Discovery of MAGL Inhibition by Lophine Derivatives: An Unexpected Finding from Chemiluminescent Assay Development
Abstract
1. Introduction
2. Results and Discussion
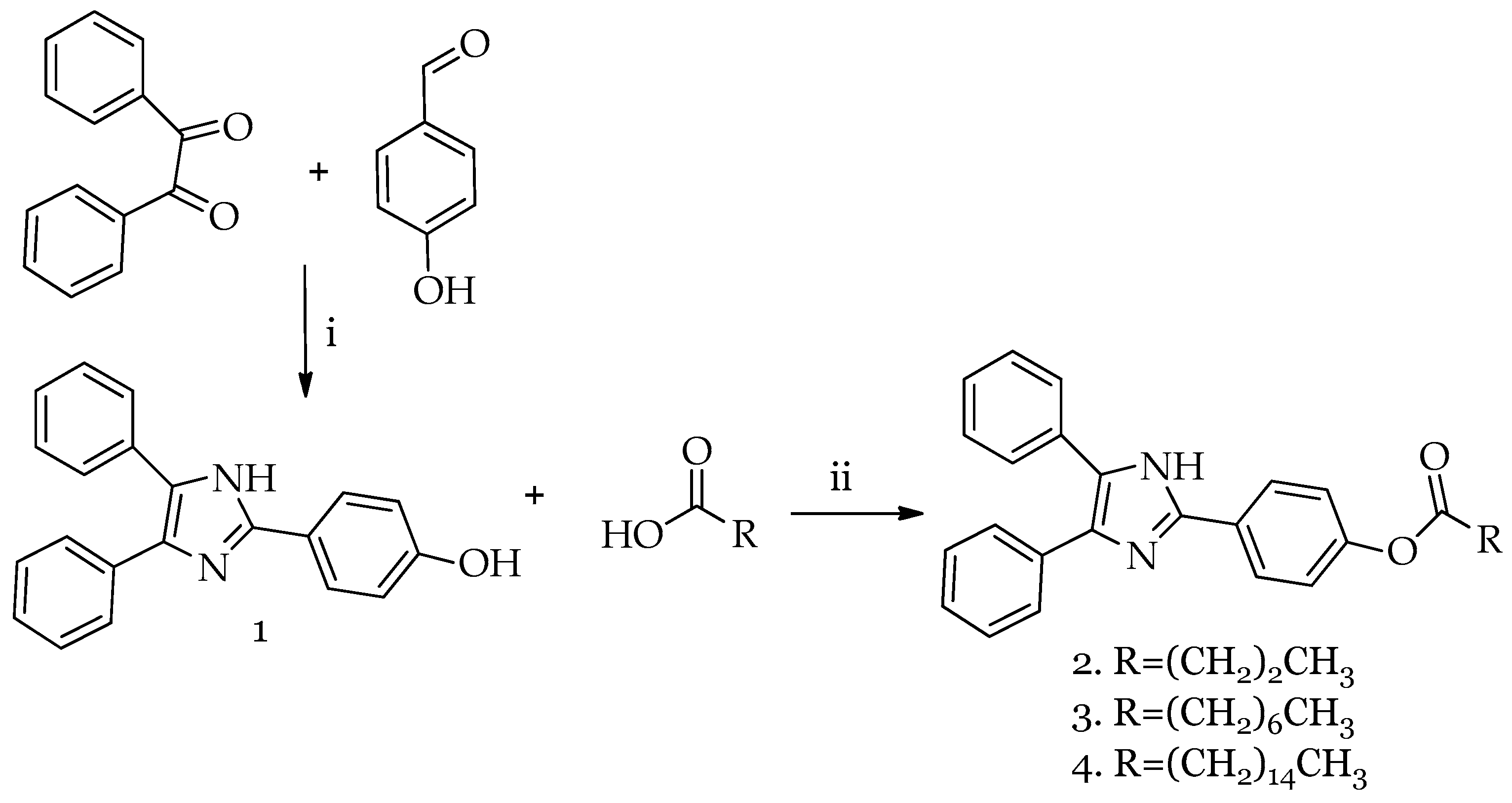
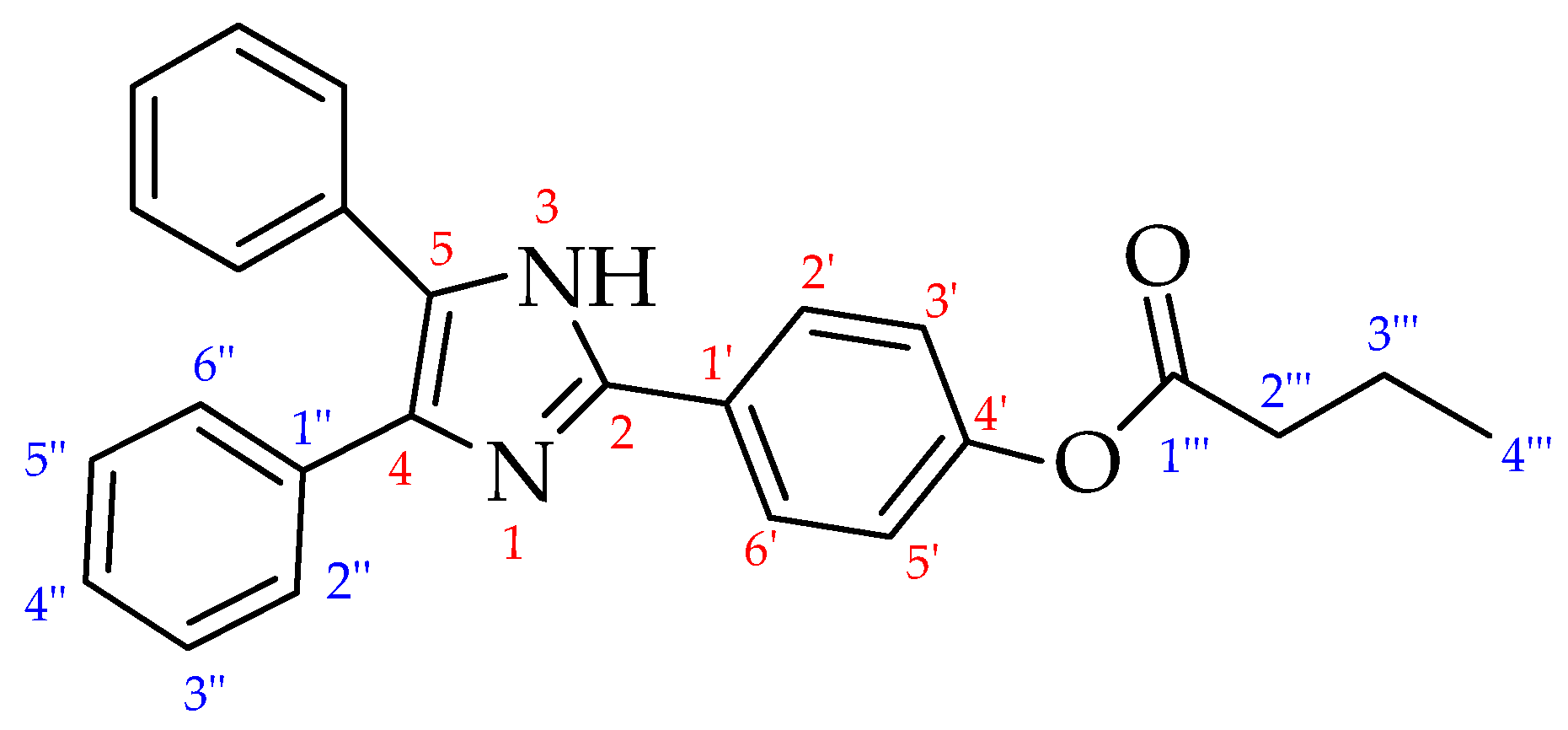
2.1. Synthesis of HDI (1) and Lophine Derivatives 2–4
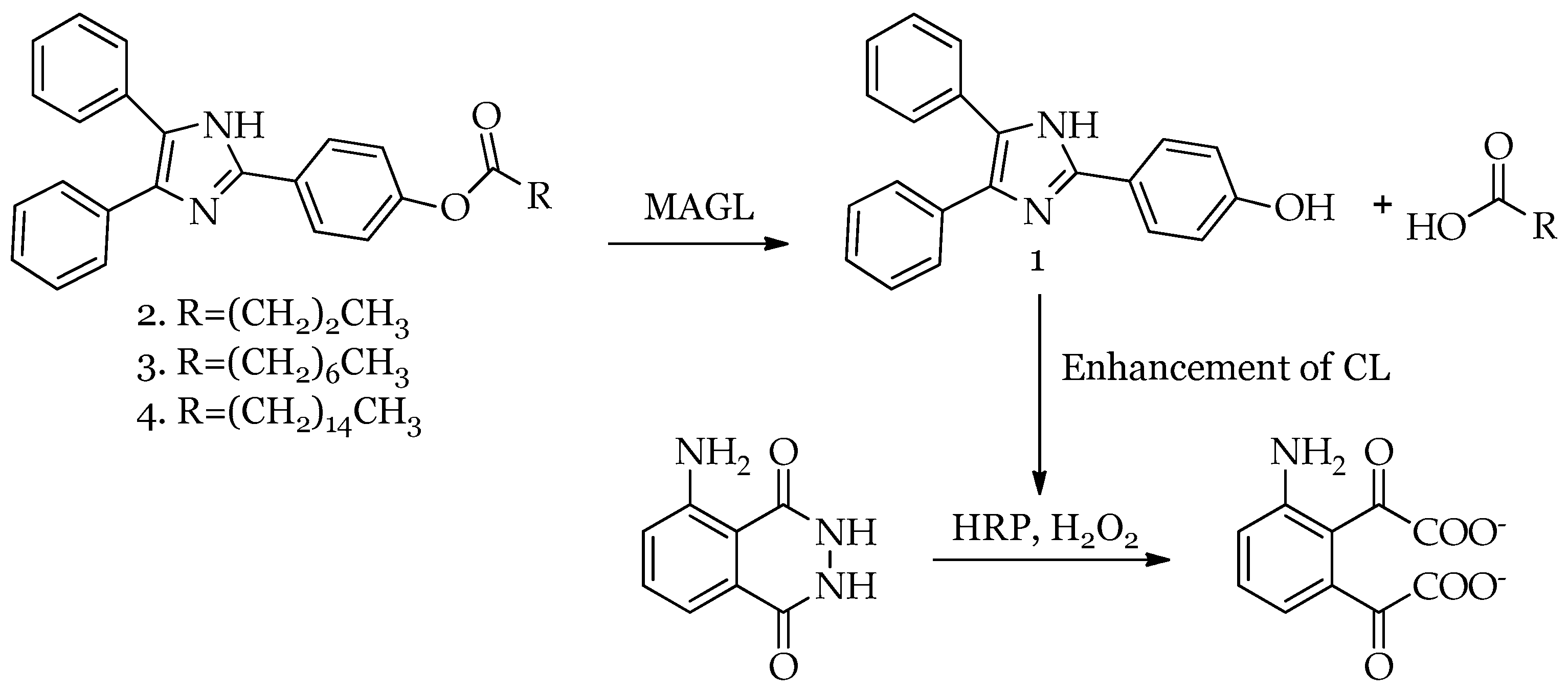
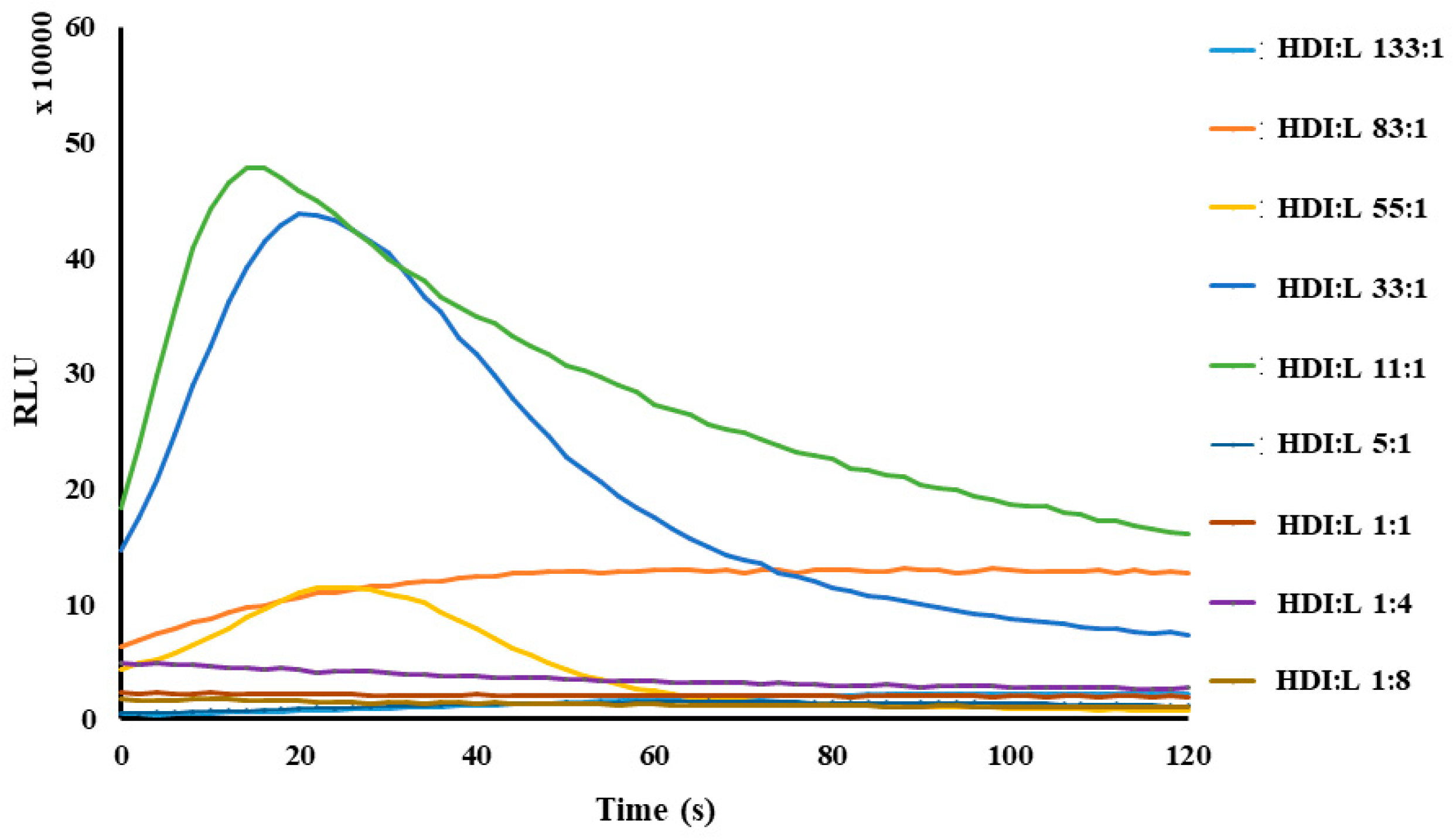
2.2. Setup of HDI–Luminol–H2O2–HRP System Conditions and Chemiluminescence Emission of Lophine Derivatives
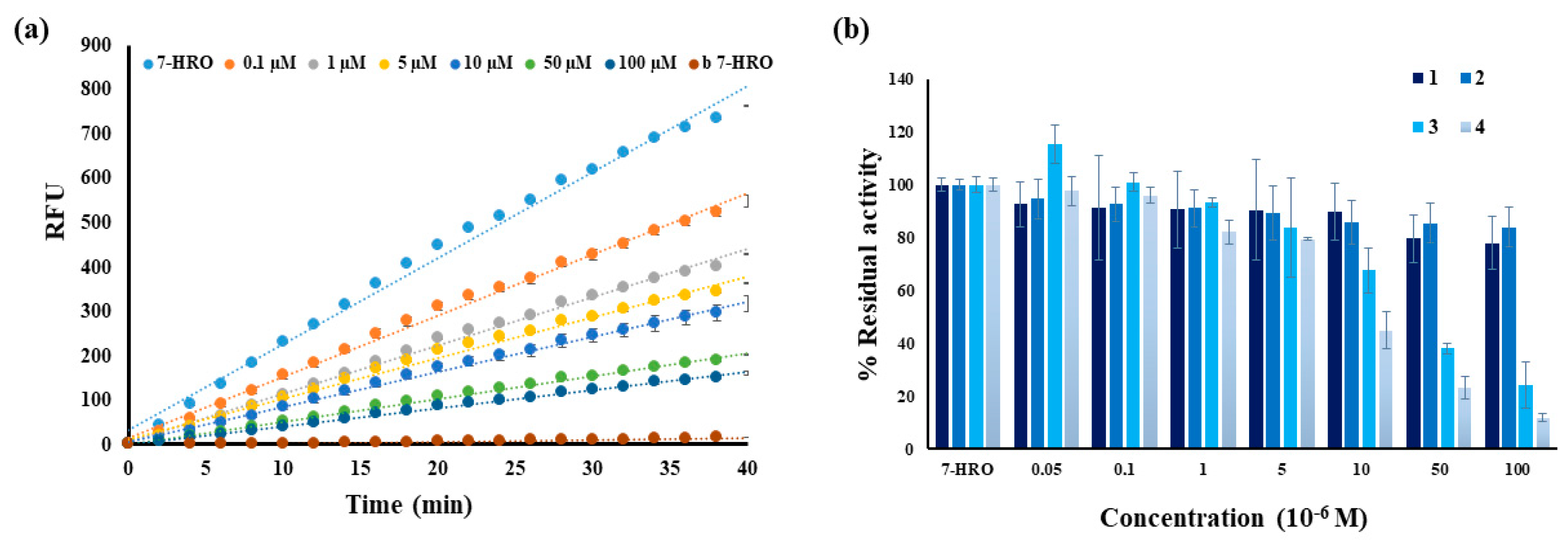
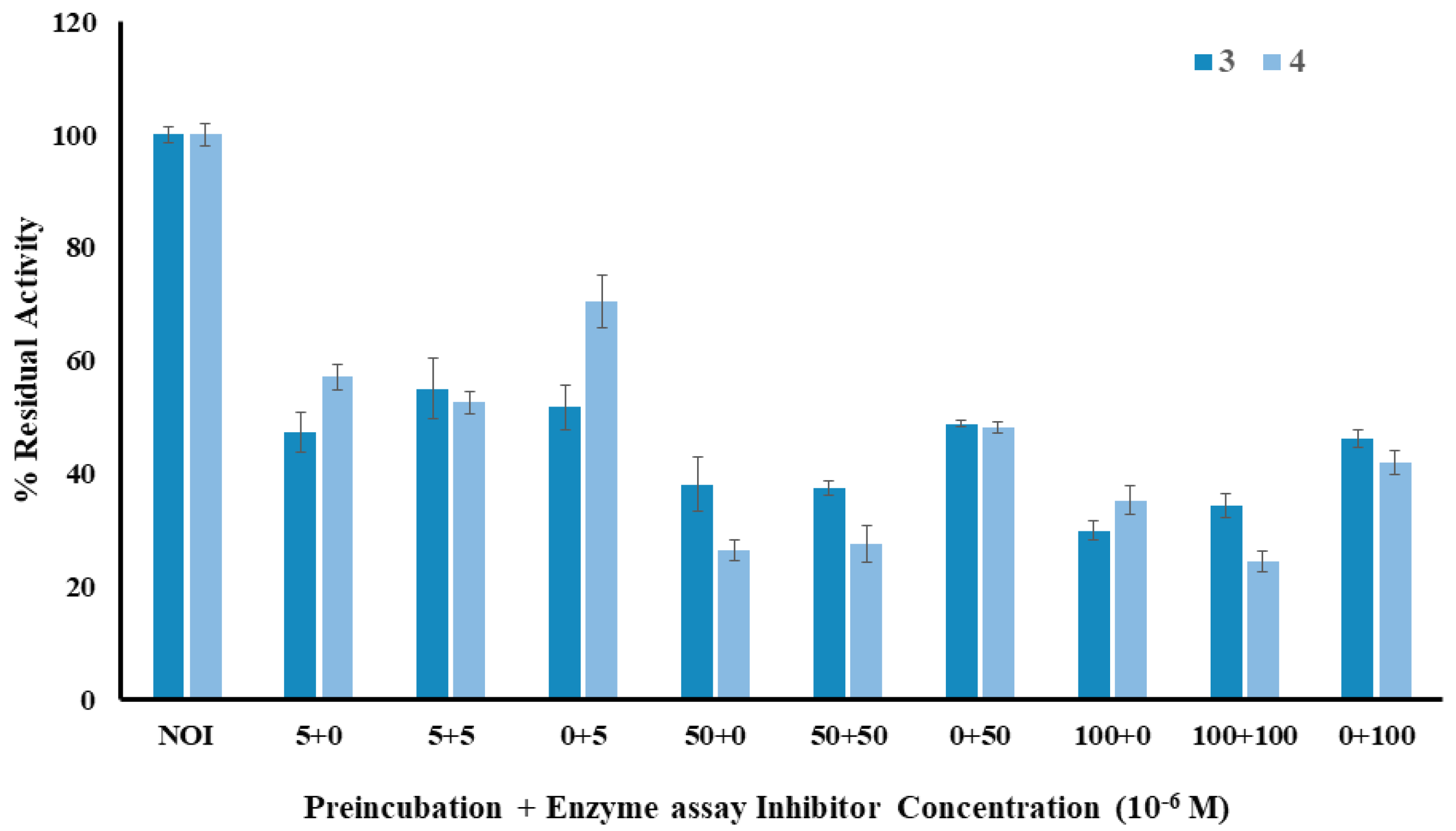
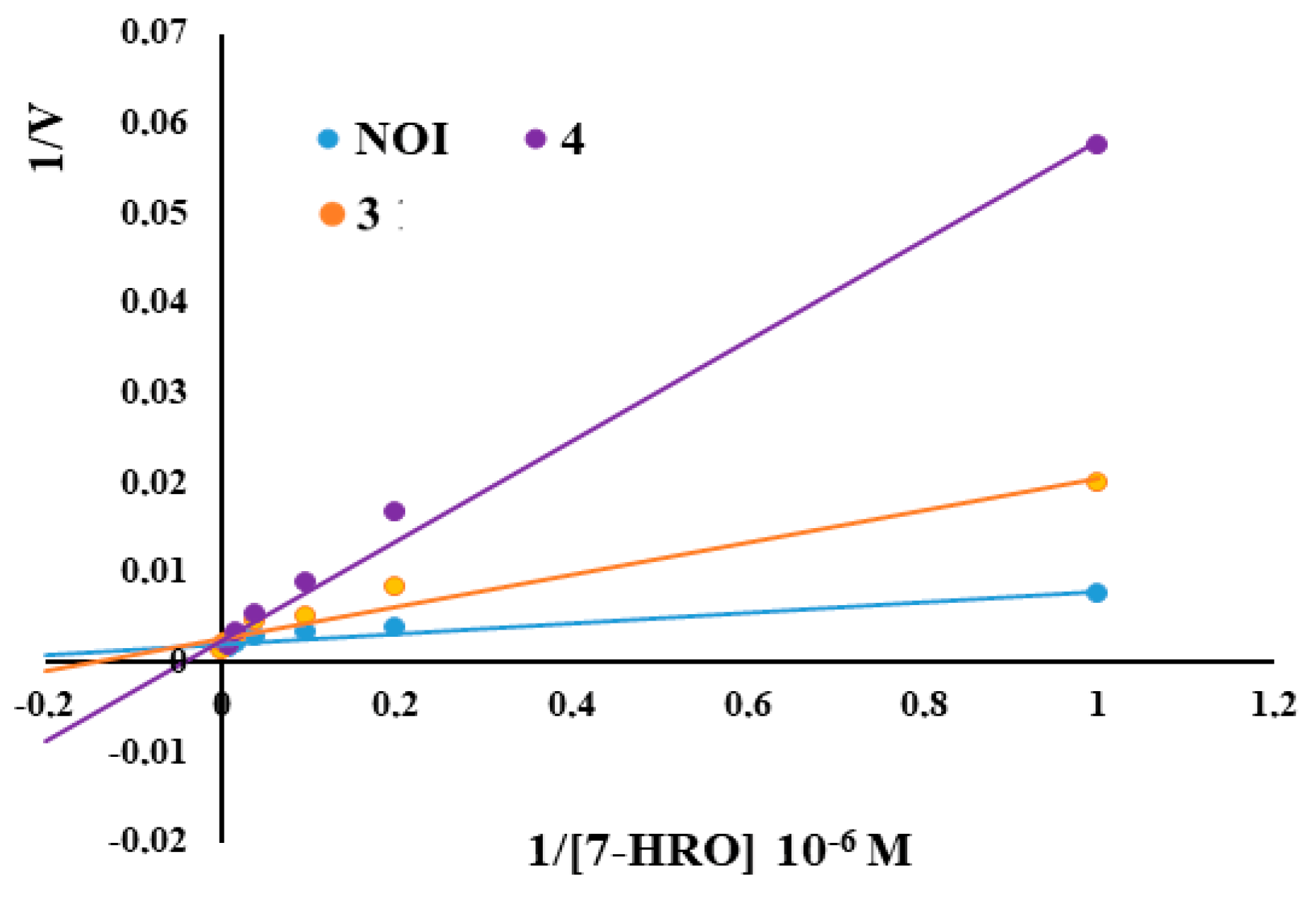
2.3. Fluorometric Assay of Lophine Derivatives 1–4
2.4. Docking Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. 2-(4-Hydroxyphenyl)-4,5-Diphenylimidazole (1) [50]
3.1.3. General Procedure for the Synthesis of Substituted Imidazoles (2–4)
3.1.4. Butyric Acid Ester of 2-(4-Hydroxyphenyl)-4,5-Diphenylimidazole (2)
3.1.5. Octanoic Acid Ester of 2-(4-Hydroxyphenyl)-4,5-Diphenylimidazole (3)
3.1.6. Palmitic Acid Ester of 2-(4-Hydroxyphenyl)-4,5-Diphenylimidazole (4)
3.2. Biochemistry
3.2.1. Chemiluminescence Experiments
3.2.2. Fluorescence Experiments
- i.
- MAGL with 7-HRO fluorescent probe (inhibitor replaced by 5 µL DMSO, representing 100% MAGL activity).
- ii.
- MAGL in the presence of 7-HRO and the tested molecules.
- iii.
- Blank molecules (10 µL of the enzyme solution replaced by 10 µL of reaction buffer).
- iv.
- 7-HRO fluorescent probe blank solution (10 µL of the enzyme solution replaced by 10 µL of reaction buffer, and inhibitor replaced by 5 µL DMSO).
3.2.3. Data Analyses
3.3. In Silico Molecular Docking Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, H.-C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef]
- Ottria, R.; Della Porta, M.; Xynomilakis, O.; Casati, S.; Cazzola, R.; Ciuffreda, P. Lipids and lipid signaling molecules in human milk and infant formula, a chemical characterization of relevant biochemical components. J. Nutr. Biochem. 2024, 126, 109580. [Google Scholar] [CrossRef]
- DiPatrizio, N.V. Endocannabinoids and the Gut-Brain Control of Food Intake and Obesity. Nutrients 2021, 13, 1214. [Google Scholar] [CrossRef]
- Rava, A.; Trezza, V. Emerging Roles of Endocannabinoids as Key Lipid Mediators for a Successful Pregnancy. Int. J. Mol. Sci. 2023, 24, 5220. [Google Scholar] [CrossRef]
- Cooray, R.; Gupta, V.; Suphioglu, C. Current Aspects of the Endocannabinoid System and Targeted THC and CBD Phytocannabinoids as Potential Therapeutics for Parkinson’s and Alzheimer’s Diseases: A Review. Mol. Neurobiol. 2020, 57, 4878–4890. [Google Scholar] [CrossRef]
- Vago, R.; Ravelli, A.; Bettiga, A.; Casati, S.; Lavorgna, G.; Benigni, F.; Salonia, A.; Montorsi, F.; Orioli, M.; Ciuffreda, P.; et al. Urine Endocannabinoids as Novel Non-Invasive Biomarkers for Bladder Cancer at Early Stage. Cancers 2020, 12, 870. [Google Scholar] [CrossRef]
- Quintero, J.-M.; Diaz, L.-E.; Galve-Roperh, I.; Bustos, R.-H.; Leon, M.-X.; Beltran, S.; Dodd, S. The endocannabinoid system as a therapeutic target in neuropathic pain: A review. Expert Opin. Ther. Targets 2024, 28, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Torres, A.M.; Morán, J. Aquaporin 4 and the endocannabinoid system: A potential therapeutic target in brain injury. Exp. Brain Res. 2024, 242, 2041–2058. [Google Scholar] [CrossRef]
- Gowatch, L.C.; Evanski, J.M.; Ely, S.L.; Zundel, C.G.; Bhogal, A.; Carpenter, C.; Shampine, M.M.; O’Mara, E.; Mazurka, R.; Barcelona, J.; et al. Endocannabinoids and Stress-Related Neurospsychiatric Disorders: A Systematic Review and Meta-Analysis of Basal Concentrations and Response to Acute Psychosocial Stress. Cannabis Cannabinoid Res. 2024, 9, 1217–1234. [Google Scholar] [CrossRef]
- Casati, S.; Giannasi, C.; Minoli, M.; Niada, S.; Ravelli, A.; Angeli, I.; Mergenthaler, V.; Ottria, R.; Ciuffreda, P.; Orioli, M.; et al. Quantitative Lipidomic Analysis of Osteosarcoma Cell-Derived Products by UHPLC-MS/MS. Biomolecules 2020, 10, 1302. [Google Scholar] [CrossRef]
- Maccarrone, M.; Di Marzo, V.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; van der Stelt, M. Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958. [Google Scholar] [CrossRef] [PubMed]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef] [PubMed]
- Batista, L.A.; Gobira, P.H.; Viana, T.G.; Aguiar, D.C.; Moreira, F.A. Inhibition of endocannabinoid neuronal uptake and hydrolysis as strategies for developing anxiolytic drugs. Behav. Pharmacol. 2014, 25, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Lauria, S.; Perrotta, C.; Casati, S.; Di Renzo, I.; Ottria, R.; Eberini, I.; Palazzolo, L.; Parravicini, C.; Ciuffreda, P. Design, synthesis, molecular modelling and in vitro cytotoxicity analysis of novel carbamate derivatives as inhibitors of Monoacylglycerol lipase. Bioorg. Med. Chem. 2018, 26, 2561–2572. [Google Scholar] [CrossRef]
- Chen, C. Inhibiting degradation of 2-arachidonoylglycerol as a therapeutic strategy for neurodegenerative diseases. Pharmacol. Ther. 2023, 244, 108394. [Google Scholar] [CrossRef]
- Vago, R.; Bettiga, A.; Salonia, A.; Ciuffreda, P.; Ottria, R. Development of new inhibitors for N-acylethanolamine-hydrolyzing acid amidase as promising tool against bladder cancer. Bioorg. Med. Chem. 2017, 25, 1242–1249. [Google Scholar] [CrossRef]
- Gil-Ordóñez, A.; Martín-Fontecha, M.; Ortega-Gutiérrez, S.; López-Rodríguez, M.L. Monoacylglycerol lipase (MAGL) as a promising therapeutic target. Biochem. Pharmacol. 2018, 157, 18–32. [Google Scholar] [CrossRef]
- Ottria, R.; Casati, S.; Rota, P.; Ciuffreda, P. 2-Arachidonoylglycerol Synthesis: Facile and Handy Enzymatic Method That Allows to Avoid Isomerization. Molecules 2022, 27, 5190. [Google Scholar] [CrossRef]
- Weiermair, T.; Svehlikova, E.; Magnes, C.; Boulgaropoulos, B.; Altendorfer-Kroath, T.; Hummer, J.; Eberl, A. Implementation and validation of a UHPLC-MS/MS method for quantification of the endocannabinoids AEA and 2-AG in cerebral interstitial fluid and plasma. J. Pharm. Biomed. Anal. 2024, 238, 115844. [Google Scholar] [CrossRef]
- Ottria, R.; Ravelli, A.; Gigli, F.; Ciuffreda, P. Simultaneous ultra-high performance liquid chromathograpy-electrospray ionization-quadrupole-time of flight mass spectrometry quantification of endogenous anandamide and related N-acylethanolamides in bio-matrices. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 958, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, Y.; Cai, N.; Liao, X.; Tang, L.; Wang, Y. Endocannabinoid Hydrolase Inhibitors: Potential Novel Anxiolytic Drugs. Drug Des. Dev. Ther. 2024, 18, 2143–2167. [Google Scholar] [CrossRef]
- Deng, H.; Li, W. Monoacylglycerol lipase inhibitors: Modulators for lipid metabolism in cancer malignancy, neurological and metabolic disorders. Acta Pharm. Sin. B 2020, 10, 582–602. [Google Scholar] [CrossRef] [PubMed]
- van Egmond, N.; Straub, V.M.; van der Stelt, M. Targeting Endocannabinoid Signaling: FAAH and MAG Lipase Inhibitors. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 441–463. [Google Scholar] [CrossRef]
- Schlosburg, J.E.; Blankman, J.L.; Long, J.Z.; Nomura, D.K.; Pan, B.; Kinsey, S.G.; Nguyen, P.T.; Ramesh, D.; Booker, L.; Burston, J.J.; et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 2010, 13, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-M.; Piomelli, D. Assay of Monoacylglycerol Lipase Activity. Methods Mol. Biol. 2023, 2576, 285–297. [Google Scholar] [CrossRef]
- Ciuffreda, P.; Xynomilakis, O.; Casati, S.; Ottria, R. Fluorescence-Based Enzyme Activity Assay: Ascertaining the Activity and Inhibition of Endocannabinoid Hydrolytic Enzymes. Int. J. Mol. Sci. 2024, 25, 7693. [Google Scholar] [CrossRef]
- Wang, Y.; Chanda, P.; Jones, P.G.; Kennedy, J.D. A fluorescence-based assay for monoacylglycerol lipase compatible with inhibitor screening. Assay Drug Dev. Technol. 2008, 6, 387–393. [Google Scholar] [CrossRef]
- Miceli, M.; Casati, S.; Ottria, R.; Di Leo, S.; Eberini, I.; Palazzolo, L.; Parravicini, C.; Ciuffreda, P. Set-Up and Validation of a High Throughput Screening Method for Human Monoacylglycerol Lipase (MAGL) Based on a New Red Fluorescent Probe. Molecules 2019, 24, 2241. [Google Scholar] [CrossRef]
- Torchi, A.; Ghamgui, H.; Cherif, S. Basic strategies for monitoring lipase activity: A review. Anal. Biochem. 2025, 696, 115659. [Google Scholar] [CrossRef]
- Yang, M.; Huang, J.; Fan, J.; Du, J.; Pu, K.; Peng, X. Chemiluminescence for bioimaging and therapeutics: Recent advances and challenges. Chem. Soc. Rev. 2020, 49, 6800–6815. [Google Scholar] [CrossRef]
- Duan, P.; Cai, F.; Luo, Y.; Chen, Y.; Zou, S. Long-term chemiluminescence signal is produced in the course of luminol oxidation catalyzed by enhancer-independent peroxidase purified from Jatropha curcas leaves. Luminescence 2015, 30, 818–822. [Google Scholar] [CrossRef]
- Zhang, Z.; Lai, J.; Wu, K.; Huang, X.; Guo, S.; Zhang, L.; Liu, J. Peroxidase-catalyzed chemiluminescence system and its application in immunoassay. Talanta 2018, 180, 260–270. [Google Scholar] [CrossRef]
- Nakashima, K. Lophine derivatives as versatile analytical tools. Biomed. Chromatogr. 2003, 17, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Alpeeva, I.S.; Soukharev, V.S.; Alexandrova, L.; Shilova, N.V.; Bovin, N.V.; Csöregi, E.; Ryabov, A.D.; Sakharov, I.Y. Cyclometalated ruthenium(II) complexes as efficient redox mediators in peroxidase catalysis. J. Biol. Inorg. Chem. 2003, 8, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, T.P.; Thorpe, G.H.G.; Carter, T.J.N.; Groucutt, C.; Kricka, L.J. Enhanced luminescence procedure for sensitive determination of peroxidase-labelled conjugates in immunoassay. Nature 1983, 305, 158–159. [Google Scholar] [CrossRef]
- Thorpe, G.H.; Kricka, L.J.; Gillespie, E.; Moseley, S.; Amess, R.; Baggett, N.; Whitehead, T.P. Enhancement of the horseradish peroxidase-catalyzed chemiluminescent oxidation of cyclic diacyl hydrazides by 6-hydroxybenzothiazoles. Anal. Biochem. 1985, 145, 96–100. [Google Scholar] [CrossRef]
- Kricka, L.J.; Cooper, M.; Ji, X. Synthesis and characterization of 4-iodophenylboronic acid: A new enhancer for the horseradish peroxidase-catalyzed chemiluminescent oxidation of luminol. Anal. Biochem. 1996, 240, 119–125. [Google Scholar] [CrossRef]
- Thorpe, G.H.; Kricka, L.J. Enhanced chemiluminescent reactions catalyzed by horseradish peroxidase. Methods Enzymol. 1986, 133, 331–353. [Google Scholar] [CrossRef]
- Ii, M.; Yoshida, H.; Aramaki, Y.; Masuya, H.; Hada, T.; Terada, M.; Hatanaka, M.; Ichimori, Y. Improved enzyme immunoassay for human basic fibroblast growth factor using a new enhanced chemiluminescence system. Biochem. Biophys. Res. Commun. 1993, 193, 540–545. [Google Scholar] [CrossRef]
- Radziszewski, B.R. Untersuchungen über Hydrobenzamid, Amarin und Lophin. Ber. Dtsch. Chem. Ges. 1877, 10, 70–75. [Google Scholar] [CrossRef]
- Chen, G.; Jin, M.; Du, P.; Zhang, C.; Cui, X.; Zhang, Y.; Wang, J.; Jin, F.; She, Y.; Shao, H.; et al. A review of enhancers for chemiluminescence enzyme immunoassay. Food Agric. Immunol. 2017, 28, 315–327. [Google Scholar] [CrossRef]
- Ichibangase, T.; Ohba, Y.; Kishikawa, N.; Nakashima, K.; Kuroda, N. Chemiluminescence assay of lipase activity using a synthetic substrate as proenhancer for luminol chemiluminescence reaction. Luminescence 2004, 19, 259–264. [Google Scholar] [CrossRef]
- La Rocca, P.; Mingione, A.; Casati, S.; Ottria, R.; Allevi, P.; Ciuffreda, P.; Rota, P. Small-Molecules as Chemiluminescent Probes to Detect Lipase Activity. Int. J. Mol. Sci. 2022, 23, 9039. [Google Scholar] [CrossRef] [PubMed]
- Lauria, S.; Casati, S.; Ciuffreda, P. Synthesis and characterization of a new fluorogenic substrate for monoacylglycerol lipase and application to inhibition studies. Anal. Bioanal. Chem. 2015, 407, 8163–8167. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.; Casati, S.; Allevi, P.; Berra, S.; Ottria, R.; Rota, P.; Branchini, B.R.; Ciuffreda, P. A New Ultrasensitive Bioluminescence-Based Method for Assaying Monoacylglycerol Lipase. Int. J. Mol. Sci. 2021, 22, 6148. [Google Scholar] [CrossRef]
- Roda, A.; Guardigli, M. Analytical chemiluminescence and bioluminescence: Latest achievements and new horizons. Anal. Bioanal. Chem. 2012, 402, 69–76. [Google Scholar] [CrossRef]
- Yang, L.; Jin, M.; Du, P.; Chen, G.; Zhang, C.; Wang, J.; Jin, F.; Shao, H.; She, Y.; Wang, S.; et al. Study on Enhancement Principle and Stabilization for the Luminol-H2O2-HRP Chemiluminescence System. PLoS ONE 2015, 10, e0131193. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Kaur, G.; Chavan, A.R.; Chahal, M.K.; Taliyan, R. Phenoxy-1,2-dioxetane-based activatable chemiluminescent probes: Tuning of photophysical properties for tracing enzymatic activities in living cells. Analyst 2024, 149, 5739–5761. [Google Scholar] [CrossRef]
- Hariharasubramanian, A.; Ravichandran, Y.D. Synthesis and studies of electrochemical properties of lophine derivatives. RSC Adv. 2014, 4, 54740–54746. [Google Scholar] [CrossRef]
- Cohen, A.S.; Dubikovskaya, E.A.; Rush, J.S.; Bertozzi, C.R. Real-time bioluminescence imaging of glycans on live cells. J. Am. Chem. Soc. 2010, 132, 8563–8565. [Google Scholar] [CrossRef] [PubMed]
- Ichibangase, T.; Hamabe, C.; Ohba, Y.; Kishikawa, N.; Nakashima, K.; Kayamori, Y.; Kang, D.; Hamasaki, N.; Kuroda, N. Study on immunocapture-chemiluminescence assay of lipase activity in a biological sample. Luminescence 2006, 21, 62–66. [Google Scholar] [CrossRef]
- Navia-Paldanius, D.; Savinainen, J.R.; Laitinen, J.T. Biochemical and pharmacological characterization of human α/β-hydrolase domain containing 6 (ABHD6) and 12 (ABHD12). J. Lipid Res. 2012, 53, 2413–2424. [Google Scholar] [CrossRef]
- Jiménez, J.; Škalič, M.; Martínez-Rosell, G.; de Fabritiis, G. KDEEP: Protein-Ligand Absolute Binding Affinity Prediction Via 3D-Convolutional Neural Networks. J. Chem. Inf. Model. 2018, 58, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Torrens-Fontanals, M.; Tourlas, P.; Doerr, S.; de Fabritiis, G. PlayMolecule Viewer: A Toolkit for the Visualization of Molecules and Other Data. J. Chem. Inf. Model. 2024, 64, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, N.; Takatani, M.; Nakashima, K.; Akiyama, S.; Ohkura, Y. Preparation and evaluation of fatty acid esters of 2-(4-hydroxyphenyl)-4,5-diphenylimidazole as fluorescent substrates for measurement of lipase activity. Biol. Pharm. Bull. 1993, 16, 220–222. [Google Scholar] [CrossRef]
- Churat, A.; Katrun, P.; Laohpongspaisan, C.; Mongkolthanaruk, W.; Champasri, C.; Moontragoon, P.; Suwannasai, N.; Sangvichien, E.; Poonsukkho, P.; McCloskey, S. Potential α-glucosidase inhibitors from cultures of Biscogniauxia capnodes SWUF15-40 fungus. J. Nat. Med. 2025. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Zivkovic, M.; Zlatanovic, M.; Zlatanovic, N.; Golubović, M.; Veselinović, A.M. The Application of the Combination of Monte Carlo Optimization Method Based QSAR Modeling and Molecular Docking in Drug Design and Development. Mini Rev. Med. Chem. 2020, 20, 1389–1402. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A.; Sindhu, J. In silico design of diacylglycerol acyltransferase-1 (DGAT1) inhibitors based on SMILES descriptors using Monte-Carlo method. SAR QSAR Environ. Res. 2019, 30, 525–541. [Google Scholar] [CrossRef]
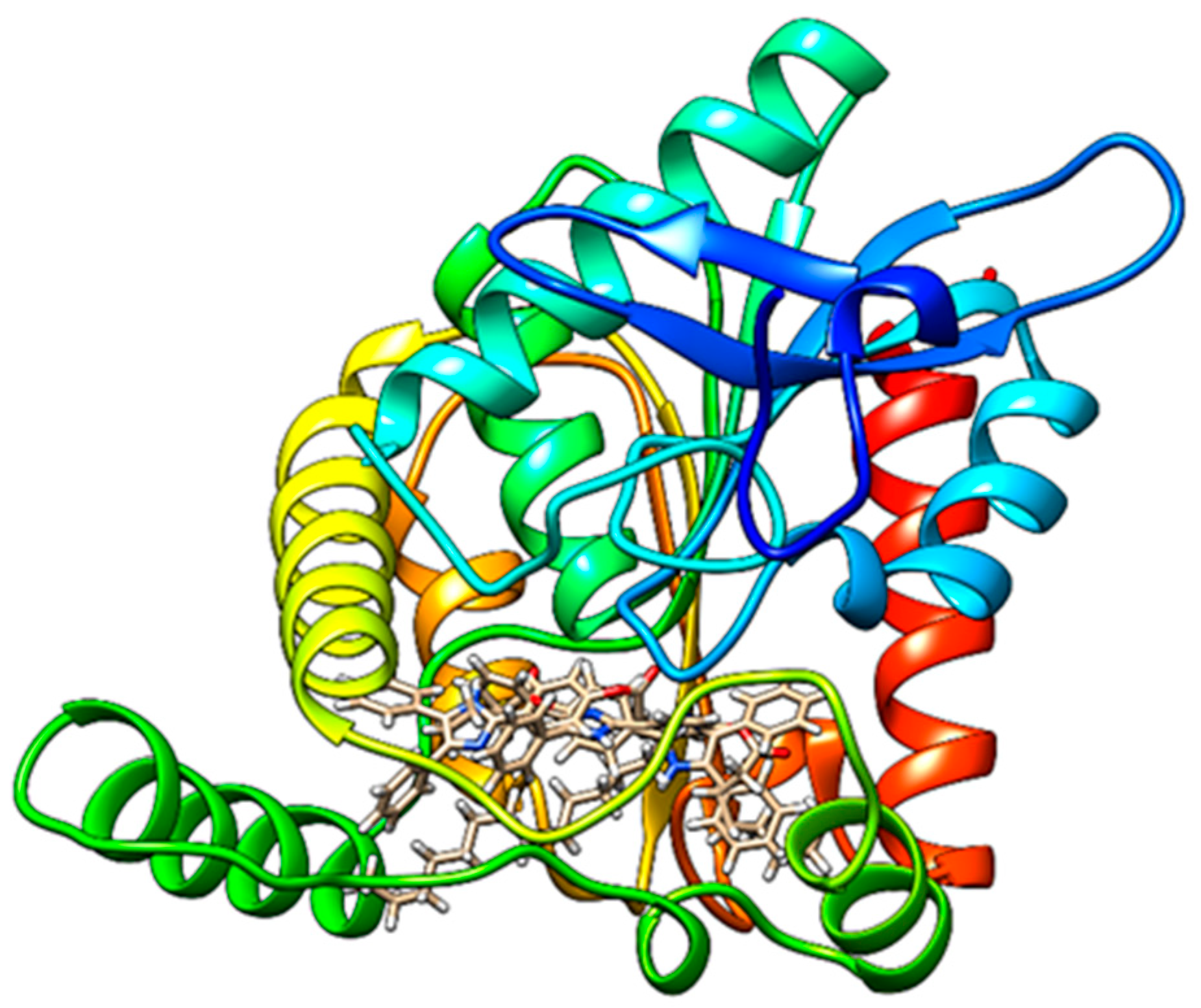
| Molecule | HBond | NoHBond 90 | Steric | VdW | Energy | MolDock Score | Rerank Score | ΔG | LE | pKd |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | −3.2594 | −5.0 | −168.812 | −21.7531 | −148.975 | −144.028 | −79.9843 | −9.5990 | −0.2415 | 7.1103 |
| 3 | −4.786 | −7.5 | −186.11 | −25.6259 | −175.106 | −161.543 | −119.866 | −10.3682 | −0.3342 | 7.6801 |
| 4 | −0.1089 | −2.5 | −174.296 | −17.7926 | −179.927 | −145.218 | −106.764 | −9.9003 | −0.3110 | 7.336 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottria, R.; Casati, S.; Xynomilakis, O.; Veselinović, A.; Ciuffreda, P. Discovery of MAGL Inhibition by Lophine Derivatives: An Unexpected Finding from Chemiluminescent Assay Development. Molecules 2025, 30, 1605. https://doi.org/10.3390/molecules30071605
Ottria R, Casati S, Xynomilakis O, Veselinović A, Ciuffreda P. Discovery of MAGL Inhibition by Lophine Derivatives: An Unexpected Finding from Chemiluminescent Assay Development. Molecules. 2025; 30(7):1605. https://doi.org/10.3390/molecules30071605
Chicago/Turabian StyleOttria, Roberta, Silvana Casati, Ornella Xynomilakis, Aleksandar Veselinović, and Pierangela Ciuffreda. 2025. "Discovery of MAGL Inhibition by Lophine Derivatives: An Unexpected Finding from Chemiluminescent Assay Development" Molecules 30, no. 7: 1605. https://doi.org/10.3390/molecules30071605
APA StyleOttria, R., Casati, S., Xynomilakis, O., Veselinović, A., & Ciuffreda, P. (2025). Discovery of MAGL Inhibition by Lophine Derivatives: An Unexpected Finding from Chemiluminescent Assay Development. Molecules, 30(7), 1605. https://doi.org/10.3390/molecules30071605






