Structural Features of Coumarin-1,2,4-Triazole Hybrids Important for Insecticidal Effects Against Drosophila melanogaster and Orius laevigatus (Fieber)
Abstract
1. Introduction
2. Results
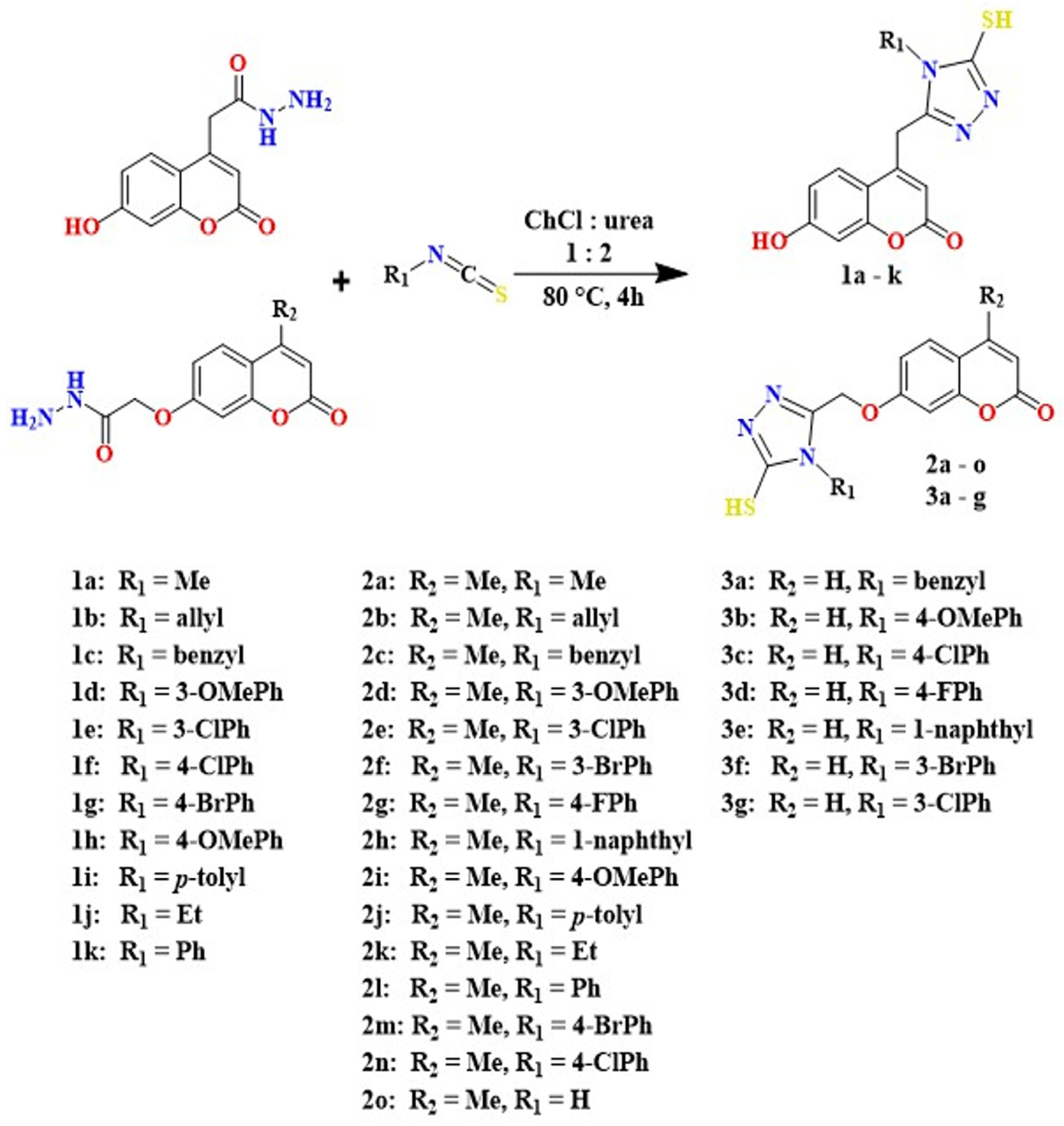
2.1. Structure of Compounds
2.2. Insecticidal Activity
2.3. Predicted Environmental Fate Properties and Ecotoxic Effects
2.4. QSAR Study of Mortality of D. melanogaster
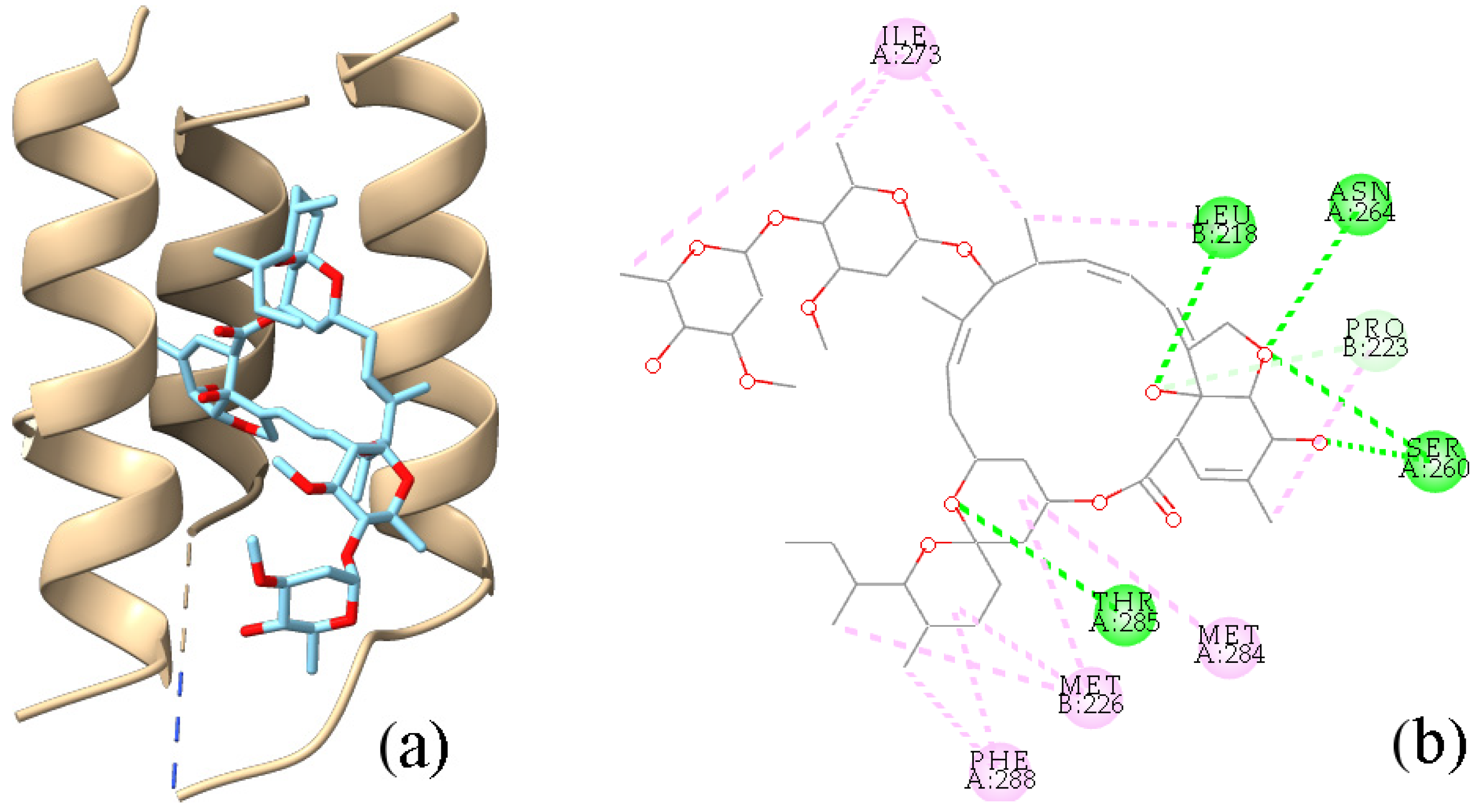
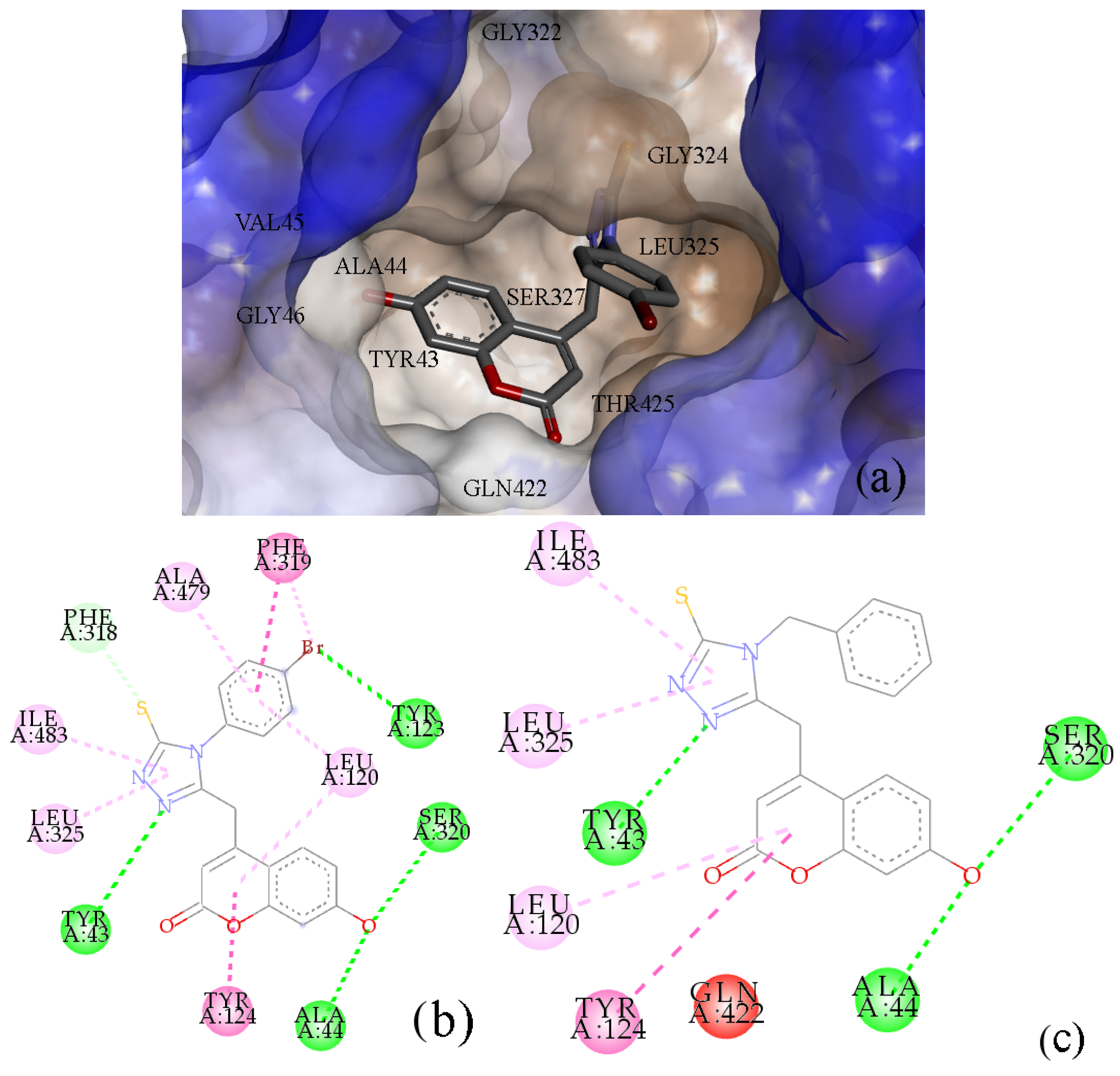
2.5. Interactions with Targets Related to Insecticidal Activities
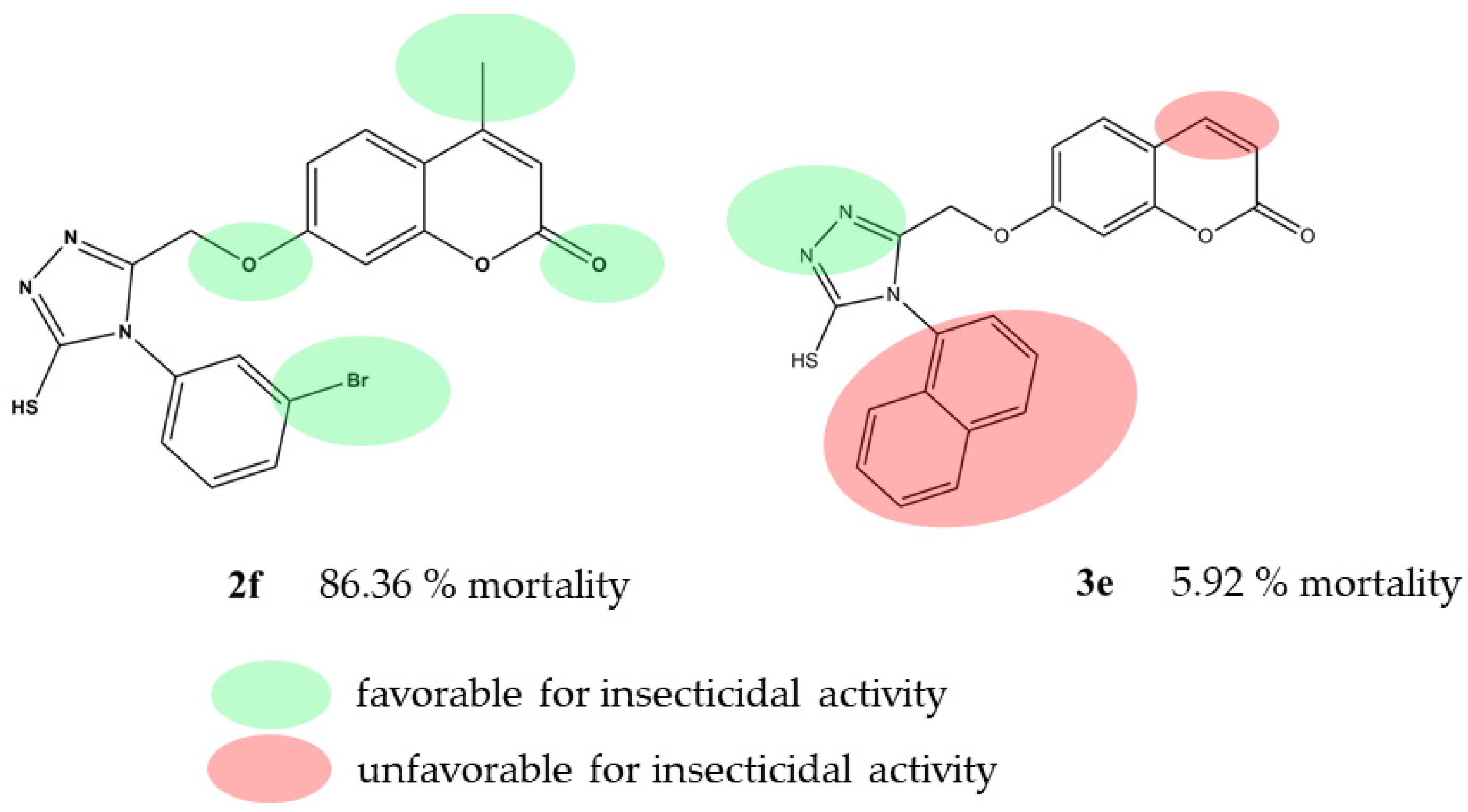
3. Discussion
4. Materials and Methods
4.1. Synthesis of Coumarin-1,2,4-Triazoles
4.2. Bioassays
4.2.1. Toxicity Assay to D. melanogaster
4.2.2. Toxicity Assay to O. laevigatus
4.3. Statistical Analysis
4.4. Computational Methods
4.4.1. Ecotoxicological and Environmental Property Calculation
4.4.2. QSAR Method
4.4.3. Molecular Docking
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| QSAR | Quantitative structure–activity relationship |
| AChE | Acetylcholinesterase |
| nAChR | Nicotinic acetylcholine receptor |
| GABA | γ-aminobutyric acid |
| GluCl | Glutamate-gated chloride channel |
| MLR | Multiple linear regression |
| ANN | Artificial neural network |
| dDAT | Drosophila melanogaster dopamine transporter |
| MLP | Multilayer perceptron |
References
- Smith, K.; Evans, D.A.; El-Hiti, G.A. Role of modern chemistry in sustainable arable crop protection. Philos. Trans. R. Soc. B 2008, 363, 623–637. [Google Scholar]
- Emameh, R.Z.; Syrjänen, L.; Barker, H.; Supuran, C.T.; Parkkila, S. Drosophila melanogaster: A model organism for controlling Dipteran vectors and pests. J. Enzyme Inhib. Med. Chem. 2015, 30, 1475–6374. [Google Scholar]
- Krittika, S.; Induhumathi, P.; Vedha Hari, B.N.; Rmya Devi, D.; Yadave, P. Evidence of nanoemulsion as an effective control measure for fruit flies Drosophila melanogaster. Sci. Rep. 2019, 9, 10578. [Google Scholar]
- Needham, A.J.; Kibart, M.; Crossley, H.; Ingham, P.W.; Foster, S.J. Drosophila melanogaster as a model host for Staphylococcus aureus infection. Microbiology 2004, 150, 2347–2355. [Google Scholar]
- Mertz, R.W.; Hesler, S.; Pfannenstiel, L.J.; Norris, R.H.; Loeb, G.; Scott, J.G. Insecticide resistance in Drosophila melanogaster in vineyards and evaluation of alternative insecticides. Pest. Manag. Sci. 2022, 78, 1272–1278. [Google Scholar] [PubMed]
- Perry, T.; Somers, J.; Yabg, Y.T.; Batterham, P. Expression of insect α6-like nicotinic acetylcholine receptors in Drosophila melanogaster highlights a high level of conservation of the receptor:spinosyn interaction, Insect. Biochem. Mol. Biol. 2015, 64, 106–115. [Google Scholar] [CrossRef]
- Scharf, M.E.; Nguyen, S.N.; Song, C. Evaluation of volatile low molecular weight insecticides using Drosophila melanogaster as a model. Pest. Manag. Sci. 2006, 62, 655–663. [Google Scholar]
- Riaz, B.; Zahoor, M.K.; Zahoor, M.A.; Majeed, H.N.; Javed, I.; Ahmad, A.; Jabeen, F.; Zulhussnain, M.; Sultana, K. Toxicity, phytochemical composition, and enzyme inhibitory activities of some indigenous weed plant extracts in fruit fly, Drosophila melanogaster. eCAM 2018, 2018, 2325659. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, S.; Wen, B.; Deng, Z.; Ding, Q.; Li, X. Characterization of ATP-binding cassette transporters associated with emamectin benzoate tolerance: From the model insect Drosophila melanogaster to the agricultural pest Spodoptera frugiperda. Pest. Manag. Sci. 2025, 81, 340–350. [Google Scholar]
- Daborn, P.J.; Lumb, C.; Harrop, T.W.R.; Blasetti, A.B.; Pasrich, S.; Morin, S.; Mitchell, S.N.; Donelly, M.J.; Müller, P.; Batterhama, P. Using Drosophila melanogaster to validate metabolism-based insecticide resistance from insect pests. Insect. Biochem. Mol. Biol. 2012, 42, 918e924. [Google Scholar]
- Bouagga, S.; Urbaneja, A.; Rambla, J.L.; Granell, A.; Pérez-Hedo, M. Orius laevigatus strengthens its role as a biological control agent by inducing plant defenses. J. Pest. Sci. 2018, 91, 55–64. [Google Scholar] [CrossRef]
- Mouratidis, A.; de Lima, A.P.; Dicke, M.; Messelink, G.J. Predator-prey interactions and life history of Orius laevig atus and O. majusculus feeding on flower and leaf-inhabiting thrips. Biol. Control 2022, 172, 104954. [Google Scholar] [CrossRef]
- Karnaš, M.; Rastija, V.; Šubarić, D.; Molnar, M. Green synthesis and acetylcholinesterase inhibition of coumarin-1,2,4-triazole hybrids. Curr. Org. Chem. 2023, 27, 883–892. [Google Scholar] [CrossRef]
- Karnaš, M.; Rastija, V.; Vrandečić, K.; Ćosić, K.; Kanižai Šarić, G.; Agić, D.; Šubarić, D.; Molnar, M. Synthesis, antifungal, antibacterial activity, and computational evaluations of some novel coumarin-1,2,4-triazole hybrid compounds. J. Taibah Univ. Sci. 2024, 18, 2331456. [Google Scholar] [CrossRef]
- Zhao, F.; Tang, X.; Liu, M.; Qin, Z.; Li, J.-Q.; Xiao, Y. Synthesis and insecticidal activity of novel 1,2,4-triazole derivatives containing trifluoroacetyl moieties. Mol. Divers. 2022, 26, 2149–2158. [Google Scholar] [PubMed]
- Zhang, L.; Liu, J.; Zhou, L.; Yan, C.; Wu, D.; Liu, M. Oriented synthesis and insecticidal activities of novel meta-diamide scaffolds incorporating with 1,2,4-triazole moiety. J. Heterocycl. Chem. 2024, 61, 1411. [Google Scholar]
- ECHA-11-R-004.2-EN; The Use of Alternatives to Testing on Animals for the REACH Regulation 2011. European Chemicals Agency: Helsinki, Finland, 2011. Available online: https://echa.europa.eu/documents (accessed on 3 March 2025).
- Baderna, D.; Faoro, R.; Selvestrel, G.; Troise, A.; Luciani, D.; Andres, S.; Benfenati, E. Defining the human-biota thresholds of toxicological concern for organic chemicals in freshwater: The proposed strategy of the LIFE VERMEER project using VEGA zools. Molecules 2021, 26, 1928. [Google Scholar]
- Nisa, N.; Dinata, R.; Arati, C.; Abdelgani-Baraka, G.A.; Bhanushree, B.; Bidanchi, R.M.; Manikandan, B.; Saeed, A.-L.; Abinash, G.; Pori, B.; et al. Computational toxicology and food safety assessment of Parkia timoriana phytoconstituents using quantitative structure-activity relationship (QSAR) modeling approaches. Indian J. Biochem. Biophys. 2023, 60, 896–918. [Google Scholar]
- Kirst, H.A. The spinosyn family of insecticides: Realizing the potential of natural products research. J. Antibiot. 2010, 63, 101–111. [Google Scholar] [CrossRef]
- Salgado, V.L. Studies on the mode of action of Spinosad: Insect symptoms and physiological correlates. Pestic. Biochem Physiol. 1998, 60, 91–102. [Google Scholar] [CrossRef]
- Bacci, L.; Lupi, D.; Savodelli, S.; Rossaro, B. A review of Spinosyns, a derivative of biological acting substances as a class of insecticides with a broad range of action against many insect pests. J. Entomol. Acarol. Res. 2016, 48, e110735. [Google Scholar] [CrossRef]
- Nguyen, J.; Ghazali, R.; Batterham, P.; Perry, T. Inhibiting the proteasome reduces molecular and biological impacts of the natural product insecticide, spinosad. Pest. Manag. Sci. 2021, 77, 3777–3786. [Google Scholar] [CrossRef] [PubMed]
- Orr, N.; Shaffner, A.J.; Richey, K.; Crouse, G.D. Novel mode of action of spinosad: Receptor binding studies demonstrating lack of interaction with known insecticidal target sites. Pestic. Biochem Physiol. 2009, 95, 1–5. [Google Scholar] [CrossRef]
- Watson, G.B. Actions of insecticidal Spinosyns on γ-aminobutyric acid responses from small-diameter cockroach neurons. Pestic. Biochem Physiol. 2001, 71, 20–28. [Google Scholar]
- Hibbs, R.; Gouaux, E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 2011, 474, 54–60. [Google Scholar] [PubMed]
- Benfenati, E.; Manganaro, A.; Gini, G. VEGA-QSAR: AI inside a platform for predictive toxicology. In Proceedings of the Workshop “Popularize Artificial Intelligence 2013”, CEUR Workshop Proceedings, Turin, Italy, 5 December 2013; Volume 1107. [Google Scholar]
- Molnar, M.; Periš, I.; Komar, M. Choline chloride based deep eutectic solvents as a tuneable medium for synthesis of coumarinyl 1,2,4-triazoles: Effect of solvent type and temperature. Eur. J. Org. Chem. 2019, 15, 2688–2694. [Google Scholar]
- Gramatica, P. On the Development and Validation of QSAR Models. In Computational Toxicology; Reisfeld, B., Mayeno, A., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 930, pp. 499–526. [Google Scholar]
- Chirico, N.; Gramatica, P. Real External Predictivity of QSAR Models: How to evaluate It? Comparison of different validation criteria and proposal of using the concordance correlation coefficient. J. Chem. Inf. Model. 2011, 51, 2320–2335. [Google Scholar]
- Tetko, I.V.; Villa, A.E.P.; Livingstone, D.J. Neural network studies. 2. Variable selection. J. Chem. Inf. Comput. Sci. 1996, 36, 794–803. [Google Scholar]
- Burlacu, C.M.; Burlacu, A.C.; Praisler, M. Sensitivity analysis of artificial neural networks identifying JWH synthetic cannabinoids built with alternative training strategies and methods. Inventions 2022, 7, 82. [Google Scholar] [CrossRef]
- Joseph, D.; Nayak, S.R.; Penmatsa, A. Structural insights into GABA transport inhibition using an engineered neurotransmitter transporter. EMBO J. 2022, 41, e110735. [Google Scholar] [CrossRef]
- Grutter, T.; Prado de Carvalho, L.; Virginie, D.; Taly, A.; Fischer, M.; Changeux, J.P. A chimera encoding the fusion of an acetylcholine-binding protein to an ion channel is stabilized in a state close to the desensitized form of ligand-gated ion channels. C. R. Biol. 2005, 328, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Rohman, N.; Ardiansah, B.; Wukirsari, T.; Judeh, Z. Recent trends in the synthesis and bioactivity of coumarin, coumarin–chalcone, and coumarin–triazole molecular hybrids. Molecules 2024, 29, 1026. [Google Scholar] [CrossRef]
- Niculescu, S.P. Artificial neural networks and genetic algorithms in QSAR. J. Mol. Struc.-Theochem. 2003, 622, 71–83. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Desheesh, M.A.; El-Zemity, S.R.; Kadous, E.A.; Fahmy, M.M.; Tawfeek, E.E. Antimicrobial activities of synthesized 3-acetyl coumarin and benzo-4-methyl coumarin. Alex. Sci. Exch. J. 2017, 38, 515–520. [Google Scholar]
- Deshmukh, M.; Pawar, P.; Joseph, M.; Phalgune, U.; Kashalkar, R.; Deshpande, N.R. Efficacy of 4-methyl-7-hydroxy coumarin derivatives against vectors Aedes aegypti and Culex quinquefasciatus. Indian J. Exp. Biol. 2008, 46, 788–792. [Google Scholar]
- Smelt, C.L.C.; Sanders, V.R.; Puinean, A.M.; Lansdell, S.J.; Goodchild, J.; Millar, N.S. Agonist and antagonist properties of an Insect GABA-gated chloride channel (RDL) are influenced by heterologous expression conditions. PLoS ONE 2021, 16, e0254251. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.M.; Dong, S.; Shen, C.; Cao, Q.H.; Song, M.Y.; He, Q.R.; Wang, X.L.; Yang, X.J.; Tang, J.J.; Gao, J.M. Furan-site bromination and transformations of fraxinellone as insecticidal agents against Mythimna separata Walker. Sci. Rep. 2018, 8, 8372. [Google Scholar] [CrossRef]
- Katsuta, H.; Nomura, M.; Wakita, T.; Daido, H.; Kobayashi, Y.; Kawahara, A.; Banba, S. Discovery of broflanilide, a novel insecticide. J. Pestic. Sci. 2019, 44, 120–128. [Google Scholar] [CrossRef]
- Omura, S.; Crump, A. Ivermectin: Panacea for resource-poor communities? Trends Parasitol. 2014, 30, 445–455. [Google Scholar] [CrossRef]
- Ueno, T.; Kumel, K. Functional characterization of dopamine transporter in vivo using Drosophila melanogaster behavioral assays. Front. Behav. Neurosci. 2014, 8, 303. [Google Scholar] [CrossRef]
- Schmidt, S.G.; Malle, M.G.; Nielsen, A.K.; Bohr, S.S.-R.; Pugh, C.F.; Nielsen, J.C.; Poulsen, I.H.; Rand, K.D.; Hatzakis, N.S.; Loland, C.J. The dopamine transporter antiports potassium to increase the uptake of dopamine. Nat. Commun. 2022, 13, 2446. [Google Scholar] [CrossRef] [PubMed]
- Studebaker, G.E.; Kring, T.J. Effects of insecticides on Orius insidiosus (Hemiptera: Anthocoridae), measured by field, greenhouse and Petri dish bioassays. Flo. Entomol. 2003, 86, 178–185. [Google Scholar] [CrossRef]
- Hocquet, A.; Langgård, M. An evaluation of the MM+ force field. J. Mol. Model. 1998, 4, 94–112. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Gramatica, P.; Chirico, N.; Papa, E.; Cassani, S.; Kovarich, S. QSARINS: A new software for the development, analysis, and validation of QSAR MLR models. J. Comput. Chem. 2013, 34, 2121–2132. [Google Scholar] [CrossRef]



| Comp. | Drosophila melanogaster | Orius laevigatus (Fieber) | ||||
|---|---|---|---|---|---|---|
| 2 Days | 4 Days | 8 Days | 24 h | 48 h | 72 h | |
| 1a | 5.28 ± 0.33 * | 8.84 ± 2.35 * | 27.06 ± 5.56 * | |||
| 1b | 14.42 ± 2.33 * | 55.15 ± 10.66 * | 95.01 ± 0.85 | 78.52 ± 1.28 | 96.30 ± 6.41 | 100.00 ± 0.00 |
| 1c | 12.56 ± 3.81 * | 52.54 ± 14.13 * | 84.91 ± 10.53 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 1d | 8.78 ± 10.56 * | 28.26 ± 13.53 * | 47.22 ± 14.97 * | |||
| 1e | 7.71 ± 10.59 * | 28.28 ± 6.04 * | 63.26 ± 16.54 * | |||
| 1f | 6.60 ± 7.74 * | 18.28 ± 9.76 * | 39.46 ± 1.81 * | |||
| 1g | 26.67 ± 21.11 * | 67.01 ± 21.86 * | 92.64 ± 7.08 | 43.33 ± 5.77 * | 64.45 ± 3.85 * | 78.57 ± 6.19 * |
| 1h | 12.82 ± 10.78 * | 50.88 ± 9.73 * | 70.58 ± 12.30 * | |||
| 1i | 4.72 ± 3.60 * | 51.68 ± 17.76 * | 86.77 ± 11.46 | 77.78 ± 19.24 | 83.33 ± 14.43 * | 86.67 ± 11.55 * |
| 1j | 8.38 ± 8.33 * | 26.23 ± 19.32 * | 56.86 ± 12.96 * | |||
| 1k | 14.98 ± 7.56 * | 60.70 ± 15.24 * | 85.58 ± 14.62 | 70.37 ± 6.41 * | 87.96 ± 0.80 | 92.59 ± 6.41 |
| 2a | 4.52 ± 0.46 * | 16.54 ± 7.06 * | 28.46 ± 12.62 * | |||
| 2b | 7.07 ± 5.99 * | 66.77 ± 27.60 * | 89.00 ± 10.10 | 78.52 ± 1.28 | 95.83 ± 7.22 | 100.00 ± 0.00 |
| 2c | 45.40 ± 28.75 * | 53.90 ± 31.87 * | 56.68 ± 30.02 * | |||
| 2d | 9.94 ± 5.30 * | 39.83 ± 8.03 * | 59.76 ± 15.24 * | |||
| 2e | 8.08 ± 6.41 * | 32.87 ±4.35 * | 68.07 ± 1.88 * | |||
| 2f | 60.30 ± 15.35 * | 86.36 ± 13.53 | 100.00 ± 0.00 | 53.33 ± 28.37 * | 61.67 ± 25.04 * | 78.57 ± 6.19 * |
| 2g | 25.06 ± 9.08 * | 29.61 ± 11.86 * | 26.21 ± 12.51 * | |||
| 2h | 11.07 ± 13.53 * | 27.55 ± 6.00 * | 54.45 ± 12.47 * | |||
| 2i | 6.62 ± 6.05 * | 23.57 ± 7.69 * | 41.77 ± 6.87 * | |||
| 2j | 15.39 ± 7.79 * | 19.35 ± 2.32 * | 20.97 ± 7.28 * | |||
| 2k | 19.84 ± 9.15 * | 35.94 ± 6.44 | 44.87 ± 5.23 * | |||
| 2l | 5.72 ± 6.32 * | 16.37 ± 5.51 * | 26.68 ± 4.27 * | |||
| 2m | 3.11 ± 4.11 * | 10.99 ± 4.91 * | 36.84 ± 7.22 * | |||
| 2n | 10.50 ± 4.61 * | 71.44 ± 6.80 * | 96.02 ± 3.46 | 96.30 ± 6.42 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 2o | 5.51 ± 2.03 * | 15.67 ± 4.90 * | 36.05 ± 10.39 * | |||
| 3a | 11.40 ± 9.06 * | 30.97 ± 17.43 * | 56.89 ± 8.76 * | |||
| 3b | 7.07 ± 5.99 * | 20.54 ± 6.23 * | 41.08 ± 2.17 * | |||
| 3c | 2.94 ± 2.56 * | 8.92 ± 4.50 * | 28.08 ± 1.58 * | |||
| 3d | 6.99 ± 8.75 * | 17.08 ± 14.17 * | 31.54 ± 17.43 * | |||
| 3e | 4.28 ± 0.10 * | 5.92 ± 2.75 * | 26.34 ± 9.32 * | |||
| 3f | 6.61 ± 3.16 * | 8.37 ± 7.63 * | 15.15 ± 11.35 * | |||
| 3g | 4.99 ± 4.32 * | 19.06 ± 6.09 * | 40.26 ± 7.98 * | |||
| spinosad | 97.87 ± 3.69 | 100.00 ± 0.00 | 100.00 ± 0.00 | 92.59 ± 12.83 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| Comp. | BCF 1 | Fish Acute Toxicity 2 | Fathead Minnow 96 h 3 | Zebrafish Embryo 4 | Daphnia magna 48 h 5 | Algae Acute Toxicity 6 | Bee Acute Toxicity 7 | Sludge Toxicity 8 |
|---|---|---|---|---|---|---|---|---|
| 1a | 0.86 | 4.6 | 5.9 | 0.78 | 6.1 | Toxic | Low | Toxic |
| 1b | 0.75 | 1.87 | 4.94 | 1.91 | 4.11 | Non-toxic | Low | Toxic |
| 1c | 1.13 | 4.49 | 5.46 | 0.93 | 4.65 | Toxic | Low | Toxic |
| 1d | 0.93 | 3.61 | 5.51 | 1.2 | 4.49 | Non-toxic | Low | Toxic |
| 1e | 1.22 | 2.83 | 5.92 | 1.1 | 4.58 | Toxic | Low | Non-toxic |
| 1f | 1.21 | 2.83 | 5.91 | 1.11 | 4.63 | Toxic | Low | Non-toxic |
| 1g | 1.26 | 2.83 | 5.98 | 1.01 | 4.75 | Toxic | Low | Toxic |
| 1h | 0.99 | 2.29 | 5.52 | 1.21 | 4.58 | Non-toxic | Low | Toxic |
| 1i | 1.19 | 4.49 | 5.79 | 1.02 | 4.56 | Toxic | Low | Non-toxic |
| 1j | 0.66 | 1.87 | 4.62 | 2.18 | 3.98 | Non-toxic | Low | Toxic |
| 1k | 1.09 | 2.83 | 5.48 | 1.03 | 4.46 | Toxic | Low | Non-toxic |
| 2a | 0.56 | 1.87 | 4.81 | 0.78 | 5.51 | Toxic | Low | Toxic |
| 2b | 0.72 | 2.83 | 5.33 | 0.34 | 5.72 | Toxic | Low | Toxic |
| 2c | 1.08 | 2.29 | 5.81 | 0.65 | 6.27 | Toxic | Low | Toxic |
| 2d | 0.86 | 4.6 | 5.9 | 0.78 | 6.1 | Toxic | Low | Toxic |
| 2e | 1.28 | 2.29 | 6.31 | 0.82 | 6.26 | Toxic | Low | Toxic |
| 2f | 1.31 | 2.29 | 6.36 | 0.73 | 6.99 | Toxic | Low | Toxic |
| 2g | 1.35 | 2.29 | 5.99 | 0.97 | 6.22 | Toxic | Low | Toxic |
| 2h | 1.3 | 4.8 | 6.49 | −0.21 | 6.52 | Toxic | Low | Toxic |
| 2i | 0.91 | 4.6 | 5.87 | 0.62 | 6.18 | Toxic | Low | Toxic |
| 2j | 1.07 | 2.29 | 6.14 | 0.33 | 6.22 | Toxic | Low | Toxic |
| 2k | 0.62 | 2.49 | 5.02 | 0.6 | 5.58 | Toxic | Low | Toxic |
| 2l | 1.02 | 4.49 | 5.84 | 0.44 | 6.11 | Toxic | Low | Toxic |
| 2m | 1.29 | 2.29 | 6.33 | 0.57 | 7.08 | Toxic | Low | Toxic |
| 2n | 1.28 | 2.29 | 6.27 | 0.66 | 6.31 | Toxic | Low | Toxic |
| 2o | 0.42 | 1.87 | 4.67 | 0.64 | 5.51 | Toxic | Low | Toxic |
| 3a | 1.03 | 2.29 | 5.48 | −0.36 | 6.24 | Toxic | Low | Toxic |
| 3b | 0.86 | 4.6 | 5.54 | −0.39 | 6.13 | Non-toxic | Low | Toxic |
| 3c | 1.07 | 2.29 | 5.94 | −0.35 | 6.29 | Toxic | Low | Toxic |
| 3d | 1.31 | 2.29 | 5.66 | −0.04 | 6.19 | Toxic | Low | Non-toxic |
| 3e | 1.27 | 4.8 | 6.16 | 0.4 | 6.46 | Toxic | Low | Non-toxic |
| 3f | 1.28 | 4.49 | 6.03 | −0.27 | 7.02 | Toxic | Low | Non-toxic |
| 3g | 0.33 | 1.87 | 4.34 | 1.52 | 5.55 | Toxic | Strong | Non-toxic |
| Network | R2training | R2test | Training Error | Test Error |
|---|---|---|---|---|
| MLP 3-4-1 | 0.78 | 0.94 | 0.02 | 0.02 |
| Network | nRCt(sp2) | PW2 | RDF075m |
|---|---|---|---|
| MLP 3-4-1 | 13.99 | 1.47 | 1.35 |
| Receptor | GluCl | dDAT | Ac-AChBP | |||||
|---|---|---|---|---|---|---|---|---|
| Binding Site | IVM | Glu | NO-711 | TII | ||||
| Comp. | Total E | Comp. | Total E | Comp. | Total E | Comp. | Total E | |
| IVM | −142.87 | 2c | −113.45 | NO-711 | −114.61 | TII | −116.98 | |
| 2d | −104.82 | 2i | −109.52 | 1h | −113.96 | 3a | −103.63 | |
| 2f | −103.21 | 2n | −107.68 | 1g | −108.60 | 2c | −102.31 | |
| 2e | −103.16 | 2b | −107.56 | 2h | −101.39 | 1a | −100.51 | |
| 3a | −99.38 | 2j | −106.94 | 1c | −98.97 | 2a | −97.81 | |
| 3c | −99.38 | 2m | −106.31 | 2c | −97.80 | 2b | −97.62 | |
| SPYNA | −79.89 | 2o | −105.96 | SPYNA | −44.16 | SPYNA | −50.70 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šubarić, D.; Rastija, V.; Karnaš Babić, M.; Agić, D.; Majić, I. Structural Features of Coumarin-1,2,4-Triazole Hybrids Important for Insecticidal Effects Against Drosophila melanogaster and Orius laevigatus (Fieber). Molecules 2025, 30, 1662. https://doi.org/10.3390/molecules30081662
Šubarić D, Rastija V, Karnaš Babić M, Agić D, Majić I. Structural Features of Coumarin-1,2,4-Triazole Hybrids Important for Insecticidal Effects Against Drosophila melanogaster and Orius laevigatus (Fieber). Molecules. 2025; 30(8):1662. https://doi.org/10.3390/molecules30081662
Chicago/Turabian StyleŠubarić, Domagoj, Vesna Rastija, Maja Karnaš Babić, Dejan Agić, and Ivana Majić. 2025. "Structural Features of Coumarin-1,2,4-Triazole Hybrids Important for Insecticidal Effects Against Drosophila melanogaster and Orius laevigatus (Fieber)" Molecules 30, no. 8: 1662. https://doi.org/10.3390/molecules30081662
APA StyleŠubarić, D., Rastija, V., Karnaš Babić, M., Agić, D., & Majić, I. (2025). Structural Features of Coumarin-1,2,4-Triazole Hybrids Important for Insecticidal Effects Against Drosophila melanogaster and Orius laevigatus (Fieber). Molecules, 30(8), 1662. https://doi.org/10.3390/molecules30081662







