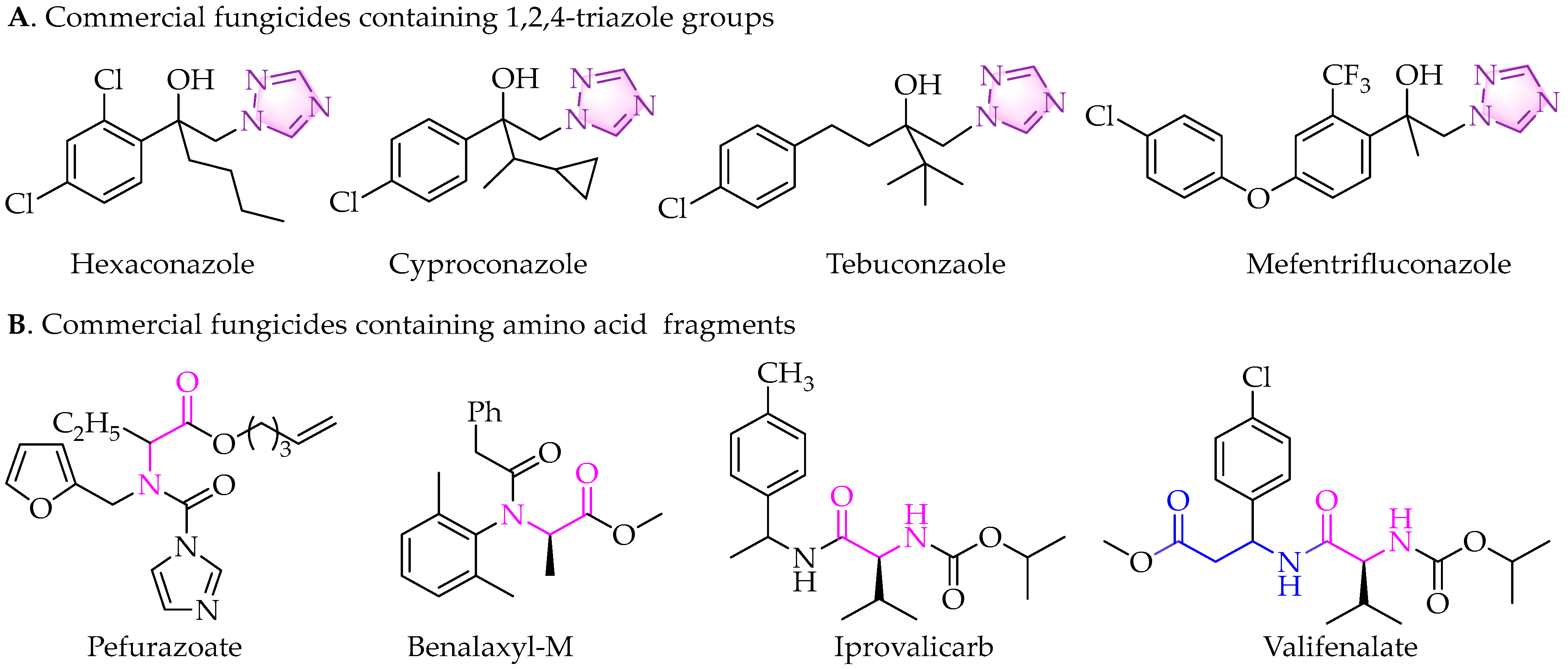
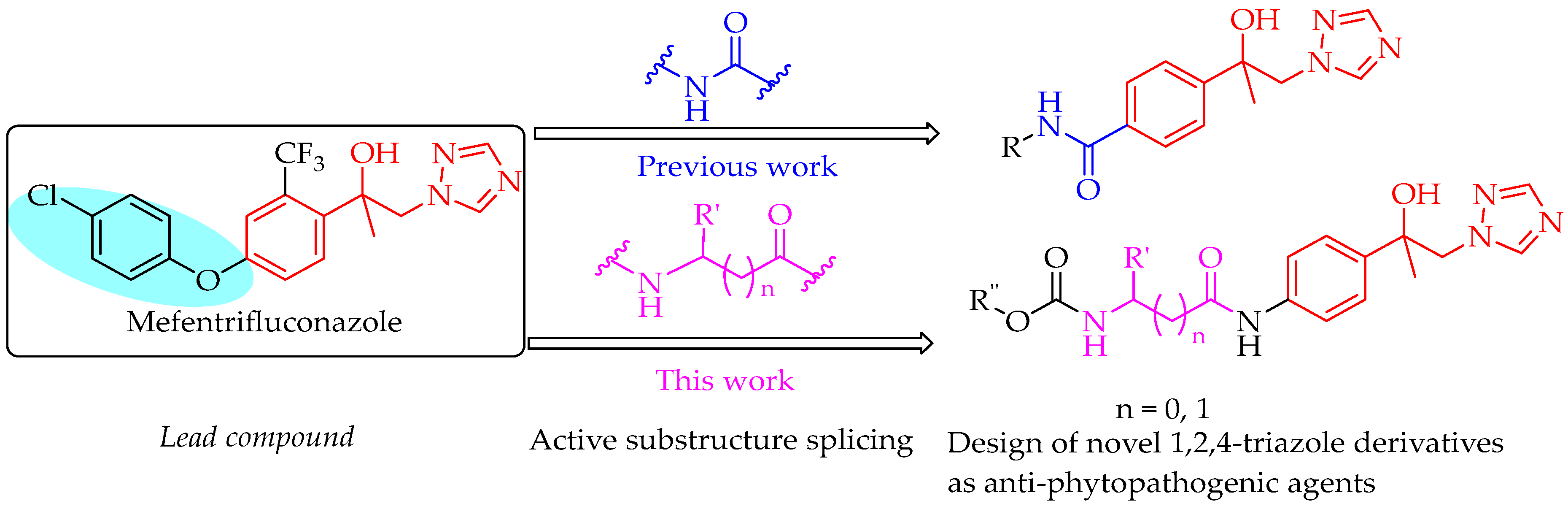
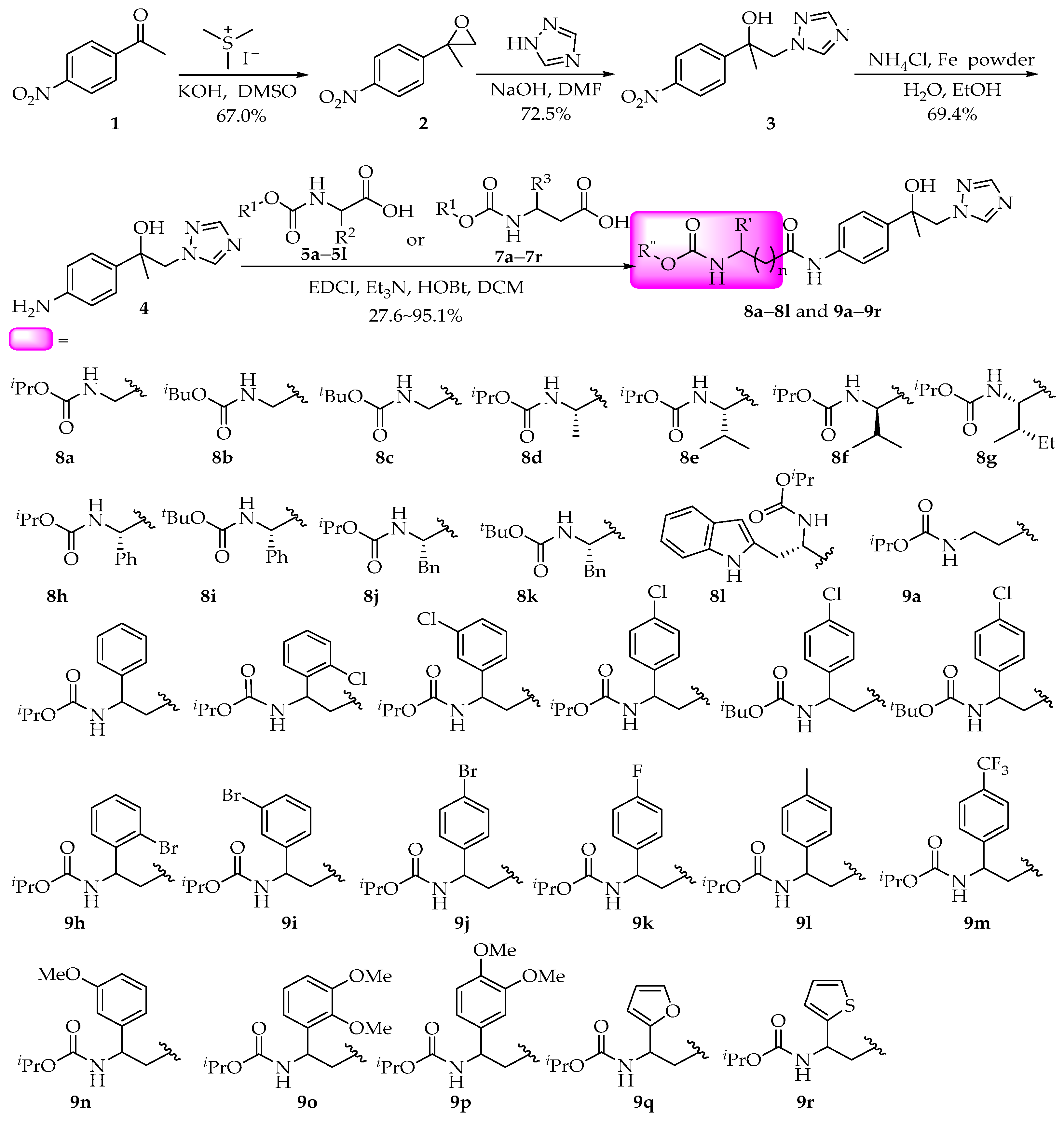4.5. Preparation of Compounds 8–9
A nitrogen-purged solution of KOH (1.529 g, 27.248 mmol) in anhydrous dimethyl sulfoxide (DMSO) (15 mL) was charged with trimethylsulfonium iodide (Me3S+I−) (7.414 g, 36.331 mmol) dissolved in anhydrous DMSO (75 mL) via cannula transfer. After 1 h of vigorous stirring at ambient temperature, compound 1 (3.000 g, 18.165 mmol) in anhydrous DMSO (30 mL) was added dropwise over 15 min. The resulting mixture was agitated for 2 h before being quenched with saturated NH4Cl solution (50 mL). Subsequent liquid–liquid extraction with EtOAc (3 × 100 mL), solvent evaporation under vacuum, and silica gel chromatography (hexane/EtOAc 3:1) afforded compound 2 as white crystals. The crude product was purified by column chromatography to obtain compound 2. Light yellow solid, 67.0% yield, m.p. 33–34 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.20 (d, J = 8.5 Hz, 2H), 7.62 (d, J = 8.4 Hz, 2H), 3.09 (d, J = 5.5 Hz, 1H), 2.82 (d, J = 5.1 Hz, 1H), 1.70 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 149.3, 147.3, 127.2, 124.0, 57.2, 56.6, 21.3.
A suspension containing compound 2 (2.000 g, 11.162 mmol), 1,2,4-triazole (3.084 g, 44.648 mmol), and NaOH (0.893 g, 22.324 mmol) in anhydrous N,N-dimethylformamide (DMF) (40 mL) was refluxed at 110 °C for 4 h under N2. Following aqueous workup with saturated NH4Cl (30 mL), the product underwent sequential extraction (EtOAc 5 × 30 mL), brine wash, and desiccation over MgSO4. Recrystallization from the mixtures of CH2Cl2/diethyl ether yielded compound 3. White solid, 72.5% yield, m.p. 148–150 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.27 (s, 1H), 8.15 (d, J = 8.4 Hz, 2H), 7.81 (s, 1H), 7.70 (d, J = 8.5 Hz, 2H), 5.90 (s, 1H), 4.47 (s, 2H), 1.49 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 154.0, 151.0, 146.9, 145.4, 127.3, 123.5, 73.3, 59.5, 27.6.
Compound 3 (3.000 g, 12.084 mmol), NH4Cl (0.646 g, 12.084 mmol), and H2O (10 mL) were added into anhydrous ethanol (60 mL). Iron powder (2.025 g, 36.252 mmol) was added, and the mixture stirred at 80 °C for 4 h. After cooling to room temperature, the solid was filtered, and the filtrate was extracted with ethyl acetate (3 × 15 mL). After evaporating the organic solvent, chromatographic purification gave compound 4 as a pale brown liquid with 69.4% yield. 1H NMR (400 MHz, DMSO-d6) δ 8.14 (s, 1H), 7.86 (s, 1H), 7.06 (d, J = 8.0 Hz, 2H), 6.49 (d, J = 7.9 Hz, 2H), 5.25 (s, 1H), 4.94 (s, 2H), 4.24 (s, 2H), 1.31 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 150.7, 147.7, 145.2, 133.5, 126.2, 113.9, 72.7, 60.5, 27.4.
To a stirred solution of compound 4 (0.218 g, 1.0 mmol) in CH2Cl2 (5 mL) was sequentially added Et3N (0.202 g, 2.0 mmol), 1-hydroxybenzotriazole (HOBt) (0.203 g, 1.5 mmol), and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) (0.288 g, 1.5 mmol). After 30 min activation, amino acid derivatives 5a–5l or 7a–7r (1.1 mmol) were introduced portions. The reaction was monitored by TLC until completion (~12 h), then subjected to aqueous extraction (H2O/EtOAc 10 mL/15 mL ×3). The organic phase was dried over Na2SO4 and concentrated to afford crude product for subsequent characterization. After evaporating the solvent, the crude product was purified by column chromatography to obtain compounds 8a–8l and 9a–9r.
Isopropyl (2-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-2-oxoethyl)carbamate (8a). White solid, 50.0% yield, m.p. 176–178 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.90 (s, 1H), 8.19 (s, 1H), 7.85 (s, 1H), 7.50 (d, J = 8.4 Hz, 2H), 7.34 (d, J = 8.3 Hz, 2H), 7.25 (t, J = 5.7 Hz, 1H), 5.49 (s, 1H), 4.79–4.72 (m, 1H), 4.37–4.29 (m, 2H), 3.75 (d, J = 6.1 Hz, 2H), 1.39 (s, 3H), 1.18 (d, J = 6.2 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 168.5, 156.9, 150.8, 145.3, 141.1, 138.0, 126.1, 119.0, 72.9, 67.6, 60.1, 44.4, 27.5, 22.5; HR-MS (ESI): calcd for C17H23N5O4 [M + H]+ 362.1823, found (ESI+) 362.1827.
Isobutyl (2-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-2-oxoethyl)carbamate (8b). White solid, 53.0% yield, m.p. 74–76 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.93 (s, 1H), 8.20 (s, 1H), 7.85 (s, 1H), 7.50 (d, J = 8.5 Hz, 2H), 7.42–7.32 (m, 3H), 5.50 (s, 1H), 4.37–4.28 (m, 2H), 3.77–3.74 (m, 4H), 1.92–1.75 (m, 1H), 1.39 (s, 3H), 0.89 (d, J = 6.7 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 168.5, 157.4, 150.8, 145.3, 141.1, 138.0, 126.1, 119.0, 72.9, 70.5, 60.1, 44.4, 28.1, 27.5, 19.4; HR-MS (ESI): calcd for C18H25N5O4 [M + H]+ 376.1980, found (ESI+) 376.1986.
Tert-butyl (2-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-2-oxoethyl)carbamate (8c). White solid, 64.0% yield, m.p. 109–111 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.89 (s, 1H), 8.20 (s, 1H), 7.85 (s, 1H), 7.50 (d, J = 8.6 Hz, 2H), 7.34 (d, J = 8.7 Hz, 2H), 7.08–7.02 (m, 1H), 5.49 (s, 1H), 4.37–4.28 (m, 2H), 3.70 (d, J = 6.1 Hz, 2H), 1.39 (s, 12H); 13C NMR (100 MHz, DMSO-d6) δ 168.6, 156.4, 150.9, 145.3, 141.1, 138.1, 126.1, 119.0, 78.6, 72.7, 60.1, 44.2, 28.7, 27.5; HR-MS (ESI): calcd for C18H25N5O4 [M + H]+ 376.1980, found (ESI+) 376.1983.
Isopropyl ((2S)-1-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-1-oxopropan-2-yl)carbamate (8d). White solid, 37.0% yield, m.p. 86–88 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.92 (s, 1H), 8.20 (s, 1H), 7.85 (s, 1H), 7.51 (d, J = 7.8 Hz, 2H), 7.32 (m, 3H), 5.50 (s, 1H), 4.75–4.70 (m, 1H), 4.37–4.28 (m, 2H), 4.19–4.11 (m, 1H), 1.39 (s, 3H), 1.26 (d, J = 6.8 Hz, 3H), 1.16 (d, J = 6.3 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 172.2, 156.1, 150.9, 145.3, 141.1, 138.2, 126.0, 119.1, 72.9, 67.4, 60.1, 51.1, 27.6, 22.6, 18.6. HR-MS (ESI): calcd for C18H25N5O4 [M + H]+ 376.1980, found (ESI+) 376.1980.
Isopropyl ((2S)-1-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (8e). White solid, 66.0%yield, m.p. 91–93 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.98 (s, 1H), 8.22 (s, 1H), 7.86 (s, 1H), 7.52 (d, J = 8.2 Hz, 2H), 7.35 (d, J = 8.3 Hz, 2H), 7.14 (d, J = 8.5 Hz, 1H), 5.49 (s, 1H), 4.78–4.71 (m, 1H), 4.37–4.29 (m, 2H), 3.94 (t, J = 8.1 Hz, 1H), 2.01–1.94 (m, 1H), 1.38 (s, 3H), 1.17 (t, J = 6.0 Hz, 6H), 0.89 (d, J = 6.8 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 171.1, 156.5, 150.9, 145.3, 141.3, 137.9, 126.1, 119.2, 72.9, 67.5, 61.4, 60.1, 30.8, 27.6, 22.6, 19.7, 19.0; HR-MS (ESI): calcd for C20H29N5O4 [M + H]+ 404.2293, found (ESI+) 404.2296.
Isopropyl ((2R)-1-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (8f). White solid, 64.0% yield, m.p. 90–92 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.98 (s, 1H), 8.22 (s, 1H), 7.86 (s, 1H), 7.52 (d, J = 8.1 Hz, 2H), 7.35 (d, J = 8.3 Hz, 2H), 7.14 (d, J = 8.5 Hz, 1H), 5.49 (s, 1H), 4.38–4.29 (m, 1H), 4.75–4.70 (m, 1H), 3.94 (t, J = 8.0 Hz, 1H), 2.03–1.94 (m, 1H), 1.38 (s, 3H), 1.17 (t, J = 6.0 Hz, 6H), 0.89 (d, J = 6.9 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 171.1, 156.5, 150.9, 145.3, 141.3, 137.9, 126.1, 119.2, 72.9, 67.5, 61.4, 60.1, 30.8, 27.6, 22.6, 19.7, 19.0; HR-MS (ESI): calcd for C20H29N5O4 [M + H]+ 404.2293, found (ESI+) 404.2292.
Isopropyl ((2S,3S)-1-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-methyl-1-oxopentan-2-yl)carbamate (8g). White solid, 56.0% yield, m.p. 85–87 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.99 (s, 1H), 8.22 (s, 1H), 7.86 (s, 1H), 7.52 (d, J = 8.2 Hz, 2H), 7.35 (d, J = 8.3 Hz, 2H), 7.15 (d, J = 7.8 Hz, 1H), 5.50 (s, 1H), 4.79–4.67 (m, 1H), 4.34–4.33 (m, 2H), 3.99–3.96 (m, 1H), 1.76 (s, 1H), 1.48 (s, 1H), 1.38 (s, 3H), 1.18–1.15 (m, 6H), 0.86–0.83 (m, 6H); 13C NMR (100 MHz, DMSO-d6) δ 171.3, 156.4, 150.8, 145.3, 141.3, 137.9, 126.1, 119.2, 72.9, 67.6, 60.2, 60.0, 36.8, 27.6, 25.1, 22.5, 15.8, 11.3; HR-MS (ESI): calcd for C21H31N5O4 [M + H]+ 418.2449, found (ESI+) 418.2446.
Isopropyl ((1S)-2-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-2-oxo-1-phenylethyl)carbamate (8h). White solid, 43.0% yield, m.p. 111–114 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.25 (s, 1H), 8.20 (s, 1H), 7.84 (s, 1H), 7.78 (d, J = 8.3 Hz, 1H), 7.50 (d, J = 4.8 Hz, 2H), 7.37–7.27 (m, 5H), 5.50 (s, 1H), 5.40 (d, J = 8.4 Hz, 1H), 4.81–4.74 (m, 1H), 4.36–4.28 (m, 1H), 1.38 (s, 3H), 1.16–1.12 (m, 6H); 13C NMR (100 MHz, DMSO-d6) δ 169.4, 156.1, 150.8, 145.3, 141.5, 138.4, 137.8, 128.9, 128.4, 127.9, 126.1, 119.1, 72.9, 67.9, 60.1, 59.2, 27.6, 22.5; HR-MS (ESI): calcd for C23H27N5O4 [M + H]+ 438.2136, found (ESI+) 438.2134.
Tert-butyl ((1S)-2-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-2-oxo-1-phenylethyl)carbamate (8i). White solid, 80.0% yield, m.p. 110–113 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.22 (s, 1H), 8.20 (s, 1H), 7.84 (s, 1H), 7.49 (d, J = 8.8 Hz, 5H), 7.37–7.27 (m, 5H), 5.49 (s, 1H), 5.34 (d, J = 8.3 Hz, 1H), 4.31 (d, J = 6.2 Hz, 2H), 1.39 (s, 9H), 1.37 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 169.5, 155.6, 150.9, 145.3, 141.5, 138.6, 137.9, 128.9, 128.3, 127.9, 126.1, 119.1, 79.0, 72.9, 60.1, 59.0, 28.7, 27.6; HR-MS (ESI): calcd for C24H29N5O4 [M + H]+ 452.2293, found (ESI+) 452.2299.
Isopropyl ((2S)-1-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-1-oxo-3-phenylpropan-2-yl)carbamate (8j). White solid, 45.0% yield, m.p. 96–98 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.06 (s, 1H), 8.21 (s, 1H), 7.86 (s, 1H), 7.51 (d, J = 8.1 Hz, 2H), 7.36–7.26 (m, 7H), 7.21–7.17 (m, 1H), 5.51 (s, 1H), 4.68–4.62 (m, 1H), 4.38–4.33 (m, 3H), 3.01–2.95 (m, 1H), 2.83 (t, J = 12.0 Hz, 1H), 1.39 (s, 3H), 1.13 (d, J = 6.2 Hz, 3H), 1.07 (d, J = 6.2 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 171.2, 156.3, 150.9, 145.3, 141.3, 138.5, 138.0, 129.8, 128.6, 126.8, 126.1, 119.2, 72.9, 67.5, 60.1, 57.3, 38.0, 27.6, 22.5; HR-MS (ESI): calcd for C24H29N5O4 [M + H]+ 452.2293, found (ESI+) 452.2294.
Tert-butyl ((2S)-1-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-1-oxo-3-phenylpropan-2-yl)carbamate (8k). White solid, 78.0% yield, m.p. 100–102 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.02 (s, 1H), 8.22 (s, 1H), 7.86 (s, 1H), 7.51 (d, J = 8.7 Hz, 2H), 7.39–7.25 (m, 6H), 7.19 (t, J = 7.1 Hz, 1H), 7.11 (d, J = 8.3 Hz, 1H), 5.50 (s, 1H), 4.34–4.28 (m, 3H), 3.01–2.96 (m, 1H), 2.86–2.80 (m, 1H), 1.39 (s, 3H), 1.32 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 171.2, 155.9, 150.9, 145.3, 141.3, 138.5, 138.0, 129.7, 128.6, 126.8, 126.1, 119.2, 78.6, 72.9, 60.1, 57.0, 38.0, 28.7, 27.6. HR-MS (ESI): calcd for C25H31N5O4 [M + H]+ 466.2449, found (ESI+) 466.2447.
Isopropyl ((2S)-1-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamate (8l). White solid, 55.0% yield, m.p. 125–127 °C; 1H NMR (400 MHz, DMSO-d6) δ 10.82 (s, 1H), 10.09 (s, 1H), 8.22 (s, 1H), 7.86 (s, 1H), 7.68 (d, J = 7.8 Hz, 1H), 7.53 (d, J = 8.2 Hz, 2H), 7.39–7.30 (m, 3H), 7.26–7.16 (m, 2H), 7.06 (t, J = 7.6 Hz, 1H), 6.98 (t, J = 7.5 Hz, 1H), 5.51 (s, 1H), 4.69–4.65 (m, 1H), 4.42–4.33 (m, 3H), 3.12 (m, 1H), 3.05–2.93 (m, 1H), 1.40 (s, 3H), 1.15 (d, J = 6.2 Hz, 3H), 1.08 (d, J = 6.2 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 171.5, 156.2, 150.9, 145.3, 141.3, 138.1, 136.5, 127.8, 126.0, 124.4, 121.4, 119.4, 119.2, 118.7, 111.8, 110.4, 72.9, 67.5, 60.1, 56.5, 28.3, 27.6, 22.5; HR-MS (ESI): calcd for C26H30N6O4 [M + H]+ 491.2402, found (ESI+) 491.2401.
Isopropyl (3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9a). Yellow viscous solid, 27.6% yield, m.p. 79–81 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.90 (s, 1H), 8.19 (s, 1H), 7.85 (s, 1H), 7.50 (d, J = 8.3 Hz, 2H), 7.32 (d, J = 8.1 Hz, 2H), 7.05 (s, 1H), 5.48 (s, 1H), 4.75–4.72 (m, 1H), 4.36–4.28 (m, 2H), 3.24 (m, 2H), 2.46 (t, J = 7.2 Hz, 2H), 1.38 (s, 3H), 1.15 (d, J = 6.2 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 169.9, 156.5, 150.8, 145.2, 140.9, 138.2, 126.0, 119.1, 79.7, 79.4, 79.0, 72.9, 60.1, 37.2, 27.5, 22.5; HR-MS (ESI): calcd for C18H25N5O4 [M + H]+ 376.1980, found (ESI+) 376.1986.
Isopropyl (3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxo-1-phenylpropyl)carbamate (9b). White solid, 41.9% yield, m.p. 113–115 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.84 (s, 1H), 8.19 (s, 1H), 7.85 (s, 1H), 7.72 (d, J = 7.7 Hz, 1H), 7.44 (d, J = 8.5 Hz, 2H), 7.31 (d, J = 8.6 Hz, 6H), 7.22 (t, J = 6.9 Hz, 1H), 5.47 (s, 1H), 5.05 (d, J = 8.2 Hz, 1H), 4.71–4.64 (m, 1H), 4.36–4.27 (m, 2H), 2.72 (d, J = 7.5 Hz, 2H), 1.37 (s, 3H), 1.15–1.10 (m, 6H); 13C NMR (100 MHz, DMSO-d6) δ 168.7, 155.6, 150.8, 145.2, 143.7, 141.1, 138.1, 128.7, 127.9, 127.4, 126.8, 126.0, 119.1, 110.1, 79.7, 79.4, 79.1, 72.9, 67.3, 60.1, 52.1, 44.0, 27.5, 22.5; HR-MS (ESI): calcd for C24H29N5O4 [M + H]+ 452.2293, found (ESI+) 452.2290.
Isopropyl (1-(2-chlorophenyl)-3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9c). White solid, 54.6% yield, m.p. 132–134 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.84 (s, 1H), 8.19 (s, 1H), 7.85 (s, 1H), 7.77 (d, J = 8.0 Hz, 1H), 7.47 (d, J = 8.1 Hz, 3H), 7.40 (d, J = 7.8 Hz, 1H), 7.36–7.32 (m, 3H), 7.26 (t, J = 7.7 Hz, 1H), 5.48 (s, 1H), 5.42 (d, J = 6.7 Hz, 1H), 4.71–4.66 (m, 1H), 4.36–4.28 (m, 2H), 1.38 (s, 3H), 1.15 (d, J = 5.6 Hz, 6H), 1.12 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 168.2, 155.5, 150.8, 145.2, 141.1, 138.1, 132.0, 129.8, 129.2, 128.0, 127.9, 126.0, 119.1, 72.9, 67.6, 60.1, 49.1, 42.1, 27.6, 22.5; HR-MS (ESI): calcd for C24H28ClN5O4 [M + H]+ 486.1903, found (ESI+) 486.1901.
Isopropyl (1-(3-chlorophenyl)-3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9d). Yellow solid, 95.1% yield, m.p. 144–146 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.87 (s, 1H), 8.19 (s, 1H), 7.85 (s, 1H), 7.82–7.71 (m, 1H), 7.41 (t, J = 11.5 Hz, 3H), 7.33–7.27(m, 5H), 5.47 (s, 1H), 5.04 (q, J = 8.0 Hz, 1H), 4.75–4.63 (m, 1H), 4.34–4.27 (m, 2H), 2.73 (d, J = 7.5 Hz, 2H), 1.37 (s, 3H), 1.16–1.11 (m, 6H); 13C NMR (100 MHz, DMSO-d6) δ 168.5, 155.7, 150.8, 146.1, 145.2, 141.1, 138.0, 133.4, 130.8, 127.6, 126.7, 126.0, 125.7, 125.1, 119.2, 110.2, 72.9, 67.6, 60.0, 51.8, 43.6, 27.5, 22.5; HR-MS (ESI): calcd for C24H28ClN5O4 [M + H]+ 486.1903, found (ESI+) 486.1901.
Isopropyl (1-(4-chlorophenyl)-3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9e). White solid, 80.9% yield, m.p. 178–181 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.85 (s, 1H), 8.19 (s, 1H), 7.85 (s, 1H), 7.74 (d, J = 8.6 Hz, 1H), 7.39–7.31 (m, 6H), 5.45 (s, 1H), 5.03 (q, J = 7.8 Hz, 1H), 4.72–4.66 (m, 1H), 4.36–4.27 (m, 2H), 2.74–2.72 (m, 2H), 1.37 (s, 3H), 1.2 (d, J = 6.4 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 168.6, 155.7, 150.7, 145.2, 142.5, 141.1, 137.9, 132.0, 128.8, 128.7, 126.0, 72.9, 67.6, 60.0, 51.6, 43.6, 27.5, 22.5; HR-MS (ESI): calcd for C24H28ClN5O4 [M + H]+ 486.1903, found (ESI+) 486.1906.
Isobutyl (1-(4-chlorophenyl)-3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9f). White solid, 50.0% yield, m.p. 168–170 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.87 (s, 1H), 8.19 (s, 1H), 7.85 (s, 2H), 7.44 (d, J = 8.4 Hz, 2H), 7.39–7.34 (m, 5H), 7.32 (d, J = 8.4 Hz, 2H), 5.48 (s, 1H), 5.03 (q, J = 7.6 Hz, 1H), 4.36–4.31(m, 2H), 3.73–3.63 (m, 2H), 2.73 (d, J = 7.7 Hz, 2H), 1.82–1.76 (m, 1H), 1.37 (s, 3H), 0.83 (d, J = 6.6 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 168.5, 156.2, 150.8, 145.2, 142.5, 141.1, 138.0, 132.0, 130.0, 128.8, 128.7, 126.0, 119.2, 72.9, 70.4, 60.0, 51.7, 43.6, 28.1, 27.5, 19.3; HR-MS (ESI): calcd for C25H30ClN5O4 [M + H]+ 500.2060, found (ESI+) 500.2061.
Tert-butyl (1-(4-chlorophenyl)-3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9g). White solid, 72.5% yield, m.p. 196–198 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.85 (s, 1H), 8.19 (s, 1H), 7.85 (s, 1H), 7.56 (d, J = 8.1 Hz, 1H), 7.44 (d, J = 8.3 Hz, 2H), 7.36–7.31 (m, 6H), 5.46 (s, 1H), 5.00 (d, J = 8.1 Hz, 1H), 4.32 (dd, J = 20.4,14.0 Hz, 2H), 2.71 (d, J = 7.5 Hz, 2H), 1.37 (s, 3H), 1.34 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ 168.5, 155.2, 150.9, 145.3, 142.9, 141.1, 138.1, 131.9, 128.8, 128.7, 126.0, 119.1, 78.5, 72.9, 60.1, 51.3, 43.8, 28.7, 27.5; HR-MS (ESI): calcd for C25H30ClN5O4 [M + H]+ 500.2060, found (ESI+) 500.2063.
Isopropyl (1-(2-bromophenyl)-3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9h). Yellow solid, 34.3% yield, m.p. 128–130 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.83 (s, 1H), 8.18 (s, 1H), 7.84 (s, 1H), 7.78 (d, J = 8.0 Hz, 1H), 7.57 (d, J = 8.0 Hz, 1H), 7.46 (t, J = 7.6 Hz, 4H), 7.33 (d, J = 8.3 Hz, 2H), 7.18 (t, J = 7.8 Hz, 1H), 5.49 (s, 1H), 5.36 (q, J = 8.0 Hz, 1H), 4.71–4.65 (m, 1H), 4.36–4.27 (m, 2H), 2.65–2.67(m, 2H), 1.38 (s, 3H), 1.15 (d, J = 5.6 Hz, 3H); 1.12 (d, J = 6.0 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 168.2, 155.5, 150.8, 145.2, 142.8, 141.1, 138.1, 133.1, 110.2, 129.4, 128.5, 128.0, 126.2, 126.0, 122.5, 119.1, 118.6, 113.9, 79.7, 79.4, 79.1, 72.9, 67.5, 60.1, 51.4, 42.1, 27.5, 22.5; HR-MS (ESI): calcd for C24H28BrN5O4 [M + H]+ 530.1398, found (ESI+) 530.1397.
Isopropyl (1-(3-bromophenyl)-3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9i). White solid, 54.9% yield, m.p. 97–99 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.87 (s, 1H), 8.19 (s, 1H), 7.84 (s, 1H), 7.77 (d, J = 8.6 Hz, 1H), 7.54 (s, 1H), 7.43 (t, J = 8.5 Hz, 3H), 7.33–7.27 (m, 4H), 5.48 (s, 1H), 5.02 (q, J = 8.1 Hz, 1H), 4.71–4.67 (m, 1H), 4.36–4.27 (m, 2H), 2.73 (d, J = 7.3 Hz, 2H), 1.37 (s, 3H), 1.15 (d, J = 5.6 Hz, 3H); 1.12 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 168.4, 155.7, 150.8, 146.4, 145.2, 141.1, 138.0, 131.0, 130.3, 129.7, 126.0, 126.0, 122.1, 119.2, 118.6, 79.7, 79.4, 79.0, 72.9, 67.6, 60.1, 51.8, 43.7, 27.5, 22.5; HR-MS (ESI): calcd for C24H28BrN5O4 [M + H]+ 530.1398, found (ESI+) 530.1395.
Isopropyl (1-(4-bromophenyl)-3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9j). White solid, 29.8% yield, m.p. 195–198 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.86 (s, 1H), 8.19 (s, 1H), 7.85 (s, 1H), 7.74 (dd, J = 23.2, 8.3 Hz, 1H), 7.51 (d, J = 8.0 Hz, 2H), 7.44 (d, J = 8.3 Hz, 2H), 7.30 (t, J = 9.7 Hz, 4H), 5.47 (s, 1H), 5.01 (d, J = 8.1 Hz, 1H), 4.70–4.67 (m, 1H), 4.36–4.27 (m, 2H), 2.72 (d, J = 7.6 Hz, 2H), 1.37 (s, 3H), 1.15 (d, J = 5.6 Hz, 3H); 1.12 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 168.5, 155.6, 150.8, 145.2, 143.1, 141.1, 138.0, 131.6, 129.2, 126.0, 120.5, 119.1, 72.9, 67.5, 60.1, 51.7, 43.6, 27.5, 22.5; HR-MS (ESI): calcd for C24H28BrN5O4 [M + H]+ 530.1398, found (ESI+) 530.1396.
Isopropyl (1-(4-fluorophenyl)-3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9k). White solid, 69.8% yield, m.p. 156–158 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.85 (s, 1H), 8.19 (s, 1H), 7.85 (s, 1H), 7.74 (t, J = 8.6 Hz, 1H), 7.44 (d, J = 8.2 Hz, 2H), 7.38–7.30 (m, 4H), 7.13 (t, J = 8.4 Hz, 2H), 5.47 (s, 1H), 5.04 (q, J = 8.2 Hz, 1H), 4.71–4.67 (m, 1H), 4.36–4.27 (m, 2H), 2.72 (d, J = 7.6 Hz, 2H), 1.37 (s, 3H), 1.15 (d, J = 5.6 Hz, 3H); 1.12 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 168.6, 161.6 (d, J = 241.0 Hz), 155.6, 150.8, 145.2, 141.1, 139.8, 138.0, 128.8 (d, J = 8.0 Hz), 126.0, 119.2, 115.4 (d, J = 21.0 Hz), 72.9, 67.5, 60.1, 51.5, 43.9, 27.5, 22.5; HR-MS (ESI): calcd for C24H29FN5O4 [M + H]+ 470.2119, found (ESI+) 470.2117.
Isopropyl (3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxo-1-(p-tolyl)propyl)carbamate (9l). White solid, 27.9% yield, m.p. 169–171 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.83 (s, 1H), 8.19 (s, 1H), 7.85 (s, 1H), 7.65 (d, J = 8.3 Hz, 1H), 7.44 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 8.3 Hz, 2H), 7.21 (d, J = 7.7 Hz, 2H), 7.10 (d, J = 7.7 Hz, 2H), 5.48 (s, 1H), 5.00 (q, J = 8.2 Hz, 1H), 4.68 (t, J = 6.8 Hz, 1H), 4.35–4.27 (m, 2H), 2.70 (d, J = 7.6 Hz, 2H), 2.25 (s, 3H), 1.37 (s, 3H), 1.15 (d, J = 5.6 Hz, 3H); 1.12 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 168.8, 155.7, 150.7, 145.2, 141.0, 140.5, 138.0, 136.6, 129.3, 126.8, 126.0, 119.2, 72.9, 67.4, 60.0, 51.9, 43.9, 27.6, 22.5, 21.1; HR-MS (ESI): calcd for C25H31N5O4 [M + H]+ 466.2449, found (ESI+) 466.2445.
Isopropyl (3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxo-1-(4-(trifluoromethyl)phenyl)propyl)carbamate (9m). White solid, 21.2% yield, m.p. 200–202 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.89 (s, 1H), 8.19 (s, 1H), 7.86 (d, J = 9.4 Hz, 2H), 7.70 (d, J = 7.9 Hz, 2H), 7.55 (d, J = 7.9 Hz, 2H), 7.45 (d, J = 8.4 Hz, 2H), 7.32 (d, J = 8.3 Hz, 2H), 5.47 (s, 1H), 5.15–5.11 (m, 1H), 4.71–4.67 (m, 1H), 4.36–4.27 (m, 2H), 2.77 (d, J = 7.5 Hz, 2H), 1.37 (s, 3H), 1.15 (d, J = 5.6 Hz, 3H); 1.12 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 168.4, 155.7, 150.8, 148.4, 145.2, 141.2, 138.0, 127.7, 126.0, 125.8, 125.7, 123.6, 119.2, 72.9, 67.6, 60.1, 51.9, 43.5, 27.5, 22.5; HR-MS (ESI): calcd for C25H28F3N5O4 [M + H]+ 520.2167, found (ESI+) 520.2173.
Isopropyl (3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-1-(3-methoxyphenyl)-3-oxopropyl)carbamate (9n). Yellow solid, 57.6% yield, m.p. 105–108 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.84 (s, 1H), 8.19 (s, 1H), 7.85 (s, 1H), 7.71 (d, J = 8.7 Hz, 1H), 7.45 (d, J = 8.4 Hz, 2H), 7.32 (d, J = 8.3 Hz, 2H), 7.21 (t, J = 7.8 Hz, 1H), 6.90 (d, J = 7.6 Hz, 2H), 6.78 (d, J = 7.9 Hz, 1H), 5.48 (s, 1H), 5.03 (m, 1H), 4.69 (t, J = 6.4 Hz, 1H), 4.364.28– (m, 2H), 3.72 (s, 3H), 2.71 (d, J = 7.4 Hz, 2H), 1.37 (s, 3H), 1.15 (d, J = 5.6 Hz, 3H); 1.12 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 168.7, 159.7, 155.7, 150.8, 145.3, 145.2, 141.1, 138.1, 129.8, 128.3, 127.8, 126.0, 125.1, 119.6, 119.2, 119.1, 118.6, 112.7, 112.6, 110.1, 79.7, 79.4, 79.1, 72.9, 67.4, 60.1, 55.4, 52.1, 44.0, 27.5, 22.5; HR-MS (ESI): calcd for C25H31N5O5 [M + H]+ 482.2398, found (ESI+) 482.2404.
Isopropyl (1-(2,3-dimethoxyphenyl)-3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9o). Yellow solid, 31.3% yield, m.p. 164–166 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.77 (s, 1H), 8.18 (s, 1H), 7.84 (s, 1H), 7.71 (d, J = 8.4 Hz, 1H), 7.47 (d, J = 8.1 Hz, 2H), 7.32 (d, J = 8.2 Hz, 2H), 7.02 (t, J = 8.0 Hz, 1H), 6.91 (t, J = 6.3 Hz, 2H), 5.47 (s, 1H), 5.40–5.34 (m, 1H), 4.70–4.65 (m, 1H), 4.36–4.28 (m, 2H), 3.78 (s, 6H), 2.77–2.52 (m, 2H), 1.38 (s, 3H), 1.13 (d, J = 5.6 Hz, 3H); 1.10 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 169.3, 155.5, 152.7, 150.8, 146.7, 145.2, 141.0, 138.2, 137.3, 128.3, 127.9, 126.2, 126.0, 125.1, 124.4, 119.6, 119.2, 119.1, 119.0, 118.2, 111.9, 110.1, 79.7, 79.4, 79.1, 72.9, 67.3, 60.6, 60.1, 56.1, 46.5, 43.2, 27.5, 22.6; HR-MS (ESI): calcd for C26H35N5O6 [M + H]+ 512.2504, found (ESI+) 512.2498.
Isopropyl (1-(3,4-dimethoxyphenyl)-3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9p). White solid, 43.9% yield, m.p. 95–98 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.83 (s, 1H), 8.19 (s, 1H), 7.85 (s, 1H), 7.61 (d, J = 8.8 Hz, 1H), 7.45 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 8.4 Hz, 2H), 6.95 (s, 1H), 6.87–6.81 (m, 2H), 5.46 (s, 1H), 5.03–4.97 (m, 1H), 4.73–4.66(m, 1H), 4.36–4.27 (m, 2H), 3.72 (s, 3H), 3.70 (s, 3H), 2.70 (d, J = 7.1 Hz, 2H), 1.37 (s, 3H), 1.16 (d, J = 5.6 Hz, 3H), 1.12 (d, J = 6.0 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ 168.8, 155.6, 150.8, 149.0, 148.2, 145.3, 141.1, 138.1, 136.0, 126.0, 119.1, 118.9, 112.0, 110.7, 72.9, 67.4, 60.1, 56.0, 55.9, 51.9, 44.2, 27.5, 22.5; HR-MS (ESI): calcd for C26H35N5O6 [M + H]+ 512.2504, found (ESI+) 512.2497.
Isopropyl (1-(furan-2-yl)-3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxopropyl)carbamate (9q). Yellow solid, 69.0% yield, m.p. 134–136 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.95 (s, 1H), 8.20 (s, 1H), 7.85 (s, 1H), 7.57 (d, J = 12.9 Hz, 2H), 7.48 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.3 Hz, 2H), 6.36 (s, 1H), 6.21 (s, 1H), 5.47 (s, 1H), 5.17–5.11 (m, 1H), 4.77–4.72 (m, 1H), 4.36–4.28 (m, 2H), 2.90–2.69 (m, 2H), 1.38 (s, 3H), 1.15 (d, J = 6.3 Hz, 6H); 13C NMR (101 MHz, DMSO-d6) δ 168.4, 155.7, 155.5, 150.8, 145.2, 142.4, 141.1, 138.1, 126.0, 119.1, 110.8, 106.1, 79.7, 79.4, 79.1, 72.9, 67.5, 60.1, 46.1, 40.9, 27.5, 22.5; HR-MS (ESI): calcd for C22H27N5O5 [M + H]+ 442.2085, found (ESI+) 442.2090.
Isopropyl (3-((4-(2-hydroxy-1-(1H-1,2,4-triazol-1-yl)propan-2-yl)phenyl)amino)-3-oxo-1-(thiophen-2-yl)propyl)carbamate (9r). Yellow solid, 51.9% yield, m.p. 86–88 °C; 1H NMR (400 MHz, DMSO-d6) δ 9.97 (s, 1H), 8.18 (s, 1H), 7.83 (s, 1H), 7.70 (d, J = 7.6 Hz, 1H), 7.44 (d, J = 8.0 Hz, 2H), 7.31–7.29 (m, 3H), 6.95–6.93 (m, 2H), 5.59 (s, 1H), 5.32–5.25 (m, 1H), 4.74–4.68 (m, 1H), 4.32 (s, 2H), 2.89–2.77 (m, 2H), 1.37 (s, 3H), 1.12 (d, J = 6.0 Hz, 6H); 13C NMR (100 MHz, DMSO-d6) δ 168.6, 155.8, 150.7, 147.2, 145.2, 141.1, 138.0, 127.3, 126.0, 124.9, 124.2, 119.3, 79.6, 79.2, 78.9, 72.9, 67.8, 60.0, 47.8, 43.6, 27.5, 22.5, 22.4; HR-MS (ESI): calcd for C22H27N5O4S [M + H]+ 458.1857, found (ESI+) 458.1851.