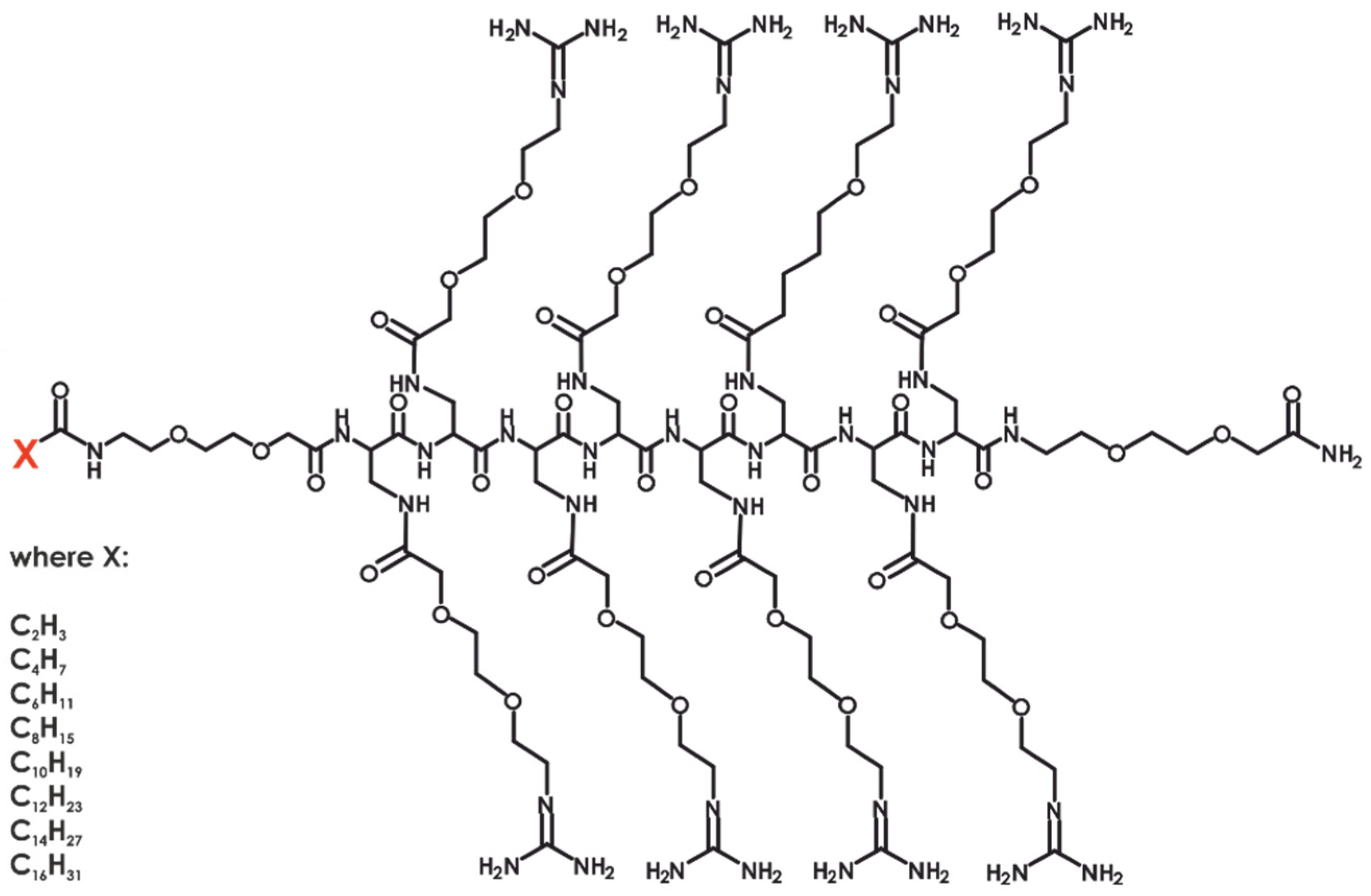
2. Results and Discussion
Following the previously described synthetic step, we obtained eight
N-modified analogs of the parent compound. All compounds were characterized using physicochemical methods, including UPLC and HR-MS (see
Table 1) and desalted.
Since we have data on the efficient DNA binding to the parent compound (
1a) [
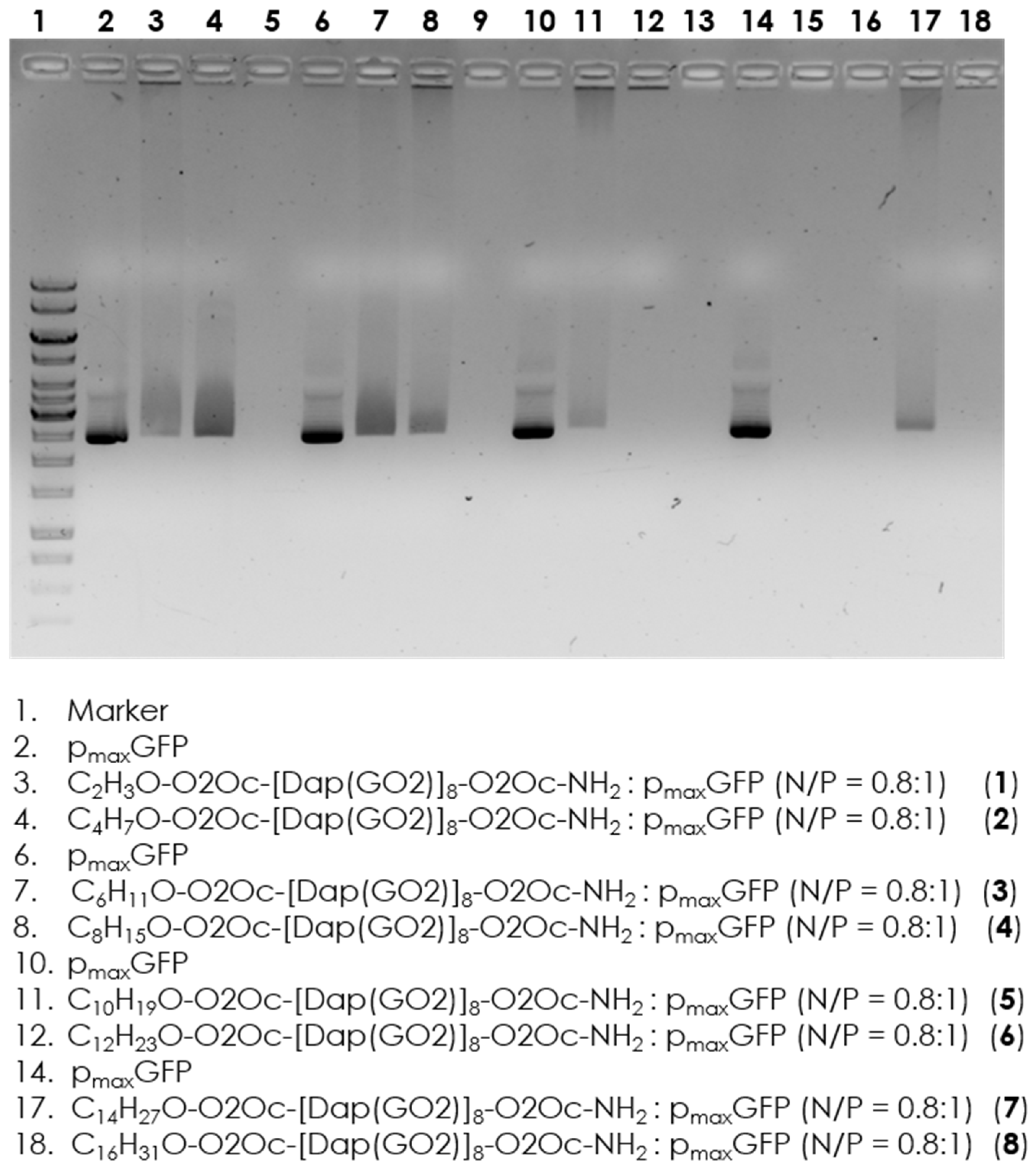
11], we determined to verify whether the attachment of fatty acids would disrupt these interactions. Electrophoretic separation of trhe formed complexes with implementation of the appropriate controls was performed. The results are shown in
Figure 2. Evidence shows that all compounds can interact with DNA at a concentration of 2 µM. Therefore, we can proceed with the next step of selecting the transfection reagent, which involves separately incubating the GFP coding plasmid with the obtained compounds.
As shown in
Figure 2, a shift in migration between the plasmid marker and the complex is observed in all wells, indicating effective interaction of all compounds with the model DNA. There is no significant difference between the individual systems where the tested compounds are present.
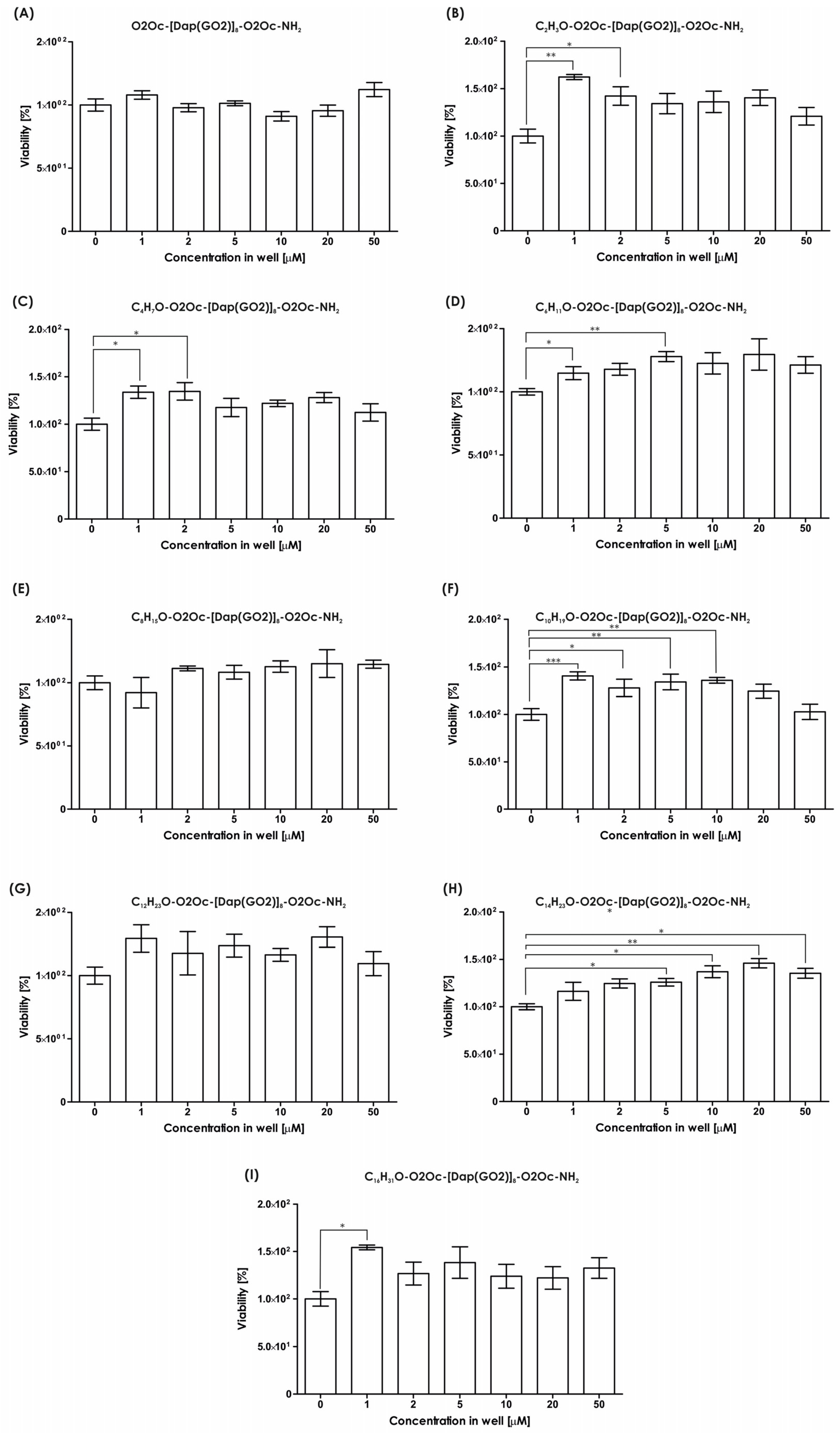
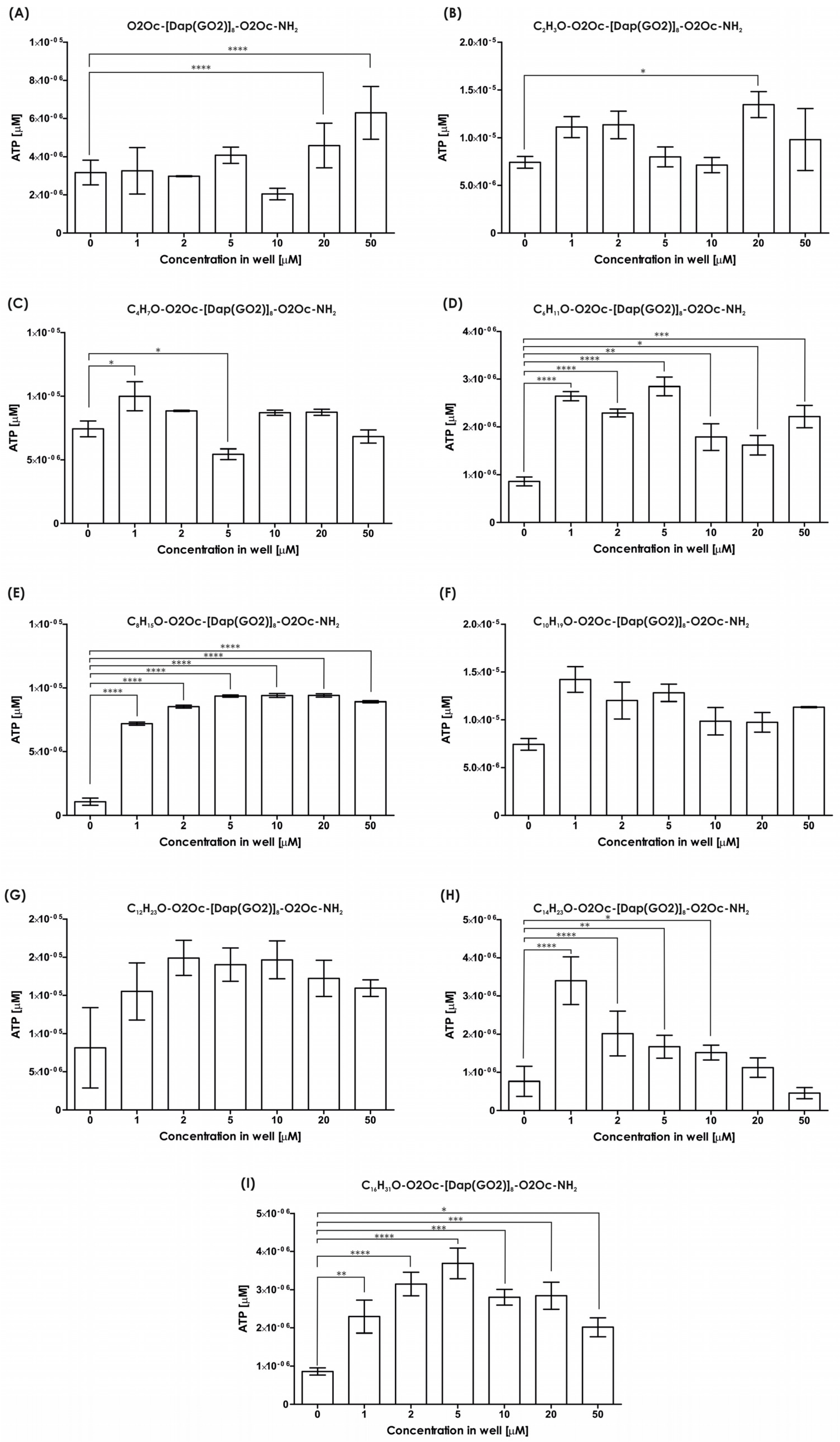
The impact of the resulting compounds on cells was evaluated using the MTT assay, and the results are illustrated in
Figure 3. The MTT test measures the activity of mitochondria to determine the number of living cells. As indicated in
Figure 3, none of the tested compounds exhibited any toxic effects in the tested concentration range up to 50 µM. Conversely, in all systems, the presence of polymers stimulates cell growth, with a statistically significant impact observed (see
Figure 3).
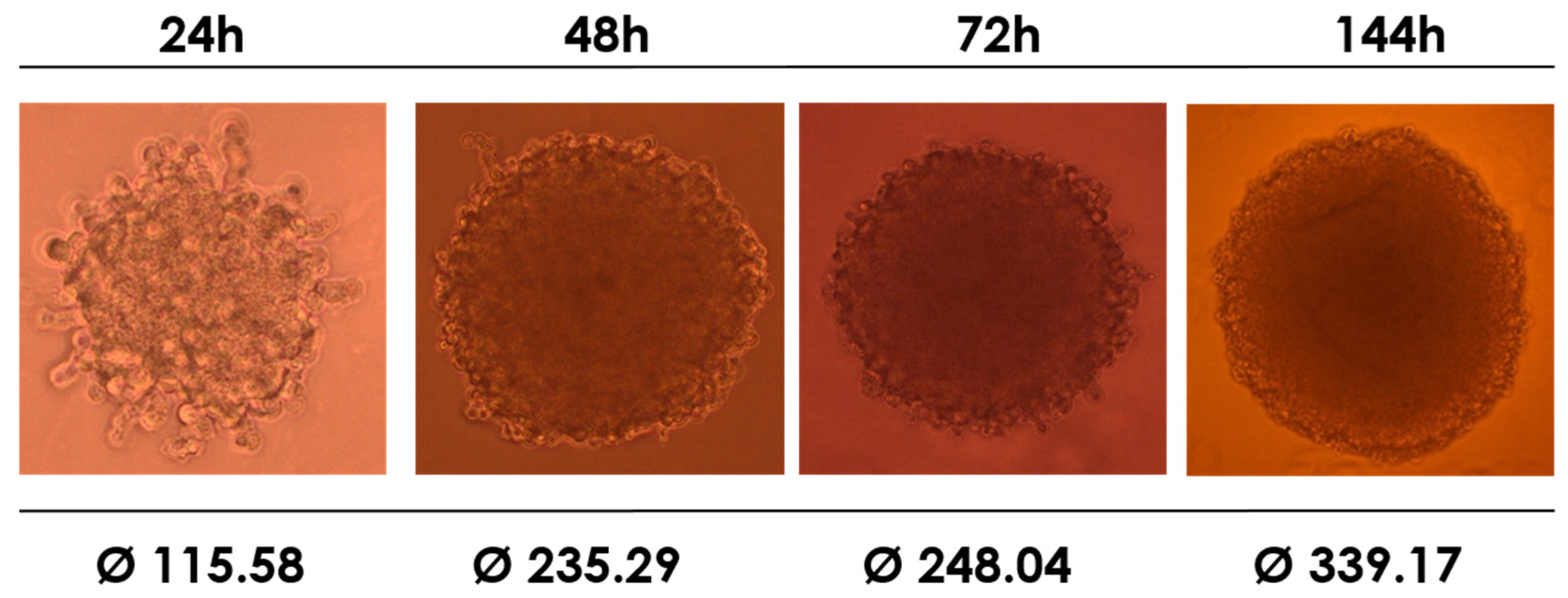
Next, we investigated the viability of the synthesized compounds against the HEK-293T cell line using cell culture in a 3D model. The cells were able to form the expected structures in a time-dependent manner. As seen in
Figure 4, the spheroids were fully formed within 72 h. It is widely acknowledged that a system that forms spheroid cells better reflects the natural environment and condition of cell growth than single-layer culturing.
All compounds underwent viability assays using 3D models. The amount of ATP was measured as a marker of living cells. We tested all compounds to determine the effect of lipidation. Several reports strongly suggest that lipidation of peptides generates more toxic molecules compared to non-modified compounds. The spheroids of the HEK-293T cells were incubated with the compounds mentioned above and cultured for 24 h. The results of the viability assay are provided in
Figure 5. The viability of the tested cells exposed to each compound (
Figure 5A–I) are not suppressed compared to the non-treated cells up to 10 µM, except for compound
1, with an acetyl moiety, which is not toxic up to 5 µM. No typical dose–response effect is observed except in
Figure 5H. The introduction of short-chain fatty acids (up to system D, which corresponds to hexanoic acid) results in a wave-like shape of the live cell number with a minimum dose of around 5 or 10 µM and two maxima at 1–2 µM and 20–50 µM, where stimulation of cell growth is observed. The presence of longer-chain fatty acids (
Figure 5E–I, except for 5H as mentioned above) strongly induces spheroid growth, with the number of cells exceeding the non-treated control several times. In conclusion, all the compounds did not suppress cell growth up to 5 µM. The effect of increased cell proliferation is not anticipated and must be considered when utilizing such compounds in living organisms. Uncontrolled stimulation of cell growth is a risk factor for cancer. However, in cell culture, increased growth of transfection-resistant cells appears to be advantageous, as it increases the yield of the final transfection product, i.e., recombinant proteins.
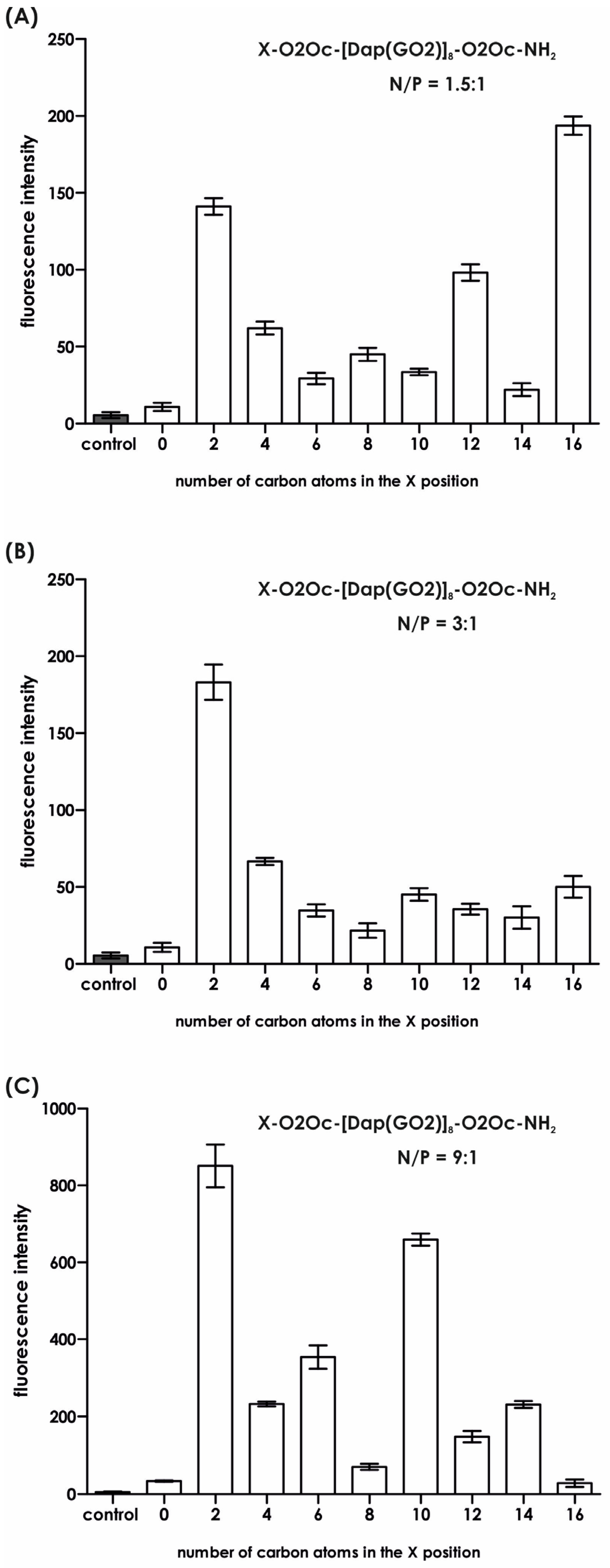
Since we found no toxic effects from the obtained compounds, we proceeded to the next stage of our research and performed the key experiment, which involved transfecting a model plasmid into the HEK-293T cell line. It is important to note that the maximum concentration of the compounds used never exceeded 5 µM, a concentration deemed safe for the cells used. The results of the GFP production efficacy are shown in
Figure 6 and
Figure 7.
Figure 6 was generated based on the mean fluorescence of the spheroids visible in
Figure 7. Significant differences were observed for all compounds in each system depending on the N/P ratio used in the experiment. For the system with the lowest N/P ratio of 1.5:1, the greatest fluorescence (due to GFP production) was observed for compound
8, followed by compound
1, with an average value in the range of 150 arbitrary units (
Figure 5A). When we doubled the N/P ratio (
Figure 6B), compound
1 became the highest with an average fluorescence of around 200. None of the other compound complexes with the plasmid exhibited at least half of the fluorescence observed in compound
1. For N/P = 9:1, which is six times the value of N/P = 1.5:1, superior fluorescence was observed for compound
1, followed by compounds
3 and
5. The other compounds did not induce significant amounts of GFP (
Figure 6 and
Figure 7). It is noteworthy that the transfection efficacy of compound
1a, the parent compound with three N-terminal amino groups, is the lowest in each series. This underscores the importance of masking a positive charge in this part of the molecule. We do not have a reasonable explanation for why a particular substituent strongly affects the transfection efficacy. In order to further illuminate this issue, an investigation was conducted into the stability of the formed complex under conditions that were identical to those used for the transfection, including the reagent buffer composition and the transfection period. Subsequently, at predetermined time points, UV spectra were recorded for each system, in addition to the controls, and are presented in
Figure S2. An analysis of the obtained results indicates that the spectra properties of the lipidated compound alone and in a complex with DNA are quite similar to each other, except for compounds
4 and
1, which do not show significant changes over time. For all other compounds, we observed a decrease in intensity over time.
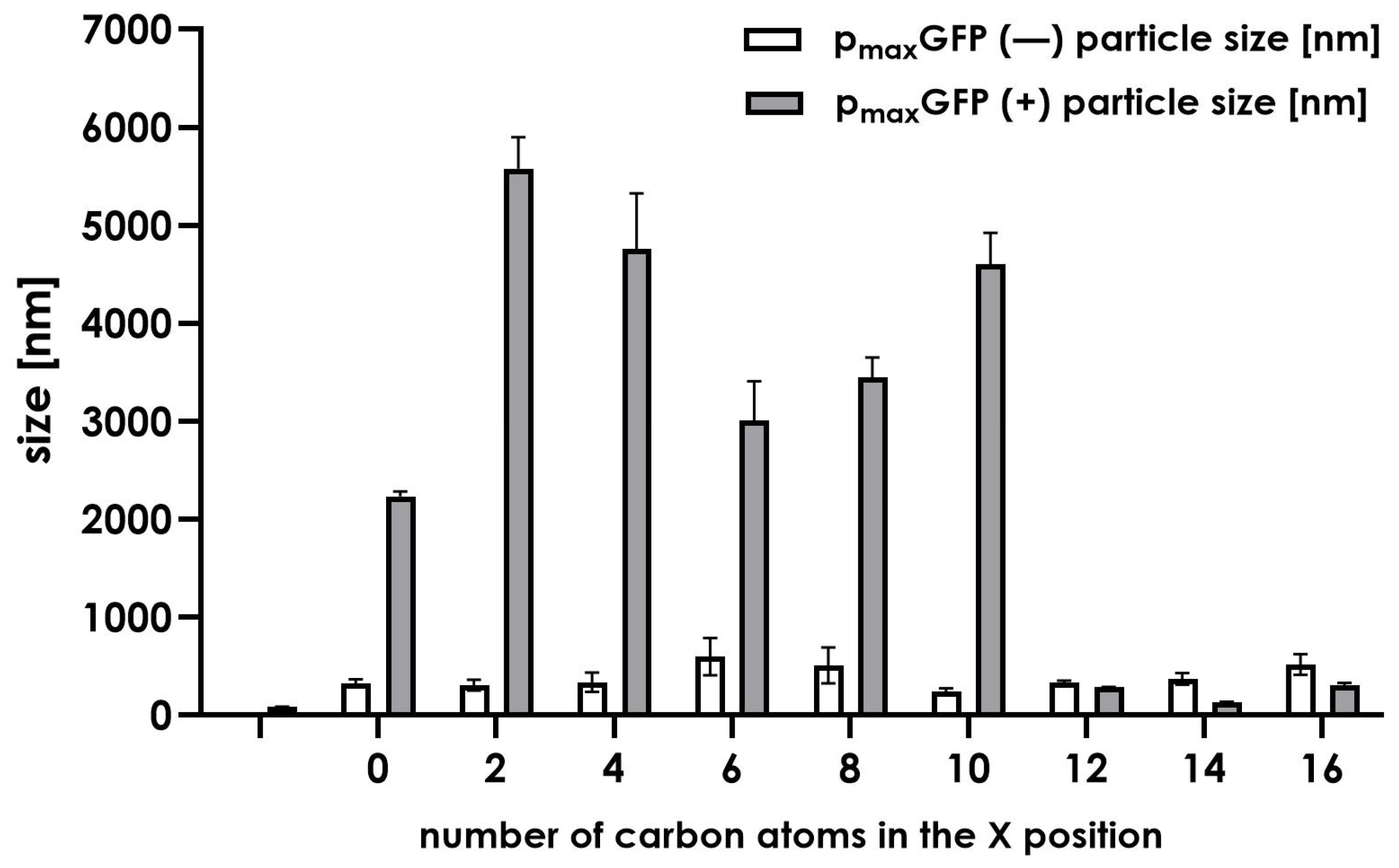
To thoroughly investigate the nature of such discrepancies, we conducted additional experiments to understand the formation of complexes between DNA and the tested compounds. We used dynamic light scattering (DLS) to determine whether the formed complexes are uniformly organized in terms of their size (
Figure 8). Additionally, we measured the zeta size of each complex to confirm the electrostatic nature of the compound–plasmid interactions (
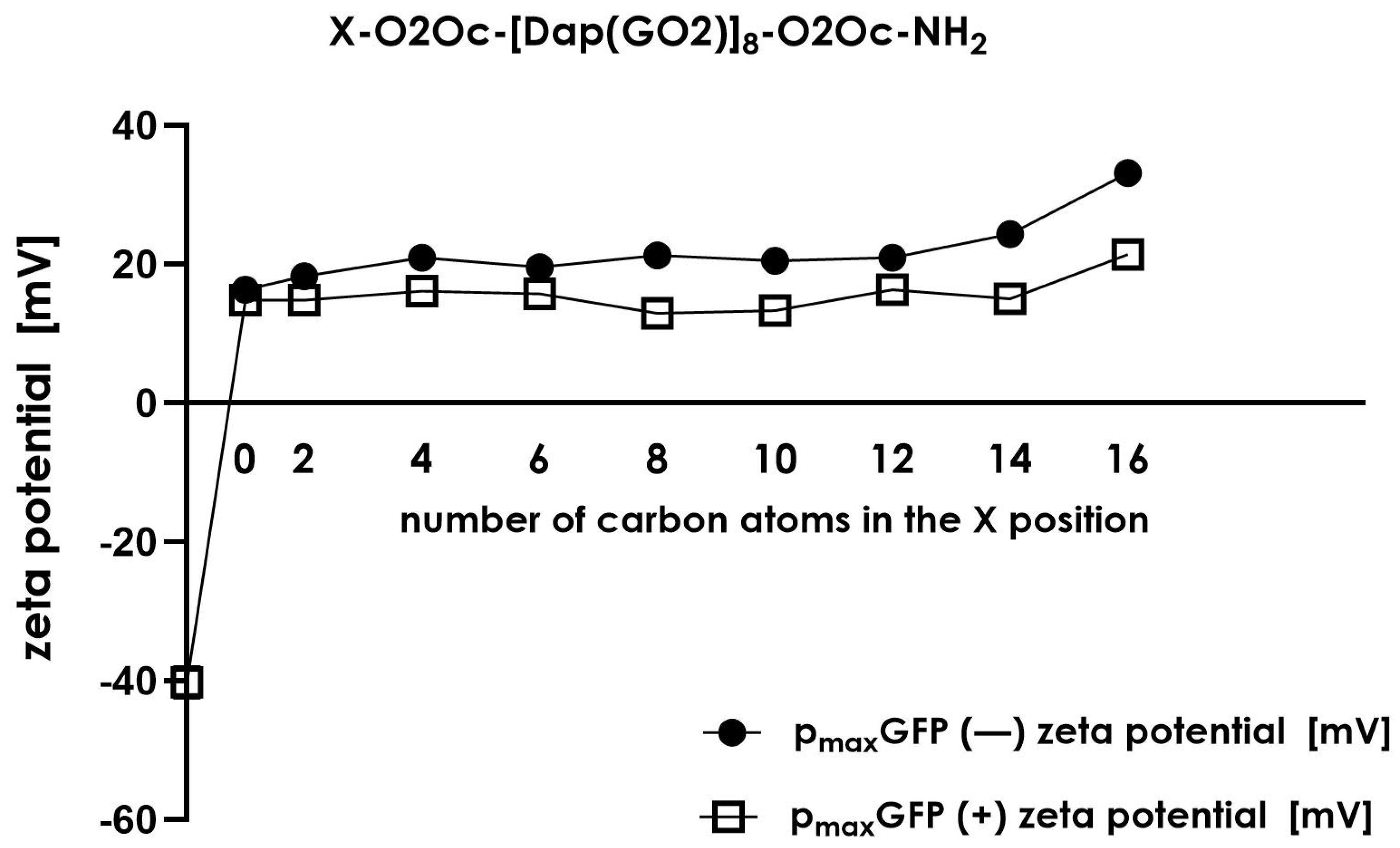
Figure 9).
Initially, we conducted several experiments using DLS to provide an overview of the average particle size of the compounds with and without model DNA plasmid. As shown in
Figure 8, the sizes of the particles formed by the tested compounds mostly range between 140 and 600 nm, indicating the presence of liposomes formed by synthesized molecules. Upon the addition of plasmid DNA (p
maxGFP), larger structures were observed. In systems
1a to
6 (corresponding to compounds
1–
5 along with a control lacking N-terminal modification), large diameter particles ranging from 2000 to almost 6000 nm were recorded. We believe this is an aggregation effect resulting from the interaction between multiple plasmids and compound instances. No large structures were observed in compounds
6–
8. The compounds alone displayed average particle sizes ranging between 300 and 600 nm. The addition of plasmid p
maxGFP caused the particle sizes to be reduced to the range of 200–400 nm. This observation suggests the condensation of DNA or DNA packing into a liposome-like structure.
Zeta size measurements provide information about the overall charge carried by the formed particles. As shown in
Figure 9, we observed a change in charge when the plasmid was incubated with the selected compound. In each case, the complex formed displayed a positive charge in contrast to the negative charge of the plasmid. The zeta size of particles in systems without DNA in the mixture displayed the greatest values (18–35 mV) compared to the charge of complexes formed in the presence of plasmid DNA (15–20 mV). This indicates that in the presence of plasmid DNA, the zeta size of all structures present in the solution reduces their charges by up to 20–30%, showing condensation or stabilization of formed particles compared to systems lacking DNA. However, the overall charge in all conditions was positive except for plasmid DNA, which was expected to be negative.
Our results are consistent with several reports indicating that introducing lipids into a polyArg sequence (preferably octamer) is an effective method for delivering various types of nucleic acids into the desired cells [
14,
15,
16]. To the best of our knowledge, there has been no comprehensive study on the effect of fatty acid chain length on the transfection efficacy of cationic lipids containing DAPEG residues. Similar research has been conducted on Arg-rich peptides [
17] or other cell-penetrating peptides (CPPs) like PeptFECT14 (an analog of transportan [
18]). Previous studies on a series of fatty acid-modified peptides have shown moderate to severe toxicity, reaching 50% at a concentration of 10 µM for fatty acids with carbon chains longer than 12 [
19]. This toxic effect is associated with increased transfection efficiency, probably due to the partial damage of the cell membrane. However, we did not observe such drastic changes since the tested compounds were non-toxic up to a concentration of 20 µM while still maintaining effective transfection capabilities.
3. Materials and Methods
3.1. Synthesis
All compounds were synthesized according to the previously described procedure [
11]. Following the final Fmoc-removal, the N-terminal lipid attachment was performed using a two-fold excess of the corresponding carboxylic acid. The compounds obtained were then detached from the resin using a mixture of TFA, phenol, water, thioanisole, and EDT (82.5:5:5:5:2.5
v/
v), lyophilized, and analyzed using RP UPLC (Shimadzu, Kyoto, Japan, software: LabSolutions LC/GC 5.54 SP2) and HR MALDI MS (Bruker Daltonics GmbH & Co. KG, Bremen, Germany, software: Compass for flexSeries 1.4).
3.2. Purification
All compounds were desalted using Speedisk® C8SPE column (BAKERBOND, Chihuahua, TX, USA). The 10 mg/mL solution was applied on the column wash by water: methanol (95:5, v/v) and eluted by water: methanol 85:15 (v/v) mixture. The obtained solution was evaporated dissolved in water and lyophilized.
3.3. DNA Interactions
The individual compounds 2–9 were mixed at a concentration of 5 × 10−5 M with 100 ng of plasmid DNA and then incubated at 24 °C. Four microliters of the GeneRuler 1 kb DNA ladder (Thermo Scientific, Waltham, MA, USA) were used. A 0.7% agarose gel was prepared (UltraPure Agarose Thermo Fisher, Osterode Am Harz, Germany) and run at 150 V for 55 min in a TAE buffer. The MidoriGreen (advanced DNA stain, Nippon Genetics Europe GmbH, Düren, Germany) was applied to visualize the DNA.
3.4. Cell Culture
The HEK-293T cell line was cultured using both 2D and 3D techniques. The non-adherent method of forming spheroids was used. Cells (750 cells per well) were seeded in a 96-well U-shaped plate BIOFLOAT (Sarstedt, Nümbrecht, Germany). The HEK-293T cells were cultured at 37 °C in 5% CO2 in Dulbecco’s modified Eagle medium (DMEM; high glucose) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin solution containing 100 units of penicillin and 100 µg/mL of streptomycin. After three days of culturing, the spheroids were formed and ready for further experiments.
3.5. Cytotoxicity
The classical MTT test was used. After 72 h of culturing, the HEK-293T cells (8000 cells per well of a 96-well plate) were incubated with increasing amounts of the appropriate compounds (0, 1, 2, 5, 10, 20, and 50 µM) in the medium described above at 37 °C with 5% CO
2. All procedures were performed as outlined in [
11].
3.6. Viability
After culturing for 72 h, spheroids of HEK-293T cells were incubated with increasing amounts of the appropriate compounds (0, 1, 2, 5, 10, 20, and 50 µM) in the medium described above at 37 °C with 5% CO2. After 24 h, the plate was incubated at room temperature (RT) for 30 min, followed by the addition of 100 μL of CellTiter Glo 3D Cell Viability Assay reagent (Promega, Fitchburg, WI, USA). The plate was then covered from light and shaken for 30 min. Next, the contents of each well were transferred to a white flat bottom plate (Nunc A/S, Thermo Fisher, Odense, Denmark) and luminescence was read at 560 nm using the CLARIOstar (BMG Labtech, Ortenberg, Germany).
3.7. Transfection Efficacy in 3D Model
Cells were cultured for 72 h under the conditions described above. Mixtures of the selected compounds and pmaxGFP plasmid in N/P ratios (charge peptidomimetic/charge pmaxGFP) of 1.5, 3:1, and 9:1 were incubated for 30 min and used immediately. An appropriate control with plasmid was employed. To each well with spheroids, 10 µL of the mixture described above was added in triplicate up to 90 µL of the full medium. The plate was then incubated at 37 °C with 5% CO2 for 5 h, washed (3 × medium), and cultured for another 72 h. After this process, the cells were inspected under a fluorescent microscope (Olympus IX51 fluorescence microscope (Olympus, Tokyo, Japan)). The molar concentration of particular components was equal to, for system 1:1.5, the concentration of each compound at 0.61 µM; for 3:1–1.21 µM; for 9:1–3.64 µM. The DNA plasmid concentration remains constant and equal at 0.46 nM.
The time-dependent transfection efficiency was tested for the selected compounds at 1.5, 3, and 5 h time points. The procedure was the same as described above.
3.8. DLS Zeta Size Measurements
Dynamic light scattering (DLS) measurements were performed using the Litesizer DIA 500 (Anton Par, Ostfildern-Scharnhausen, Germany). The concentration of the compounds in water was 2.5 mg/mL, with the inclusion of a DNA plasmid to maintain a charge-to-ratio of 1.5/1 N/P. For system 1:1.5, the concentration of each compound was 0.61 µM; for 3:1–1.21 µM; for 9:1–3.64 µM. The DNA plasmid concentration remains constant and equal at 0.46 nM. The particle size series mode was used in a cuvette with a volume of one quart. Each run was recorded at 25 °C for 10 s.
3.9. Zeta Size Measurement
Zeta sizing was performed using the same apparatus mentioned above. The concentration of each compound in water with DNA complexes was consistent with the DLS measurements (Anton Paar GmbH, Graz, Austria). The Univette low-volume cuvette was used. Voltage was adjusted automatically.
3.10. Complex Stability Assessment
The analysis of all obtained complexes was conducted using a Nanodrop 2000c UV-Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA) at designated time points from 0 to 5 h (0, 0.5, 1, 2.5, and 5). The concentration of the individual components (DNA and the compound) was used as a reference. The concentration of the complexes remained constant in relation to the transfection experiments.