Mo-W18O49/ZnIn2S4 Composites Synthesized by Metal Doping for Photocatalytic Hydrogen Evolution
Abstract
1. Introduction
2. Results and Discussion
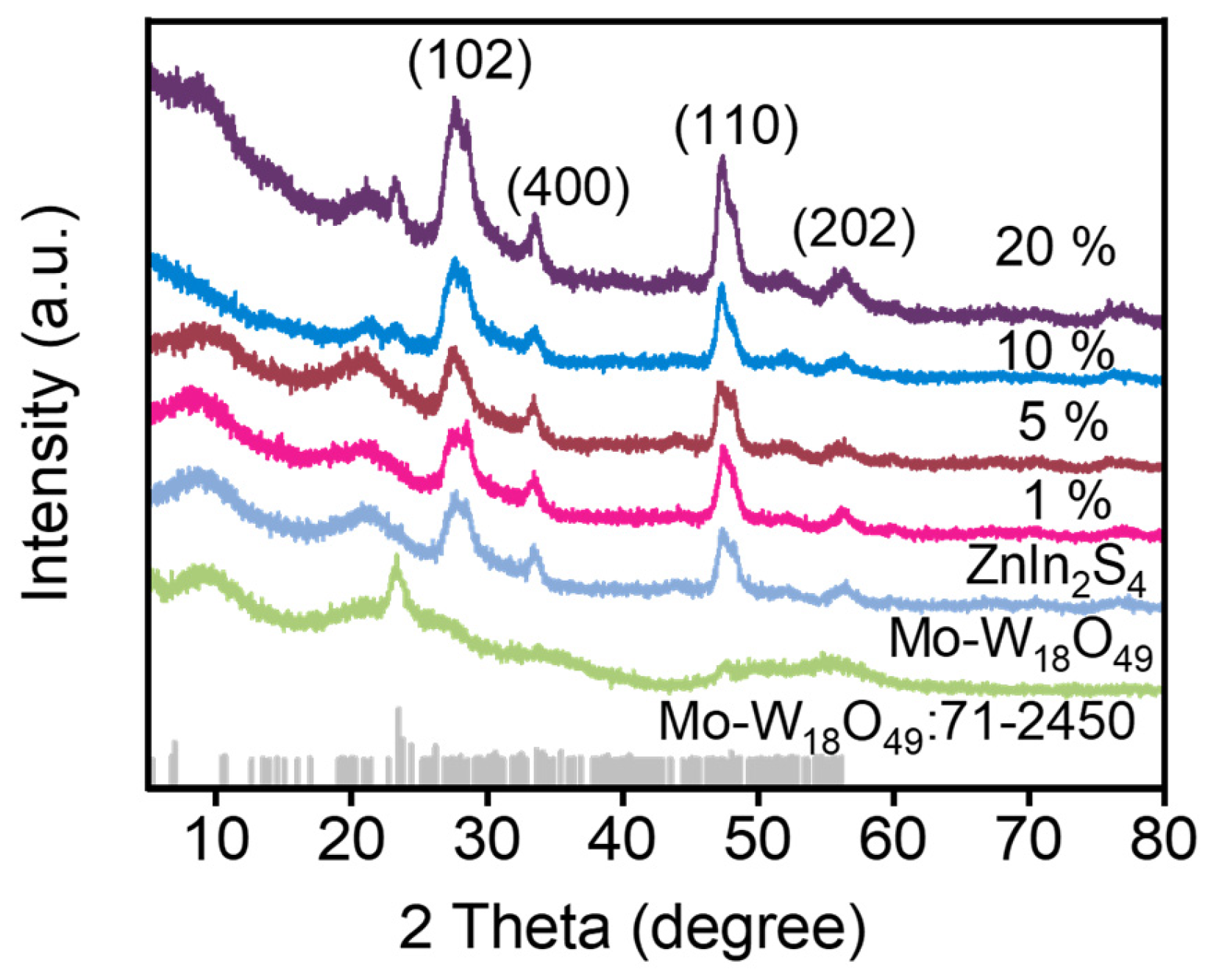
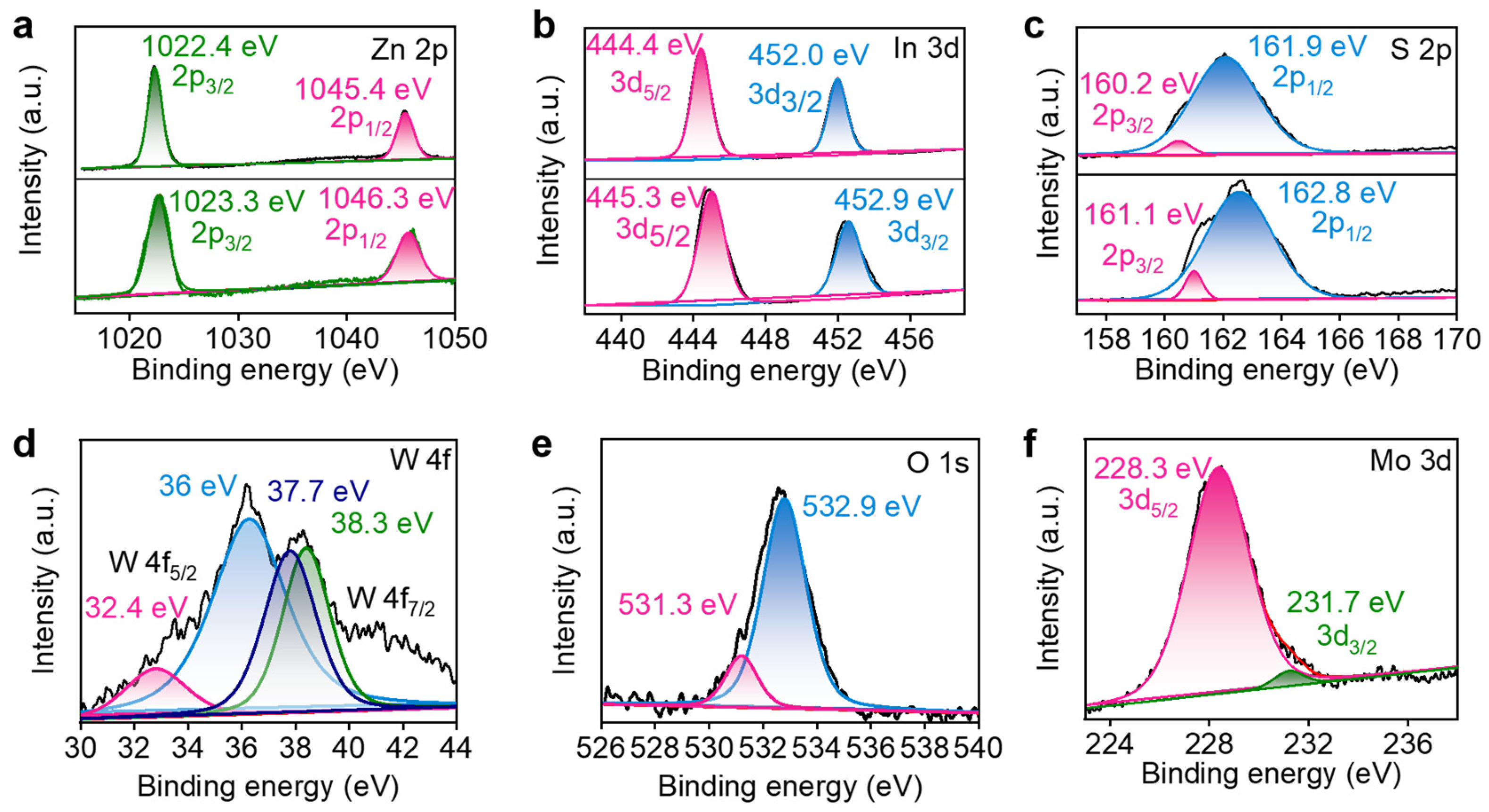
2.1. Structural and Morphological Characterization of Photocatalysts
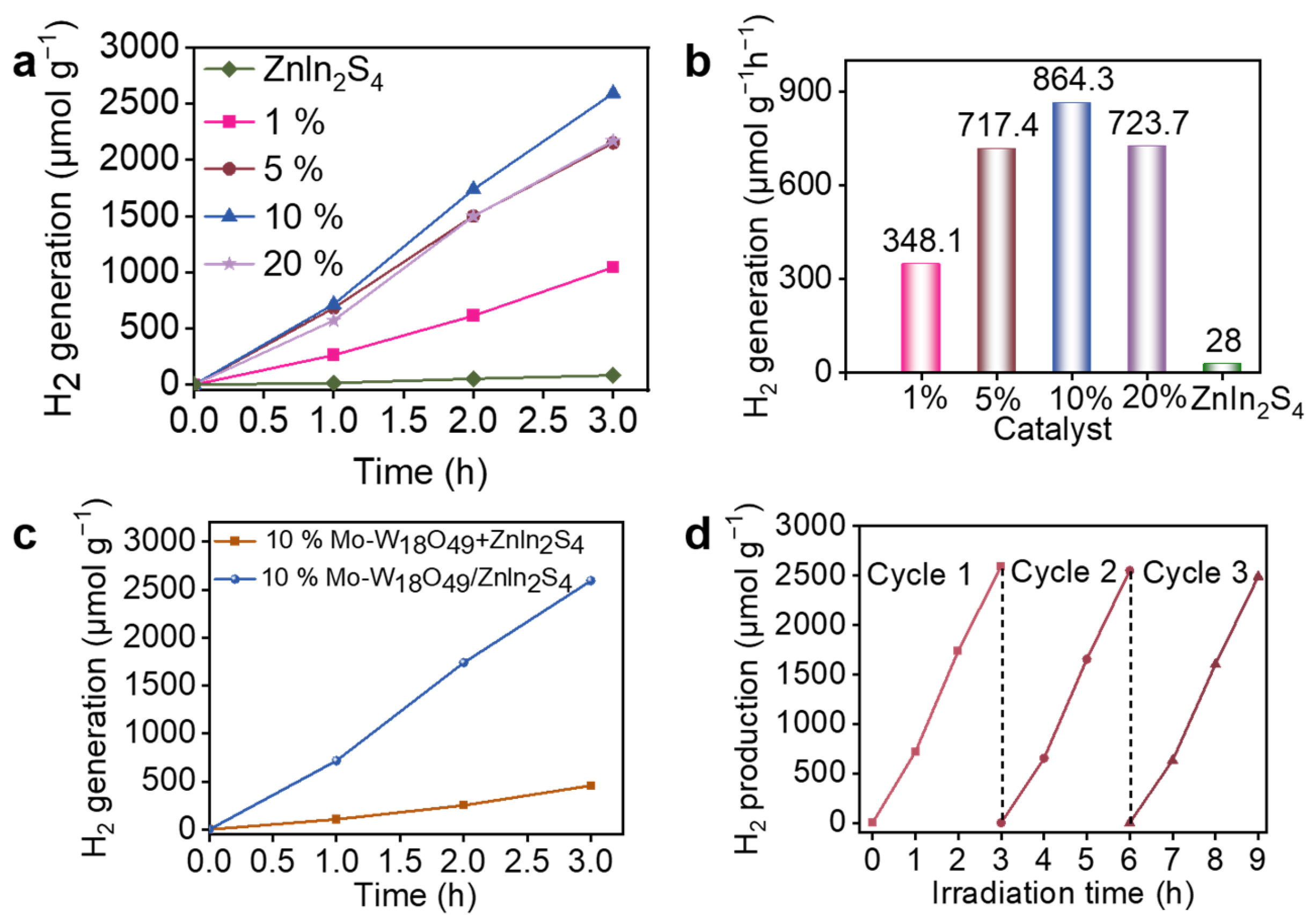
2.2. Photocatalytic Hydrogen Evolution Performance
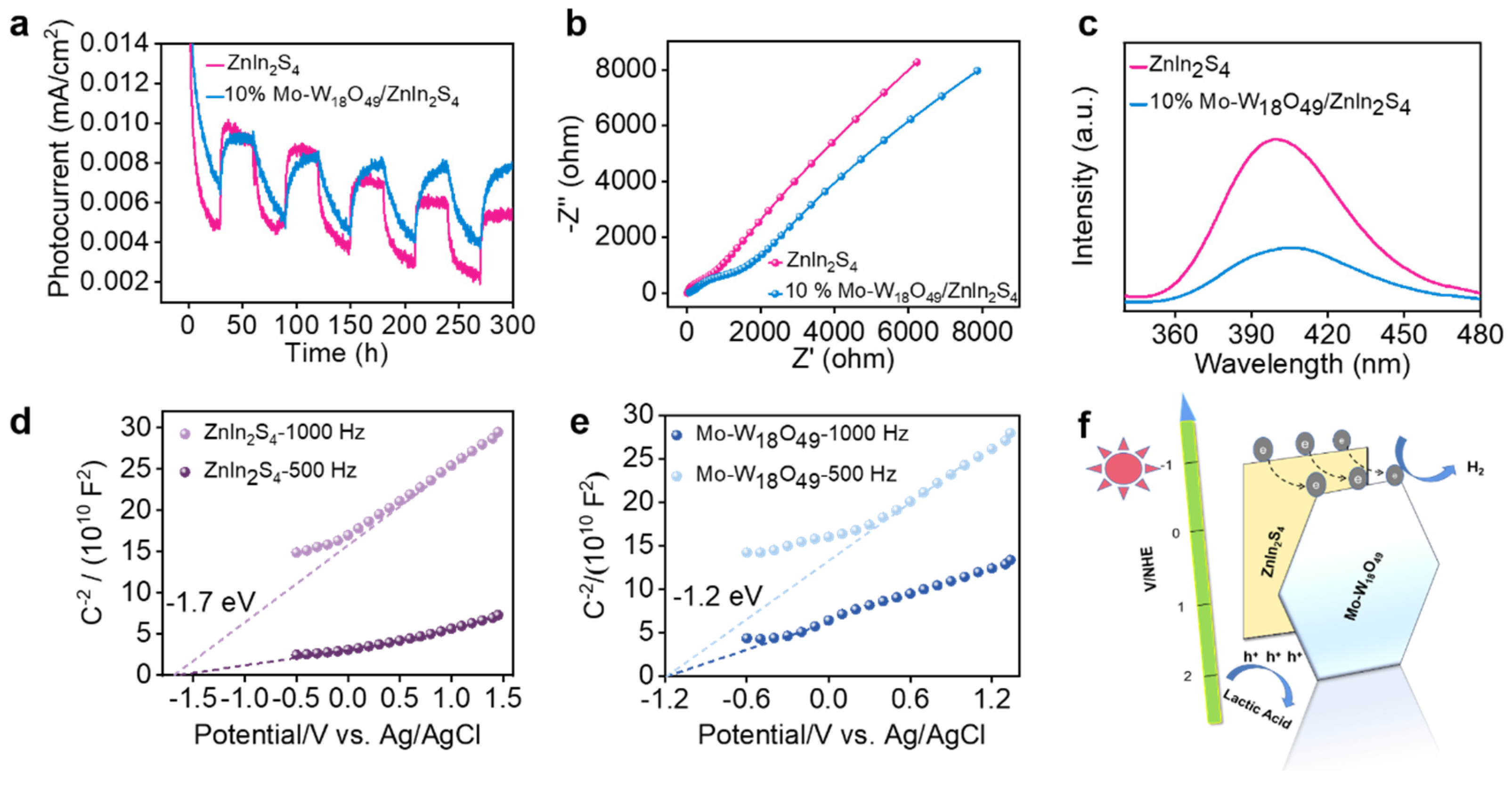
2.3. Explore the Mechanism of Photocatalytic Hydrogen Evolution
3. Experiment
3.1. Materials and Methods
3.2. Characterization
3.3. Photocatalytic Reaction Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Gudiño, L.; Bedia, J.; Belver, C. Solar photocatalytic hydrogen production through metal sulfide/UiO-66-NH2 heterojunctions. Sep. Purif. Technol. 2025, 353, 128663. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, C.; Wang, Z.; Zhang, J.; Dai, K. One-step synthesis of seamlessly contacted non-precious metal cocatalyst modified CdS hollow nanoflowers spheres for photocatalytic hydrogen production. J. Mater. Sci. Technol. 2024, 195, 146–154. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Ren, J.; Xue, Y.; Tian, J. Synergy of atom doping and defect construction in marigold-like Zn3In2S6 for improved photocatalytic hydrogen production. Mater. Today Phys. 2024, 46, 101484. [Google Scholar] [CrossRef]
- Gong, G.; Liu, Y.; Mao, B.; Tan, L.; Yang, Y.; Shi, W. Ag doping of Zn-In-S quantum dots for photocatalytic hydrogen evolution: Simultaneous bandgap narrowing and carrier lifetime elongation. Appl. Catal. B Environ. 2017, 216, 11–19. [Google Scholar] [CrossRef]
- Li, X.; You, J.; Li, J.; Wang, Z.; Zhao, Y.; Xu, J.; Duan, M.; Zhang, H. Progress of copper-based nanocatalysts in advanced oxidation degraded organic pollutants. ChemCatChem 2024, 16, e202301108. [Google Scholar] [CrossRef]
- Hu, X.; Song, J.; Luo, J.; Zhang, H.; Sun, Z.; Li, C.; Zheng, S.; Liu, Q. Single-atomic Pt sites anchored on defective TiO2 nanosheets as a superior photocatalyst for hydrogen evolution. J. Energy Chem. 2021, 62, 1–10. [Google Scholar] [CrossRef]
- Dhiman, P.; Rana, G.; Kumar, A.; Sharma, G.; Vo, D.N.; Naushad, M. ZnO-based heterostructures as photocatalysts for hydrogen generation and depollution: A review. Environ. Chem. Lett. 2022, 20, 1047–1081. [Google Scholar] [CrossRef]
- Xie, X.; Fan, Y.; Tian, W.; Zhang, M.; Cai, J.; Zhang, X.; Ding, J.; Liu, Y.; Lu, S. Construction of Ru/WO3 with hetero-interface structure for efficient hydrogen evolution reaction. J. Energy Chem. 2023, 83, 150–157. [Google Scholar] [CrossRef]
- Shen, J.; Luo, C.; Qiao, S.; Chen, Y.; Tang, Y.; Xu, J.; Fu, K.; Yuan, D.; Tang, H.; Zhang, H.; et al. Single-atom Cu channel and N-vacancy engineering enables efficient charge separation and transfer between C3N4 interlayers for boosting photocatalytic hydrogen production. ACS Catal. 2023, 13, 6280–6288. [Google Scholar] [CrossRef]
- He, F.; Wang, S.; Lu, Y.; Dong, P.; Zhang, Y.; Lin, F.; Liu, X.; Wang, Y.; Zhao, C.; Wang, S.; et al. Unification of hot spots and catalytic sites on isolated boron centers in porous carbon nitride nanosheets for efficient photocatalytic oxygen evolution. Nano Energy 2023, 116, 108800. [Google Scholar] [CrossRef]
- Wang, X.; Gong, J.; Dong, Y.; An, S.; Zhang, X.; Tian, J. Energy band engineering of hydroxyethyl group grafted on the edge of 3D g-C3N4 nanotubes for enhanced photocatalytic H2 production. Mater. Today Phys. 2022, 27, 100806. [Google Scholar] [CrossRef]
- Sun, J.; Yang, J.; Liang, J.; Tu, L.; Bin, Y.; Hou, Y. Construction of microspherical flower-like Zn3In2S6-BGQDs/AgBr S-scheme heterojunction for photocatalytic elimination of nitrofurazone and Cr (VI). Sep. Purif. Technol. 2022, 299, 121563. [Google Scholar] [CrossRef]
- Zhou, W.; Li, F.; Yang, X.; Yang, W.; Wang, C.; Cao, R.; Zhou, C.; Tian, M. Peanut-chocolate-ball-inspired construction of the interface engineering between CdS and intergrown Cd: Boosting both the photocatalytic activity and photocorrosion resistance. J. Energy Chem. 2023, 76, 75–89. [Google Scholar] [CrossRef]
- Huang, R.; Qin, Z.; Shen, L.; Lv, G.; Tao, F.; Wang, J.; Gao, Y. Interface engineering of InP/ZnS core/shell quantum dots by the buffer monolayer for exceptional photocatalytic H2 evolution. J. Mater. Chem. A 2023, 11, 6217–6225. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Chen, J.; Xing, X.; Wang, Y.; Zhang, Y.; Yang, M.; Zhang, G. Confined synthesis of MoS2 with rich Co-doped edges for enhanced hydrogen evolution performance. J. Energy Chem. 2022, 70, 18–26. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, X.; Ma, Z.; Li, Y.; Yue, S.; Liu, X.; Zhang, J. W5+–W5+ pair induced LSPR of W18O49 to sensitize ZnIn2S4 for full-spectrum solar-light-driven photocatalytic hydrogen evolution. Adv. Funct. Mater. 2022, 32, 2203638. [Google Scholar] [CrossRef]
- Su, L.; Wang, P.; Wang, J.; Zhang, D.; Wang, H.; Li, Y.; Zhan, S.; Gong, J. Pt–Cu interaction induced construction of single Pt sites for synchronous electron capture and transfer in photocatalysis. Adv. Funct. Mater. 2021, 31, 2104343. [Google Scholar] [CrossRef]
- Du, C.; Yan, B.; Lin, Z.; Yang, G. Enhanced carrier separation and increased electron density in 2D heavily N-doped ZnIn2S4 for photocatalytic hydrogen production. J. Mater. Chem. A 2020, 8, 207–217. [Google Scholar] [CrossRef]
- Kang, F.; Shi, C.; Zhu, Y.; Eqi, M.; Shi, J.; Teng, M.; Huang, Z.; Si, C.; Jiang, F.; Hu, J. Dual-functional marigold-like Zn Cd1-xS homojunction for selective glucose photoreforming with remarkable H2 coproduction. J. Energy Chem. 2023, 79, 158–167. [Google Scholar] [CrossRef]
- Bariki, R.; Kumar Pradhan, S.; Panda, S.; Kumar Nayak, S.; Majhi, D.; Das, K.; Mishra, B.G. In-situ synthesis of structurally oriented hierarchical UiO-66(–NH2)/CdIn2S4/CaIn2S4 heterostructure with dual S-scheme engineering for photocatalytic renewable H2 production and asulam degradation. Sep. Purif. Technol. 2023, 314, 123558. [Google Scholar] [CrossRef]
- Janani, R.; V, R.P.; Singh, S.; Rani, A.; Chang, C. Hierarchical ternary sulfides as effective photocatalyst for hydrogen generation through water splitting: A review on the performance of ZnIn2S4. Catalysts 2021, 11, 277. [Google Scholar] [CrossRef]
- Luan, J.; Chen, J. Photocatalytic water splitting for hydrogen production with novel Y2MSbO7 (M = Ga, In, Gd) under visible light irradiation. Materials 2012, 5, 2423–2438. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, L.; Zhang, J.; Wang, F.; Xie, Y.; Shang, X.; Gu, Y.; Zhao, H.; Wang, X. In situ constructing interfacial contact MoS2/ZnIn2S4 heterostructure for enhancing solar photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 233, 112–119. [Google Scholar] [CrossRef]
- Li, A.; Peng, Z.; Fu, X. Exfoliated, mesoporous W18O49/g-C3N4 composites for efficient photocatalytic H2 evolution. Solid State Sci. 2020, 106, 106298. [Google Scholar] [CrossRef]
- Zhong, X.; Sun, Y.; Chen, X.; Zhuang, G.; Li, X.; Wang, J.G. Mo doping induced more active sites in urchin-like W18O49 nanostructure with remarkably enhanced performance for hydrogen evolution reaction. Adv. Funct. Mater. 2016, 26, 5778–5786. [Google Scholar] [CrossRef]
- Ling, G.Z.S.; Kok, S.H.W.; Zhang, P.; Chiah, Z.J.; Tan, L.L.; Chen, B.; Ong, W.J. In situ construction of fuzzy sea-urchin ZnIn2S4/W18O49: Leveraging interfacial Z-scheme redox sites toward cooperative electron–hole utilization in photocatalysis. Adv. Funct. Mater. 2025, 35, 2409320. [Google Scholar] [CrossRef]
- Liu, X.; Liu, E.; Wang, Z.; Zhang, W.; Dou, M.; Yang, H.; An, C.; Li, D.; Dou, J. Engineering S-scheme W18O49/ZnIn2S4 heterojunction by CoxP nanoclusters for enhanced charge transfer capability and solar hydrogen evolution. Nano Res. 2024, 17, 8095–8103. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, B.; Cherif, M.; Yu, H.; Zhang, Q.; Vidal, F.; Wang, X.; Ding, F.; Sun, Y.; Ma, D.; et al. Atomic insights for Ag interstitial/substitutional doping into ZnIn2S4 nanoplates and intimate coupling with reduced graphene oxide for enhanced photocatalytic hydrogen production by water splitting. Appl. Catal. B Environ. 2020, 279, 119403. [Google Scholar] [CrossRef]
- Pudkon, W.; Kaowphong, S.; Pattisson, S.; Miedziak, P.J.; Bahruji, H.; Davies, T.E.; Morgan, D.J.; Hutchings, G.J. Microwave synthesis of ZnIn2S4/WS2 composites for photocatalytic hydrogen production and hexavalent chromium reduction. Catal. Sci. Technol. 2019, 9, 5698–5711. [Google Scholar] [CrossRef]
- Bo, L.; He, K.; Tan, N.; Gao, B.; Feng, Q.; Liu, J.; Wang, L. Photocatalytic oxidation of trace carbamazepine in aqueous solution by visible-light-driven ZnIn2S4: Performance and mechanism. J. Environ. Manag. 2017, 190, 259–265. [Google Scholar] [CrossRef]
- Bhavani, P.; Praveen Kumar, D.; Jeong, S.; Kim, E.H.; Park, H.; Hong, S.; Gopannagari, M.; Amaranatha Reddy, D.; Song, J.K.; Kim, T.K. Multidirectional-charge-transfer urchin-type Mo-doped W18O49 nanostructures on CdS nanorods for enhanced photocatalytic hydrogen evolution. Catal. Sci. Technol. 2018, 8, 1880–1891. [Google Scholar] [CrossRef]
- Zou, X.; Yuan, C.; Cui, Y.; Dong, Y.; Chen, D.; Ge, H.; Ke, J. Construction of zinc-indium-sulfide/indium oxide step-scheme junction catalyst for enhanced photocatalytic activities of pollutant degradation and hydrogen generation. Sep. Purif. Technol. 2021, 266, 118545. [Google Scholar] [CrossRef]
- Xiong, M.; Chai, B.; Yan, J.; Fan, G.; Song, G. Few-layer WS2 decorating ZnIn2S4 with markedly promoted charge separation and photocatalytic H2 evolution activity. Appl. Surf. Sci. 2020, 514, 145965. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Jiang, H.; Chen, J.; Liu, Y.; Liang, Q.; Zhou, M.; Li, Z.; Zhou, Y. Combined effects of octahedron NH2 -UiO-66 and flowerlike ZnIn2S4 microspheres for photocatalytic dye degradation and hydrogen evolution under visible light. J. Phys. Chem. C 2019, 123, 18037–18049. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, K.; Wang, L.; Bai, B.; Liu, H.; Wang, Q. Aminated flower-like ZnIn2S4 coupled with benzoic acid modified g-C3N4 nanosheets via covalent bonds for ameliorated photocatalytic hydrogen generation. Appl. Catal. B Environ. 2020, 268, 118462. [Google Scholar] [CrossRef]
- Ye, L.; Fu, J.; Xu, Z.; Yuan, R.; Li, Z. Facile one-pot solvothermal method to synthesize sheet-on-sheet reduced graphene oxide (RGO)/ZnIn2S4 nanocomposites with superior photocatalytic performance. ACS Appl. Mater. Interfaces 2014, 6, 3483–3490. [Google Scholar] [CrossRef]
- Xiong, M.; Yan, J.; Chai, B.; Fan, G.; Song, G. Liquid exfoliating CdS and MoS2 to construct 2D/2D MoS2/CdS heterojunctions with significantly boosted photocatalytic H2 evolution activity. J. Mater. Sci. Technol. 2020, 56, 179–188. [Google Scholar] [CrossRef]
- Liu, X.; Dou, M.; Guang, Y.; Erkang, L.; Zeming, L.; Baochen, H.; Yang, H.; Li, D.; Dou, J. Modulation of catalyst interfacial electric field and charge transfer promotes NiS-modiffed CoWO4/ZnIn2S4 S-scheme heterojunction for efficient photocatalytic hydrogen evolution. Sep. Purif. Technol. 2025, 254, 128680. [Google Scholar]
- Zhao, M.; Liu, S.; Chen, D.; Zhang, S.; Carabineiro, S.A.C.; Lv, K. A novel S-scheme 3D ZnIn2S4/WO3 heterostructure for improved hydrogen production under visible light irradiation. Chin. J. Catal. 2022, 43, 2615–2624. [Google Scholar] [CrossRef]
- Lv, X.; Liang, Y.; Jiang, X.; Sun, T.; Yang, H.; Bai, L.; Wei, D.; Wang, W.; Ji, C.; Yang, L. Z-scheme heterojunction WO3/ZnIn2S4 solar absorber for wastewater remediation. Ceram. Int. 2024, 50, 9489–9498. [Google Scholar] [CrossRef]
- Yang, M.; Zhan, X.; Ou, D.; Wang, L.; Zhao, L.; Yang, H.; Liao, Z.; Yang, W.; Ma, G.; Hou, H. Efficient visible-light-driven hydrogen production with ag-doped flower-like ZnIn2S4 microspheres. Rare Met. 2025, 44, 1024–1041. [Google Scholar] [CrossRef]
- Pan, B.; Wu, Y.; Rhimi, B.; Qin, J.; Huang, Y.; Yuan, M.; Wang, C. Oxygen-doping of ZnIn2S4 nanosheets towards boosted photocatalytic CO2 reduction. J. Energy Chem. 2021, 57, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Cui, M.; Li, X.; Niu, L.; Wu, X.; Chen, Y.; Li, X.; Zhu, H.; Wang, D.; et al. Z-type ZnIn2S4 homojunction for high performance photocatalytic hydrogen evolution. Chem. Eng. J. 2025, 507, 160370. [Google Scholar] [CrossRef]
- Wei, L.; Chen, Y.; Zhao, J.; Li, Z. Preparation of NiS/ZnIn2S4 as a superior photocatalyst for hydrogen evolution under visible light irradiation. Beilstein J. Nanotechnol. 2013, 4, 949–955. [Google Scholar] [CrossRef]
- Shen, C.; Wen, X.; Fei, Z.; Liu, Z.; Mu, Q. Novel Z-scheme W18O49/CeO2 heterojunction for improved photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2020, 579, 297–306. [Google Scholar] [CrossRef]
- Ying, Z.; Chen, S.; Zhang, S.; Peng, T.; Li, R. Efficiently enhanced N2 photofixation performance of sea-urchin-like W18O49 microspheres with Mn-doping. Appl. Catal. B Environ. 2019, 254, 351–359. [Google Scholar] [CrossRef]
- Ren, D.; Shen, R.; Jiang, Z.; Lu, X.; Li, X. Highly efficient visible-light photocatalytic H2 evolution over 2D–2D CdS/Cu7S4 layered heterojunctions. Chin. J. Catal. 2020, 41, 31–40. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Li, J.; Zhang, G. Boosting interfacial charge separation of Ba5Nb4O15/g-C3N4 photocatalysts by 2D/2D nanojunction towards efficient visible-light driven H2 generation. Appl. Catal. B Environ. 2020, 263, 117730. [Google Scholar] [CrossRef]
- Li, C.; Che, H.; Yan, Y.; Liu, C.; Dong, H. Z-scheme AgVO3/ZnIn2S4 photocatalysts: “one stone and two birds” strategy to solve photocorrosion and improve the photocatalytic activity and stability. Chem. Eng. J. 2020, 398, 125523. [Google Scholar] [CrossRef]
- Mishra, P.; Acharya, L.; Parida, K. A comparison study between novel ternary retrieval NiFe2O4@p-doped g-C3N4 and Fe3O4@p-doped g-C3N4 nanocomposite in the field of photocatalysis, H2 energy production and super capacitive property. Mater. Today Proc. 2021, 35, 281–288. [Google Scholar] [CrossRef]
- Yang, C.; Li, Q.; Xia, Y.; Lv, K.; Li, M. Enhanced visible-light photocatalytic CO2 reduction performance of ZnIn2S4 microspheres by using CeO2 as cocatalyst. Appl. Surf. Sci. 2019, 464, 388–395. [Google Scholar] [CrossRef]
- Guo, W.; Lian, X.; Nie, Y.; Hu, M.; Wu, L.; Gao, H.; Wang, T. Facile growth of β-Cu2V2O7 thin films and characterization for photoelectrochemical water oxidation. Mater. Lett. 2020, 258, 126842. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Liu, Y.; Yang, J.; Wuren, T.; Duan, H.; Tan, Z.; Yu, S. Mo-W18O49/ZnIn2S4 Composites Synthesized by Metal Doping for Photocatalytic Hydrogen Evolution. Molecules 2025, 30, 1563. https://doi.org/10.3390/molecules30071563
Sun R, Liu Y, Yang J, Wuren T, Duan H, Tan Z, Yu S. Mo-W18O49/ZnIn2S4 Composites Synthesized by Metal Doping for Photocatalytic Hydrogen Evolution. Molecules. 2025; 30(7):1563. https://doi.org/10.3390/molecules30071563
Chicago/Turabian StyleSun, Ruiqin, Yue Liu, Jiamei Yang, Tuoya Wuren, Haochen Duan, Zhibing Tan, and Shiyong Yu. 2025. "Mo-W18O49/ZnIn2S4 Composites Synthesized by Metal Doping for Photocatalytic Hydrogen Evolution" Molecules 30, no. 7: 1563. https://doi.org/10.3390/molecules30071563
APA StyleSun, R., Liu, Y., Yang, J., Wuren, T., Duan, H., Tan, Z., & Yu, S. (2025). Mo-W18O49/ZnIn2S4 Composites Synthesized by Metal Doping for Photocatalytic Hydrogen Evolution. Molecules, 30(7), 1563. https://doi.org/10.3390/molecules30071563






