang, X.; Wang, X. Carbapenem-Resistant Pseudomonas aeruginosa Infections in Critically Ill Children: Prevalence, Risk Factors, and Impact on Outcome in a Large Tertiary Pediatric Hospital of China. Front. Public Health 2023, 11, 1088262. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Howell, P.L. Biosynthesis of the Pseudomonas aeruginosa Extracellular Polysaccharides, Alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 167. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Jackson, K.D.; Landry, R.M.; Parsek, M.R.; Wozniak, D.J. Analysis of Pseudomonas aeruginosa Conditional psl Variants Reveals Roles for the psl Polysaccharide in Adhesion and Maintaining Biofilm Structure Postattachment. J. Bacteriol. 2006, 188, 8213–8221. [Google Scholar] [CrossRef]
- Irie, Y.; Borlee, B.R.; O’Connor, J.R.; Hill, P.J.; Harwood, C.S.; Wozniak, D.J.; Parsek, M.R. Self-Produced Exopolysaccharide Is a Signal That Stimulates Biofilm Formation in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2012, 109, 20632–20636. [Google Scholar] [CrossRef]
- Liu, Y.; Gloag, E.S.; Hill, P.J.; Parsek, M.R.; Wozniak, D.J. Interbacterial Antagonism Mediated by a Released Polysaccharide. J. Bacteriol. 2022, 204, e00076-22. [Google Scholar] [CrossRef]
- Gheorghita, A.A.; Wozniak, D.J.; Parsek, M.R.; Howell, P.L. Pseudomonas aeruginosa Biofilm Exopolysaccharides: Assembly, Function, and Degradation. FEMS Microbiol. Rev. 2023, 47, 6. [Google Scholar] [CrossRef]
- DiGiandomenico, A.; Warrener, P.; Hamilton, M.; Guillard, S.; Ravn, P.; Minter, R.; Camara, M.M.; Venkatraman, V.; Macgill, R.S.; Lin, J.; et al. Identification of Broadly Protective Human Antibodies to Pseudomonas aeruginosa Exopolysaccharide Psl by Phenotypic Screening. J. Exp. Med. 2012, 209, 1273–1287. [Google Scholar] [CrossRef]
- Jones, C.J.; Wozniak, D.J. Psl Produced by Mucoid Pseudomonas aeruginosa Contributes to the Establishment of Biofilms and Immune Evasion. mBio 2017, 8, e00864-17. [Google Scholar] [CrossRef]
- Zegans, M.E.; DiGiandomenico, A.; Ray, K.; Naimie, A.; Keller, A.E.; Stover, C.K.; Lalitha, P.; Srinivasan, M.; Acharya, N.R.; Lietman, T.M. Association of Biofilm Formation, Psl Exopolysaccharide Expression, and Clinical Outcomes in Pseudomonas aeruginosa Keratitis: Analysis of Isolates in the Steroids for Corneal Ulcers Trial. JAMA Ophthalmol. 2016, 134, 383–389. [Google Scholar] [CrossRef]
- Gonzaga, Z.J.C.; Merakou, C.; DiGiandomenico, A.; Priebe, G.P.; Rehm, B.H.A. A Pseudomonas aeruginosa-Derived Particulate Vaccine Protects against P. aeruginosa Infection. Vaccines 2021, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mo, K.F.; Wang, Q.; Stover, C.K.; DiGiandomenico, A.; Boons, G.J. Epitope Mapping of Monoclonal Antibodies Using Synthetic Oligosaccharides Uncovers Novel Aspects of Immune Recognition of the Psl Exopolysaccharide of Pseudomonas aeruginosa. Chemistry 2013, 19, 17425–17431. [Google Scholar] [CrossRef] [PubMed]
- Demeter, F.; Chang, M.D.-T.; Lee, Y.-C.; Borbás, A.; Herczeg, M. An Efficient Synthesis of the Pentasaccharide Repeating Unit of Pseudomonas aeruginosa Psl Exopolysaccharide. Synlett 2020, 31, 469–474. [Google Scholar] [CrossRef]
- Shinefield, H.R. Overview of the Development and Current Use of CRM(197) Conjugate Vaccines for Pediatric Use. Vaccine 2010, 28, 4335–4339. [Google Scholar] [CrossRef]
- Khatuntseva, E.A.; Nifantiev, N.E. Cross-Reacting Material (CRM197) as a Carrier Protein for Carbohydrate Conjugate Vaccines Targeted at Bacterial and Fungal Pathogens. Int. J. Biol. Macromol. 2022, 218, 775–798. [Google Scholar] [CrossRef]
- Crich, D.; Sun, S. Direct Chemical Synthesis of β-Mannopyranosides and Other Glycosides via Glycosyl Triflates. Tetrahedron 1998, 54, 8321–8348. [Google Scholar] [CrossRef]
- Gucchait, A.; Shit, P.; Misra, A.K. Concise Synthesis of a Tetrasaccharide Related to the Repeating Unit of the Cell Wall O-Antigen of Salmonella enterica O60. Tetrahedron 2020, 76, 34. [Google Scholar] [CrossRef]
- van der Ven, J.G.M.; Kamerling, J.P.; Vliegenthart, J.F.G. Synthesis of a Fucosylated and a Non-Fucosylated Core Structure of Xylose-Containing Carbohydrate Chains from N-Glycoproteins. Carbohydr. Res. 1994, 2, 45–62. [Google Scholar] [CrossRef][Green Version]
- Fujikawa, K.; Imamura, A.; Ishida, H.; Kiso, M. Synthesis of a GM3 Ganglioside Analogue Carrying a Phytoceramide Moiety by Intramolecular Glycosylation as a Key Step. Carbohydr. Res. 2008, 343, 2729–2734. [Google Scholar] [CrossRef]
- Si, A.; Sucheck, S.J. Synthesis of Aminooxy Glycoside Derivatives of the Outer Core Domain of Pseudomonas aeruginosa Lipopolysaccharide. Front. Mol. Biosci. 2021, 8, 750502. [Google Scholar] [CrossRef]
- Veeneman, G.H.; van Leeuwen, S.H.; van Boom, J.H. Iodonium Ion Promoted Reactions at the Anomeric Centre. II. An Efficient Thioglycoside-Mediated Approach toward the Formation of 1,2-Trans Linked Glycosides and Glycosidic Esters. Tetrahedron Lett. 1990, 31, 1331–1334. [Google Scholar] [CrossRef]
- Oikawa, Y.; Yoshioka, T.; Yonemitsu, O. Specific Removal of O-Methoxybenzyl Protection by DDQ Oxidation. Tetrahedron Lett. 1982, 23, 885–888. [Google Scholar] [CrossRef]
- Crich, D. Mechanism of a Chemical Glycosylation Reaction. Acc. Chem. Res. 2010, 43, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Crich, D. Chemistry of Glycosyl Triflates: Synthesis of β-Mannopyranosides. J. Carbohydr. Chem. 2007, 21, 663–686. [Google Scholar] [CrossRef]
- Bock, K.; Pedersen, C. A Study of 13C-H Coupling Constants in Hexopyranoses. J. Chem. Soc. 1974, 2, 293. [Google Scholar]
- Schmidt, R.R. New Methods for the Synthesis of Glycosides and Oligosaccharides—Are There Alternatives to the Koenigs-Knorr Method? Angew. Chem. Int. Ed. Engl. 1986, 25, 212–235. [Google Scholar] [CrossRef]
- Poirier, D. Description of Chemical Synthesis, Nuclear Magnetic Resonance Characterization and Biological Activity of Estrane-Based Inhibitors/Activators of Steroidogenesis. Molecules 2023, 28, 8. [Google Scholar] [CrossRef]
- Ghosh, S.; Trabbic, K.R.; Shi, M.; Nishat, S.; Eradi, P.; Kleski, K.A.; Andreana, P.R. Chemical Synthesis and Immunological Evaluation of Entirely Carbohydrate Conjugate Globo H-PS A1. Chem. Sci. 2020, 11, 13052–13059. [Google Scholar] [CrossRef]
- Donahue, T.C.; Zong, G.; O’Brien, N.A.; Ou, C.; Gildersleeve, J.C.; Wang, L.X. Synthesis and Immunological Study of N-Glycan-Bacteriophage Qβ Conjugates Reveal Dominant Antibody Responses to the Conserved Chitobiose Core. Bioconjug. Chem. 2022, 33, 1350–1362. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- De Leo, V.; Milano, F.; Agostiano, A.; Catucci, L. Recent Advancements in Polymer/Liposome Assembly for Drug Delivery: From Surface Modifications to Hybrid Vesicles. Polymers 2021, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Thanvi, R.; Nada, S.; Dissanayake, R.; Vartak, A.; Sebilleau, C.O.; Alom, N.E.; Prestwich, E.G.; Wall, K.A.; Sucheck, S.J. Synthesis and Evaluation of a Self-Adjuvanting Pseudomonal Vaccine Based on Major Outer Membrane Porin OprF Epitopes Formulated with Low-Toxicity QS-21-Containing Liposomes. Bioconjug. Chem. 2023, 34, 893–910. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.J.; Whitters, D.; Sevastsyanovich, Y.R.; Heath, J.N.; Pravin, J.; Goodall, M.; Browning, D.F.; O’Shea, M.K.; Cranston, A.; De Soyza, A.; et al. Increased Severity of Respiratory Infections Associated with Elevated Anti-LPS IgG2 Which Inhibits Serum Bactericidal Killing. J. Exp. Med. 2014, 211, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Konradsson, P.; Udodong, U.E.; Fraser-Reid, B. Iodonium Promoted Reactions of Disarmed Thioglycosides. Tetrahedron Lett. 1990, 31, 4313–4316. [Google Scholar] [CrossRef]
- Mong, T.K.; Shiau, K.S.; Lin, Y.H.; Cheng, K.C.; Lin, C.H. A Concise Synthesis of Single Components of Partially Sulfated Oligomannans. Org. Biomol. Chem. 2015, 13, 11550–11560. [Google Scholar] [CrossRef]
- Crich, D.; Sun, S.; Brunckova, J. Chemistry of 1-Alkoxy-1-Glycosyl Radicals: The Manno- and Rhamnopyranosyl Series. Inversion of α- to β-Pyranosides and the Fragmentation of Anomeric Radicals. J. Org. Chem. 1996, 61, 605–615. [Google Scholar] [CrossRef]
- Crich, D.; Li, W.; Li, H. Direct Chemical Synthesis of the β-Mannans: Linear and Block Syntheses of the Alternating β-(1→3)-β-(1→4)-Mannan Common to Rhodotorula glutinis, Rhodotorula mucilaginosa, and Leptospira biflexa. J. Am. Chem. Soc. 2004, 126, 15081–15086. [Google Scholar] [CrossRef]
- Dhénin, S.G.Y.; Moreau, V.; Nevers, M.-C.; Créminon, C.; Djedaïni-Pilard, F. Sensitive and Specific Enzyme Immunoassays for Antigenic Trisaccharide from Bacillus anthracis Spores. Org. Biomol. Chem. 2009, 7, 5184–5199. [Google Scholar] [CrossRef]
- Polat, T.; Wong, C.-H. Anomeric Reactivity-Based One-Pot Synthesis of Heparin-Like Oligosaccharides. J. Am. Chem. Soc. 2007, 129, 12795–12800. [Google Scholar] [CrossRef]
- Hossain, M.K.; Vartak, A.; Karmakar, P.; Sucheck, S.J.; Wall, K.A. Augmenting Vaccine Immunogenicity through the Use of Natural Human Anti-Rhamnose Antibodies. ACS Chem. Biol. 2018, 13, 2130–2142. [Google Scholar] [CrossRef]

| Formulation | DPMG | DMPC | Cholesterol | Pam3CysSK4 | QS-21 |
|---|---|---|---|---|---|
| mol % | 4.4 | 40 | 54.2 | 1.5 | |
| Amount (mg) | 0.909 | 8.135 | 6.288 | 0.733 | 0.010 |
| Molecular weight (g/mol) | 688.8 | 677.9 | 386.7 | 1631 | 1990 |
| Samples | DPMG (mg) | DMPC (mg) | Cholesterol (mg) | Pam3CysSK4 (mg) | QS-21 (mg) | 16 (mg) |
|---|---|---|---|---|---|---|
| QS-21 liposome (Control) | 0.909 | 8.135 | 6.288 | 0.733 | 0.01 | - |
| QS-21 + Psl-CRM197 liposome | 0.909 | 8.135 | 6.288 | 0.733 | 0.01 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokolo, U.C.; Dissanayake, R.; Ghosh, S.; Nada, S.; Obadawo, B.S.; Prestwich, E.G.; Wall, K.A.; Sucheck, S.J. Synthesis of the Pentasaccharide Unit of the Pseudomonas aeruginosa Exopolysaccharide Psl Conjugation with CRM197, and Evaluation of Antigenicity in a QS-21/Pam3CSK4-Liposomal Formulation. Molecules 2025, 30, 1720. https://doi.org/10.3390/molecules30081720
Bokolo UC, Dissanayake R, Ghosh S, Nada S, Obadawo BS, Prestwich EG, Wall KA, Sucheck SJ. Synthesis of the Pentasaccharide Unit of the Pseudomonas aeruginosa Exopolysaccharide Psl Conjugation with CRM197, and Evaluation of Antigenicity in a QS-21/Pam3CSK4-Liposomal Formulation. Molecules. 2025; 30(8):1720. https://doi.org/10.3390/molecules30081720
Chicago/Turabian StyleBokolo, Uzoamaka Clara, Ravindika Dissanayake, Samir Ghosh, Shadia Nada, Babatunde S. Obadawo, Erin G. Prestwich, Katherine A. Wall, and Steven J. Sucheck. 2025. "Synthesis of the Pentasaccharide Unit of the Pseudomonas aeruginosa Exopolysaccharide Psl Conjugation with CRM197, and Evaluation of Antigenicity in a QS-21/Pam3CSK4-Liposomal Formulation" Molecules 30, no. 8: 1720. https://doi.org/10.3390/molecules30081720
APA StyleBokolo, U. C., Dissanayake, R., Ghosh, S., Nada, S., Obadawo, B. S., Prestwich, E. G., Wall, K. A., & Sucheck, S. J. (2025). Synthesis of the Pentasaccharide Unit of the Pseudomonas aeruginosa Exopolysaccharide Psl Conjugation with CRM197, and Evaluation of Antigenicity in a QS-21/Pam3CSK4-Liposomal Formulation. Molecules, 30(8), 1720. https://doi.org/10.3390/molecules30081720









