Advancing Thermal Energy Storage: Synthesis and Thermal Performance of Silica-Encapsulated Paraffin PCMs
Abstract
1. Introduction
2. Results and Discussion
2.1. Spectroscopy by FTIR
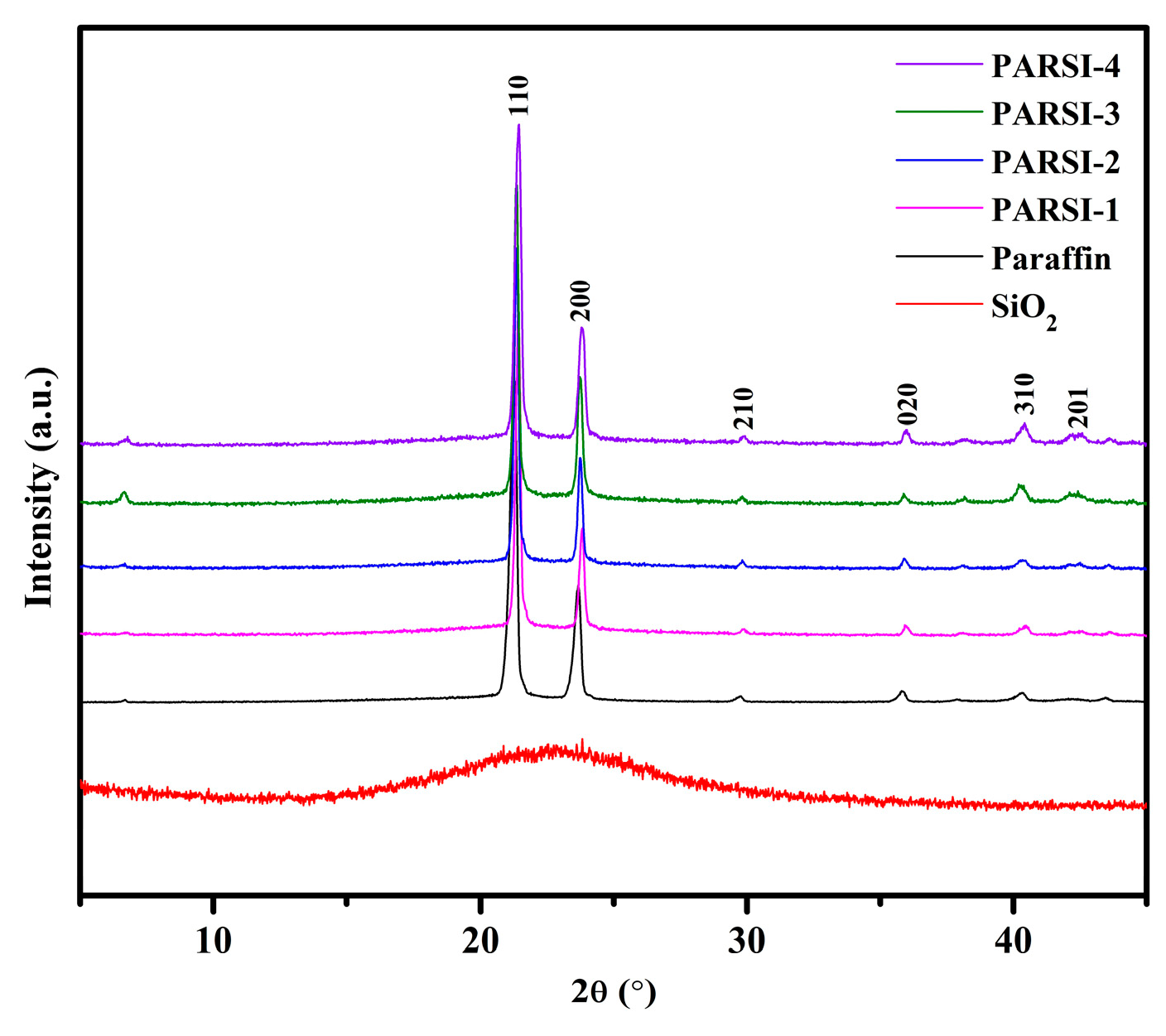
2.2. XRD Analysis of the NPCMs
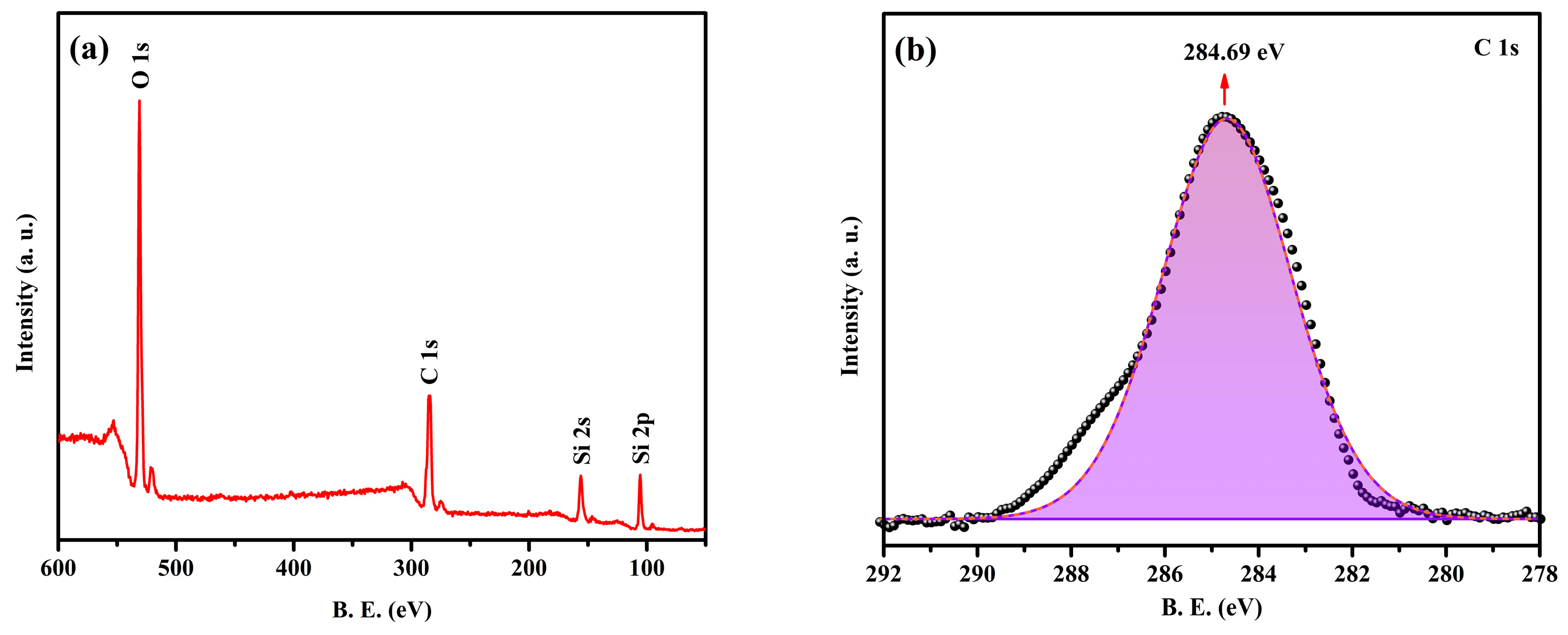
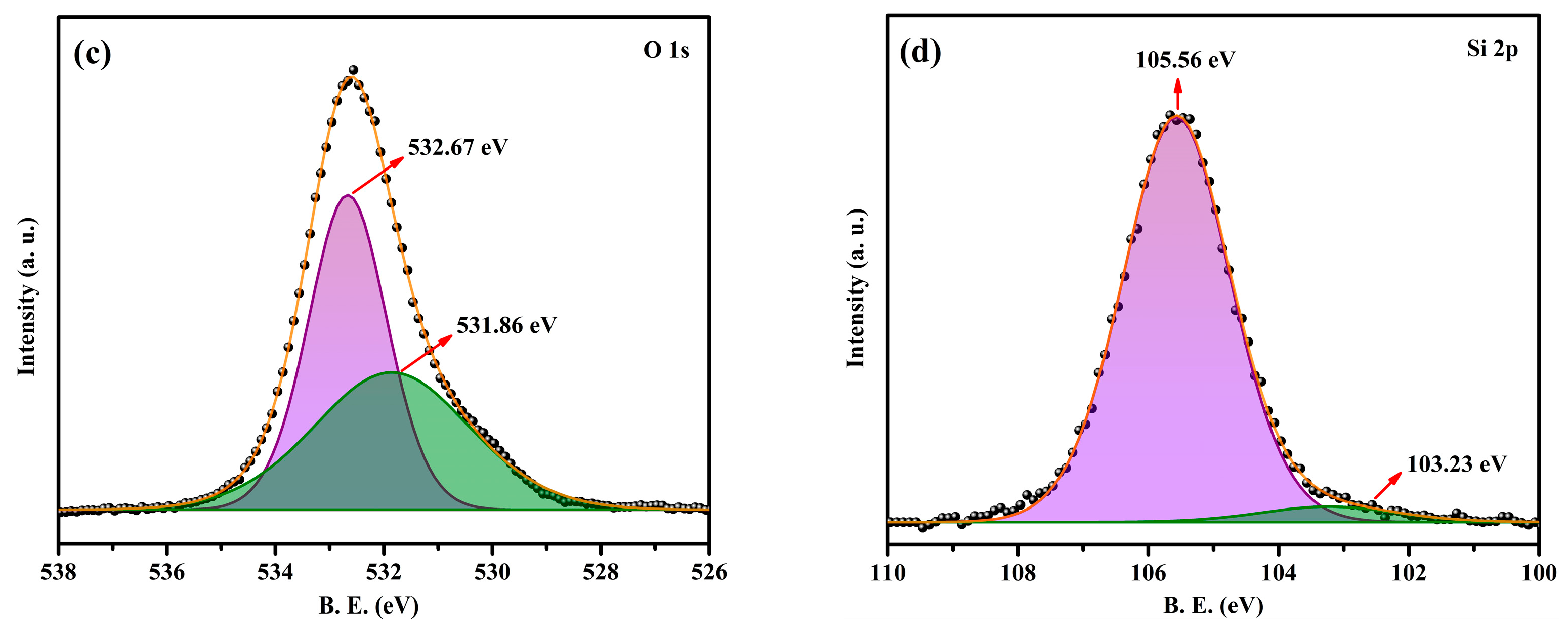
2.3. XPS Analysis of the NPCMs
2.4. Morphological Features of the NPCMs
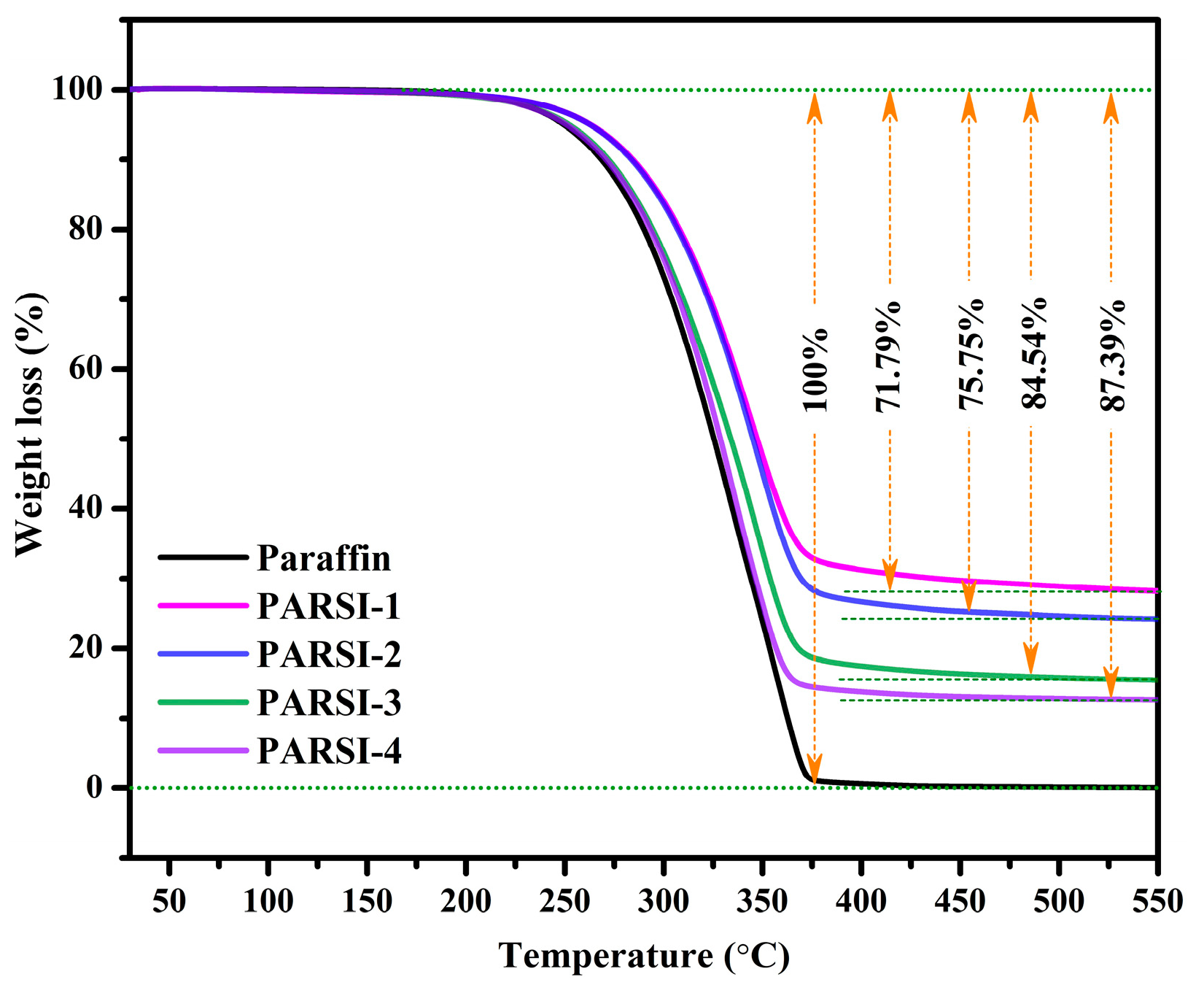
2.5. Thermal Stability Evaluation of the NPCMs
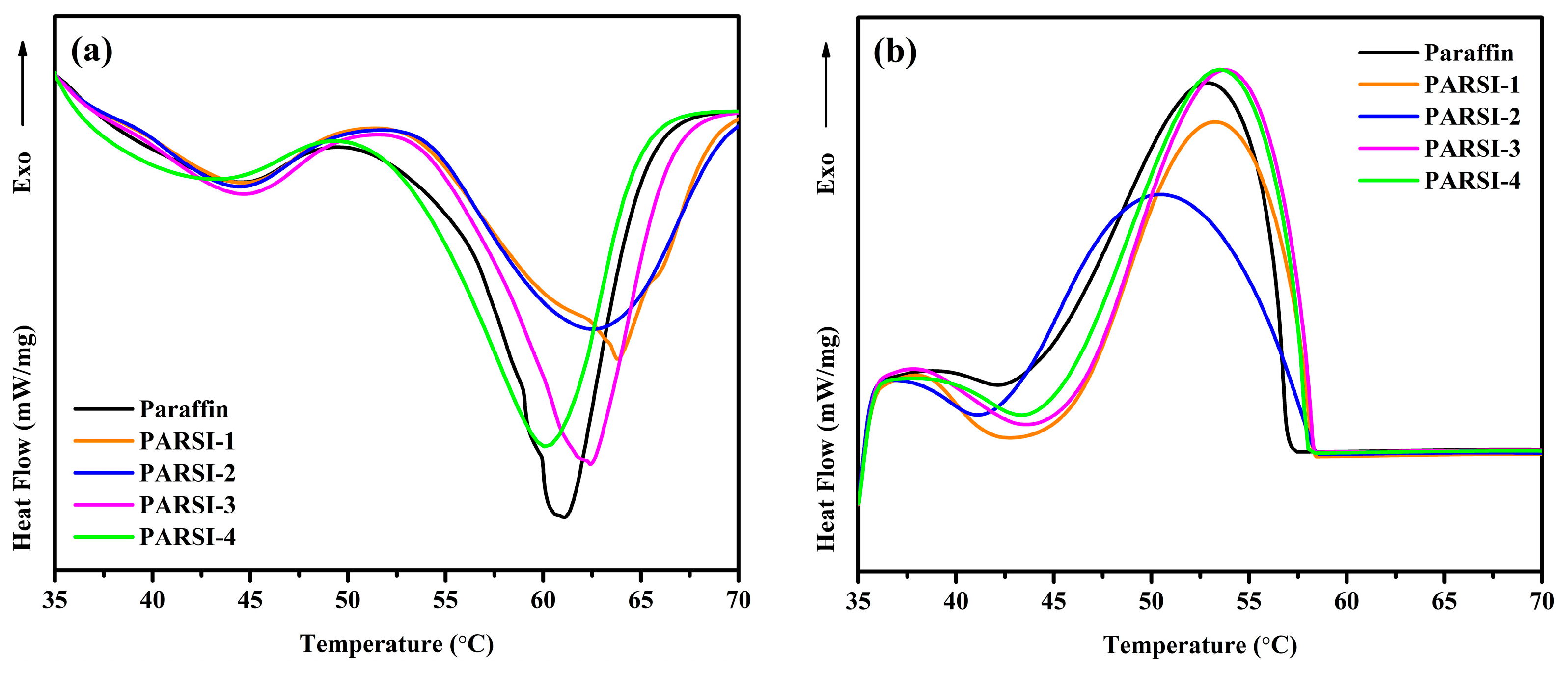
2.6. Heat Storage Capability of the NPCMs
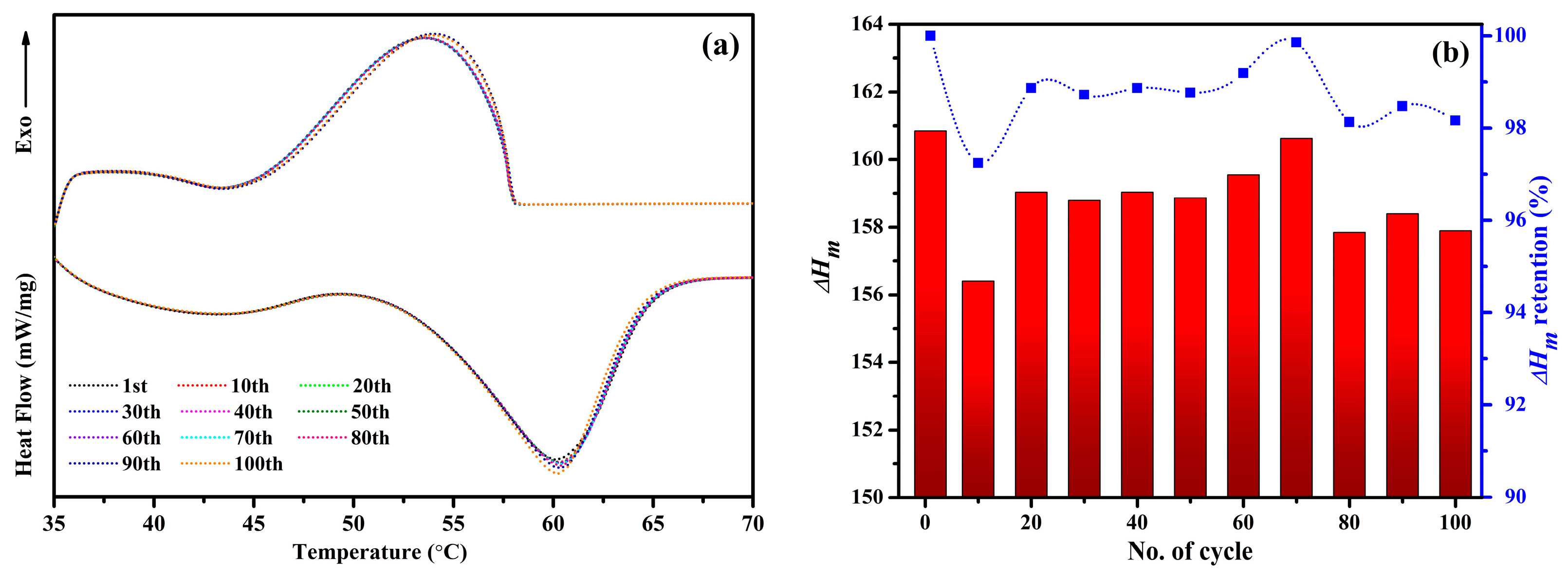
2.7. Thermal Cycle Endurance of the NPCMs
2.8. Leakage Resistance Performance
3. Materials and Methods
3.1. Materials
3.2. Encapsulation Technique of Paraffin@SiO2 NPCMs
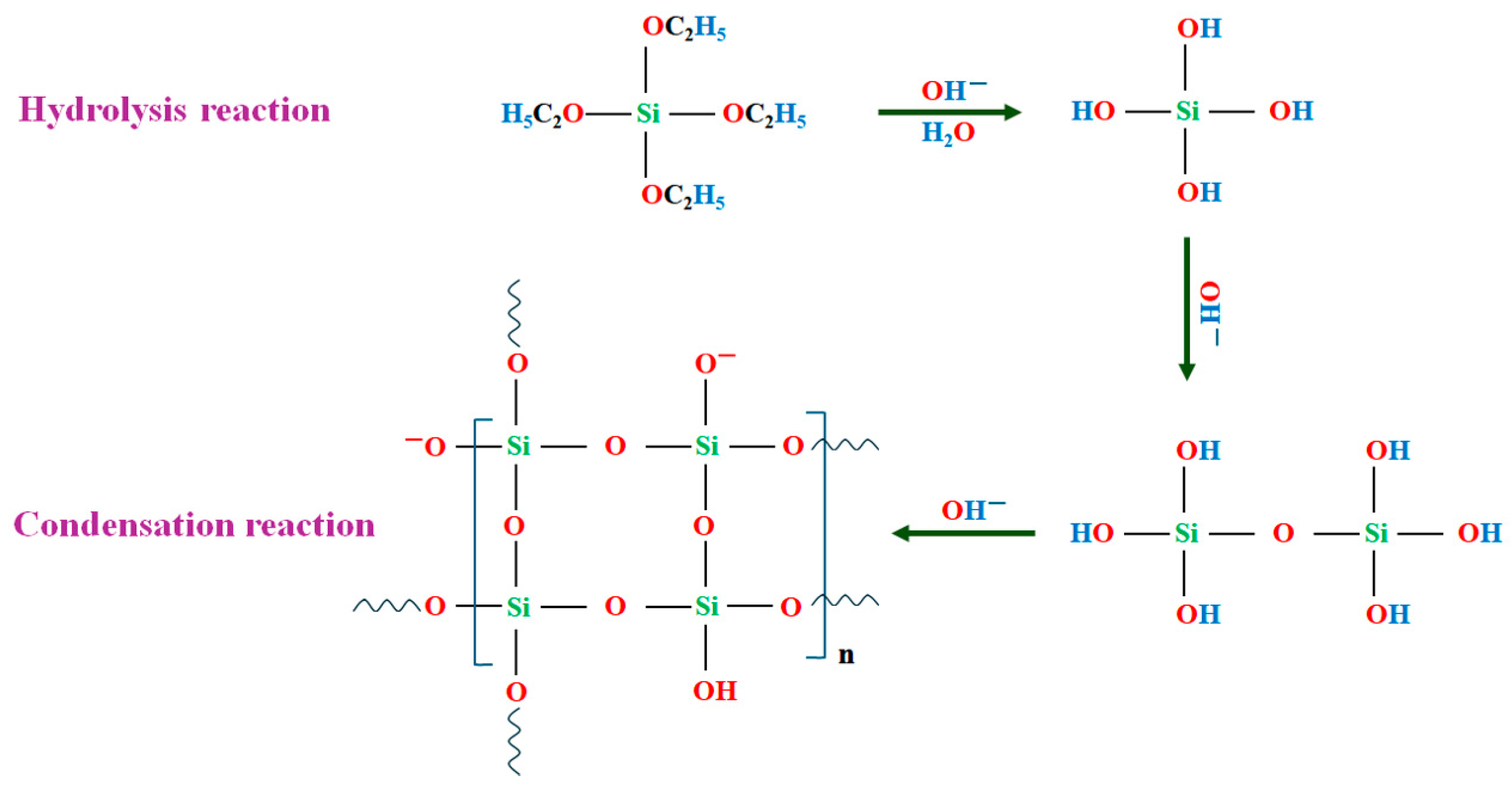
3.3. Mechanism of Encapsulation
3.4. Characterization Techniques
3.4.1. Microscopy
3.4.2. X-Ray Diffraction
3.4.3. Spectroscopy
3.4.4. Thermal Analysis
3.4.5. Leakage Test
4. Conclusions
- FTIR and XRD analyses confirm the presence of Si–O–Si and Si–OH bonds, indicating the successful encapsulation of paraffin within the SiO2 shell without any chemical interaction between the core and shell materials. The amorphous nature of the SiO2 shell is validated by XRD results.
- The XPS analysis further affirms the presence of O 1s and Si 2p peaks, supporting the successful encapsulation of paraffin within the SiO2 shell and corroborating the FTIR and XRD findings.
- The SEM analysis reveals a well-defined core–shell structure with uniform spherical particles, and the particle sizes vary depending on composition. The smallest average particle size of 190 nm is observed for the PARSI-4 sample.
- The TGA results indicate that SiO2 encapsulation provides effective protection against thermal degradation, where thicker SiO2 shells offer enhanced thermal stability, ensuring better thermal resistance.
- The thermal properties of NPCMs improve with an increasing core–shell ratio, with the PARSI-4 sample exhibiting the highest melting (160.86 J/g) and solidifying (153.93 J/g) enthalpies, demonstrating superior heat storage capacity.
- The encapsulation ratio and encapsulation efficiency increase with the core–shell ratio, where the PARSI-4 sample achieves the highest ER (87.83%) and EE (87.04%), ensuring efficient paraffin holding within the SiO2 shell.
- After 100 DSC thermal cycles, the PARSI-4 sample retains 98.16% of its initial enthalpy, highlighting its excellent thermal reliability and long-term applicability for thermal energy storage systems.
- The SiO2 shell effectively prevents paraffin leakage, ensuring shape stability and durability even at elevated temperatures, demonstrating the successful encapsulation and practical feasibility of NPCMs in thermal energy storage applications.
- This novel single-pot sol–gel synthesis enables the economical fabrication of nano-sized silica-encapsulated paraffin with a high surface area, enhancing heat transfer, thermal stability, and storage efficiency. This innovative approach offers a scalable solution for advanced TES applications, supporting sustainable energy goals by improving PCM performance and reducing dependence on fossil fuels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishak, S.; Mandal, S.; Lgaz, H.; Atinafu, D.G.; Mohammad Harmay, N.S.; Lee, H.-S.; Abdul Shukor Lim, N.; Abdullah, M.M.A.B.; Yang, H.-M. Microscopic molecular insights of different carbon chain fatty acids on shape-stabilized phase change composite. J. Therm. Anal. Calorim. 2024, 149, 9203–9221. [Google Scholar] [CrossRef]
- United Nations Economic Commission for Africa. Intergenerational Relations: Creating A World for All Ages So That No One Is Left Behind; ESCAP: Bangkok, Thailand, 2024. [Google Scholar]
- Mizutani, N.; Rijal, H.B.; Aqilah, N.; Khadka, S. Thermal Environment and Comfort in Japanese Dwellings During Summer. Atmosphere 2025, 16, 157. [Google Scholar] [CrossRef]
- Hilmi, M. Middle East Journal of Agriculture Research Volume: 14|Issue: 01|Jan.–Mar.|2025. Middle East J. 2025, 14, 9–42. [Google Scholar]
- Ray, A.; Bhonsle, A.K.; Singh, J.; Trivedi, J.; Atray, N. Examining alternative carbon resources for sustainable energy generation: A comprehensive review. Next Energy 2025, 6, 100194. [Google Scholar] [CrossRef]
- Rahman, A.; Murad, S.W.; Mohsin, A.; Wang, X. Does renewable energy proactively contribute to mitigating carbon emissions in major fossil fuels consuming countries? J. Clean. Prod. 2024, 452, 142113. [Google Scholar] [CrossRef]
- Ishak, S.; Mandal, S.; Lee, H.-S.; Singh, J.K. pH-controlled synthesis of sustainable lauric acid/SiO2 phase change material for scalable thermal energy storage. Sci. Rep. 2021, 11, 15012. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, K.; Zhang, H.; Liu, H.; Wu, D.; Wang, X. Development of poly (ethylene glycol)/silica phase-change microcapsules with well-defined core-shell structure for reliable and durable heat energy storage. Sol. Energy Mater. Sol. Cells 2021, 225, 111069. [Google Scholar] [CrossRef]
- Mandal, S.; Ishak, S.; Singh, J.K.; Lee, D.-E.; Park, T. Synthesis and application of paraffin/silica phase change nanocapsules: Experimental and numerical approach. J. Energy Storage 2022, 51, 104407. [Google Scholar] [CrossRef]
- Salihi, M.; El Mastouri, M.; El Fiti, M.; Harmen, Y.; Chebak, A.; Jama, C.; Chhiti, Y. Calcium carbonate-shelled microencapsulated phase change materials in cement mortar: A pathway to enhancing energy efficiency in building envelopes. J. Energy Storage 2025, 110, 115306. [Google Scholar] [CrossRef]
- Maka, A.O.; Ghalut, T.; Elsaye, E. The pathway toward decarbonisation and net-zero emissions by 2050: The role of solar energy technology. Green Technol. Sustain. 2024, 2, 100107. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Luo, J.-W.; Yaji, K.; Chen, L.; Tao, W.-Q. Data-driven multi-fidelity topology design of fin structures for latent heat thermal energy storage. Appl. Energy 2025, 377, 124596. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Shukla, S.K. A comprehensive review of the thermal performance in energy efficient building envelope incorporated with phase change materials. J. Energy Storage 2024, 79, 110128. [Google Scholar] [CrossRef]
- Seyitini, L.; Belgasim, B.; Enweremadu, C.C. Solid state sensible heat storage technology for industrial applications–A review. J. Energy Storage 2023, 62, 106919. [Google Scholar] [CrossRef]
- Sarath, K.; Osman, M.F.; Mukhesh, R.; Manu, K.; Deepu, M. A review of the recent advances in the heat transfer physics in latent heat storage systems. Therm. Sci. Eng. Prog. 2023, 42, 101886. [Google Scholar] [CrossRef]
- Luo, J.-W.; Chen, L.; Wang, M.; Xia, Y.; Tao, W. Particle-scale study of coupled physicochemical processes in Ca(OH)2 dehydration using the lattice Boltzmann method. Energy 2022, 250, 123835. [Google Scholar] [CrossRef]
- Geng, L.; Wang, J.; Yang, X.; Jiang, J.; Li, R.; Yan, Y.; Zhao, J.; Liu, C. Synergistic enhancement of phase change materials through three-dimensional porous layered covalent triazine framework/expanded graphite composites for solar energy storage and beyond. Chem. Eng. J. 2024, 487, 150749. [Google Scholar] [CrossRef]
- Khademi, A.; Shank, K.; Mehrjardi, S.A.A.; Tiari, S.; Sorrentino, G.; Said, Z.; Chamkha, A.J.; Ushak, S. A brief review on different hybrid methods of enhancement within latent heat storage systems. J. Energy Storage 2022, 54, 105362. [Google Scholar] [CrossRef]
- Kiyak, B.; Oztop, H.F.; Biswas, N.; Selimefendigil, F. Effects of geometrical configurations on melting and solidification processes in phase change materials. Appl. Therm. Eng. 2025, 258, 124726. [Google Scholar] [CrossRef]
- Mandal, S. Advancements in Phase Change Materials: Stabilization Techniques and Applications. Prabha Mater. Sci. Lett. 2024, 3, 254–267. [Google Scholar] [CrossRef]
- Talebizadeh Sardari, P.; Mohammed, H.I.; Mahdi, J.M.; Ghalambaz, M.; Gillott, M.; Walker, G.S.; Grant, D.; Giddings, D. Localized heating element distribution in composite metal foam-phase change material: Fourier’s law and creeping flow effects. Int. J. Energy Res. 2021, 45, 13380–13396. [Google Scholar] [CrossRef]
- Lamba, R.; Montero, F.J.; Rehman, T.-U.; Singh, S.; Manikandan, S. PCM-based hybrid thermal management system for photovoltaic modules: A comparative analysis. Environ. Sci. Pollut. Res. 2024, 31, 46397–46416. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, M.; Khalili, Z. Enhancing photovoltaic solar panel performance with integration of PCM-based spectral filter and self-cleaning coating. J. Build. Eng. 2024, 94, 110019. [Google Scholar] [CrossRef]
- Elamin, A.E.A. Thermal management of photovoltaic thermal (PVT) system for improving electrical performance. J. Therm. Anal. Calorim. 2024, 149, 12417–12427. [Google Scholar] [CrossRef]
- Afaynou, I.; Faraji, H.; Choukairy, K.; Arshad, A.; Arıcı, M. Heat transfer enhancement of phase-change materials (PCMs) based thermal management systems for electronic components: A review of recent advances. Int. Commun. Heat Mass Transf. 2023, 143, 106690. [Google Scholar] [CrossRef]
- Kim, S.; Stavins, R.A.; Garimella, V.S.; Koronio, E.; Shockner, T.; Ziskind, G.; Miljkovic, N.; King, W.P. Cooling high power electronics using dynamic phase change material. Int. J. Heat Mass Transf. 2025, 237, 126433. [Google Scholar] [CrossRef]
- El Moutaouakil, L. Hybrid Cooling System for High-Power Electronics Using Pcms and Wavy Surfaces. 2025. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5088554 (accessed on 15 March 2025).
- Mandal, S.; Ishak, S.; Lee, D.-E.; Park, T. Optimization of eco-friendly Pinus resinosa biochar-dodecanoic acid phase change composite for the cleaner environment. J. Energy Storage 2022, 55, 105414. [Google Scholar] [CrossRef]
- Marinković, M.; Nikolić, R.; Savović, J.; Gadžurić, S.; Zsigrai, I. Thermochromic complex compounds in phase change materials: Possible application in an agricultural greenhouse. Sol. Energy Mater. Sol. Cells 1998, 51, 401–411. [Google Scholar] [CrossRef]
- Elawady, N.; Bekheit, M.; Sultan, A.A.; Radwan, A. Energy assessment of a roof-integrated phase change materials, long-term numerical analysis with experimental validation. Appl. Therm. Eng. 2022, 202, 117773. [Google Scholar] [CrossRef]
- Alkan, C.; Alakara, E.H.; Aksoy, S.A.; Demir, İ. Cement mortar composites including 1-tetradecanol@ PMMA Pickering emulsion particles for thermal energy management of buildings. Chem. Eng. J. 2023, 476, 146843. [Google Scholar] [CrossRef]
- Lopez-Arias, M.; Francioso, V.; Velay-Lizancos, M. High thermal inertia mortars: New method to incorporate phase change materials (PCMs) while enhancing strength and thermal design models. Constr. Build. Mater. 2023, 370, 130621. [Google Scholar] [CrossRef]
- Ishak, S.; Yio, M.; Moon, J.; Mandal, S.; Sasui, S.; Lim, N.H.A.S.; Wang, X.-Y.; Wang, Y.-S.; Abdullah, M.M.A.B.; Shek, P.N. Hydration and microstructural development of cement pastes incorporating diatomaceous earth, expanded perlite, and shape-stabilized phase change materials (SSPCMs). Constr. Build. Mater. 2025, 468, 140483. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Li, S.-T.; Shao, Y.-W.; Jin, X.-Z.; Qi, X.-D.; Yang, J.-H.; Zhou, Z.-W.; Wang, Y. Melamine foam/reduced graphene oxide supported form-stable phase change materials with simultaneous shape memory property and light-to-thermal energy storage capability. Chem. Eng. J. 2020, 379, 122373. [Google Scholar] [CrossRef]
- Anand, A.; Mansor, M.; Sharma, K.; Shukla, A.; Sharma, A.; Siddiqui, M.I.H.; Sadasivuni, K.K.; Priyadarshi, N.; Twala, B. A comprehensive review on eutectic phase change materials: Development, thermophysical properties, thermal stability, reliability, and applications. Alex. Eng. J. 2025, 112, 254–280. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, C.; Du, X. Research on the optimization control strategy of a battery thermal management system based on serpentine liquid cooling combined with phase change material. J. Power Sources 2025, 630, 236127. [Google Scholar] [CrossRef]
- Liu, Z.; Huadan, C.; Wang, B.; Li, P. Coupling optimization of protruding fin and PCM in hybrid cooling system and cycle strategy matching for lithium-ion battery thermal management. Int. J. Therm. Sci. 2025, 207, 109372. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Zhao, K.; Wang, J.; Xie, H. Preparation of PW@ CaCO3 phase change microcapsules modified by ZnO nanoparticles with excellent photocatalytic and thermal properties. J. Energy Storage 2024, 77, 109942. [Google Scholar] [CrossRef]
- Li, Q.; Wang, S.; Zhou, M.; Lu, X.; Qiao, G.; Li, C.; Wu, Y. A review of imidazolium ionic liquid-based phase change materials for low and medium temperatures thermal energy storage and their applications. Green Energy Resour. 2023, 1, 100010. [Google Scholar] [CrossRef]
- Gogoi, M.; Das, B.; Patowari, P.K. Characterization and performance analysis of modified phase change material with paraffin wax and waste exhaust carbon particles for thermal energy storage. J. Energy Storage 2025, 108, 115068. [Google Scholar] [CrossRef]
- Harmen, Y.; Chhiti, Y.; El Fiti, M.; Salihi, M.; Jama, C. Eccentricity analysis of annular multi-tube storage unit with phase change material. J. Energy Storage 2023, 64, 107211. [Google Scholar] [CrossRef]
- El Fiti, M.; Salihi, M.; Harmen, Y.; Chhiti, Y.; Chebak, A.; Bousalham, S.; Alaoui, F.E.M.H.; Achak, M.; Jama, C. Thermal performance enhancement of a longitudinal finned latent thermal energy storage unit. In Proceedings of the 2021 9th International Renewable and Sustainable Energy Conference (IRSEC), Virtual, 23–27 November 2021; pp. 1–6. [Google Scholar]
- Lin, X.; Qiu, C.; Wang, K.; Zhang, Y.; Wan, C.; Fan, M.; Wu, Y.; Sun, W.; Guo, X. Biomimetic bone tissue structure: An ultrastrong thermal energy storage wood. Chem. Eng. J. 2023, 457, 141351. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M. Nanoencapsulation of phase change materials (PCMs) and their applications in various fields for energy storage and management. Adv. Colloid Interface Sci. 2020, 283, 102226. [Google Scholar] [CrossRef]
- Said, Z.; Pandey, A.; Tiwari, A.K.; Kalidasan, B.; Jamil, F.; Thakur, A.K.; Tyagi, V.; Sarı, A.; Ali, H.M. Nano-enhanced phase change materials: Fundamentals and applications. Prog. Energy Combust. Sci. 2024, 104, 101162. [Google Scholar] [CrossRef]
- Schossig, P.; Henning, H.-M.; Haussmann, T.; Raicu, A. Encapsulated phase-change materials integrated into constructions materials. In Proceedings of the International Conference on Thermal Energy Storage, Warsaw, Poland, 1–4 September 2003. [Google Scholar]
- Salunkhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew. Sustain. Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Hua, W.; Chen, Z. Optimization of a silicon-based microencapsulation technology of phase change material without organic solvents. J. Energy Storage 2023, 64, 107122. [Google Scholar] [CrossRef]
- Ismail, A.; Zhou, J.; Aday, A.; Davidoff, I.; Odukomaiya, A.; Wang, J. Microencapsulation of bio-based phase change materials with silica coated inorganic shell for thermal energy storage. J. Build. Eng. 2023, 67, 105981. [Google Scholar] [CrossRef]
- Krump, H.; Alexy, P.; Luyt, A. Preparation of a maleated Fischer–Tropsch paraffin wax and FTIR analysis of grafted maleic anhydride. Polym. Test. 2005, 24, 129–135. [Google Scholar] [CrossRef]
- Mandal, S.; Ishak, S.; Adnin, R.J.; Lee, D.-E.; Park, T. An approach to utilize date seeds biochar as waste material for thermal energy storage applications. J. Energy Storage 2023, 68, 107739. [Google Scholar] [CrossRef]
- Hanif, F.; Zhang, Y.; Lu, R.; Tang, B. Enhanced encapsulation of paraffin wax in delignified porous wood framework for sustainable heat regulation in buildings. J. Energy Storage 2025, 110, 115290. [Google Scholar] [CrossRef]
- Mochane, M.; Luyt, A. Preparation and properties of polystyrene encapsulated paraffin wax as possible phase change material in a polypropylene matrix. Thermochim. Acta 2012, 544, 63–70. [Google Scholar] [CrossRef]
- Mandal, S.; Ishak, S.; Ariffin, M.A.M.; Lee, D.-E.; Park, T. Effect of pore structure on the thermal stability of shape-stabilized phase change materials. J. Mater. Res. Technol. 2023, 25, 465–479. [Google Scholar] [CrossRef]
- Sharifah, I.; Nurul, A.T.; Khairusshima, M.N. Thermal modelling and analysis of batik canting design. Procedia Eng. 2017, 184, 326–333. [Google Scholar] [CrossRef]
- Bucio, A.; Moreno-Tovar, R.; Bucio, L.; Espinosa-Dávila, J.; Anguebes-Franceschi, F. Characterization of beeswax, candelilla wax and paraffin wax for coating cheeses. Coatings 2021, 11, 261. [Google Scholar] [CrossRef]
- Mandal, S.; Ishak, S.; Lee, D.; Park, T. Shape-stabilized orange peel/myristic acid phase change materials for efficient thermal energy storage application. Energy Rep. 2022, 8, 9618–9628. [Google Scholar] [CrossRef]
- Valentini, F.; Dorigato, A.; Pegoretti, A.; Tomasi, M.; Sorarù, G.D.; Biesuz, M. Si3N4 nanofelts/paraffin composites as novel thermal energy storage architecture. J. Mater. Sci. 2021, 56, 1537–1550. [Google Scholar] [CrossRef]
- Fredi, G.; Dirè, S.; Callone, E.; Ceccato, R.; Mondadori, F.; Pegoretti, A. Docosane-organosilica microcapsules for structural composites with thermal energy storage/release capability. Materials 2019, 12, 1286. [Google Scholar] [CrossRef]
- Coward, J.L. FTIR spectroscopy of synthesized racemic nonacosan-10-ol: A model compound for plant epicuticular waxes. J. Biol. Phys. 2010, 36, 405–425. [Google Scholar] [CrossRef]
- Cao, W.; Xijun, L.; Yuwei, W.; Yong, Z.; Xianxin, H.; Congzhi, F. Study on Photodegradation Activity of Fe3O4@ SiO2@ TiO2-Ce/rGO Magnetic Photocatalyst. 2021. Available online: https://www.researchsquare.com/article/rs-338828/v1 (accessed on 2 April 2025).
- Netzahual-Lopantzi, Á.; Sánchez-Ramírez, J.F.; Jiménez-Pérez, J.L.; Cornejo-Monroy, D.; López-Gamboa, G.; Correa-Pacheco, Z.N. Study of the thermal diffusivity of nanofluids containing SiO2 decorated with Au nanoparticles by thermal lens spectroscopy. Appl. Phys. A 2019, 125, 588. [Google Scholar] [CrossRef]
- Jung, Y.; You, J.-O.; Youm, K.-H. Synthesis of monodispersed paraffin microcapsules by polycondensation using membrane emulsification and their latent heat properties. Macromol. Res. 2015, 23, 1004–1011. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Tian, Q.; Ma, Z.; Zhu, M. Making graphene oxide (GO)-cladded SiO2 spheres (SiO2@ GO) as inorganic fillers for dental restorative resin composites. Dent. Mater. 2023, 39, 1076–1084. [Google Scholar] [CrossRef]
- Mandal, S.; Kailath, A.J. Enhanced plasticity of Cu-Zr-Ti bulk metallic glass and its correlation with fragility. Metall. Mater. Trans. A 2019, 50, 199–208. [Google Scholar] [CrossRef]
- Mandal, S.; Sivakumar, B.; Singhbabu, Y.; Bandyopadhyay, N.; Chattopadhyay, P.; Kailath, A.J. Electrochemical behavior of Cu60Zr25Ti15 bulk metallic glass with the addition of Nb and Mo. J. Mater. Eng. Perform. 2019, 28, 6874–6884. [Google Scholar] [CrossRef]
- Mandal, S.; Lee, D.-E.; Park, T. Crystallization kinetics of Cu60Zr25Ti15 and (Cu60Zr25Ti15)95Ni5 bulk metallic glasses by differential scanning calorimetry (DSC). J. Therm. Anal. Calorim. 2021, 145, 467–474. [Google Scholar] [CrossRef]
- Huo, J.; Wang, Z.; Guo, H.; Xu, X.; Zhang, T.; Ji, X.; Zhou, X.; Zhang, H. Energy storage and hydrophobicity characteristics of cement-based materials containing paraffin-pumice at low air pressure. J. Energy Storage 2024, 77, 109973. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Wang, J.; Sun, L.; Xie, T.; Yang, K.; Li, Z. Preparation and characterization of high efficiency microencapsulated phase change material based on paraffin wax core and SiO2 shell derived from sodium silicate precursor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126905. [Google Scholar] [CrossRef]
- Peng, C.; Liu, Y.; Yu, C.; Zhao, Y. A synaptic transistor with a stacked layer of SiNx and SiO2 deposited from hexamethyldisiloxane/O2. Appl. Phys. Lett. 2025, 126, 022901. [Google Scholar] [CrossRef]
- Ishak, S.; Mandal, S.; Lee, H.-S.; Singh, J.K. Effect of core-shell ratio on the thermal energy storage capacity of SiO2 encapsulated lauric acid. J. Energy Storage 2021, 42, 103029. [Google Scholar] [CrossRef]
- Rajagopalan, K.; Ramasubramanian, B.; Manojkumar, K.; Ramakrishna, S.; Marappan, P.; Saminathan, R.K. Organo-metallic electrolyte additive for regulating hydrogen evolution and self-discharge in Mg–air aqueous battery. New J. Chem. 2022, 46, 19950–19962. [Google Scholar] [CrossRef]
- Ghufran, M.; Huitink, D. Synthesis of silica-encapsulated myristic acid phase-change-assisted nanocapsules for thermal management applications. Emergent Mater. 2024, 7, 1429–1443. [Google Scholar] [CrossRef]
- Ghufran, M.; Huitink, D. Synthesis of nano-size paraffin/silica-based encapsulated phase change materials of high encapsulation ratio via sol–gel method. J. Mater. Sci. 2023, 58, 7673–7689. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, T.; Li, Y.; Li, Y.; He, F.; Chen, Z.; Zhu, L.; He, C.; Yang, W. Paraffin@ Silica Microencapsulated Phase Change Materials with Improved Anti-Leakage Properties. ChemistrySelect 2022, 7, e202202930. [Google Scholar] [CrossRef]
- Fang, Y.; Wei, H.; Liang, X.; Wang, S.; Liu, X.; Gao, X.; Zhang, Z. Preparation and thermal performance of silica/n-tetradecane microencapsulated phase change material for cold energy storage. Energy Fuels 2016, 30, 9652–9657. [Google Scholar] [CrossRef]
- Ishak, S.; Mandal, S.; Lee, H.-S.; Lee, D.-E.; Chen, Z. Structure-property correlation of thermally activated nano-size phase change material in the cementitious system. J. Build. Eng. 2023, 66, 105871. [Google Scholar] [CrossRef]
- Hu, J.; He, J.; He, Y.; Fang, X.; Zhang, Z. Promising candidate for thermal management: Thermophysical and mechanical properties of h-BN/polyurea@ n-eicosane phase change microcapsules. Sol. Energy Mater. Sol. Cells 2025, 281, 113310. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; Wu, D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: Synthesis, microstructure, and performance evaluation. Appl. Energy 2014, 114, 632–643. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Fabrication and performances of microencapsulated phase change materials based on n-octadecane core and resorcinol-modified melamine–formaldehyde shell. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 129–138. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Synthesis and properties of microencapsulated n-octadecane with polyurea shells containing different soft segments for heat energy storage and thermal regulation. Sol. Energy Mater. Sol. Cells 2009, 93, 1366–1376. [Google Scholar] [CrossRef]
- Liu, H.; Niu, J.; Wang, X.; Wu, D. Design and construction of mesoporous silica/n-eicosane phase-change nanocomposites for supercooling depression and heat transfer enhancement. Energy 2019, 188, 116075. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, S.; Li, T.; Yang, L.; Li, D.; Bai, H.; Wang, X. Review on thermal properties with influence factors of solid–liquid organic phase-change micro/nanocapsules. Energies 2024, 17, 604. [Google Scholar] [CrossRef]
- Ishak, S.; Lgaz, H.; Mandal, S.; Adnin, R.J.; Lee, D.-E.; Lee, H.-S.; Harmay, N.S.M.; Abdullah, M.M.A.B.; Wang, X.-Y.; Yang, H.-M. Multi-technique investigation on the surface interaction of diatomaceous earth with organic phase change material: Experimental and molecular dynamics aspects. J. Mol. Liq. 2023, 391, 123292. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Zhao, Y.; Ling, Z.; Zhang, Z.; Fang, X. Compatible paraffin@ SiO2 microcapsules/polydimethylsiloxane composites with heat storage capacity and enhanced thermal conductivity for thermal management. Compos. Sci. Technol. 2022, 218, 109192. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Papadokostaki, K.G.; Favvas, E.P.; Efthimiadou, E.K.; Karellas, S. Preparation and investigation of distinct and shape stable paraffin/SiO2 composite PCM nanospheres. Energy Convers. Manag. 2018, 168, 382–394. [Google Scholar] [CrossRef]
- Luo, R.; Wang, S.; Wang, T.; Zhu, C.; Nomura, T.; Akiyama, T. Fabrication of paraffin@ SiO2 shape-stabilized composite phase change material via chemical precipitation method for building energy conservation. Energy Build. 2015, 108, 373–380. [Google Scholar] [CrossRef]
- Kang, L.; Ren, L.; Niu, H.; Lv, R.; Guo, H.; Bai, S. Paraffin@ SiO2 microcapsules-based phase change composites with enhanced thermal conductivity for passive battery cooling. Compos. Sci. Technol. 2022, 230, 109756. [Google Scholar] [CrossRef]
- Yuan, K.; Chen, Q.; Zhang, A.; Xiao, N.; Zou, X.; Lin, Z. Efficient thermal energy conversion and storage enabled by hybrid graphite nanoparticles/silica-encapsulated phase-change microcapsules. J. Mater. Chem. A 2024, 12, 2456–2464. [Google Scholar] [CrossRef]
- Şahan, N.; Paksoy, H. Determining influences of SiO2 encapsulation on thermal energy storage properties of different phase change materials. Sol. Energy Mater. Sol. Cells 2017, 159, 1–7. [Google Scholar] [CrossRef]
- Li, B.; Liu, T.; Hu, L.; Wang, Y.; Gao, L. Fabrication and properties of microencapsulated paraffin@ SiO2 phase change composite for thermal energy storage. ACS Sustain. Chem. Eng. 2013, 1, 374–380. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Shi, J. Synthesis and properties of phase change microcapsule with SiO2-TiO2 hybrid shell. Sol. Energy 2018, 167, 158–164. [Google Scholar] [CrossRef]
- He, F.; Wang, X.; Wu, D. Phase-change characteristics and thermal performance of form-stable n-alkanes/silica composite phase change materials fabricated by sodium silicate precursor. Renew. Energy 2015, 74, 689–698. [Google Scholar] [CrossRef]
- He, F.; Wang, X.; Wu, D. New approach for sol–gel synthesis of microencapsulated n-octadecane phase change material with silica wall using sodium silicate precursor. Energy 2014, 67, 223–233. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wu, D. Silica encapsulation of n-octadecane via sol–gel process: A novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J. Colloid Interface Sci. 2010, 343, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liang, S.; Wang, H.; Zhang, K.; Jia, X.; Tian, C.; Zhou, Y.; Wang, J. Morphological control and thermal properties of nanoencapsulated n-octadecane phase change material with organosilica shell materials. Energy Convers. Manag. 2016, 119, 151–162. [Google Scholar] [CrossRef]
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.; Wu, D. Design and synthesis of magnetic microcapsules based on n-eicosane core and Fe3O4/SiO2 hybrid shell for dual-functional phase change materials. Appl. Energy 2014, 134, 456–468. [Google Scholar] [CrossRef]
- Sun, K.; Liu, H.; Wang, X.; Wu, D. Innovative design of superhydrophobic thermal energy-storage materials by microencapsulation of n-docosane with nanostructured ZnO/SiO2 shell. Appl. Energy 2019, 237, 549–565. [Google Scholar] [CrossRef]
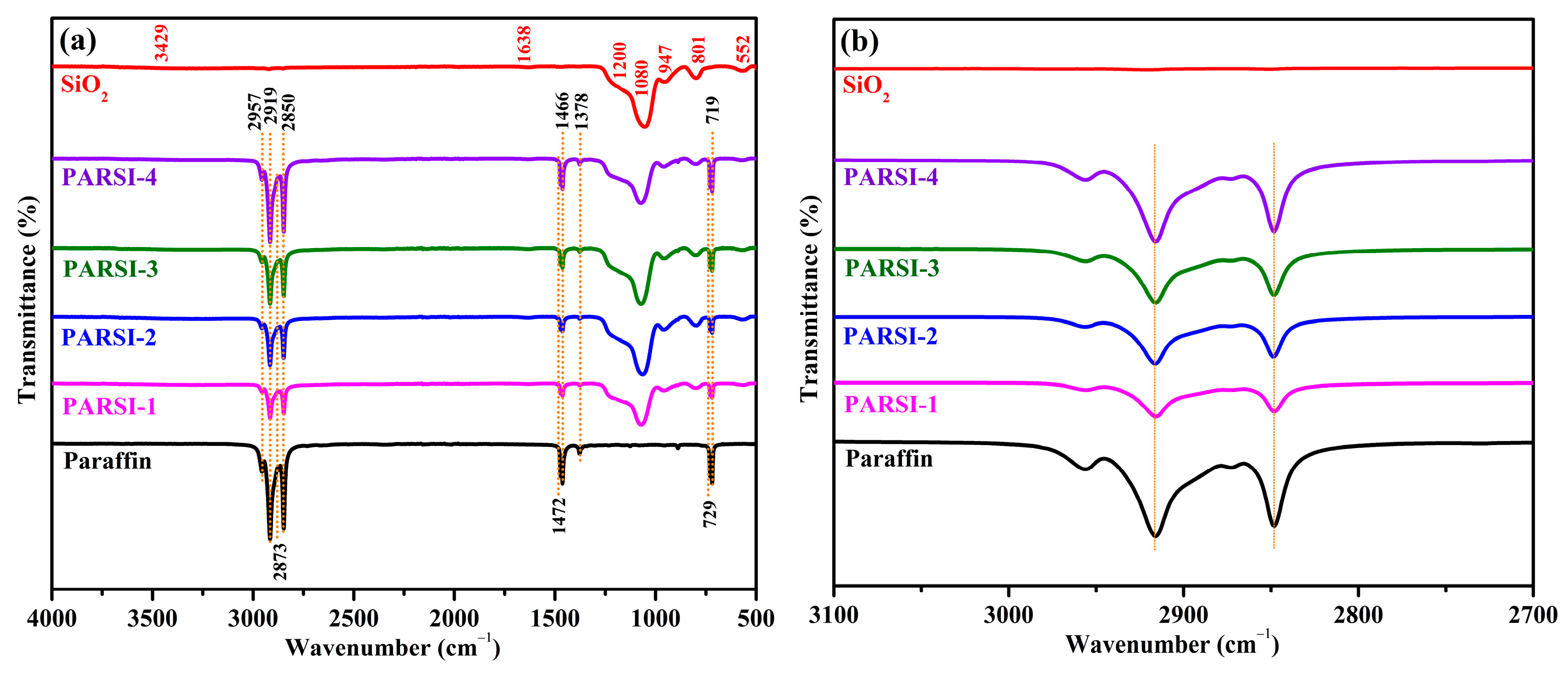


| Wavenumber (cm−1) | Vibration Type | Assignment |
|---|---|---|
| 2957 | asymmetric stretching | –CH3 |
| 2919 | asymmetric stretching | –CH2 |
| 2873 | symmetric stretching | –CH3 |
| 2850 | symmetric stretching | –CH2 |
| 1472 | in plane deformation | –CH2 |
| asymmetric in-plane deformation | –CH3 | |
| 1466 | in plane deformation | –CH2 |
| asymmetric in-plane deformation | –CH3 | |
| 1378 | symmetric carbon-hydrogen bending absorption | –CH3 |
| 719/729 | rocking | –CH2 |
| 3429 | stretching | –OH |
| 1638 | bending | –OH |
| 1200 | in-plane asymmetric stretching | Si–O–Si |
| 1080 | Out of plane asymmetric stretching | Si–O–Si |
| 947 | stretching | Si–OH |
| 801 | symmetric stretching | Si–O–Si |
| 552 | longitudinal optical vibration | Si–O–Si |
| Sample ID | Elements (Mass Norm.%) | ||
|---|---|---|---|
| C | O | Si | |
| PARSI-1 | 43.54 | 39.40 | 17.06 |
| PARSI-2 | 48.89 | 37.17 | 13.94 |
| PARSI-3 | 52.34 | 35.11 | 12.55 |
| PARSI-4 | 60.09 | 30.13 | 9.78 |
| Sample ID | Tm (°C) | ∆Hm (J/g) | Ts (°C) | ∆Hs (J/g) | ER% | EE% | η % |
|---|---|---|---|---|---|---|---|
| Paraffin | 61.08 ± 0.5 | 183.14 ± 1.2 | 53.09 ± 0.7 | 178.51 ± 1.4 | - | - | - |
| PARSI-1 | 63.83 ± 0.4 | 130.13 ± 1.6 | 53.30 ± 0.3 | 126.71 ± 1.7 | 71.05 ± 0.41 | 71.02 ± 0.40 | 99.95 |
| PARSI-2 | 62.68 ± 0.6 | 137.63 ± 0.9 | 50.70 ± 0.2 | 131.57 ± 2.1 | 75.15 ± 0.02 | 74.44 ± 0.31 | 99.05 |
| PARSI-3 | 62.42 ± 0.1 | 152.98 ± 1.4 | 53.87 ± 0.6 | 147.49 ± 1.8 | 83.53 ± 0.22 | 83.08 ± 0.28 | 99.46 |
| PARSI-4 | 60.05 ± 0.3 | 160.86 ± 1.5 | 53.50 ± 0.3 | 153.93 ± 1.1 | 87.83 ± 0.25 | 87.04 ± 0.09 | 99.10 |
| Serial No. | Core Material | Shell Material | ∆Hm (J/g) | ∆Hs (J/g) | ER% | EE% | Refs. |
|---|---|---|---|---|---|---|---|
| 1. | Paraffin | SiO2 | 94.4 | 93.2 | 74.51 | 75.58 | [70] |
| 2. | Paraffin | SiO2 | 161.4 | 158.1 | - | 80 | [86] |
| 3. | Paraffin | SiO2 | 156.6 | 158.1 | 78.2 | 78.4 | [87] |
| 4. | Paraffin | SiO2 | 117.7 | 110.8 | 61.88 | 61.84 | [88] |
| 5. | Paraffin | SiO2 | 189.4 | - | - | 81.3 | [89] |
| 6. | Paraffin | SiO2 | 191.67 | 188.84 | 75.97 | 75.82 | [76] |
| 7. | Paraffin | SiO2 | 91.9 | 89.7 | - | 52.7 | [90] |
| 8. | Paraffin | SiO2 | 79.2 | 83.5 | 42.33 | 44.13 | [78] |
| 9. | Paraffin | SiO2 | 13 | - | 11 | - | [91] |
| 10. | Paraffin | SiO2 | 45.5 | 43.8 | 31.7 | 31.1 | [92] |
| 11. | Paraffin | SiO2 | 94.4 | 93.2 | 74.51 | 75.58 | [70] |
| 12. | Paraffin | SiO2–TiO2 | 93.70 | - | 39.80 | - | [93] |
| 13. | Heptadecane | SiO2 | 60.25 | 61.37 | 30.94 | 30.48 | [94] |
| 14. | Octadecane | SiO2 | 73.52 | 72.18 | 35.62 | 35.18 | [94] |
| 15. | Octadecane | SiO2 | 87.46 | 84.89 | 41.83 | 41.45 | [95] |
| 16. | Octadecane | SiO2 | 123.00 | 125.40 | 57.50 | 57.70 | [96] |
| 17. | Octadecane | SiO2 | 109.9 | 103.4 | 53.8 | - | [97] |
| 18. | Octadecane | SiO2 | 109.5 | 98.85 | 51.5 | 49.3 | [98] |
| 19. | Nonadecane | SiO2 | 74.78 | 80.79 | 41.12 | 40.64 | [94] |
| 20. | Eicosane | SiO2 | 81.21 | 78.63 | 32.97 | 32.51 | [94] |
| 21. | Eicosane | SiO2 | 170.20 | 169.60 | 71.78 | 71.72 | [99] |
| 22. | Docosane | SiO2 | 181.5 | - | 78.6 | 80.8 | [100] |
| 23. | Paraffin | SiO2 | 160.86 | 153.93 | 87.83 | 87.04 | This study |
| Sample | Core/Shell Ratio | Ethanol (mL) | Paraffin (g) | TEOS (mL) | CTAB (g) | Ammonia Water (mL) |
|---|---|---|---|---|---|---|
| PARSI-1 | 1:1 | 50 | 1 | 1 | 1 | 6 |
| PARSI-2 | 2:1 | 2 | ||||
| PARSI-3 | 3:1 | 3 | ||||
| PARSI-4 | 4:1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnin, R.J.; Lee, H.-S. Advancing Thermal Energy Storage: Synthesis and Thermal Performance of Silica-Encapsulated Paraffin PCMs. Molecules 2025, 30, 1698. https://doi.org/10.3390/molecules30081698
Adnin RJ, Lee H-S. Advancing Thermal Energy Storage: Synthesis and Thermal Performance of Silica-Encapsulated Paraffin PCMs. Molecules. 2025; 30(8):1698. https://doi.org/10.3390/molecules30081698
Chicago/Turabian StyleAdnin, Raihana Jannat, and Han-Seung Lee. 2025. "Advancing Thermal Energy Storage: Synthesis and Thermal Performance of Silica-Encapsulated Paraffin PCMs" Molecules 30, no. 8: 1698. https://doi.org/10.3390/molecules30081698
APA StyleAdnin, R. J., & Lee, H.-S. (2025). Advancing Thermal Energy Storage: Synthesis and Thermal Performance of Silica-Encapsulated Paraffin PCMs. Molecules, 30(8), 1698. https://doi.org/10.3390/molecules30081698







